Abstract
Reverse phase protein microarrays (RPMA) are designed for quantitative, multiplexed analysis of proteins, and their posttranslational modified forms, from a limited amount of sample. To correct for sample to sample variability due to the number of cells in each lysate and the presence of extracellular proteins or red blood cells, a normalization method is required that is independent of these potentially confounding parameters. We adopted a gene microarray algorithm for use with RPMA to optimize the proteomic data normalization process and developed a systematic approach to RPMA processing and analysis, tailored to the study set. Our approach capitalizes on the gene microarray algorithms geNorm and NormFinder to identify the normalization parameter with the lowest variability across a proteomic sample set. Seven analytes (ssDNA, glyceraldehyde 3-phosphate dehydrogenase, α/β-tubulin, mitochondrial ribosomal protein L11, ribosomal protein L13a, β-actin, and total protein) were compared across sample sets including cell lines, tissues subjected to laser capture microdissection, and blood-contaminated tissues. We examined normalization parameters to correct for red blood cell content. We show that single-stranded DNA (ssDNA) is proportional to total non-red blood cell content and is a suitable RPMA normalization parameter. Simple modifications to RPMA processing allow flexibility in using ssDNA- or protein-based normalization molecules.
Keywords: reverse phase protein microarray, normalization, geNorm, NormFinder, ssDNA
Reverse phase protein microarray (RPMA) is a quantitative, multiplexed array of heterogeneous mixtures of cellular proteins derived from cells, serum, or body fluids (1–3). Levels of proteins or post-translationally modified proteins are detected by probing the array with specific, validated antibodies directed against target proteins (4). Similar to oligonucleotide microarrays, RPMA data analysis begins with image analysis and spot finding using software that generates raw pixel intensity values for each array spot. The pixel intensity is directly proportional to the amount of protein per spot. RPMA technology provides quantitative information regarding the state of cellular signaling cascades derived from cellular samples or from proteins shed into body fluids (3).
Ideally, protein analyte levels should reflect the tissue’s physiologic state at the time of procurement. Tissue samples are highly heterogeneous with regard to cellular and extracellular elements, biological state, disease state, originating organ, and level of contamination by blood. Due to this heterogeneity and the unknown contribution of cellular and extracellular components, including blood, data normalization methods should be used (5, 6). RPMA data normalization corrects spot intensity values through the use of a reference factor (e.g., total protein) wherein non-specific staining is first subtracted out and then spot values are divided by the reference factor, allowing differing RPMA data sets to be directly compared.
Quality control metrics and normalization algorithms have been extensively evaluated for gene microarrays (minimum information about a microarray experiment; MIAME) (7–10), but RPMA analysis is not a direct recapitulation of gene microarray analysis. RPMAs differ from gene microarrays because RMPAs are printed with heterogeneous protein mixtures, and can have an unknown contaminating serum or extracellular protein component. Furthermore, RPMAs are routinely detected with a single-wavelength fluorescent or chromogenic marker rather than dual fluorescent markers (2,3).
Reverse phase protein microarrays represent a unique, complex normalization issue. Tissue samples of equal volume can contain a different number of target cells mixed with different levels of blood, extracellular matrix, or extracellular fluid. Thus, samples with equal total protein can contain vastly different numbers of target cells (Supplementary figure S1). Therefore, when RPMA is applied to complex tissue samples procured by laser capture micro-dissection (LCM; 11) or from whole tissue specimens, or to blood or bone marrow aspirates, the total protein in each sample or the volume of each sample may not reflect accurate reference factors for normalization because a variable amount of non-cell derived protein is present in each sample.
Based on this need, we have developed additional reference factors for normalization in an attempt to more accurately normalize each sample to the cellular content as a common scale. We have chosen several protein reference analytes that are derived from different cellular compartments. In particular, we have developed and verified single-stranded (denatured) DNA content as a normalization factor that correlates with total cell number. We have also adapted an algorithm used for gene microarrays to RPMA analytes in order to determine the best normalization analyte showing the greatest reduction in RPMA sample to sample variability.
Erythrocytes (red blood cells (RBCs)) are devoid of a nucleus, therefore cellular DNA content—which is proportional to total nucleated cell content of the sample—is a potential new normalization molecule for blood-contaminated samples. Our multi-analyte RPMA normalization process capitalizes on quality metrics proposed for gene microarrays while providing a means to determine the optimal normalization analytes for each RPMA study set.
We demonstrated the utility of these normalization methods in several tissue types, including blood-contaminated metastatic tumor samples, by evaluating the following reference molecules: (i) total protein, (ii) β-actin, (iii) single-stranded DNA (ssDNA), (iv) glyceraldehyde 3-phosphate dehydrogenase (GAPDH), (v) α/β-tubulin (microtubule subunits), (vi) mitochondrial ribosomal protein L11 (MRPL11), and (vii) ribosomal protein L13a (RPL13a) (Supplementary Table 1). Additionally, we created RPMA Analysis Suite (RAS), a dedicated macro tool (VBA Excel macro) for RPMA data reduction that we designed to maintain data reduction steps while permitting flexibility in array design and normalization options.
Materials and methods
Sample collection and preparation
ssDNA from herring sperm (Sigma, St Louis, MO) was used as the ssDNA standard. Calf liver 18S + 28S ribosomal RNA (rRNA; Sigma) was used as control RNA. Spiked-in samples were prepared by adding 2–8µg of herring sperm DNA, or calf liver 18S + 28S rRNA, to 50µL of sample lysate.
RPMI 8226 and U266 cell lines (ATCC, Manassas, VA, USA) were used to create cell lysates from a specific number of cells. RPMI 8226 and U266 cells were maintained as suspension cultures in RPMI 1640 medium (ATCC) at 37°C, 5% CO2, and 70% humidity. Cells were counted in a hemacytometer, then a known number of cells were removed from culture and lysed with protein extraction buffer: 45% T-PER (Pierce, Rockford, IL), 45% Novex Tris-Glycine SDS Sample Buffer (2X) (Invitrogen, Carlsbad, CA), 10% TCEP Bond Breaker (Pierce), and heated at 100°C for 5 min.
Peripheral blood for preparing enriched RBC samples was obtained by venipuncture from a healthy volunteer with informed consent. EDTA anti-coagulated peripheral blood was spun twice at 200× g for 10 min. The buffy coat and plasma were discarded after each centrifugation step to enrich the RBC fraction. RBCs were counted in a hemacytometer and a known number of cells were incubated in protein extraction buffer for 10 min then heated at 100°C for 5 min (RBC lysate). Mixtures of RPMI 8226 cells and RBCs were prepared by mixing a known number of cells of each type in the ratio of 10:1 and 1:10. Cells were lysed in protein extraction buffer for 10 min then heated at 100°C for 5 min.
Bone metastasis and normal muscle tissue samples were collected at the Istituto Ortopedico Rizzoli (IOR), IRCCS, Bologna, Italy, under an IRB-approved protocol with informed consent. Specimens were snap-frozen and maintained at −80°C. Samples were placed in protein extraction buffer and lysis was performed using Adaptive Focus Acoustic (AFA) technology (Covaris) at 20% duty factor, 275 pick incident power, and 200 cycles per burst for 90 s.
RPMA construction
RPMA were printed with whole cell lysates, ssDNA, and RNA controls, in duplicate or triplicate. Lysates were printed on glass-backed nitrocellulose array slides (SCHOTT Nexterion, Germany) in a 2-fold dilution series using an Aushon 2470 arrayer equipped with 350µm pins (Aushon Biosystems, Billerica, MA, USA). After printing, the slides were either baked for 2 h at 80°C and then stored, or stored without baking, with desiccant (Drierite, W. A. Hammond, Xenia, OH, USA) at −20°C prior to use.
Slides were treated with ReBlot mild solution (Millipore, Billerica, MA, USA) for 15 min and washed twice in PBS. The slides were blocked (I-Block, Applied Biosystems) for 2 h before immunostaining. Immunostaining was performed on an automated slide stainer according to the manufacturer’s instructions (Autostainer CSA kit, Dako, Carpinteria, CA, USA). Each array was probed with a single polyclonal or monoclonal primary antibody (Table 1) for 30 min. Negative control slides were incubated with antibody diluent (Dako). Secondary antibody was goat anti-rabbit IgG H + L (1:7500) (Vector Laboratories, Burlingame, CA, USA) or rabbit anti-mouse IgG (1:10) (Dako). (5,12,13) Subsequent protein detection was performed with a diaminobenzidine according to the manufacturer’s instructions (Dako).
Table 1.
Antibodies used with reverse phase protein microarrays.
| Antibody | Manufacturer |
|---|---|
| Anti Akt Ser473 | Cell Signaling Technology (Danvers, MA) |
| Anti Akt Thr308 | Cell Signaling Technology (Danvers, MA) |
| Anti BAD Ser112 | Cell Signaling Technology (Danvers, MA) |
| Anti p38 Thr180 | Cell Signaling Technology (Danvers, MA) |
| Anti Cleaved Caspase 3 | Cell Signaling Technology (Danvers, MA) |
| Anti EGFR Tyr1148 | Cell Signaling Technology (Danvers, MA) |
| Anti GSK3αβ Ser219 | Cell Signaling Technology (Danvers, MA) |
| Anti IkappaB Ser32 | Cell Signaling Technology (Danvers, MA) |
| Anti IRS-1 Ser612 | Cell Signaling Technology (Danvers, MA) |
| Anti MARCKS Ser152/156 | Cell Signaling Technology (Danvers, MA) |
| Anti ERK Thr202/Tyr204 | Cell Signaling Technology (Danvers, MA) |
| Anti CREB Ser133 | Cell Signaling Technology (Danvers, MA) |
| Anti STAT1 Tyr701 | Cell Signaling Technology (Danvers, MA) |
| Anti MRPL 11 | Cell Signaling Technology (Danvers, MA) |
| Anti RPL 13 a | Cell Signaling Technology (Danvers, MA) |
| Anti GAPDH | Cell Signaling Technology (Danvers, MA) |
| Anti α/β-Tubulin | Cell Signaling Technology (Danvers, MA) |
| Anti β-Actin | Cell Signaling Technology (Danvers, MA) |
| Anti ssDNA | Immuno-Biological Laboratories Co (IBL) (Gunma, Japan) |
| Anti β-Arrestin | Cell Signaling Technology (Danvers, MA) |
| Anti IL1β | Cell Signaling Technology (Danvers, MA) |
Total protein microarray staining
Total protein staining was performed using Sypro Ruby Protein blot stain (Invitrogen) according to the manufacturer’s instructions and scanned with a NovaRay CCD imager (Alpha Innotech, San Leonardo, CA, USA) equipped with a Cy3 filter.
Imaging and data analysis
The immunostained slides were scanned on a UMAX 2100XL flatbed scanner using the following settings: white balance 255, black 0, middle tone 1.37, 600dpi, 14 bit. Spot intensity was analyzed by Image Quant v5.2 software (Molecular Dynamics). Data reduction was performed with a VBA Excel macro, RPMA Analysis Suite (RAS) (http://capmm.gmu.edu/rpma-analysis-suite). To normalize data, the relative intensity value for each endpoint for each spot was divided by the relative intensity value for the selected normalization molecule (or the geometric mean of a group of analytes) for the corresponding spot.
Selection of analytes for RPMA normalization
The suitability (stability) of seven normalization analytes (ssDNA, total protein, GAPDH, MRPL11, RPL13a, α/β-tubulin, and β-actin) and 13 protein analytes (Akt Ser473, Akt Thr308, BAD Ser112, p38 Thr180, Cleaved Caspase 3 Asp198, EGFR Tyr1148, GSK3 a/b Ser219, IKappaB Ser32, IRS1 Ser612, MARCKS Ser152/156, ERK Thr202/Tyr204, CREB Ser133, and STAT1 Tyr701) was evaluated by geNorm (7) and NormFinder (8).
Statistics
The Mann-Whitney t-test on medians (GraphPad Prism v5.03, GraphPad Software, Inc.) was applied to groups of samples from the multiple myeloma core biopsies data set normalized by ssDNA, total protein, and β-actin for comparison of three normalization methods.
Results and discussion
Normalization of spot intensity values is a major issue in microarray data analysis. A priori selection of the normalization parameters may not take into account potential sources of contamination and/or variability. In the present study, we introduce the novel use of ssDNA alone or in combination with cellular proteins as normalization molecules which can aid in selecting the optimal normalization parameter for a given RPMA study set.
In order to standardize and streamline data analysis, we have automated part of the data reduction/normalization processes by writing a VBA Excel macro, RAS. RAS was designed to standardize the data reduction steps after image acquisition and raw pixel intensity generation, while permitting flexibility in RPMA array design and normalization parameters. RAS operational features are: (i) removal of flagged spots from the downstream analysis; (ii) correction of pixel intensities below zero; (iii) quality control filters based on replicate spot CV and spot intensity versus background; (iv) subtraction of non-specific signal; and (v) normalization to user-specified endpoints or the geometric mean of several endpoints.
To measure nucleic acids (ssDNA) or proteins on a nitrocellulose membrane, we developed a microarray treatment strategy to ensure ssDNA binding without disrupting protein binding. Nitrocellulose does not bind double-stranded DNA (14,15) but ssDNA provides an ionic interaction between negatively charged phosphate groups of the nucleic acid and the positively charged nitrocellulose. DNA in our protein whole cell lysates assumes the single- stranded conformation because denaturing reagents (TCEP and SDS) used in the protein extraction buffer and heating cause DNA strand scission (16,17). The first RPMA processing modification we made was to eliminate alkaline pre-treatment (ReBlot) of the RPMA prior to immunostaining for any microarray that was destined to be stained with anti-ssDNA. Alkaline pre-treatment is often performed to ensure that the immobilized proteins are fully denatured, which provides optimal linear protein conformation for antibody binding. However, alkaline solutions can remove DNA from the nitrocellulose due to strong charge interactions (14,15) between the alkali and the DNA.
The second RPMA processing modification we made was to ensure that the ssDNA was bound tightly to the nitrocellulose. After printing, baking the RPMA nitrocellulose slide at 80°C for 2 h ensures that the ssDNA fragments become firmly attached to the nitrocellulose matrix (15).
The effect of baking on protein antigenicity could hypothetically affect immunostaining. We performed a series of replicate experiments to compare antibody binding/reactivity on baked and unbaked reverse phase protein microarrays. A total of 74 identical arrays (37 baked and 37 non baked), containing ssDNA as a positive control, RNA as a negative control, and several different cell and tissue lysates, were stained with Sypro Ruby for total protein (Supplementary Figure S2A), probed with antibodies directed against a phosphorylated protein (phospho-Akt Ser473; Supplementary Figure S2B), or non-phosphorylated protein (β-actin; Supplementary Figure S2C). Baking reverse phase protein microarrays retains protein antigenicity/reactivity as shown by equivalent staining intensity between baked and unbaked array slides.
The third RPMA processing modification was to optimize a detection method for ssDNA. The most common fluorescent dyes for DNA detection, such as DAPI, SYBR GREEN II, and acridine orange (18,19), cannot discern DNA from RNA and the autofluorescence of nitrocellulose overlaps with the emission wavelength of each of these dyes (20).
ssDNA antibodies are commercially available which react with random sequences of ssDNA of all species (21–25). ssDNA is conventionally used in immunohistochemical apoptosis assays because anti-ssDNA binds ssDNA produced during apoptosis but it will not bind to DNA/protein complexes generated during S-phase (26–28).
To demonstrate ssDNA antibody specificity and the absence of RNA cross-reactivity we printed RPMAs with ssDNA, rRNA, bovine serum albumin (BSA), ssDNA spiked into BSA, rRNA spiked into BSA, tissue protein lysates, lysates derived from a known number of cells, and tissue and cell protein lysates devoid of DNA (DNA precipitated out by ethanol). ssDNA signal was detectable only in those samples containing ssDNA when the array was probed with anti-ssDNA antibody. No staining was observed in RNA samples either alone or in BSA, in BSA alone, or in lysates lacking DNA (Figure 1A). Moreover, we demonstrated that ssDNA staining intensity is directly proportional to DNA amount and cell number in the sample. Samples with increasing amounts of spiked-in ssDNA or larger numbers of cell input exhibited increasing relative intensity when probed with ssDNA antibody (Figure 1B). A comparison of ssDNA levels between RPMI 8226 cells lines that were heat treated to induce apoptosis or untreated cells revealed no difference between total ssDNA on the RPMA, indicating that our sample extraction and RPMA processing produced ssDNA independent of the state of the cell (data not shown).
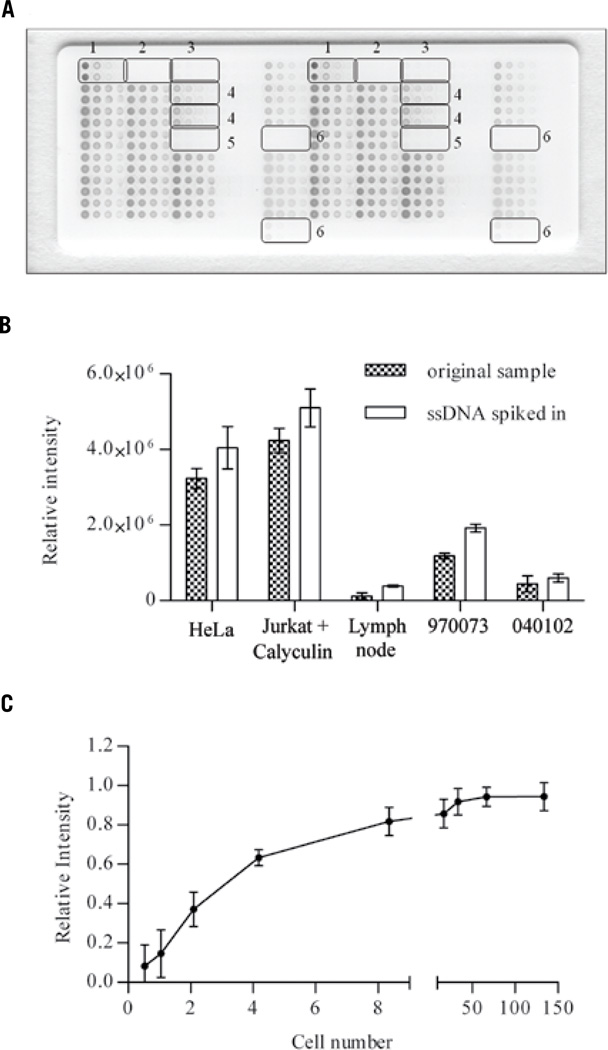
Figure 1. Anti-ssDNA antibody binds ssDNA in a specific and reproducible way.
(A) Anti-ssDNA antibody reacts exclusively with samples containing ssDNA only (1) or ssDNA spiked into BSA (4). No signal is observable in samples containing RNA only (2), BSA only (3), or RNA spiked into BSA (5), and tissue lysate samples in which DNA was depleted by ethanol precipitation (6). (B) ssDNA staining intensity increases in a directly proportional fashion when ssDNA is spiked into cell line or tissue lysates (empty columns) compared with the original samples (black columns). (C) Dose response curve: RPMA ssDNA staining intensity is proportional to cell number and is highly precise. The average spot intensity of 15 different arrays comprised of RPMI 8226 cell lysates was plotted against cell number (between arrays n = 15; mean ± sd). The curve was fit in GraphPad Prism 5 using the equation for one site specific binding with Hill Slope; goodness of fit R2 = 0.9481.
Inter-slide ssDNA staining reproducibility was assessed using 50 sequentially printed arrays comprised of cellular control lysates, ssDNA positive controls, and ribosomal RNA-negative controls. The amount of ssDNA between slide 1 (first printed) and slide 50 (last printed) showed high precision across a printing run (CV <10%). Between run precision was assessed using 15 different staining sessions for 30 identical sequentially printed protein microarrays. Each array was prepared with U266 cell lysates of known cell number, printed in 12 point, 2-fold dilution curves. One RPMA was probed with anti-ssDNA antibody and one was probed with antibody diluent instead of the primary antibody which served as a control for non-specific binding. The curve representing the antibody binding reaction for each sample for each array exhibited high reproducibility. (Figure 1C)
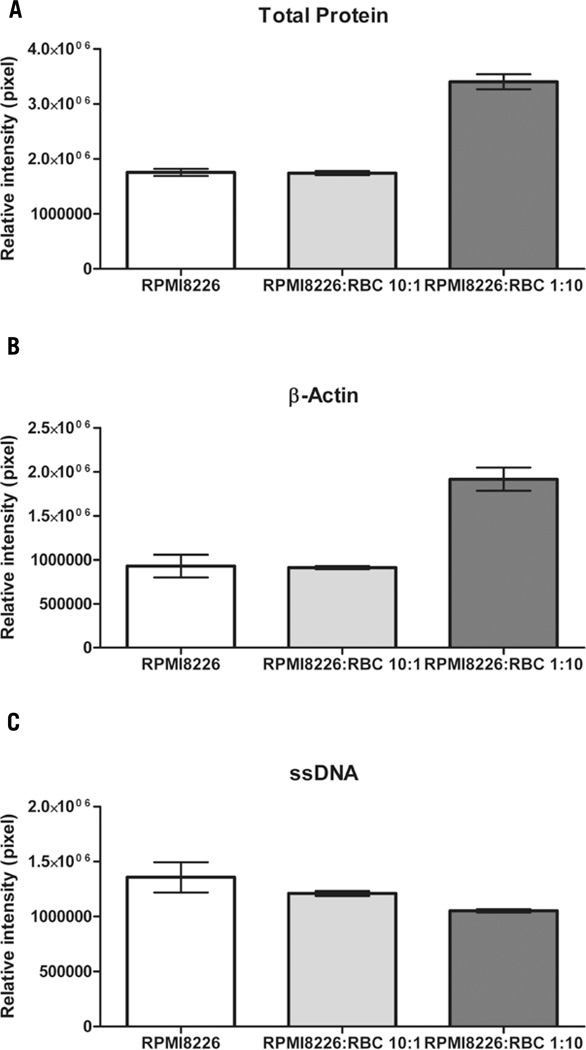
To demonstrate the contribution of red blood cells to total protein and β-actin levels but not ssDNA in a blood contaminated sample, we built an array with: i) RPMI 8226 lysate, as control nucleated cells; i) mixed lysate containing RPMI 8226:RBCs in a ratio 10:1; and iii) mixed lysate containing RPMI 8266:RBCs in a ratio 1:10. Triplicate arrays were stained for total protein, β-actin, or ssDNA. For an equal number of RPMI 8226 cells in the lysates, the 3 lysates showed no statistical difference in the ssDNA content, while β-actin and total protein were significantly higher in the RPMI 8226:RBCs 1:10 lysate, thus highlighting the substantial contribution of red blood cells to β-actin and total protein levels. (Figure 2)
Figure 2. Red blood cells (RBCs) contribute to total protein and β-actin levels, but not ssDNA levels.
The same number of RPMI8226 cells is represented in each sample, while a different amount of RBCs is present in different samples: RPMI8226 contains no RBCs; RPMI 8226:RBCs 10:1 contains 1 RBC per 10 RPMI8226 cells; and RPMI 8226:RBCs 1:10 contains 10 RBCs per one RPMI8226 cell. (A) Total protein staining intensities for each of the sample lysate are plotted: RPMI 8226:RBCs 1:10 shows a much higher amount of total protein because of the RBCs contribution. (RPMI8226 mean = 1.755e+006, sem = 64607; RPMI 8226:RBCs 10:1 mean = 1.740e+006, sem = 36571; and RPMI 8226:RBCs 1:10 mean = 3.405e+006, sem = 137234). (B) β-actin staining intensities for each sample are shown: RPMI 8226:RBCs 1:10 has a much higher β-actin protein expression because of the RBCs contribution. (RPMI8226 mean = 927622, sem = 129134; RPMI 8226:RBCs 10:1 mean = 910435, sem = 18488; RPMI 8226:RBCs 1:10 mean = 1.916e+006, sem = 132528).(C) ssDNA staining intensities for each sample are plotted. The three samples show no significant difference in the amount of ssDNA, demonstrating that RBCs do not contribute ssDNA to the sample. (RPMI8226 mean = 1.357e+006, sem = 137517; RPMI 8226:RBCs 10:1 mean = 1.210e+006, sem = 22443; RPMI 8226:RBCs 1:10 mean = 1.053e+006, sem = 15132).
The RBC proteome and interactome have been published in a comprehensive review by D’Alessandro et al. (29). Theoretically, contaminating RBCs may contribute proteins that correspond to analyte proteins investigated in the experimental samples. Thus, an RBC protein lysate could be used as a control sample to exclude from the study those proteins that are positive in the RBC sample. Another method for quantifying the amount of blood contamination is the use of an antibody against a specific RBC protein to measure the relative intensity in each sample for that specific RBC marker. In addition to blood-contaminated samples, peripheral blood mononuclear cell (PBMC) preparations could benefit from ssDNA normalization methods. Although efficient lymphocyte extraction from whole blood is well established, there is a certain amount of residual protein contamination due to immunoglobulins, albumin and other abundant proteins. (30–32)
Normalization of spot intensity data occurs prior to curve-fitting. Real Time RT-PCR studies commonly use two different algorithms, geNorm (7) and NormFinder (8), which use geometric averaging, for determining the most stable genes for normalization. Analogous to RT-PCR or gene microarray normalization, the optimal normalization molecule can be determined for RPMA data sets. Different RPMA data sets, such as microdissected samples, cell culture samples, and homogeneous/heterogeneous tissues, may benefit from systematic selection of normalization molecules. A single molecule selected a priori will not necessarily represent the most suitable normalization analyte for all RPMA study sets.
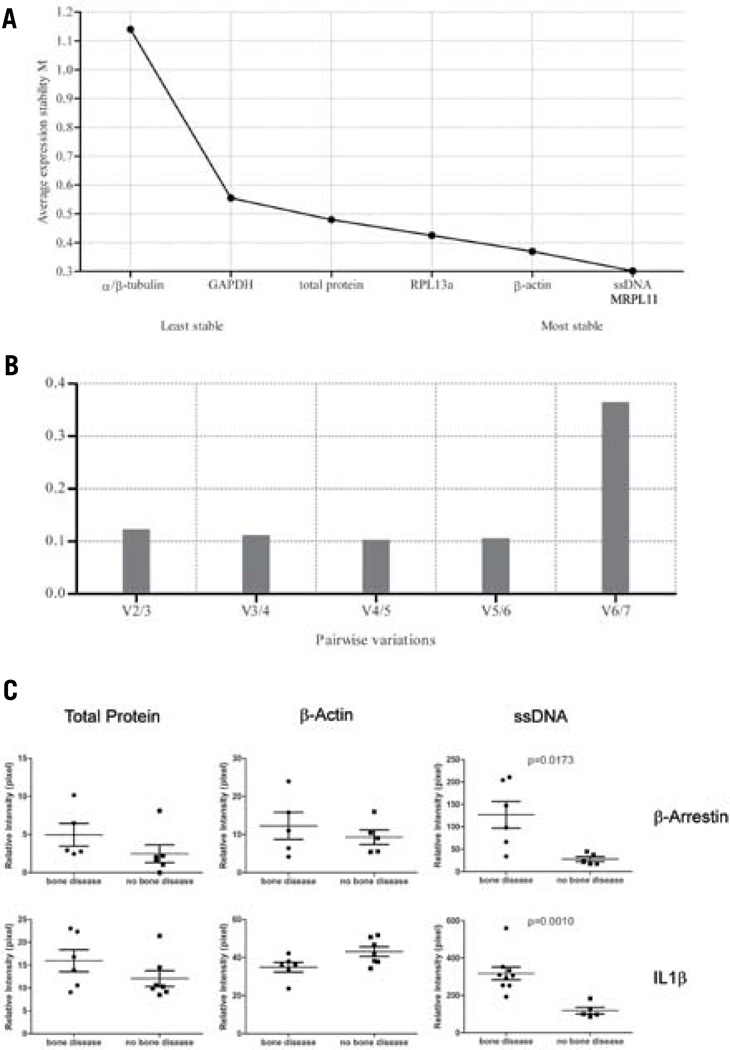
We applied this concept of determining normalization molecules ex post facto in clinical tissue samples. The expression level of total protein, β-actin, ssDNA, α/β-tubulin, MRPL11, RPL13a, and GAPDH was determined by RPMA in bone metastasis samples contaminated by RBCs. The stability of the normalization analytes was calculated using both geNorm and NormFinder algorithms (Figure 3A–B). ssDNA and MRPL11 showed the least variability in the RBC-contaminated bone metastasis samples. Similar normalization comparisons were performed with a variety of tissue types (Supplementary Table 1).
Figure 3. geNorm and NormFinder algorithms are suitable for finding a normalization analyte for RPMAs.
(A) An example of protein expression stability ranking of the seven normalization analytes in a group of bone metastasis specimens. A lower rank indicates a more stable analyte, while a higher rank indicates a less stable analyte. (B) An example of pairwise variation analysis to determine the least number of analytes required for data normalization. The shortest column indicates the optimal number of analytes to use for normalization. Any column less than 1.5 indicates a number of analytes sufficient for reliable normalization. (C) Bone marrow core biopsy sample data normalized using three different methods—total protein, β-actin, and ssDNA—for two different proteins, β-arrestin and IL1β. ssDNA normalization in each case demonstrated better discrimination between the bone disease group and the no bone disease group, compared with total protein or β-actin normalization (Mann-Whitney t-test: β-arrestin, P = 0.0173; IL1β, P = 0.0010).
Fifteen bone marrow aspirate core biopsies from patients diagnosed with multiple myeloma with or without bone disease were analyzed for β-arrestin and IL1β protein expression. Data were normalized alternatively by ssDNA, β-actin, or total protein. ssDNA normalization demonstrated a higher resolution in uncovering differences between groups that would otherwise be obscured by the effect of blood contamination. (Figure 3C)
This study highlights the utility of data normalization for RPMA. We demonstrated the advantages of data set normalization using ssDNA for blood-contaminated samples and the application of functional genomic algorithms for determining the geometric mean of the most stable molecules in a data set. RPMA technology has advanced for use in clinical trials for predicting and monitoring individual patients’ response to treatments (3). The normalization approach we describe individualizes the choice of normalization parameters for a given data set, thereby reducing potential bias caused by sample-to-sample variability.
Supplementary Material
Method summary.
Reverse phase protein microarrays (RPMA) are widely used to measure a large number of protein analytes from a small clinical sample. Data normalization for this technology is problematic for complex samples contaminated with blood and non cellular proteins. To address this need, we adopted gene microarray algorithms to RPMA processing and analysis, tailored to the study set, to compare seven normalization analytes across sample sets including cell lines, tissues subjected to laser capture microdissection and blood contaminated tissues. Specific normalization analytes were found to be advantageous for classes of samples sets. ssDNA was found optimal for samples contaminated with blood.
Acknowledgments
Competing interests
The authors declare no competing interests.
Footnotes
Supplementary material for this article is available at www.BioTechniques.com/article/113926.
References
- 1.Paweletz CP, Charboneau L, Bichsel VE, Simone NL, Chen T, Gillespie JW, Emmert-Buck MR, Roth MJ, et al. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA, Espina V, Mehta AI, Calvert V, Rosenblatt K, Geho D, Munson PJ, Young L, et al. Protein microarrays: meeting analytical challenges for clinical applications. Cancer Cell. 2003;3:217–225. doi: 10.1016/s1535-6108(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 3.Mueller C, Liotta LA, Espina V. Reverse phase protein microarrays advance to use in clinical trials. Mol. Oncol. 2010;4:461–481. doi: 10.1016/j.molonc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espina V, Wulfkuhle JD, Calvert VS, Petricoin EF, 3rd, Liotta LA. Reverse phase protein microarrays for monitoring biological responses. Methods Mol. Biol. 2007;383:321–336. doi: 10.1007/978-1-59745-335-6_21. [DOI] [PubMed] [Google Scholar]
- 5.VanMeter AJ, Rodriguez AS, Bowman ED, Jen J, Harris CC, Deng J, Calvert VS, Silvestri A, et al. Laser capture micro-dissection and protein microarray analysis of human non-small cell lung cancer: differential epidermal growth factor receptor (EGPR) phosphorylation events associated with mutated EGFR compared with wild type. Mol. Cell. Proteomics. 2008;7:1902–1924. doi: 10.1074/mcp.M800204-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmert-Buck MR, Bonner RF, Smith PD, Chuagui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 7.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen CL, Jensen JL, Ørntoft TF. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 9.Akilesh S, Shaffer DJ, Roopenian D. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 2003;13:1719–1727. doi: 10.1101/gr.533003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal. Biochem. 2002;309:293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 11.Jacquet R, Hillyer J, Landis WJ. Analysis of connective tissues by laser capture microdissection and reverse transcriptase-polymerase chain reaction. Anal. Biochem. 2005;337:22–34. doi: 10.1016/j.ab.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Bordeaux J, Welsh A, Agarwal S, Killiam E, Baguero M, Hanna J, Anagnostou V, Rimm D. Antibody validation. Biotechniques. 2010;48:197–209. doi: 10.2144/000113382. Erratum in: 2010. Biotechniques 48: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Björling E, Uhlén M. Antibodypedia - a portal for sharing antibody and antigen validation data. Mol Cell Proteomics. 2008;7:2028–2037. doi: 10.1074/mcp.M800264-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Amiss T, Presnell SC. Nucleic Acid Blotting Techniques, theory and practice. In: Colemann WB, Tsongalis GJ, editors. Molecular Diagnostic For the Clinical Laboratorian. Totowa, NJ: Humana Press; 2006. pp. 31–36. [Google Scholar]
- 15.Easteal S, McLeod N, Reed K. The science: principle of DNA profiling. In: Easteal S, McLeod N, Reed K, editors. DNA profiling: principles, pitfalls and potential. Switzerland: Harwood Academic Publishers, Chur; 1991. pp. 42–64. [Google Scholar]
- 16.Bode VC. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J. Mol. Biol. 1967;26:125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- 17.Pividori MI, Alegret S. DNA adsorption strategies. In: Wittmann C, editor. Immobilization of DNA on Chips I. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 2005. pp. 10–14. [Google Scholar]
- 18.Olive PL. The Comet Assay, an overview of techniques. In: Didenko VV, editor. Situ Detection of DNA Damage, methods and protocols. Totowa, NJ: Humana Press; 2002. pp. 179–194. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DB, Hallberg KB. General techniques for detecting and quantifying microbial life in mineral-oxidizing environments. In: Rawlings DE, Johnson DB, editors. Biomining. Heidelberg, Germany: Springer-Verlag; 2007. pp. 236–262. [Google Scholar]
- 20.Walter JG, Stahl F, Reck M, Praulich I, Nataf Y, Hollas M, Pflanz K, Melzner D, et al. Protein microarrays: Reduced autofluorescence and improved LOD. Eng. Life Sci. 2010;10:103–108. [Google Scholar]
- 21.Naruse I, Keino H, Kawarada Y. Antibody against single-stranded DNA detects both programmed cell death and drug-induced apoptosis. Histochemistry. 1994;101:73–78. doi: 10.1007/BF00315834. [DOI] [PubMed] [Google Scholar]
- 22.Kawarada Y, Miura N, Sugiyama T. Antibody against single-stranded DNA useful for detecting apoptotic cells recognizes hexadeoxynucleotides with various base sequences. J. Biochem. 1998;123:492–498. doi: 10.1093/oxfordjournals.jbchem.a021963. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe I, Toyoda M, Okuda J, Tenjo T, Tanaka K, Yamamoto T, Kawasaki H, Sugiyama T, et al. Detection of apoptotic cells in human colorectal cancer by two different in situ methods: Antibody against single-stranded DNA and terminal deoxynu-cleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) methods. Jpn. J. Cancer Res. 1999;90:188–193. doi: 10.1111/j.1349-7006.1999.tb00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi S, Iwase H, Kawarada Y, Miura N, Sugiyama T, Iwata H, Hara Y, Omoto Y, et al. Detection of DNA fragmentation in human breast cancer tissue by an antibody specific to single-stranded DNA. Breast Cancer. 1998;5:47–52. doi: 10.1007/BF02967414. [DOI] [PubMed] [Google Scholar]
- 25.Maeda M, Sugiyama T, Akai F, Jikihara I, Hayashi Y, Takagi H. Single-stranded DNA as an immunocytochemical marker for apoptotic change of ischemia in gerbil hippocampus. Neurosci. Lett. 1998;240:69–72. doi: 10.1016/s0304-3940(97)00901-4. [DOI] [PubMed] [Google Scholar]
- 26.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Apoptosis in breast carcinomas detected with monoclonal antibody to single-stranded DNA: relation to bcl-2 expression, Hormone receptors, and lymph node metastases. Clin Cancer Res. 1997;3:465–471. [PubMed] [Google Scholar]
- 27.Korkolopoulou PA, Konstantinidou AE, Patsouris ES, Christodoulou PN, Thomas-Tsagli EA, Davaris PS. Detection of apoptotic cells in archival tissue from diffuse astrocytomas using a monoclonal antibody to single-stranded DNA. J. Pathol. 2001;193:377–382. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH812>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Monoclonal antibody to single-stranded DNA is a specific and cellular marker of apoptosis. Exp Cell Res. 1996;226:387–397. doi: 10.1006/excr.1996.0240. [DOI] [PubMed] [Google Scholar]
- 29.D’Alessandro A, Righetti PG, Zolla L. The red blood cell proteome and inter-actome: un update. J.Proteome Res. 2010;9:144–163. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- 30.Walsh FS, Barber BH, Crumpton MJ. Preparation of inside-out vesicles of pig lymphocyte plasma membrane. Biochemistry. 1976;15:3557–3563. doi: 10.1021/bi00661a025. [DOI] [PubMed] [Google Scholar]
- 31.Owen MJ, Barber BH, Faulkes RA, Crumpton MJ. Albumin is associated with the inner surface of the lymphocyte plasma membrane. Biochem. Soc. Trans. 1978;6:920–922. doi: 10.1042/bst0060920. [DOI] [PubMed] [Google Scholar]
- 32.Owen MJ, Barber BH, Faulkes RA, Crumpton MJ. Albumin associated with purified pig lymphocyte plasma membrane. Biochem. J. 1980;192:49–57. doi: 10.1042/bj1920049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.