Abstract
The control of vascular resistance and tissue perfusion reflect coordinated changes in the diameter of feed arteries and the arteriolar networks they supply. Against a background of myogenic tone and metabolic demand, vasoactive signals originating from perivascular sympathetic and sensory nerves are integrated with endothelium-derived signals to produce vasodilation or vasoconstriction. PVNs release adrenergic, cholinergic, peptidergic, purinergic, and nitrergic neurotransmitters that lead to SMC contraction or relaxation via their actions on SMCs, ECs, or other PVNs. ECs release autacoids that can have opposing actions on SMCs. Respective cell layers are connected directly to each other through GJs at discrete sites via MEJs projecting through holes in the IEL. Whereas studies of intercellular communication in the vascular wall have centered on endothelium-derived signals that govern SMC relaxation, attention has increasingly focused on signaling from SMCs to ECs. Thus, via MEJs, neurotransmission from PVNs can evoke distinct responses from ECs subsequent to acting on SMCs. To integrate this emerging area of investigation in light of vasomotor control, the present review synthesizes current understanding of signaling events that originate within SMCs in response to perivascular neurotransmission in light of EC feedback. Though often ignored in studies of the resistance vasculature, PVNs are integral to blood flow control and can provide a physiological stimulus for myoendothelial communication. Greater understanding of these underlying signaling events and how they may be affected by aging and disease will provide new approaches for selective therapeutic interventions.
Keywords: sympathetic nerves, sensory nerves, cell-cell communication, Ca2+ signaling
INTRODUCTION
The local control of blood flow is integral to homeostasis of tissues and organ systems throughout the body. The entire vasculature is lined by ECs with vessels controlling blood flow magnitude and distribution (the focus of our present discussion) encircled by SMCs that are surrounded by an adventitia that often contains a meshwork of PVNs. These nerve fibers typically consist of sympathetic efferent axons that may be complemented by sensory (and in some cases parasympathetic) axons (33, 111) (Table 1, Figure 1). Each source of innervation can modulate vasomotor function through multiple signaling pathways that we explore in this review. While our discussion centers on events occurring within the blood vessel wall, it should be recognized that neural control of the circulation (primarily via the SNS) is integral to regulating systemic blood pressure and cardiac output (224).
Table. Visualization of perivascular nerves in different vascular beds.
A summary of studies using immunological methods to visualize perivascular nerves in different vascular beds. References are grouped according to vessels studied, animal species and markers used. Sympathetic nerve markers: TH = tyrosine hydroxylase, NPY = neuropeptide Y, GA = glutaraldehyde. Sensory nerve markers: CGRP = calcitonin gene-related peptide, SP = substance P, VIP = vasoactive inhibitory peptide, Nitrergic nerve markers: nNOS = neuronal nitric oxide synthase, NADPHd = nicotinamide adenine dinucleotide phosphate-diaphorase, Total nerve markers: PGP9.5 = protein gene product 9.5. For all categories, MISC indicates use of a marker other than those listed.
| Vascular Bed | Species | Sympathetic | Sensory | Parasympathetic | Nitroxidergic | Total Nerves | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| TH | NPY | GA | MISC | CGRP | SP | MISC | VIP | MISC | nNOS | NADPHd | PGP9.5 | ||
| Mesenteric | Rat | (44, 165) | (44, 68, 113, 236) | (84, 201) | (44, 68, 113, 139, 165, 235, 236) | (235, 236) | (68) | (236) | (151) | ||||
| Mouse | (172) | ||||||||||||
| Human | (18, 19, 52) | (18, 19, 52) | (18, 19, 52) | (18, 19, 52) | (18, 19, 52) | (18, 52) | (18, 19, 52) | ||||||
| Guinea Pig | (56) | (56) | (56) | ||||||||||
| Hamster | (227) | (227) | (227) | (227) | (286) | ||||||||
| Dog | |||||||||||||
| Toad | (196) | (196) | (196) | (196) | (196) | (196) | (58) | ||||||
|
| |||||||||||||
| Cerebral | Rat | (7) | (87, 206) | (7, 87) | (66) | (5, 249) | (5, 170) | (7, 87) | |||||
| Mouse | (135) | (135) | (135) | ||||||||||
| Human | (67) | (67) | (245) | (67) | (67) | (67) | |||||||
| Monkey | (258) | (258) | |||||||||||
| Rabbit | (290) | (290) | (290) | (290) | (290) | ||||||||
| Guinea Pig | (197) | (197) | (197) | (9, 197) | (9) | ||||||||
| Cat | (188) | (188) | (146) | ||||||||||
| Dog | (286) | ||||||||||||
| Pig | (287) | (287) | |||||||||||
|
| |||||||||||||
| Femoral | Rat | (206) | |||||||||||
| Mouse | (172) | ||||||||||||
| Guinea Pig | (56) | (56) | (56) | ||||||||||
| Dog | (286) | ||||||||||||
|
| |||||||||||||
| Carotid | Mouse | (172) | |||||||||||
| Guinea Pig | (56) | (56) | (56) | ||||||||||
| Toad | (196) | (196) | (196) | (196) | (196) | (196) | |||||||
|
| |||||||||||||
| Skin | |||||||||||||
| Rat | (226) | (226) | |||||||||||
| Toad | (196) | (196) | (196) | (196) | (196) | (196) | (129) | ||||||
|
| |||||||||||||
| Renal | Guinea Pig | (56) | (56) | (56) | |||||||||
| Hamster | (227) | (227) | (227) | (227) | |||||||||
|
| |||||||||||||
| Coronary | Rat | (239) | (239) | (239) | |||||||||
| Human | (100) | (100) | (100) | (100) | (100) | (100) | |||||||
|
| |||||||||||||
| Nasal Mucosa | Rat | (153) | |||||||||||
| Human | (153) | ||||||||||||
|
| |||||||||||||
| Eye | Rat | (13, 20) | (20) | (229) | (13) | (13, 79) | (79) | ||||||
| Human | (79) | (79) | |||||||||||
| Pig | (257) | ||||||||||||
|
| |||||||||||||
| Forepaw | Dog | (262) | (262) | (262) | (262) | ||||||||
|
| |||||||||||||
| Lip Arteries | Rat | (130) | |||||||||||
|
| |||||||||||||
| Cremaster | Rat | (77) | |||||||||||
|
| |||||||||||||
| Spino-trapezius | Rat | (184) | |||||||||||
|
| |||||||||||||
| Spiral Modiolar | Guinea Pig | (268) | (268) | ||||||||||
|
| |||||||||||||
| Intra-redicular | Rat | (147) | (147) | (147) | (147) | (147) | (147) | ||||||
|
| |||||||||||||
| Gracilis | Mouse | (172) | |||||||||||
|
| |||||||||||||
| Lingual | Guinea Pig | (108) | (108) | (108) | |||||||||
|
| |||||||||||||
| Pancreas | Mouse | (167) | (167) | ||||||||||
|
| |||||||||||||
| Prostate | Pig | (220) | |||||||||||
|
| |||||||||||||
| Retractor | Hamster | (95, 173) | (95) | (95) | (95) | (95) | |||||||
Figure 1. Perivascular sympathetic and sensory nerves surrounding a mouse mesenteric artery.

Z-stack of immunofluorescent confocal slices taken through one wall a first-order mesenteric artery of a C57BL/6 mouse. Sympathetic nerves labeled for tyrosine hydroxylase are shown in red, sensory nerves labeled for CGRP are labeled in green and overlapping regions are shown in yellow. Scale bar = 100 μm.
Typically, the activation of sympathetic PVNs causes vasoconstriction whereas activation of sensory or parasympathetic PVNs causes vasodilation. In addition to classical neurotransmitters such as NE and ACh, concomitant release of co-transmitters and neuromodulator substances can further influence vascular function (Figure 2). Respective compounds are first packaged into synaptic vesicles. As action potentials propagate along the efferent axon, depolarization of the presynaptic membrane leads to Ca2+ influx, vesicular fusion and neurotransmitter exocytosis en passant from varicosities (174). Once released at the vascular neuroeffector junction, these agents diffuse to receptors located on SMCs, ECs and other PVNs (38, 60, 136) (Figures 2 and 3). The primary goal of this review is to examine PVNs in light of these signaling events as they pertain to vasomotor control. Aspects of this comprehensive literature are based on particular vascular beds (e.g., brain, gut, skeletal muscle, skin). While our goal is to develop functional relationships that can be applied to resistance networks throughout the body, current knowledge is often based upon particular experimental models and protocols. Thus, regional variations are considered in light of tissue specificity.
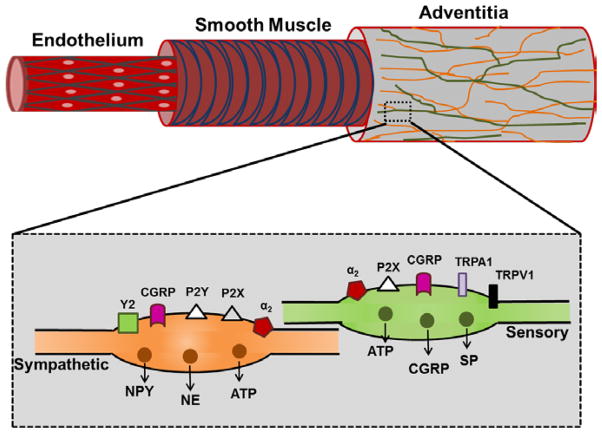
Figure 2. Anatomical location of perivascular sympathetic and sensory nerves.
Perivascular nerves are located in the adventitia and do not make direct contact with SMCs or ECs. Varicosities along efferent sympathetic and sensory nerve axons release multiple neurotransmitters and contain multiple receptors (see text for details) that contribute to presynaptic regulation of neurotransmitter release. While perivascular parasympathetic and nitrergic nerves are present on many vessels, We focus on sympathetic and sensory PVNs here for clarity.
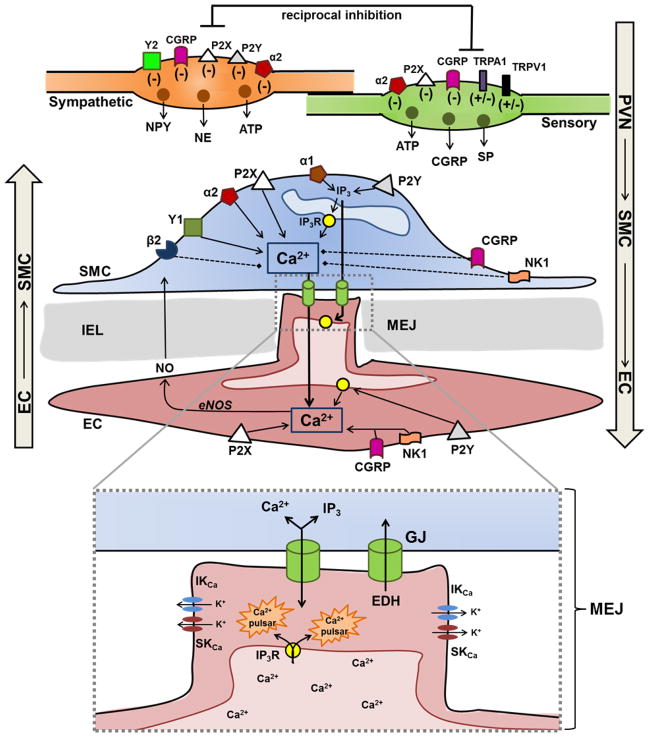
Figure 3. Perivascular nerve-mediated regulation of myoendothelial signaling.
TOP: Depiction of transmitters released from sympathetic and sensory nerve varicosities and where these compounds can act to regulate intercellular (myoendothelial) communication in the wall of resistance vessels. For respective varicosities, symbols indicate whether activation of the receptor increases (+) or decreases (−) neurotransmitter release. For SMCs and ECs, receptor activation leads to an increase (solid arrow) or decrease (dashed line) in [Ca2+]i and/or IP3. These second messengers can then diffuse through myoendothelial GJs and initiate signaling in the heterologous cell. BOTTOM: Inset (dotted line) indicates local signals that occur within MEJs in response to Ca2+ or IP3 entering from SMCs. In turn, Ca2+ released from IP3Rs on the ER within endothelial projections can activate IKCa and SKCa locally, with EDH providing negative feedback to attenuate SMC contraction. Note that signals originating within ECs (EDH, NO, Ca2+ and IP3) can diffuse into SMCs, thus heterocellular signaling at MEJs is bidirectional in nature.
Heterocellular communication through MEJs as mediators of vasomotor control was introduced ~50 years ago based upon exquisite ultrastructural studies of microvessels within the fascia of rabbit skeletal muscle (223). In addition to documenting perivascular innervation of resistance networks, these classic experiments illustrated that cellular projections through the IEL provide discrete sites of contact positioned to enable direct signaling between ECs and SMCs, particularly as arteries branched into progressively smaller arterioles. Some 20 years later, heterocellular signaling through GJs in arterioles was proposed to coordinate vasodilation along arterioles in the hamster cheek pouch (238). Such behavior was later confirmed using electrophysiological measurements in pressurized feed arteries of the cheek pouch retractor muscle (70). Following classic studies identifying the essential role of the endothelium in promoting SMC relaxation of the rabbit aorta (83), studies of heterocellular communication in the vascular wall have centered on the nature and actions of signals originating within ECs that are transmitted to SMCs, e.g., NO- and EDH-mediated relaxation [see Reviews (8, 61, 85, 246)] (Figure 3). However, from a holistic perspective, it is essential to recognize that heterocellular signaling in the wall of resistance microvessels is bi-directional in nature. Indeed, a growing body of evidence points to myoendothelial coupling through GJs as being integral to neuroeffector signaling (Figure 3).
The ability of SMCs to evoke responses in underlying ECs originally focused on [Ca2+]i dynamics in arterioles isolated from the hamster cheek pouch (62) and cremaster muscle (62, 125, 285). Complementary studies using cell culture and arterial preparations implicated concomitant heterocellular (myoendothelial) diffusion of IP3 (121, 156). In turn, the rise in EC [Ca2+]i can stimulate NO production and hyperpolarization (42) to thereby attenuate SMC contraction (260) (Figure 3). Thus, as investigators have focused on the functional microdomain of MEJs (121, 164, 253), it has become evident that EDH may serve both as a signal originating in ECs that initiates SMC relaxation and as a mechanism for providing negative feedback in response to the activation of SMCs (62, 125, 156, 260, 261). Remarkably, these studies have routinely been performed using a pharmacological approach; e.g., applying phenylephrine to activate α1ARs on SMCs. While these ARs are activated physiologically by NE released from sympathetic PVNs (184, 267), there is a paucity of information relating the physiological activation of SMCs (e.g., via PVNs) to EC Ca2+ signaling. Recent findings from isolated rat mesenteric arteries have identified EC Ca2+ signals (pulsars) in response to electrical stimulation of sympathetic nerves (198) with evidence supporting EDH in attenuating SMC contraction. In light of this emerging area of investigation, a complementary goal of this review is to consider the role of SMCs in effecting EC feedback subsequent to the activation of PVNs.
INNERVATION OF BLOOD VESSELS
Histochemical and immunolabeling techniques have enabled identification of the presence and origin of PVN fibers. While appropriate markers identify respective sources of innervation (Figure 1), the density, pattern and composition of PVNs can vary with vascular bed, vessel diameter and animal species (Table 1); representative examples are given in context throughout this discussion. Most studies have not quantified nerve density and - even where it has been measured - differences in immunological markers, preparation and analytical techniques between laboratories make quantitative comparisons difficult. It should be recognized that, in addition to variations in the density and origins of innervation, differences in the size and location of NMJs relative to the vessel wall can also impact vasomotor responses to the activation of PVNs. For example, when compared to diffusion distances for neurotransmission in smaller resistance vessels (e.g., ~100 nm for vessels with diameter < 150 μm), large arteries have up to ten-fold greater distances (e.g., several hundred nm) between sites of neurotransmitter release and adjacent SMCs (14, 45, 174), thereby increasing diffusion time while reducing the effective chemical concentration at receptors. Nevertheless, such regional heterogeneity in the anatomy and composition of PVNs (Table 1), along with variations in receptor expression and effector signaling pathways, contribute towards tuning vasomotor control according to the particular needs of specific vessels and vascular beds.
Sympathetic Innervation
Sympathetic nerves account for the largest proportion of innervation in the resistance vasculature and have been associated with nearly every vascular bed studied across animal species (Table 1). Reaction of glutaraldehyde with catecholamines or immunostaining for TH or NPY has been most commonly used for their identification. Perivascular sympathetic nerves arise from postganglionic efferent axons, with their cell bodies located in the paravertebral ganglia (186). Efferent sympathetic axons form a plexus within the adventitia (84) and typically follow the arterial supply, entering the tissue along feed arteries, coursing along arterioles and terminating along the precapillary arterioles (223). Regional differences in the pattern of sympathetic PVNs are consistent with corresponding differences in the role of respective vascular beds. For example, in skeletal muscle, only precapillary vessels are innervated (78, 95, 184) whereas in the mesentery, the veins are innervated as well (84, 172). From a physiological perspective, whereas the regulation of tissue blood flow and perfusion pressure occur via precapillary resistance vessels in both vascular beds, veins in the splanchnic circulation serve as a reservoir of blood that can be mobilized by SNA in times of physical stress (225). Although arteries and resistance vessels of the brain are innervated by noradrenergic axons originating in the superior cervical ganglia of the SNS, SNA typically has little effect on cerebral blood flow. However, during hypertension, sympathetic vasoconstriction may serve as a protective mechanism to preserve the integrity of the blood brain barrier, protect capillary and venous pressures, and to thereby prevent edema formation (reviewed in (47)).
Individual axons rarely make direct contact with SMCs (125) and do not penetrate the vessel wall irrespective of the number of SMC layers present (14, 112, 174). Unlike classical synapses (e.g., at the NMJ of skeletal muscle), there is not a single site of neurotransmitter release from sympathetic nerves. Instead, neurotransmitter is released ‘en passant’ from varicosities along the efferent axons (Figure 2). While many of these varicosities are not directly associated with SMCs, sympathetic NMJs (when present) typically occur within 100 nm from SMCs (174, 175). In contrast to discrete activation of individual cells (e.g., at the NMJs of skeletal muscle), this functional anatomy results in dispersed actions of neurotransmitter molecules as they diffuse to their receptors. In arteries and arterioles (Figure 2), activation of ARs on SMCs typically (e.g., in skeletal muscle) results in vasoconstriction however the onset and duration of action are variable (111). With increased thickness of the media, neurotransmitter is unable to reach deeper layers of SMCs thus homocellular coupling though GJs plays an important role in coordinating SMC activation throughout the vessel wall (14, 187). In addition to NE, SNA releases two cotransmitters, ATP and NPY (35), and the proportion of cotransmitter release relative to that of NE can modulate the time course and magnitude of vasoconstriction (278). Vascular responses to SNA can also vary with the content and composition of vesicles released from specific axon varicosities (21, 250), the frequency and firing pattern of action potentials [i.e., single versus bursts (26)], and according to the size and location of vessel branches within resistance networks (184, 267, 289).
Adrenergic neuroeffector signaling
NE is the primary neurotransmitter released by sympathetic PVNs (17). NE is synthesized in nerve fibers from its tyrosine precursor through the actions of the enzyme TH and stored in vesicles along with its co-transmitters (112). ARs are subtypes of GPCRs. Upon release, NE binds to postsynaptic αARs and βARs on SMCs, where it activates signaling until it is removed. The majority of NE released undergoes reuptake into presynaptic nerve terminals by the NE transporter with a fraction undergoing degradation (e.g., by monoamine oxidase) (112, 266). The activation of αARs causes constriction, whereas βAR activation evokes vasodilation (33, 99, 191). Activating the α1 subtype of ARs on the postjunctional membrane of SMCs stimulates PLC through Gq with ensuing production of IP3 leading to the intracellular release of Ca2+ from IP3 receptors in the SR (189). The actions of Gq are also linked to receptor operated Ca2+ channels, thereby leading to Ca2+ entry through TRPC3 and TRPC6 channels (110). In contrast, α2ARs are expressed both on pre- and postjunctional membranes. Postjunctionally, α2ARs are coupled to Gi protein-mediated signaling leading to diminished adenylyl cyclase activity, with a fall in [cAMP] (43) leading to increased [Ca2+]i via a reduction in PKA-mediated phosphorylation of Ca2+ channels (IP3Rs) in the SR (254) and of L-type Ca2+ channels in the plasma membrane (281). Contraction of SMCs is also increased through cAMP-mediated increases in the activity of myosin light chain kinase and through Ca2+ sensitization (215). The activation of prejunctional α2ARs on nerve fibers (98, 99) provides negative feedback by stimulating reuptake of NE released during sympathetic neurotransmission along with reducing transmitter release (99, 266).
Functional heterogeneity of αAR responses
Whereas α1ARs often predominate in mediating sympathetic vasoconstriction (211), the expression and relative contributions of α1ARs versus α2ARs to sympathetic vasoconstriction can vary with vascular bed, vessel branch order and animal species. For example, using selective AR agonists and antagonists in rat (75, 205) and mouse (191) cremaster muscle preparations, α1ARs were found to dominate sympathetic constriction of proximal (first-order) arterioles, while α2ARs contributed more to constriction of second- and third-order arterioles. This functional pattern of αAR subtype distribution is reversed in the mouse gluteus maximus muscle, where constriction mediated by α2ARs predominates in first-order arterioles while constriction mediated by α1ARs predominates in third-order arterioles (191). α1ARs are also dominant in constriction of multiple branches of mouse mesenteric arteries in vivo (275). Intra-arterial infusion of subtype-selective agents into the human forearm revealed that α2ARs contribute more to basal vasomotor tone than do α1ARs (57). However, in response to regional activation of α1ARs, increases in vascular resistance were greater in the calf than in the forearm (208). Separate studies in human thigh muscles suggest that α1ARs but not α2ARs are critical for sympathetic constriction of conduit arteries (280). While the functional expression of AR subtypes can vary between vascular beds, the ability of smaller downstream arterioles to consistently “escape” from sympathetic constriction while the larger upstream vessels do not (4, 23, 184, 205, 267) may have more to do with local actions of vasodilator metabolites than with AR subtype distribution.
Variability in the expression and/or role of α1AR and α2AR subtypes further contributes to the functional heterogeneity in adrenergic signaling. In rat and human skeletal muscle arteries (128, 288) and rat hindlimb arteries (291), α1AARs appear to be the predominant isoform mediating sympathetic vasoconstriction. In contrast, α1DARs appear more important in hamster cremaster muscle arterioles (125), rat mesenteric arteries (51, 118), rat thoracic aorta (118) and rat pulmonary arteries (118). However, both α1AR subtypes appear equally important in mediating sympathetic constriction of rat retinal arterioles (192). Elucidating the functional role of α2ARs is more complex because in addition to variations in activity based on subtype expression, the role of prejunctional α2ARs in modulating neuroeffector signaling through the NE transporter can vary with the level of SNA (111). Because of these challenges along with a lack of more specific pharmacological agents, the characterization of different α2AR subtypes relies largely upon the molecular expression of mRNA rather than functional studies. An earlier review (99) provides a comprehensive analysis of studies characterizing the expression and function of both α1AR and α2AR subtypes in a wide variety of vascular beds. More recent studies have defined the expression of α1AR subtypes in SMCs of hamster arterioles while confirming the lack of α1AR expression in ECs (125). In light of such methods to isolate respective cell types from individual microvessels, definitive studies of receptor subtype expression can now be extended to SMCs and ECs in microvessels from other vascular beds.
βARs promote vasodilation
Whereas αARs typically function as the predominant effectors of sympathetic control, βARs may also contribute to the regulation of blood flow (99, 214). In contrast to the αARs, activation of βARs leads to vasodilation (81, 85, 191). This action provides the potential for βARs to play an important role in the regulation of tone in many blood vessels, although the exact role of βARs in resistance vessels remains unclear. The βARs (primarily β1 and β2 in the peripheral vasculature (32, 99)) are located on SMCs, where agonist binding leads to activation of adenylyl cyclase through Gs, increased cAMP and, ultimately, SMC relaxation through reductions in intracellular Ca2+ (148, 209). βAR activation also lead to vasodilation through SMC hyperpolarization, likely via activation of KATP channels (82, 86). Though it remains controversial, expression of β2ARs on ECs may also contribute to vasodilation (119) through NO-dependent mechanisms (214). Thus, whereas removal of endothelium has been shown to reduce vessel relaxation to βAR agonists (31, 94, 97, 131, 259), others have found no role for endothelial βARs (49, 64, 190, 233). Unfortunately, variable experimental conditions, species differences and the diversity of vessels used in respective experiments make direct comparisons between studies difficult. Further, because the majority of these studies were performed using larger conduit arteries, their findings may not apply to βARs in the regulation of small arteries and arterioles; e.g., where myoendothelial signaling is paramount (70, 107, 260). For example, even though arterioles are exquisitely sensitive to βAR activation (81, 191), the predominant action of NE released from PVNs of resistance vessels during SNA is consistently constriction that increases with stimulation frequency (106, 184, 267) and this relationship is maintained when βARs are inhibited with propranolol (191). Thus, the physiological role of βARs in governing the resistance vasculature remains to be established.
Role of NPY as an adrenergic co-transmitter
In addition to ATP (discussed below, see Purinergic Signaling), NPY is the second major neurotransmitter co-released from sympathetic nerve terminals (33). In the rat cremaster muscle, NPY immunoreactivity often appeared to be co-localized with that of TH throughout the arteriolar network (78). As shown in cutaneous vessels of the ear in developing Guinea pigs, NPY is expressed in subpopulations of sympathetic (TH-immunoreactive) neurons prior to innervation of target tissue (195), thus NPY expression is not dependent upon contact of nerve fibers with the vasculature. NPY is synthesized in sympathetic neurons, transported along the axon (80) and may be stored within and released from vesicles separate from those containing NE (142, 178). During SNA, evidence suggests that NPY can be packaged and coreleased with ATP from a single pool of “dense cored” vesicles (53). As with ATP, the corelease of NPY depends upon the intensity of SNA. Thus under experimental conditions, NPY is released during higher stimulation frequencies (180); e.g., those associated with cardiovascular stress and/or dysfunction (115) and vasoconstriction. Once released, NPY binds to one of six receptor subtypes (Y1-Y6) (162), with Y1 being the primary post-junctional receptor expressed on vascular SMCs (154) (Figure 3). Nevertheless, Y2 receptors on SMCs have been implicated in mediating vasoconstriction in mouse cutaneous microvessels (46). Binding of NPY to Gα-coupled Y1 or Y2 receptors on SMCs [(and ventricular myocytes (109)] increases PLC activity, thereby increasing IP3 production and intracellular Ca2+ (29). In cultured SMCs, NPY increased the phosphorylation of myosin light chain (169). Whereas these actions alone produce vasoconstriction, a key role of NPY is to potentiate the vasoconstrictor effects of αAR activation by NE (65, 270).
The actions of NPY are terminated upon its enzymatic degradation (115). Thus vasoconstrictions induced solely by NPY are of longer duration than those induced by NE (115), attributable to the slower degradation of NPY when compared to the active reuptake of catecholamines (177). While these signaling pathways have been defined under experimental conditions, it remains unclear whether NPY contributes significantly to vasomotor control under resting physiological conditions (115). Through suppressing neurotransmitter release, the activation of prejunctional Y2 receptors (Figure 2) may also attenuate sympathetic vasoconstriction, as shown in canine (283) and porcine (181) splenic arteries and guinea pig submucosal arterioles (149). However, because there have been few studies using specific receptor antagonists (2, 179, 182), the relative contribution of Y1- versus Y2-mediated signaling events towards modulating sympathetic vasoconstriction remains unclear. As the role of NPY appears to vary with vascular bed, animal species and gender (50, 54, 123, 124), defining the precise actions of NPY in vasomotor control in vivo remains complicated by its synergistic effects on adrenergic vasoconstriction.
Parasympathetic, cholinergic and nitrergic innervation
Parasympathetic PVNs originate in the CNS with most cell bodies located in ganglia (17, 102). While the terminals of these PVNs release ACh as neurotransmitter, the presence and functional role of parasympathetic PVNs is poorly-defined relative to those of sympathetic or sensory PVNs (111, 251, 265). In part, this is attributable to the difficulty in interpreting immunological studies of parasympathetic innervation, as VIP, the most commonly-used marker for parasympathetic nerves (Table 1), can also be associated with non-cholinergic nerves (66, 188). As shown in cats, VIP is distributed widely throughout the cephalic arterial supply, where it mediates atropine-resistant relaxation of SMCs in responses to parasympathetic nerve stimulation (89). In the brain, activation of parasympathetic nerves evokes vasodilation and increases cerebral blood flow (47). In some vascular beds, parasympathetic nerves may play a minor role in vasomotor function (15, 25, 111, 204), with no presence or functional role in other vessels (193, 251).
An intriguing example of the multiplicity of vascular innervation is cholinergic vasodilation mediated by the sympathetic nervous system. Using pharmacological interventions while evoking SNA, atropine-sensitive (i.e., muscarinic receptor-mediated) vasodilation has been most clearly associated with the vascular supply to skeletal muscle in dogs and cats (264). Comparative studies indicated similar responses in related species (e.g., fox and jackal), however, there was no evidence for their presence or function in humans or primates (264). Where it is present, sympathetic cholinergic vasodilation in skeletal muscle may serve as a feed forward mechanism for directing blood flow in anticipation of exercise, e.g., as a component of the autonomic fight-or-flight reflex. It is also possible that ACh (or CGRP) released at the motor endplate of skeletal muscle (95) plays a similar role in promoting vasodilation coincident with the activation of muscle fibers (274) but such actions also remain controversial (6). Other vascular beds that have been associated with cholinergic innervation of arteries and arterioles in dogs and cats include those supplying the tongue, reproductive organs, heart and gastrointestinal tract (234). The origins of such innervation have occasionally been attributed to ganglion cells within tissues (234) or even the vascular wall itself (117). Signaling events initiated by ACh acting on the vasculature (e.g., EDH) have been well described (8, 83, 85) and are beyond the focus of this discussion.
Nitrergic (i.e., nitroxidergic) nerves are present in many vascular beds (Table 1) and contribute to PVN-mediated vasodilation via NO produced within nerve terminals that contain nNOS (33), including some sensory and parasympathetic PVNs. Thus unlike other neurotransmitters, NO is not stored in and then released from synaptic vesicles (however it production is also dependent upon Ca2+ influx into the nerve terminal). Instead, it is synthesized by nNOS as described for NO produced via eNOS in ECs (83), and NO released from PVNs diffuses into SMCs and activates soluble guanylate cyclase to generate cGMP and produce vasodilation (55), consistent with downstream actions of NO generated by eNOS. Nitrergic nerves can also modulate vasomotor activity through interacting with other PVNs. For example, in rat mesenteric arteries, nitrergic nerves localize with sympathetic nerves and their release of NO inhibits adrenergic vasoconstriction, presumably by diminishing the release of NE (105, 151). Nitrergic-cholinergic interactions producing vasodilation have been demonstrated in porcine ciliary arteries (257) and monkey cerebral arteries (258). Nevertheless, despite numerous studies demonstrating the presence of nitrergic nerves in the vasculature (Table 1), the physiological role of NO as a neurotransmitter remains to be resolved in the resistance vasculature. While there is a lack of definitive evidence for the presence of nNOS within sympathetic PVNs, additional studies are required to define the role of NO as a cotransmitter in sensory and parasympathetic PVNs.
Sensory Innervation
The presence of sensory PVNs has been characterized in a wide variety of vascular beds across several animal species including humans (Table 1). In contrast to sympathetic nerves, the cell bodies of sensory nerves lie in the dorsal root ganglia (114, 122). Immunostaining for the CGRP and SP peptides synthesized in these neurons typically identify perivascular sensory nerves (95), although other markers are occasionally used (see Table 1). In addition to coursing diffusely through surrounding tissue (95), efferent axons of sensory nerves can also localize to form a plexus surrounding blood vessels (Figure 1). However the distances between their varicosities and SMCs can exceed 500 nm (176); i.e., several-fold greater than those associated with perivascular sympathetic nerves (174). Unlike sympathetic nerves, sensory nerves are capable of both antidromic and orthodromic conduction, thereby enabling their participation in local axon reflexes independent of efferent signaling from the cell body (152, 284). Thus, noxious stimuli experienced in the tissue, such as chemical or mechanical irritation, extremes in temperature or pH can cause antidromic stimulation of sensory nerves, leading to neurotransmitter release and vasodilation (41, 152) in addition to the sensation of pain. While CGRP is the primary neurotransmitter (30), SP and ATP are released as cotransmitters (140). Collectively, the release of these agents underlies nonadrenergic - noncholinergic vasodilation (36).
Peptidergic neuroeffector signaling
Calcitonin gene related peptide
CGRP is synthesized in both central and peripheral sensory neurons, transported along axons (134) and packaged into vesicles along with SP and ATP (30). Because CGRP does not undergo reuptake, its actions are terminated through degradation (30). Once released, CGRP can bind to one of its two G protein-coupled receptor subtypes, CGRP1 and CGRP2, with the former mediating most cardiovascular effects including relaxation of vascular SMCs (12). CGRP1 is associated with RAMP1, which is required for ligand binding and specificity (240). The predominant action is vasodilation mediated by an increase in cAMP, with PKA activating K+ channels (e.g., KATP and BKCa) (28, 199, 222, 273). The resulting hyperpolarization of SMCs evokes closure of voltage-gated Ca2+ channels, lowering intracellular [Ca2+]i to promote relaxation (Figure 3). While such direct effects on SMCs occur in the majority of vascular beds, an endothelium-dependent pathway for CGRP in promoting vasodilation has been demonstrated in aorta (96), mammary artery (216) and pulmonary artery (279) that results from cAMP- and PKA-mediated increases in NO production. Despite the consistency of vasodilation observed in response to CGRP, its effect on Ca2+ signaling remains unclear. In SMCs from human umbilical veins, CGRP exposure was linked to reductions in both Ca2+ influx through the plasma membrane and release of Ca2+ from internal stores (59). In cultured skeletal muscle cells, exposure to CGRP increased IP3 levels, an effect that was attributed to crosstalk between cAMP and phosphoinositide signaling (163). The actions of CGRP have yet to be resolved in the context of vasomotor control.
Substance P
Substance P is a neurokinin that is synthesized in dorsal root ganglia, transported along axons and contained in vesicles within sensory nerve terminals (276). Upon release, SP exerts its effects through binding to postjunctional G-protein coupled tachykinin (i.e., NK) receptors located on ECs (30). Like CGRP, SP does not undergo reuptake and continues exerting its actions until it is degraded enzymatically (276). Three NK receptor isoforms have been identified (NK1-3), with NK1 having the highest affinity for SP. Exogenous SP applied within the vessel lumen is a potent NO-dependent vasodilator (1, 138, 277). Its binding to NK1 receptors on ECs increases [Ca2+]i to activate eNOS (29) (Figure 3); either endothelial denudation or scavenging NO inhibited SP-mediated dilation of mesenteric arteries (24). When released from PVNs, SP increases vascular permeability through its alteration of EC structure and function (88, 200, 292) in conjunction with activation of mast cells (27, 29). Nevertheless, the physiological role of SP in the resistance vasculature remains controversial as its levels in the microcirculation may not be sufficient to affect vessel diameter or permeability (27). For hepatic (210) and mesenteric (140, 166) arteries, exogenous SP had no effect on vessel diameter while exposure of the same vessels to CGRP produced vasodilation. The latter findings suggest that SP released from the abluminal perivascular sensory nerves has little effect on adjacent SMCs. Thus, it appears unlikely that SP released as a neurotransmitter contributes substantively to vasomotor control. Conversely, SP that gains access to the vessel lumen may contribute to signaling from ECs to SMCs subsequent to elevating EC [Ca2+]i (Figure 3).
Purinergic neurotransmission
Multiple sources and receptors for ATP
Arising from both sensory and sympathetic nerves, purinergic signaling encompasses an array of mechanisms involved in the mediation of vascular function (37). As first shown in rabbit ear arteries (116), ATP is released upon stimulation of sensory nerves. However, it is difficult to resolve the actions of ATP released from sensory nerves versus that released from sympathetic nerves or other physiological sources which include ECs, erythrocytes and other non-neuronal cells (71, 171). Purinergic receptor expression varies between vascular beds (111, 217) and some innervated vessels may express multiple receptor subtypes on sympathetic nerves, sensory nerves, SMCs and ECs (Figure 3). Such multiplicity of receptor expression further complicates the difficulty in determining specifically where ATP exerts direct effects on blood vessels and how its actions relate to vasomotor control. A recent review (39) outlines the historical and current controversies surrounding the study of purinergic signaling in the vasculature, highlighting the need for more work in this field. However, even when selective agonists and antagonists become available for respective purinergic receptor subtypes, the challenge remains to identify the source(s) of vasoactive ATP under physiological conditions. Nevertheless, because ATP can be released from multiple sources, we now address purinergic signaling in the vasculature.
Purinergic neuroeffector signaling is multifaceted
Since the co-release of neurotransmitters was first proposed (34), it has become accepted that ATP is released along with NE during SNA (144). Free ATP can activate two types of P2 receptors, P2X and P2Y, located on vascular cells and nerves (35). The P2X receptors on SMCs are intrinsic cation channels that, when activated, allow influx of Ca2+ and/or Na+ to cause a rapid and transient depolarization known as an excitatory junction potential (39, 112). In turn, depolarization activates L-type Ca2+ channels to increase SMC [Ca2+]i (155). As a result of acute desensitization of P2X receptors and the rapid degradation of ATP, this purinergic response contributes more to the initiation than to the maintenance of sympathetic vasoconstriction (35, 171). There are seven P2X subtypes (P2X1-7), with P2X1 being primarily responsible for purinergic signaling in vascular SMCs (38, 158). Expression of P2X receptors on ECs has also been reported (103, 282) and linked to vasodilation (3, 104), however these receptors are far more likely to be activated by luminal ATP [e.g., released from erythrocytes in response to low PO2 (69) or ECs in response to shear stress (171)] rather than by ATP released from sympathetic nerve terminals. The activation of P2X receptors on SMCs can also produce vasodilation through mechanisms that remain unclear but are independent of the endothelium (218). Given this diversity of responses, it should not be surprising that the activation of P2X can result in biphasic vasomotor responses. For example, P2X receptors located on ECs of the mesenteric artery were linked to a transient vasoconstriction followed by prolonged vasodilation (104). In the rat femoral artery, ATP evoked dilation via P2X receptors on ECs or constriction via P2X receptors on SMCs (143). Nevertheless, because vasomotor responses of feed arteries and arterioles to SNA are abolished by phentolamine (a nonselective αAR antagonist) (191, 267) the functional expression of P2X receptors and their role in sympathetic neural control of the resistance vasculature require further elucidation of their physiological signficance.
Free ATP can also bind to P2Y receptors on ECs, which express five of the eight known isoforms (P2Y1, P2Y2, P2Y4, P2Y8, P2Y11) (219, 271). In contrast to the ionotropic nature of P2X receptors, the P2Y receptors are metabotropic. Thus binding of ATP leads to activation of PLC with production of IP3 stimulating internal release of Ca2+ and the activation of eNOS to promote SMC relaxation via the generation of NO (37). While these effects have been defined for ATP released from ECs in response to shear stress (40), it is not clear whether ATP released from PVNs actually reaches ECs to activate P2Y receptors. In response to PVN stimulation, the activation of P2Y receptors on SMCs has been linked to constriction of coronary arteries (243). The expression of P2Y receptors has also been reported in cultured SMCs derived from the aorta (72, 93, 271), with their activation resulting in distinct IP3-dependent Ca2+ signals that vary with the P2Y receptor isoform expressed (92). Studies of isolated SMCs have linked increased P2Y receptor expression to their growth in culture, consistent with P2Y receptor activation leading to SMC proliferation in the arterial wall (72, 241). The latter findings support a role for ATP released during SNA in promoting SMC growth and proliferation (73, 248), which may thereby contribute to the etiology of hypertension.
Confounding factors to resolve
Complicating the resolution of the physiological actions of ATP released from PVNs, the magnitude and duration of the purinergic component of sympathetic vasoconstriction in resistance vessels is affected not only by P2X and P2Y actions in SMCs and ECs but also by the expression of these receptors on sympathetic and sensory nerve terminals (Figure 2), where their activation can facilitate both constriction and dilation (38). A recent review explores the heterogeneity of purinergic receptors on perivascular nerves as well as SMCs and ECs (217). Suffice to say that the presence of both P2X and P2Y receptors (each with different subtypes) in ECs and SMCs and the lack of correspondingly specific pharmacological agents have made it difficult to isolate the specific actions of respective receptors in light of vasomotor control. Nevertheless, purinergic constriction (mediated primarily via P2X receptors) is consistently more pronounced in resistance arteries and arterioles than in larger conduit arteries (74, 90, 91, 221). Such regional heterogeneity in the actions of ATP suggests that purinergic signaling pathways could serve as selective targets for pharmacological agents acting at defined branches within the vascular tree. Whereas the breakdown products of ATP are also vasoactive (e.g., adenosine via P1 receptors), the actions of such “vasodilator metabolites” are beyond the focus of the present discussion.
Feedback between sympathetic and sensory nerves
In addition to their effects on SMCs and ECs, sympathetic and sensory PVNs interact through negative feedback to regulate the efficacy of neuroeffector signaling (Figure 3). For example, the activity of sensory nerves can reduce sympathetic vasoconstriction via prejunctional inhibition of noradrenergic neurotransmission. In segments of rabbit ear arteries (194) and in arterioles of the guinea pig submucosa (47, 48), pretreatment with the sensory neurotoxin capsaicin (which binds to TRPV1 receptors leading to desensitization) transiently enhanced vasoconstriction to electrical stimulation of PVNs but not to NE applied externally. In isolated rat mesenteric arteries, the inhibition of CGRPergic nerve function potentiated vasoconstriction to SNA (136, 203) however recent intravital studies in mice have shown this effect to be lost with aging (275). Conversely, treatment with CGRP or SP (i.e., sensory neurotransmitters) reduced the amplitude of neurally-evoked vasoconstrictions (47, 48).
The preceding findings collectively suggest that vasodilator (sensory) nerve activity can inhibit sympathetic vasoconstriction via prejunctional actions on sympathetic nerve terminals (Figure 3) without altering downstream signaling pathways initiated by NE (150). In rat mesenteric arterial rings, the activation of TRPA1 channels on sensory nerve terminals led to relaxation (10, 213), presumably through enhanced Ca2+ influx promoting exocytosis and release of CGRP which, in turn, inhibited the release of NE (63). TRPV1 channels appear to play a similar role (141), as supported by impaired dilation of mesenteric arteries isolated from TRPV1-null mice upon stimulation of sensory PVNs (272). However, it appears unlikely that the activities of TRPV1 and TRPA1 channels are coupled (10, 63). Instead, respective channels represent distinct targets that can mediate CGRP release and thereby influence vasomotor control.
In a reciprocal manner, sympathetic PVNs can inhibit the activity of sensory PVNs (136, 203). As shown in rat mesenteric arteries, NE acting on prejunctional α2ARs of sensory nerve terminals impairs the release of CGRP (137) (Figure 3). Further, NPY (a sympathetic co-transmitter; above) has been found to inhibit dilation of rat mesenteric arteries mediated by stimulation of sensory PVNs (202) although the mechanism remains to be resolved. Experiments performed on the rat vas deferens suggest that ATP released from sympathetic nerves binds to P2Y receptors on sensory nerves to inhibit CGRP release (60). However the potential role for ATP in modulating sensory nerve activity has not been studied in the vasculature. Nevertheless, P2Y receptors localized to sympathetic PVNs were found to respond to ATP by inhibiting transmitter release (22). From earlier discussion, the ATP exerting such prejunctional effects could arise from either sympathetic or sensory nerve activation. Thus, P2Y receptors may contribute indirectly (i.e., by reducing NE release) to the purinergic component of sensory nerve-mediated vasodilation. In addition, sensory nerves may exhibit autoinhibition. For example, in the presence of guanethidine (to block adrenergic neurotransmission), application of exogenous CGRP decreased vasodilation of mesenteric arteries during PVN stimulation (203), implying the presence of prejunctional CGRP receptors on sensory nerve terminals. In a complementary manner, ATP released from either sympathetic or sensory PVNs may bind to prejunctional P2X receptors that act to inhibit further release of sensory neurotransmitters (38). While the crosstalk between respective PVNs nerves appears integral to vasomotor control [e.g., in mesenteric arteries (136, 202, 203); Figure 1], these relationships require further investigation in the microcirculation to resolve their role in the local control of tissue blood flow.
Roles for perivascular nerves in myoendothelial communication
Myoendothelial signaling initiated by adrenergic receptor activation
Adrenergic signaling initiated through SNA plays a critical role in governing the control of blood flow by small arteries and arterioles (106, 183, 267). Growing evidence implicates signaling from SMCs to ECs as an integral component of vasomotor control intrinsic to these resistance vessels (Figure 3). Thus, myoendothelial GJs enable the direct transmission of electrical and chemical signals between SMCs and ECs within the vessel wall (107, 145) (Figure 3). As first reported in hamster cheek pouch arterioles, activation of α1ARs with PE increased SMC [Ca2+]i with an ensuing rise of EC [Ca2+]i leading to activation of eNOS and the release of NO (62). These findings suggested that signaling from SMCs to ECs occurs via heterocellular diffusion of a second messenger which thereby provides feedback to moderate vasoconstriction. Ensuing studies in cremaster arterioles (125, 263, 285) found similar increases in EC [Ca2+]i that were initiated by stimulation of α1ARs on SMCs. Confirming the lack of α1AR expression or function in ECs ruled out direct effects of PE on the endothelium (125). Studies in cremaster muscle arterioles have also linked PE-induced increases in EC [Ca2+]i to the initiation of conducted vasodilation (285), indicating that interactions between SMCs and ECs initiated through αAR activation have functional implications both at local sites and throughout resistance networks.
In a co-culture model of ECs and SMCs derived from vessels of the cremaster muscle, both Ca2+ and IP3 were found to diffuse from SMCs to ECs upon α1AR stimulation, with each having differential effects on EC [Ca2+]i (121). Supporting the idea that increases in SMC [Ca2+]i lead to EC responses via MEJs are findings that purported blockers of GJs inhibit EC Ca2+ responses to adrenergic stimulation (121). While these studies point to the diffusion of second messenger(s) from SMCs to ECs, its identity (e.g., Ca2+ vs. IP3) has not been ascertained in native microvessels and remains a key issue to resolve in the context of blood flow control. It should also be recognized that the co-culture model is has pronounced differences in ultrastructure when compared to the vessel wall. For example, it lacks an IEL and contains far more myoendothelial contacts than occur in vivo. Thus caution and appropriate controls are advised when applying findings from vascular cell culture models to intact vessels (253).
In isolated strips of rat mesenteric arteries, [Ca2+]i increased within ECs following elevations of [Ca2+]i within SMCs responding to PE or high-K+ depolarizing solution (156). Pharmacological inhibition of IP3 signaling in SMCs prevented these EC Ca2+ signals, suggesting that IP3 could diffuse from SMCs to ECs via MEJs. In pressurized rat mesenteric arteries, Ca2+ signals within ECs appeared spontaneously, increased in frequency upon SMC stimulation with PE, and were diminished when IP3Rs, voltage-gated Ca2+ channels, or GJs were inhibited (133). These observations are consistent with constitutive intercellular communication from SMCs to ECs that can increase upon SMC stimulation. Because high K+ depolarization (which acts independent of PLC or IP3) caused similar increases in EC Ca2+ signals, the diffusion of Ca2+ (vs. IP3) was proposed to serve as the likely second messenger from SMC to EC (133). While such studies collectively support the idea of SMC-to-EC communication via diffusion of a second messenger, it remains unclear whether IP3, Ca2+ or both are important to myoendothelial signaling in the vessel wall under physiological conditions. Resolving this issue will provide important insight into which signaling pathways regulate myoendothelial Ca2+ signaling and may thereby enable determination of whether and/or how these pathways may be altered (and treated) with vascular disease.
The development of the Cx40BAC-GCaMP2 transgenic mouse model represents a significant advancement towards understanding intercellular communication with respect to EC Ca2+ signaling (256). In these animals, the ECs of arteries and arterioles selectively express a fluorescent GFP-based Ca2+ indicator by linking its expression to that of Connexin40, a constitutive subunit of EC GJs. Thus visualization of EC Ca2+ signals is enabled without the need for fluorescent dyes that may alter intercellular signaling through Ca2+ buffering and/or dye sequestration (207). Recently, opened mesenteric artery preparations from GCaMP2 mice were studied en face to define Ca2+ “pulsars” in the endothelium (164). These localized events were characterized as spontaneous, IP3-dependent Ca2+ signals within ECs that are associated with holes in the IEL (164) (see Figure 3), highlighting their potential role in mediating intercellular signaling through MEJs. A subsequent study confirmed this correlation and linked the regulation of Ca2+ pulsars to sympathetic nerve stimulation, proposing that pulsars can provide negative feedback to attenuate vasoconstriction (198). Thus, by increasing Ca2+ within EC projections, the activation of IKCa and SKCa channels evokes hyperpolarization that, in turn, spreads back into SMCs via myoendothelial GJs (120, 121, 133, 164, 231, 260). Thus Ca2+ signaling from SMCs to ECs through MEJs is implicated as a mechanism for providing negative feedback to oppose sympathetic vasoconstriction (Figure 3).
Myoendothelial signaling initiated by purinergic receptor activation
Purinergic-mediated Ca2+ signals may represent another mechanism through which PVNs mediate intercellular communication between ECs and SMCs. Unfortunately, few studies have investigated the effect of P2 receptor activation on SMC Ca2+ signaling or intercellular communication. Nevertheless, Ca2+ imaging of rat mesenteric arteries has revealed that the activation of P2X1 receptors on SMCs produces jCaTs (159) near varicosities of sympathetic PVNs (157) and that these Ca2+ signaling events can be elicited by PVN stimulation (158). While jCaTs are spatially restricted within SMCs, their occurrence can lead to global elevations in SMC [Ca2+]i mesenteric arteries (289), consistent with their role in promoting Ca2+-induced Ca2+ release from IP3 receptors in SMCs of renal arteries (212). In the juxtaglomerular apparatus of the kidney, purinergic signaling plays an important role in tubuloglomerular feedback through GJs, as the purported blocking of GJs prevented such feedback and reduced renal blood flow autoregulation (255). One explanation for such actions is that SMC Ca2+ derived from purinergic neurotransmission could no longer move though GJs to coordinate cellular function. Thus, purinergic signaling associated with SNA (particularly jCaTs) could also result in SMC-to-EC signaling via the diffusion of Ca2+ and/or IP3 through MEJs. Nonadrenergic signaling initiated by PVNs may thereby contribute further to vasomotor control through myoendothelial signaling.
Myoendothelial signaling initiated by peptidergic signaling
Peptidergic signaling is initiated via sympathetic nerves through NPY and the activation of Y1 receptors may further contribute to myoendothelial signaling (Figure 3). For example, in cardiac myocytes and vascular SMCs, exposure to NPY increases [Ca2+]i (126, 127). Such actions in the resistance vasculature would promote Ca2+ diffusion through MEJs to initiate feedback signaling in ECs as discussed above. Whereas the activation Y1 receptors can increase [IP3]i and [Ca2+]i in cardiac myocytes (109), it appears more likely that the effects of NPY in the vessel wall reflect augmentation of Ca2+ transients caused by activation of α1ARs (278). Further, NPY may contribute to purinergic receptor-mediated jCaTs through activating nonspecific cation channels (101, 244). While the correspondence between jCaTs and myoendothelial signaling remains to be tested in the vasculature, the actions of NPY as a perivascular cotransmitter appear likely to contribute to intercellular signaling and vasomotor control in at least some vascular beds.
In addition to inhibiting sympathetic vasoconstriction by suppressing neurotransmission during SNA, CGRP released from sensory nerves may also influence vascular function by reducing myoendothelial signaling. This effect may be explained by CGRP-mediated activation of PKA in SMCs leading to phosphorylation of connexin protein subunits within myoendothelial GJs (160, 161, 252). In the pregnant uterine vasculature, CGRP-dependent dilations are impaired by the GJ uncoupler carbenoxolone (269). It is also possible that this effect of carbenoxolone results from its non-specific inhibition of ion channels that initiate EC hyperpolarization (11). Nevertheless, and in light of classic studies illustrating vasodilation mediated by the axon reflex of sensory nerves (152), further experiments are needed to determine the functional role of CGRP in the microcirculation along with the associated signaling events underlying vasomotor control.
Regional heterogeneity in myoendothelial coupling and intercellular signaling
Just as variations in perivascular nerves, neurotransmitters and their receptors underlie regional differences in the nature of effector signaling on SMCs and ECs, variation in the presence of MEJs and expression of myoendothelial GJs likely contribute to regional heterogeneity in neuroeffector signaling. For example, in dye transfer studies, the ECs and SMCs of rat mesenteric arteries appear well-coupled to each other through GJs (185), while those in hamster cremaster arterioles appear poorly coupled (242). Heterocellular coupling in hamster cheek pouch arterioles has reported to be both robust in vitro (168) and absent in vivo (237), highlighting the potential influence of experimental conditions. Differences between species and/or regional differences in vessel size, prevalence of MEJs and fenestrae in the IEL can all contribute to regional differences in the regulation of vascular function (232), e.g., by determining how efficiently second messengers can diffuse between SMCs and ECs (107). Thus smaller resistance arteries and arterioles tend to have more myoendothelial contacts (223) when compared to larger conduit arteries (228), consistent with greater prevalence of myoendothelial signaling (e.g., EDH) in the resistance vasculature when compared to flow-mediated and NO-dependent dilation of larger conduit arteries (16). Further complexity arises from heterogeneity in the expression (230) and regulation (e.g., through phosphorylation and nitrosylation) of connexin isoforms comprising GJs, including those at MEJs (76, 107, 160, 161, 252). Such complexity argues against a “unifying principal” for neuromodulation of myoendothelial signaling while pointing to the need for greater understanding of its complexities.
PERSPECTIVE
The induction and modulation of sympathetic vasoconstriction and sensory nerve-mediated vasodilation have been well-characterized. However the underlying signaling events remain unclear, particularly in the context of myoendothelial feedback. Intercellular communication in the arterial wall has long focused on the role of NO (and other diffusible autocoids) in mediating SMC relaxation. More recently, the role of EDH in governing SMC [Ca2+]i and vascular tone via direct electrical coupling through myoendothelial GJs has gained recognition as an independent yet complementary signaling pathway mediating vasodilation (8, 85). Recent studies have provided critical insight into the importance of MEJs as signaling microdomains that can regulate intercellular communication as well as vasomotor tone (133, 156, 198, 247, 253, 260) (Figure 3). Remarkably, though integral to the physiological regulation of vasoconstriction and vasodilation, the role of PVNs in coordinated signaling between SMCs and ECs remains poorly studied and, therefore, poorly understood. Recent evidence from isolated mesenteric arteries indicates that local Ca2+ signals in ECs can result from stimulating sympathetic PVNs (198). This behavior is consistent with earlier findings in isolated arterioles that α1AR activation on SMCs evoked Ca2+ signaling in ECs (62, 125, 285). Whereas Ca2+ and IP3 have been identified as candidates based upon studies of α1AR activation, virtually nothing is known about the role of other intercellular second messengers [e.g., cAMP (132)] or neuroeffector signaling pathways in either initiating or modulating heterocellular communication through MEJs. In future studies, the utilization of new recording techniques and improved pharmacological tools will help to determine the roles of each transmitter released from perivascular sympathetic and sensory nerves on both SMC-to-EC signaling and the resulting effects on vasomotor function. Resolving such direct and indirect signaling events and how they interact in the vessel wall will provide new insight into the multiplicity of roles that PVNs exert during vasomotor control, how such actions vary between vascular beds and branch orders, and how effective responses are modulated through intercellular communication. In turn, this new knowledge can be applied towards developing more selective therapeutic interventions for targeting the treatment of vascular disease.
Acknowledgments
The authors thank Dr. Paul Fadel for critical review and discussion of this manuscript. Research in the authors’ laboratory is supported by National Institutes of Health grants R37-HL041026 and R01-HL086483.
Grant support: NIH R01-HL086483 and R37-HL041026
Abbreviations
- ACh
acetylcholine
- AR
adrenergic receptor
- BKCa
large conductance calcium-activated potassium channel
- [Ca2+]i
intracellular calcium concentration
- CGRP
calcitonin gene-related peptide
- EC
endothelial cell
- EDH
endothelium-dependent hyperpolarization
- eNOS
endothelial nitric oxide synthase
- GFP
green fluorescent protein
- GJ
gap junction
- GPCR
G-protein coupled receptor
- IEL
internal elastic lamina
- IKCa
intermediate conductance calcium-activated potassium channel
- IP3
inositol 1,4,5 trisphosphate
- IP3R
inositol 1,4,5 trisphosphate receptor
- jCaT
junctional calcium transient
- KATP
ATP-sensitive potassium channel
- Kir
inwardly rectifying potassium channel
- MEJ
myoendothelial junction
- NADPH-d
Nicotinamide adenine dinucleotide phosphate-diaphorase
- NE
norepinephrine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NPY
neuropeptide Y
- NMJ
neuromuscular junction
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PVN
perivascular nerve
- RAMP
receptor activated modifying protein
- SKCa
small conductance calcium-activated potassium channel
- SMC
smooth muscle cell
- SNA
sympathetic nerve activity
- SNS
sympathetic nervous system
- SP
substance P
- SR
sarcoplasmic reticulum
- TH
tyrosine hydroxylase
- TRP
transient receptor potential
- VIP
vasoactive inhibitory peptide
References
- 1.Abdelrahman AM, Pang CC. Effect of substance P on venous tone in conscious rats. J Cardiovasc Pharmacol. 2005;45:49–52. doi: 10.1097/00005344-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Abounader R, Villemure JG, Hamel E. Characterization of neuropeptide Y (NPY) receptors in human cerebral arteries with selective agonists and the new Y1 antagonist BIBP 3226. Br J Pharmacol. 1995;116:2245–2250. doi: 10.1111/j.1476-5381.1995.tb15060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkayed F, Boudaka A, Shiina T, Takewaki T, Shimizu Y. P2X purinoceptors mediate an endothelium-dependent hyperpolarization in longitudinal smooth muscle of anterior mesenteric artery in young chickens. Br J Pharmacol. 2009;158:888–895. doi: 10.1111/j.1476-5381.2009.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- 5.Ando K, Mishima Y, Sakai M. Development of nitric oxide synthase-immunoreactive nerves in the cerebral arteries of the rat. J Vet Med Sci. 2004;66:933–940. doi: 10.1292/jvms.66.933. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RB, Laughlin MH. Atropine: no effect on exercise muscle hyperemia in conscious rats. J Appl Physiol. 1986;61:679–682. doi: 10.1152/jappl.1986.61.2.679. [DOI] [PubMed] [Google Scholar]
- 7.Aukes AM, Bishop N, Godfrey J, Cipolla MJ. The influence of pregnancy and gender on perivascular innervation of rat posterior cerebral arteries. Reprod Sci. 2008;15:411–419. doi: 10.1177/1933719107314067. [DOI] [PubMed] [Google Scholar]
- 8.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 2011;202:271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barroso CP, Edvinsson L, Zhang W, Cunha e Sá M, Springall DR, Polak JM, Gulbenkian S. Nitroxidergic innervation of guinea pig cerebral arteries. J Auton Nerv Syst. 1996;58:108–114. doi: 10.1016/0165-1838(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol. 2012;166:774–787. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell D, McDermott BJ. Calcitonin gene-related peptide in the cardiovascular system: characterization of receptor populations and their (patho)physiological significance. Pharmacol Rev. 1996;48:253–288. [PubMed] [Google Scholar]
- 13.Bergua A, Schrödl F, Neuhuber WL. Vasoactive intestinal and calcitonin gene-related peptides, tyrosine hydroxylase and nitrergic markers in the innervation of the rat central retinal artery. Exp Eye Res. 2003;77:367–374. doi: 10.1016/s0014-4835(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 14.Bevan JA. Some bases of differences in vascular response to sympathetic activity. Circ Res. 1979;45:161–171. doi: 10.1161/01.res.45.2.161. [DOI] [PubMed] [Google Scholar]
- 15.Bevan JA, Brayden JE. Nonadrenergic neural vasodilator mechanisms. Circ Res. 1987;60:309–326. doi: 10.1161/01.res.60.3.309. [DOI] [PubMed] [Google Scholar]
- 16.Bevan JA, Halpern W, Mulvany MJ University of Vermont. Center for Vascular Research. The Resistance vasculature. Totowa, N.J: Humana Press; 1991. p. xii.p. 476. [Google Scholar]
- 17.Biaggioni I. Primer on the autonomic nervous system. Amsterdam ; Boston: Elsevier Academic Press; 2012. p. xxv.p. 703. 741 p. of plates. [Google Scholar]
- 18.Birch D, Knight GE, Boulos PB, Burnstock G. Analysis of innervation of human mesenteric vessels in non-inflamed and inflamed bowel--a confocal and functional study. Neurogastroenterol Motil. 2008;20:660–670. doi: 10.1111/j.1365-2982.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- 19.Birch DJ, Turmaine M, Boulos PB, Burnstock G. Sympathetic innervation of human mesenteric artery and vein. J Vasc Res. 2008;45:323–332. doi: 10.1159/000119095. [DOI] [PubMed] [Google Scholar]
- 20.Björklund H, Hökfelt T, Goldstein M, Terenius L, Olson L. Appearance of the noradrenergic markers tyrosine hydroxylase and neuropeptide Y in cholinergic nerves of the iris following sympathectomy. J Neurosci. 1985;5:1633–1640. doi: 10.1523/JNEUROSCI.05-06-01633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair DH, Lin YQ, Bennett MR. Differential sensitivity to calcium and osmotic pressure of fast and slow ATP currents at sympathetic varicosities in mouse vas deferens. Auton Neurosci. 2003;105:45–52. doi: 10.1016/S1566-0702(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 22.Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- 23.Boegehold MA, Johnson PC. Response of arteriolar network of skeletal muscle to sympathetic nerve stimulation. Am J Physiol. 1988;254:H919–928. doi: 10.1152/ajpheart.1988.254.5.H919. [DOI] [PubMed] [Google Scholar]
- 24.Bolton TB, Clapp LH. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986;87:713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boysen NC, Dragon DN, Talman WT. Parasympathetic tonic dilatory influences on cerebral vessels. Auton Neurosci. 2009;147:101–104. doi: 10.1016/j.autneu.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol. 2003;284:H2007–2014. doi: 10.1152/ajpheart.01061.2002. [DOI] [PubMed] [Google Scholar]
- 27.Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997;37:133–152. doi: 10.1016/s0162-3109(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 28.Brain SD, Cambridge H. Calcitonin gene-related peptide: vasoactive effects and potential therapeutic role. Gen Pharmacol. 1996;27:607–611. doi: 10.1016/0306-3623(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 29.Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;147 (Suppl 1):S202–211. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 31.Brawley L, Shaw AM, MacDonald A. Beta 1-, beta 2- and atypical beta-adrenoceptor-mediated relaxation in rat isolated aorta. Br J Pharmacol. 2000;129:637–644. doi: 10.1038/sj.bjp.0703091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briones AM, Daly CJ, Jimenez-Altayo F, Martinez-Revelles S, Gonzalez JM, McGrath JC, Vila E. Direct demonstration of beta1- and evidence against beta2- and beta3-adrenoceptors, in smooth muscle cells of rat small mesenteric arteries. Br J Pharmacol. 2005;146:679–691. doi: 10.1038/sj.bjp.0706369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- 35.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 36.Burnstock G. Purinergic cotransmission. F1000 Biol Rep. 2009;1:46. doi: 10.3410/B1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnstock G. Purinergic Neurotransmission and Nucleotide Receptors. In: Robertson D, editor. Primer on the Autonomic Nerve System. USA: Elsevier Academic Press; 2012. pp. 87–93. [Google Scholar]
- 38.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 39.Burnstock G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194 ( Pt 3):335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg. 1994;47:527–543. doi: 10.1016/0007-1226(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 42.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 43.Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 44.Cao X, Demel SL, Quinn MT, Galligan JJ, Kreulen D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton Neurosci. 2009;151:90–97. doi: 10.1016/j.autneu.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christ GJ, Spray DC, el-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- 46.Chu DQ, Cox HM, Costa SK, Herzog H, Brain SD. The ability of neuropeptide Y to mediate responses in the murine cutaneous microvasculature: an analysis of the contribution of Y1 and Y2 receptors. Br J Pharmacol. 2003;140:422–430. doi: 10.1038/sj.bjp.0705452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipolla MJ. The Cerebral Circulation. In: Granger DN, Granger J, editors. Integrated Syatems Physiology: from molecule to function. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [Google Scholar]
- 48.Coffa FP, Kotecha N. Modulation of sympathetic nerve activity by perivascular sensory nerves in the arterioles of the guinea-pig small intestine. J Auton Nerv Syst. 1999;77:125–132. [PubMed] [Google Scholar]
- 49.Cohen RA, Shepherd JT, Vanhoutte PM. Neurogenic cholinergic prejunctional inhibition of sympathetic beta adrenergic relaxation in the canine coronary artery. J Pharmacol Exp Ther. 1984;229:417–421. [PubMed] [Google Scholar]
- 50.Coney AM, Marshall JM. Contribution of alpha2-adrenoceptors and Y1 neuropeptide Y receptors to the blunting of sympathetic vasoconstriction induced by systemic hypoxia in the rat. J Physiol. 2007;582:1349–1359. doi: 10.1113/jphysiol.2007.132563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, McGrath JC. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- 52.De Fontgalland D, Wattchow DA, Costa M, Brookes SJ. Immunohistochemical characterization of the innervation of human colonic mesenteric and submucosal blood vessels. Neurogastroenterol Motil. 2008;20:1212–1226. doi: 10.1111/j.1365-2982.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 53.De Potter WP, Partoens P, Schoups A, Llona I, Coen EP. Noradrenergic neurons release both noradrenaline and neuropeptide Y from a single pool: the large dense cored vesicles. Synapse. 1997;25:44–55. doi: 10.1002/(SICI)1098-2396(199701)25:1<44::AID-SYN6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.De Potter WP, Partoens P, Strecker S. Noradrenaline storing vesicles in sympathetic neurons and their role in neurotransmitter release: an historical overview of controversial issues. Neurochem Res. 1997;22:911–919. doi: 10.1023/a:1022458322406. [DOI] [PubMed] [Google Scholar]
- 55.Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 56.Dhall U, Cowen T, Haven AJ, Burnstock G. Perivascular noradrenergic and peptide-containing nerves show different patterns of changes during development and ageing in the guinea-pig. J Auton Nerv Syst. 1986;16:109–126. doi: 10.1016/0165-1838(86)90003-2. [DOI] [PubMed] [Google Scholar]
- 57.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donald JA, Broughton BR. Nitric oxide control of lower vertebrate blood vessels by vasomotor nerves. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:188–197. doi: 10.1016/j.cbpa.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Dong YL, Vegiraju S, Yallampalli C. Ca2+ signaling in human fetoplacental vasculature: effect of CGRP on umbilical vein smooth muscle cytosolic Ca2+ concentration. Am J Physiol Heart Circ Physiol. 2005;289:H960–967. doi: 10.1152/ajpheart.00059.2005. [DOI] [PubMed] [Google Scholar]
- 60.Donoso MV, Hermosilla D, Navarrete C, Álvarez P, Lillo JG, Huidobro-Toro JP. Reciprocal sympatho-sensory control: functional role of nucleotides and calcitonin gene-related peptide in a peripheral neuroeffector junction. Neuroscience. 2012;203:216–229. doi: 10.1016/j.neuroscience.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 61.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74:226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 62.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckly AE, Stoclet JC, Lugnier C. Isoprenaline induces endothelium-independent relaxation and accumulation of cyclic nucleotides in the rat aorta. Eur J Pharmacol. 1994;271:237–240. doi: 10.1016/0014-2999(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 65.Edvinsson L, Ekblad E, Håkanson R, Wahlestedt C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol. 1984;83:519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edvinsson L, Elsås T, Suzuki N, Shimizu T, Lee TJ. Origin and Co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech. 2001;53:221–228. doi: 10.1002/jemt.1086. [DOI] [PubMed] [Google Scholar]
- 67.Edvinsson L, Gulbenkian S, Barroso CP, Cunha e Sá M, Polak JM, Mortensen A, Jørgensen L, Jansen-Olesen I. Innervation of the human middle meningeal artery: immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides. 1998;19:1213–1225. doi: 10.1016/s0196-9781(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 68.Eguchi S, Tezuka S, Hobara N, Akiyama S, Kurosaki Y, Kawasaki H. Vanilloid receptors mediate adrenergic nerve- and CGRP-containing nerve-dependent vasodilation induced by nicotine in rat mesenteric resistance arteries. Br J Pharmacol. 2004;142:1137–1146. doi: 10.1038/sj.bjp.0705773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 70.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 71.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erlinge D, Hou M, Webb TE, Barnard EA, Möller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- 73.Erlinge D, Yoo H, Edvinsson L, Reis DJ, Wahlestedt C. Mitogenic effects of ATP on vascular smooth muscle cells vs. other growth factors and sympathetic cotransmitters. Am J Physiol. 1993;265:H1089–1097. doi: 10.1152/ajpheart.1993.265.4.H1089. [DOI] [PubMed] [Google Scholar]
- 74.Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faber JE. In situ analysis of alpha-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res. 1988;62:37–50. doi: 10.1161/01.res.62.1.37. [DOI] [PubMed] [Google Scholar]
- 76.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fleming BP, Barron KW, Howes TW, Smith JK. Response of the microcirculation in rat cremaster muscle to peripheral and central sympathetic stimulation. Circ Res. 1987;61:II26–31. [PubMed] [Google Scholar]
- 78.Fleming BP, Gibbins IL, Morris JL, Gannon BJ. Noradrenergic and peptidergic innervation of the extrinsic vessels and microcirculation of the rat cremaster muscle. Microvasc Res. 1989;38:255–268. doi: 10.1016/0026-2862(89)90004-6. [DOI] [PubMed] [Google Scholar]
- 79.Flügel C, Tamm ER, Mayer B, Lütjen-Drecoll E. Species differences in choroidal vasodilative innervation: evidence for specific intrinsic nitrergic and VIP-positive neurons in the human eye. Invest Ophthalmol Vis Sci. 1994;35:592–599. [PubMed] [Google Scholar]
- 80.Fried G, Lundberg JM, Theodorsson-Norheim E. Subcellular storage and axonal transport of neuropeptide Y (NPY) in relation to catecholamines in the cat. Acta Physiol Scand. 1985;125:145–154. doi: 10.1111/j.1748-1716.1985.tb07701.x. [DOI] [PubMed] [Google Scholar]
- 81.Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol. 1975;228:791–796. doi: 10.1152/ajplegacy.1975.228.3.791. [DOI] [PubMed] [Google Scholar]
- 82.Fujii K, Onaka U, Goto K, Abe I, Fujishima M. Impaired isoproterenol-induced hyperpolarization in isolated mesenteric arteries of aged rats. Hypertension. 1999;34:222–228. doi: 10.1161/01.hyp.34.2.222. [DOI] [PubMed] [Google Scholar]
- 83.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 84.Furness JB, Marshall JM. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol. 1974;239:75–88. doi: 10.1113/jphysiol.1974.sp010556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garland CJ, Yarova PL, Jiménez-Altayó F, Dora KA. Vascular hyperpolarization to β-adrenoceptor agonists evokes spreading dilatation in rat isolated mesenteric arteries. Br J Pharmacol. 2011;164:913–921. doi: 10.1111/j.1476-5381.2011.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gavazzi I, Boyle KS, Cowen T. Extracellular matrix molecules influence innervation density in rat cerebral blood vessels. Brain Res. 1996;734:167–174. [PubMed] [Google Scholar]
- 88.Ghabriel MN, Lu MX, Leigh C, Cheung WC, Allt G. Substance P-induced enhanced permeability of dura mater microvessels is accompanied by pronounced ultrastructural changes, but is not dependent on the density of endothelial cell anionic sites. Acta Neuropathol. 1999;97:297–305. doi: 10.1007/s004010050988. [DOI] [PubMed] [Google Scholar]
- 89.Gibbins IL, Brayden JE, Bevan JA. Perivascular nerves with immunoreactivity to vasoactive intestinal polypeptide in cephalic arteries of the cat: distribution, possible origins and functional implications. Neuroscience. 1984;13:1327–1346. doi: 10.1016/0306-4522(84)90301-4. [DOI] [PubMed] [Google Scholar]
- 90.Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132:1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gitterman DP, Evans RJ. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br J Pharmacol. 2000;131:1561–1568. doi: 10.1038/sj.bjp.0703760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Govindan S, Taylor CW. P2Y receptor subtypes evoke different Ca2+ signals in cultured aortic smooth muscle cells. Purinergic Signal. 2012;8:763–777. doi: 10.1007/s11302-012-9323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Govindan S, Taylor EJ, Taylor CW. Ca(2+) signalling by P2Y receptors in cultured rat aortic smooth muscle cells. Br J Pharmacol. 2010;160:1953–1962. doi: 10.1111/j.1476-5381.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grace GC, Macdonald PS, Dusting GJ. Cyclic nucleotide interactions involved in endothelium-dependent dilatation in rat aortic rings. Eur J Pharmacol. 1988;148:17–24. doi: 10.1016/0014-2999(88)90449-9. [DOI] [PubMed] [Google Scholar]
- 95.Grasby DJ, Morris JL, Segal SS. Heterogeneity of vascular innervation in hamster cheek pouch and retractor muscle. J Vasc Res. 1999;36:465–476. doi: 10.1159/000025689. [DOI] [PubMed] [Google Scholar]
- 96.Gray DW, Marshall I. Human alpha-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol. 1992;107:691–696. doi: 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gray DW, Marshall I. Novel signal transduction pathway mediating endothelium-dependent beta-adrenoceptor vasorelaxation in rat thoracic aorta. Br J Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guimarães S, Figueiredo IV, Caramona MM, Moura D, Paiva MQ. Prejunctional alpha2A-autoreceptors in the human gastric and ileocolic arteries. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:207–211. doi: 10.1007/pl00005244. [DOI] [PubMed] [Google Scholar]
- 99.Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- 100.Gulbenkian S, Saetrum Opgaard O, Ekman R, Costa Andrade N, Wharton J, Polak JM, Queiroz e Melo J, Edvinsson L. Peptidergic innervation of human epicardial coronary arteries. Circ Res. 1993;73:579–588. doi: 10.1161/01.res.73.3.579. [DOI] [PubMed] [Google Scholar]
- 101.Gustafsson H, Nilsson H. Endothelium-independent potentiation by neuropeptide Y of vasoconstrictor responses in isolated arteries from rat and rabbit. Acta Physiol Scand. 1990;138:503–507. doi: 10.1111/j.1748-1716.1990.tb08878.x. [DOI] [PubMed] [Google Scholar]
- 102.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 103.Hansen MA, Dutton JL, Balcar VJ, Barden JA, Bennett MR. P2X (purinergic) receptor distributions in rat blood vessels. J Auton Nerv Syst. 1999;75:147–155. doi: 10.1016/s0165-1838(98)00189-1. [DOI] [PubMed] [Google Scholar]
- 104.Harrington LS, Mitchell JA. Novel role for P2X receptor activation in endothelium-dependent vasodilation. Br J Pharmacol. 2004;143:611–617. doi: 10.1038/sj.bjp.0706004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, Takayama F, Gomita Y, Kawasaki H. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther. 2006;316:490–497. doi: 10.1124/jpet.105.094656. [DOI] [PubMed] [Google Scholar]
- 106.Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of alpha1- and alpha2-adrenoreceptor activation. J Physiol. 2005;563:541–555. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation. 2009;16:307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henrich M, Haberberger RV, Hempelmann G, Kummer W. Quantitative immunohistochemical investigation of the intrinsic vasodilator innervation of the guinea pig lingual artery. Auton Neurosci. 2003;103:72–82. doi: 10.1016/s1566-0702(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 109.Heredia MeP, Delgado C, Pereira L, Perrier R, Richard S, Vassort G, Bénitah JP, Gómez AM. Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2+ sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J Mol Cell Cardiol. 2005;38:205–212. doi: 10.1016/j.yjmcc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Hill AJ, Hinton JM, Cheng H, Gao Z, Bates DO, Hancox JC, Langton PD, James AF. A TRPC-like non-selective cation current activated by alpha 1-adrenoceptors in rat mesenteric artery smooth muscle cells. Cell Calcium. 2006;40:29–40. doi: 10.1016/j.ceca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 112.Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- 113.Hobara N, Goda M, Hashikawa N, Jin X, Zamami Y, Takatori S, Kawasaki H. Role of angiontensin receptors in remodeling perivascular nerves. Yakugaku Zasshi. 2010;130:1421–1425. doi: 10.1248/yakushi.130.1421. [DOI] [PubMed] [Google Scholar]
- 114.Hobara N, Nakamura A, Ohtsuka A, Narasaki M, Shibata K, Gomoita Y, Kawasaki H. Distribution of adrenomedullin-containing perivascular nerves in the rat mesenteric artery. Peptides. 2004;25:589–599. doi: 10.1016/j.peptides.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 115.Hodges GJ, Jackson DN, Mattar L, Johnson JM, Shoemaker JK. Neuropeptide Y and neurovascular control in skeletal muscle and skin. Am J Physiol Regul Integr Comp Physiol. 2009;297:R546–555. doi: 10.1152/ajpregu.00157.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.HOLTON P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Honig CR, Frierson JL. Neurons intrinsic to arterioles initiate postcontraction vasodilation. Am J Physiol. 1976;230:493–507. doi: 10.1152/ajplegacy.1976.230.2.493. [DOI] [PubMed] [Google Scholar]
- 118.Hussain MB, Marshall I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol. 1997;122:849–858. doi: 10.1038/sj.bjp.0701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. Beta(2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–355. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- 120.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121:3664–3673. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 122.Ishikawa H, Honda T, Toriyama K, Torii S, Sugiura Y. Origin and course of nerves immunoreactive for calcitonin gene-related peptide surrounding the femoral artery in rat. Anat Embryol (Berl) 2003;207:299–305. doi: 10.1007/s00429-003-0359-9. [DOI] [PubMed] [Google Scholar]
- 123.Jackson DN, Milne KJ, Noble EG, Shoemaker JK. Gender-modulated endogenous baseline neuropeptide Y Y1-receptor activation in the hindlimb of Sprague-Dawley rats. J Physiol. 2005;562:285–294. doi: 10.1113/jphysiol.2004.076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jackson DN, Noble EG, Shoemaker JK. Y1- and alpha1-receptor control of basal hindlimb vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;287:R228–233. doi: 10.1152/ajpregu.00723.2003. [DOI] [PubMed] [Google Scholar]
- 125.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol. 2008;155:514–524. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jacques D, Abdel-Samad D. Neuropeptide Y (NPY) and NPY receptors in the cardiovascular system: implication in the regulation of intracellular calcium. Can J Physiol Pharmacol. 2007;85:43–53. doi: 10.1139/Y06-106. [DOI] [PubMed] [Google Scholar]
- 127.Jacques D, Sader S, El-Bizri N, Chouffani S, Hassan G, Shbaklo H. Neuropeptide Y induced increase of cytosolic and nuclear Ca2+ in heart and vascular smooth muscle cells. Can J Physiol Pharmacol. 2000;78:162–172. [PubMed] [Google Scholar]
- 128.Jarajapu YP, Coats P, McGrath JC, Hillier C, MacDonald A. Functional characterization of alpha(1)-adrenoceptor subtypes in human skeletal muscle resistance arteries. Br J Pharmacol. 2001;133:679–686. doi: 10.1038/sj.bjp.0704130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jennings BL, Donald JA. Neurally-derived nitric oxide regulates vascular tone in pulmonary and cutaneous arteries of the toad, Bufo marinus. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1640–1646. doi: 10.1152/ajpregu.00057.2008. [DOI] [PubMed] [Google Scholar]
- 130.Kaji A, Shigematsu H, Fujita K, Maeda T, Watanabe S. Parasympathetic innervation of cutaneous blood vessels by vasoactive intestinal polypeptide-immunoreactive and acetylcholinesterase-positive nerves: histochemical and experimental study on rat lower lip. Neuroscience. 1988;25:353–362. doi: 10.1016/0306-4522(88)90031-0. [DOI] [PubMed] [Google Scholar]
- 131.Kamata K, Miyata N, Kasuya Y. Involvement of endothelial cells in relaxation and contraction responses of the aorta to isoproterenol in naive and streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 1989;249:890–894. [PubMed] [Google Scholar]
- 132.Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008;131:293–305. doi: 10.1085/jgp.200709934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kashihara Y, Sakaguchi M, Kuno M. Axonal transport and distribution of endogenous calcitonin gene-related peptide in rat peripheral nerve. J Neurosci. 1989;9:3796–3802. doi: 10.1523/JNEUROSCI.09-11-03796.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kawaja MD. Sympathetic and sensory innervation of the extracerebral vasculature: roles for p75NTR neuronal expression and nerve growth factor. J Neurosci Res. 1998;52:295–306. doi: 10.1002/(SICI)1097-4547(19980501)52:3<295::AID-JNR6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 136.Kawasaki H. Regulation of vascular function by perivascular calcitonin gene-related peptide-containing nerves. Jpn J Pharmacol. 2002;88:39–43. doi: 10.1254/jjp.88.39. [DOI] [PubMed] [Google Scholar]
- 137.Kawasaki H, Nuki C, Saito A, Takasaki K. Adrenergic modulation of calcitonin gene-related peptide (CGRP)-containing nerve-mediated vasodilation in the rat mesenteric resistance vessel. Brain Res. 1990;506:287–290. doi: 10.1016/0006-8993(90)91263-g. [DOI] [PubMed] [Google Scholar]
- 138.Kawasaki H, Nuki C, Saito A, Takasaki K. Role of calcitonin gene-related peptide-containing nerves in the vascular adrenergic neurotransmission. J Pharmacol Exp Ther. 1990;252:403–409. [PubMed] [Google Scholar]
- 139.Kawasaki H, Saito A, Takasaki K. Age-related decrease of calcitonin gene-related peptide-containing vasodilator innervation in the mesenteric resistance vessel of the spontaneously hypertensive rat. Circ Res. 1990;67:733–743. doi: 10.1161/01.res.67.3.733. [DOI] [PubMed] [Google Scholar]
- 140.Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- 141.Kawasaki H, Takatori S, Zamami Y, Koyama T, Goda M, Hirai K, Tangsucharit P, Jin X, Hobara N, Kitamura Y. Paracrine control of mesenteric perivascular axo-axonal interaction. Acta Physiol (Oxf) 2011;203:3–11. doi: 10.1111/j.1748-1716.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 142.Kelly RB. Storage and release of neurotransmitters. Cell. 1993;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 143.Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985;107:161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- 144.Kennedy C, Saville VL, Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986;122:291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- 145.Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, Welsh DG, Plane F. Endothelial feedback and the myoendothelial projection. Microcirculation. 2012;19:416–422. doi: 10.1111/j.1549-8719.2012.00187.x. [DOI] [PubMed] [Google Scholar]
- 146.Kimura T, Yu JG, Edvinsson L, Lee TJ. Cholinergic, nitric oxidergic innervation in cerebral arteries of the cat. Brain Res. 1997;773:117–124. doi: 10.1016/s0006-8993(97)00889-5. [DOI] [PubMed] [Google Scholar]
- 147.Kobayashi S, Mwaka ES, Meir A, Uchida K, Takeno K, Miyazaki T, Kubota M, Nakajima H, Nomura E, Yoshizawa H, Baba H. Vasomotion of intraradicular microvessels in rat. Spine. 2009;34:990–997. doi: 10.1097/BRS.0b013e3181a100bf. [DOI] [PubMed] [Google Scholar]
- 148.Kornfeld M, Salomonsson M, Gutierrez A, Persson AE. The influence of beta-adrenergic activation on noradrenergic alpha1 activation of rabbit afferent arterioles. Pflugers Arch. 2000;441:25–31. doi: 10.1007/s004240000382. [DOI] [PubMed] [Google Scholar]
- 149.Kotecha N. Modulation of submucosal arteriolar tone by neuropeptide Y Y2 receptors in the guinea-pig small intestine. J Auton Nerv Syst. 1998;70:157–163. doi: 10.1016/s0165-1838(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 150.Kotecha N, Neild TO. Actions of vasodilator nerves on arteriolar smooth muscle and neurotransmitter release from sympathetic nerves in the guinea-pig small intestine. J Physiol. 1995;489 ( Pt 3):849–855. doi: 10.1113/jphysiol.1995.sp021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koyama T, Hatanaka Y, Jin X, Yokomizo A, Fujiwara H, Goda M, Hobara N, Zamami Y, Kitamura Y, Kawasaki H. Altered function of nitrergic nerves inhibiting sympathetic neurotransmission in mesenteric vascular beds of renovascular hypertensive rats. Hypertens Res. 2010;33:485–491. doi: 10.1038/hr.2010.48. [DOI] [PubMed] [Google Scholar]
- 152.Krogh A, Harrop GA, Rehberg PB. Studies on the physiology of capillaries: III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922;56:179–189. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kulkarni AP, Getchell TV, Getchell ML. Neuronal nitric oxide synthase is localized in extrinsic nerves regulating perireceptor processes in the chemosensory nasal mucosae of rats and humans. J Comp Neurol. 1994;345:125–138. doi: 10.1002/cne.903450110. [DOI] [PubMed] [Google Scholar]
- 154.LG . Multiple receptors and multiple actions. In: LG, SRB, editors. Neuropeptide Y and Drug Development. San Diego, CA: Academic; 1997. [Google Scholar]
- 155.Lagaud GJ, Stoclet JC, Andriantsitohaina R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J Physiol. 1996;492 ( Pt 3):689–703. doi: 10.1113/jphysiol.1996.sp021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lamboley M, Pittet P, Koenigsberger M, Sauser R, Bény JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium. 2005;37:311–320. doi: 10.1016/j.ceca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 157.Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003;549:801–808. doi: 10.1113/jphysiol.2003.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic postjunctional Ca2+ transients in mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3106–3113. doi: 10.1152/ajpheart.00466.2006. [DOI] [PubMed] [Google Scholar]
- 159.Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- 160.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 161.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38:141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 163.Laufer R, Changeux JP. Calcitonin gene-related peptide and cyclic AMP stimulate phosphoinositide turnover in skeletal muscle cells. Interaction between two second messenger systems. J Biol Chem. 1989;264:2683–2689. [PubMed] [Google Scholar]
- 164.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci. 2010;154:66–73. doi: 10.1016/j.autneu.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 166.Li YJ, Duckles SP. Effect of endothelium on the actions of sympathetic and sensory nerves in the perfused rat mesentery. Eur J Pharmacol. 1992;210:23–30. doi: 10.1016/0014-2999(92)90647-m. [DOI] [PubMed] [Google Scholar]
- 167.Lindsay TH, Halvorson KG, Peters CM, Ghilardi JR, Kuskowski MA, Wong GY, Mantyh PW. A quantitative analysis of the sensory and sympathetic innervation of the mouse pancreas. Neuroscience. 2006;137:1417–1426. doi: 10.1016/j.neuroscience.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 168.Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- 169.Lobaugh LA, Blackshear PJ. Neuropeptide Y stimulation of myosin light chain phosphorylation in cultured aortic smooth muscle cells. J Biol Chem. 1990;265:18393–18399. [PubMed] [Google Scholar]
- 170.Loesch A, Belai A, Burnstock G. An ultrastructural study of NADPH-diaphorase and nitric oxide synthase in the perivascular nerves and vascular endothelium of the rat basilar artery. J Neurocytol. 1994;23:49–59. doi: 10.1007/BF01189816. [DOI] [PubMed] [Google Scholar]
- 171.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Long JB, Segal SS. Quantifying perivascular sympathetic innervation: regional differences in male C57BL/6 mice at 3 and 20 months. J Neurosci Methods. 2009;184:124–128. doi: 10.1016/j.jneumeth.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Looft-Wilson RC, Haug SJ, Neufer PD, Segal SS. Independence of connexin expression and vasomotor conduction from sympathetic innervation in hamster feed arteries. Microcirculation. 2004;11:397–408. doi: 10.1080/10739680490457782. [DOI] [PubMed] [Google Scholar]
- 174.Luff SE. Ultrastructure of sympathetic axons and their structural relationship with vascular smooth muscle. Anat Embryol (Berl) 1996;193:515–531. doi: 10.1007/BF00187924. [DOI] [PubMed] [Google Scholar]
- 175.Luff SE, McLachlan EM. Frequency of neuromuscular junctions on arteries of different dimensions in the rabbit, guinea pig and rat. Blood Vessels. 1989;26:95–106. doi: 10.1159/000158758. [DOI] [PubMed] [Google Scholar]
- 176.Luff SE, Young SB, McLachlan EM. Ultrastructure of substance P-immunoreactive terminals and their relation to vascular smooth muscle cells of rat small mesenteric arteries. J Comp Neurol. 2000;416:277–290. [PubMed] [Google Scholar]
- 177.Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- 178.Lundberg JM, Franco-Cereceda A, Lou YP, Modin A, Pernow J. Differential release of classical transmitters and peptides. Adv Second Messenger Phosphoprotein Res. 1994;29:223–234. doi: 10.1016/s1040-7952(06)80018-2. [DOI] [PubMed] [Google Scholar]
- 179.Lundberg JM, Modin A. Inhibition of sympathetic vasoconstriction in pigs in vivo by the neuropeptide Y-Y1 receptor antagonist BIBP 3226. Br J Pharmacol. 1995;116:2971–2982. doi: 10.1111/j.1476-5381.1995.tb15952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lundberg JM, Stjarne L. Neuropeptide Y (NPY) depresses the secretion of 3H-noradrenaline and the contractile response evoked by field stimulation, in rat vas deferens. Acta Physiol Scand. 1984;120:477–479. doi: 10.1111/j.1748-1716.1984.tb07410.x. [DOI] [PubMed] [Google Scholar]
- 181.Malmström RE, Lundberg JO, Weitzberg E. Autoinhibitory function of the sympathetic prejunctional neuropeptide Y Y(2) receptor evidenced by BIIE0246. Eur J Pharmacol. 2002;439:113–119. doi: 10.1016/s0014-2999(02)01371-7. [DOI] [PubMed] [Google Scholar]
- 182.Malmström RE, Modin A, Lundberg JM. SR 120107A antagonizes neuropeptide Y Y1 receptor mediated sympathetic vasoconstriction in pigs in vivo. Eur J Pharmacol. 1996;305:145–154. doi: 10.1016/0014-2999(96)00164-1. [DOI] [PubMed] [Google Scholar]
- 183.Marshall J, Tandon H. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 186.McLachlan EM. Transmission of signals through sympathetic ganglia--modulation, integration or simply distribution? Acta Physiol Scand. 2003;177:227–235. doi: 10.1046/j.1365-201X.2003.01075.x. [DOI] [PubMed] [Google Scholar]
- 187.Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974;242:143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miao FJ, Lee TJ. Cholinergic and VIPergic innervation in cerebral arteries: a sequential double-labeling immunohistochemical study. J Cereb Blood Flow Metab. 1990;10:32–37. doi: 10.1038/jcbfm.1990.4. [DOI] [PubMed] [Google Scholar]
- 189.Minneman KP. Alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+ Pharmacol Rev. 1988;40:87–119. [PubMed] [Google Scholar]
- 190.Molenaar P, Malta E, Jones CR, Buxton BF, Summers RJ. Autoradiographic localization and function of beta-adrenoceptors on the human internal mammary artery and saphenous vein. Br J Pharmacol. 1988;95:225–233. doi: 10.1111/j.1476-5381.1988.tb16568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moore AW, Jackson WF, Segal SS. Regional heterogeneity of α-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol. 2010;588:4261–4274. doi: 10.1113/jphysiol.2010.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mori A, Hanada M, Sakamoto K, Nakahara T, Ishii K. Noradrenaline contracts rat retinal arterioles via stimulation of α(1A)- and α(1D)-adrenoceptors. Eur J Pharmacol. 2011;673:65–69. doi: 10.1016/j.ejphar.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 193.Morita Y, Hardebo JE, Bouskela E. Influence of cerebrovascular parasympathetic nerves on resting cerebral blood flow, spontaneous vasomotion, autoregulation, hypercapnic vasodilation and sympathetic vasoconstriction. J Auton Nerv Syst. 1994;49 (Suppl):S9–14. doi: 10.1016/0165-1838(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 194.Moritoki H, Takase H, Tanioka A. Dual effects of capsaicin on responses of the rabbit ear artery to field stimulation. Br J Pharmacol. 1990;99:152–156. doi: 10.1111/j.1476-5381.1990.tb14669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Morris JL, Anderson RL, Gibbins IL. Neuropeptide Y immunoreactivity in cutaneous sympathetic and sensory neurons during development of the guinea pig. J Comp Neurol. 2001;437:321–334. doi: 10.1002/cne.1286. [DOI] [PubMed] [Google Scholar]
- 196.Morris JL, Gibbins IL, Campbell G, Murphy R, Furness JB, Costa M. Innervation of the large arteries and heart of the toad (Bufo marinus) by adrenergic and peptide-containing neurons. Cell Tissue Res. 1986;243:171–184. doi: 10.1007/BF00221866. [DOI] [PubMed] [Google Scholar]
- 197.Nakakita K. Peptidergic innervation in the cerebral blood vessels of the guinea pig: an immunohistochemical study. J Cereb Blood Flow Metab. 1990;10:819–826. doi: 10.1038/jcbfm.1990.138. [DOI] [PubMed] [Google Scholar]
- 198.Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H594–602. doi: 10.1152/ajpheart.00773.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 200.Nguyen LS, Villablanca AC, Rutledge JC. Substance P increases microvascular permeability via nitric oxide-mediated convective pathways. Am J Physiol. 1995;268:R1060–1068. doi: 10.1152/ajpregu.1995.268.4.R1060. [DOI] [PubMed] [Google Scholar]
- 201.Nilsson H, Goldstein M, Nilsson O. Adrenergic innervation and neurogenic response in large and small arteries and veins from the rat. Acta Physiol Scand. 1986;126:121–133. doi: 10.1111/j.1748-1716.1986.tb07795.x. [DOI] [PubMed] [Google Scholar]
- 202.Nuki C, Kawasaki H, Takasaki K. Effect of neuropeptide Y on vasodilation mediated by calcitonin gene-related peptide (CGRP)-containing nerves in the mesenteric resistance vessel of the rat. Jpn J Pharmacol. 1990;53:125–128. doi: 10.1254/jjp.53.125. [DOI] [PubMed] [Google Scholar]
- 203.Nuki C, Kawasaki H, Takasaki K, Wada A. Pharmacological characterization of presynaptic calcitonin gene-related peptide (CGRP) receptors on CGRP-containing vasodilator nerves in rat mesenteric resistance vessels. J Pharmacol Exp Ther. 1994;268:59–64. [PubMed] [Google Scholar]
- 204.Ogawa F, Hanamitsu M, Ayajiki K, Aimi Y, Okamura T, Shimizu T. Effect of nitric oxide synthase inhibitor on increase in nasal mucosal blood flow induced by sensory and parasympathetic nerve stimulation in rats. Ann Otol Rhinol Laryngol. 2010;119:424–430. doi: 10.1177/000348941011900610. [DOI] [PubMed] [Google Scholar]
- 205.Ohyanagi M, Faber JE, Nishigaki K. Differential activation of alpha 1- and alpha 2-adrenoceptors on microvascular smooth muscle during sympathetic nerve stimulation. Circ Res. 1991;68:232–244. doi: 10.1161/01.res.68.1.232. [DOI] [PubMed] [Google Scholar]
- 206.Omar NM, Marshall JM. Age-related changes in the sympathetic innervation of cerebral vessels and in carotid vascular responses to norepinephrine in the rat: in vitro and in vivo studies. J Appl Physiol. 2010;109:314–322. doi: 10.1152/japplphysiol.01251.2009. [DOI] [PubMed] [Google Scholar]
- 207.Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. Chemical calcium indicators. Methods. 2008;46:143–151. doi: 10.1016/j.ymeth.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol. 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- 209.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 210.Phillips JK, Hickey H, Hill CE. Heterogeneity in mechanisms underlying vasodilatory responses in small arteries of the rat hepatic mesentery. Auton Neurosci. 2000;83:159–170. doi: 10.1016/S1566-0702(00)00175-2. [DOI] [PubMed] [Google Scholar]
- 211.Piascik MT, Soltis EE, Piascik MM, Macmillan LB. Alpha-adrenoceptors and vascular regulation: molecular, pharmacologic and clinical correlates. Pharmacol Ther. 1996;72:215–241. doi: 10.1016/s0163-7258(96)00117-9. [DOI] [PubMed] [Google Scholar]
- 212.Povstyan OV, Harhun MI, Gordienko DV. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br J Pharmacol. 2011;162:1618–1638. doi: 10.1111/j.1476-5381.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemény A, Materazzi S, Nassini R, Helyes Z, Szolcsányi J, Pintér E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol. 2012;689:56–64. doi: 10.1016/j.ejphar.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 214.Queen LR, Ferro A. Beta-adrenergic receptors and nitric oxide generation in the cardiovascular system. Cell Mol Life Sci. 2006;63:1070–1083. doi: 10.1007/s00018-005-5451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Raat NJ, Wetzels GE, De Mey JG. Calcium-contraction relationship in rat mesenteric arterial smooth muscle. Effects of exogenous and neurogenic noradrenaline. Pflugers Arch. 1998;436:262–269. doi: 10.1007/s004240050631. [DOI] [PubMed] [Google Scholar]
- 216.Raddino R, Pelà G, Manca C, Barbagallo M, D’Aloia A, Passeri M, Visioli O. Mechanism of action of human calcitonin gene-related peptide in rabbit heart and in human mammary arteries. J Cardiovasc Pharmacol. 1997;29:463–470. doi: 10.1097/00005344-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 217.Ralevic V. Purines as neurotransmitters and neuromodulators in blood vessels. Curr Vasc Pharmacol. 2009;7:3–14. doi: 10.2174/157016109787354123. [DOI] [PubMed] [Google Scholar]
- 218.Ralevic V. The involvement of smooth muscle P2X receptors in the prolonged vasorelaxation response to purine nucleotides in the rat mesenteric arterial bed. Br J Pharmacol. 2002;135:1988–1994. doi: 10.1038/sj.bjp.0704663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 220.Recio P, Orensanz LM, Martínez MP, Navarro-Dorado J, Bustamante S, García-Sacristán A, Prieto D, Hernández M. Noradrenergic vasoconstriction of pig prostatic small arteries. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:397–406. doi: 10.1007/s00210-007-0227-x. [DOI] [PubMed] [Google Scholar]
- 221.Ren LM, Nakane T, Chiba S. Purinergic and adrenergic transmission and their presynaptic modulation in canine isolated perfused splenic arteries. Eur J Pharmacol. 1996;295:61–68. doi: 10.1016/0014-2999(95)00654-0. [DOI] [PubMed] [Google Scholar]
- 222.Reslerova M, Loutzenhiser R. Renal microvascular actions of calcitonin gene-related peptide. Am J Physiol. 1998;274:F1078–1085. doi: 10.1152/ajprenal.1998.274.6.F1078. [DOI] [PubMed] [Google Scholar]
- 223.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 224.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 225.Rowell LB. Importance of scintigraphic measurements of human splanchnic blood volume. J Nucl Med. 1990;31:160–162. [PubMed] [Google Scholar]
- 226.Ruocco I, Cuello AC, Parent A, Ribeiro-da-Silva A. Skin blood vessels are simultaneously innervated by sensory, sympathetic, and parasympathetic fibers. J Comp Neurol. 2002;448:323–336. doi: 10.1002/cne.10241. [DOI] [PubMed] [Google Scholar]
- 227.Saitongdee P, Milner P, Loesch A, Knight G, Burnstock G. Electron-immunocytochemical studies of perivascular nerves of mesenteric and renal arteries of golden hamsters during and after arousal from hibernation. J Anat. 1999;195 ( Pt 1):121–130. doi: 10.1046/j.1469-7580.1999.19510121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 229.Sandow SL, Hill CE. Physiological and anatomical studies of the development of the sympathetic innervation to rat iris arterioles. J Auton Nerv Syst. 1999;77:152–163. [PubMed] [Google Scholar]
- 230.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60:643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 231.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19:403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 233.Satake N, Shibata M, Shibata S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br J Pharmacol. 1996;119:505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schenk EA, el-Badawi A. Dual innervation of arteries and arterioles. A histochemical study. Z Zellforsch Mikrosk Anat. 1968;91:170–177. doi: 10.1007/BF00364308. [DOI] [PubMed] [Google Scholar]
- 235.Scott TM, Drodge KH, Foote J. Peptidergic nerve involvement in the control of endothelium-dependent vascular relaxation. Artery. 1992;19:211–224. [PubMed] [Google Scholar]
- 236.Scott TM, Robinson J, Foote J. The peptidergic innervation of the developing mesenteric vascular bed in the rat. J Anat. 1989;162:177–183. [PMC free article] [PubMed] [Google Scholar]
- 237.Segal SS, Bény JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992;263:H1–7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 238.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 239.Sequeira IM, Haberberger RV, Kummer W. Atrial and ventricular rat coronary arteries are differently supplied by noradrenergic, cholinergic and nitrergic, but not sensory nerve fibres. Ann Anat. 2005;187:345–355. doi: 10.1016/j.aanat.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 240.Sexton PM, Albiston A, Morfis M, Tilakaratne N. Receptor activity modifying proteins. Cell Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 241.Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- 242.Siegl D, Koeppen M, Wölfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 243.Simonsen U, Triguero D, García-Sacristán A, Prieto D. Cholinergic modulation of non-adrenergic, non-cholinergic relaxation in isolated, small coronary arteries from lambs. Pflugers Arch. 1999;438:177–186. doi: 10.1007/s004240050896. [DOI] [PubMed] [Google Scholar]
- 244.Sjöblom-Widfeldt N, Gustafsson H, Nilsson H. Transmitter characteristics of small mesenteric arteries from the rat. Acta Physiol Scand. 1990;138:203–212. doi: 10.1111/j.1748-1716.1990.tb08834.x. [DOI] [PubMed] [Google Scholar]
- 245.Smoliar E, Smoliar A, Belkin VS. Innervation of human trigeminal nerve blood vessels. Cells Tissues Organs. 1999;165:40–44. doi: 10.1159/000016672. [DOI] [PubMed] [Google Scholar]
- 246.Socha MJ, Behringer EJ, Segal SS. Calcium and electrical signalling along endothelium of the resistance vasculature. Basic Clin Pharmacol Toxicol. 2012;110:80–86. doi: 10.1111/j.1742-7843.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Southwell BR, Chamley-Campbell JH, Campbell GR. Tropic interactions between sympathetic nerves and vascular smooth muscle. J Auton Nerv Syst. 1985;13:343–354. doi: 10.1016/0165-1838(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 249.Stanarius A, Seidel B, Wolf G. Neuronal nitric oxide synthase in the vasculature of the rat brain: an immunocytochemical study using the tyramide signal amplification technique. J Neurocytol. 1998;27:731–736. doi: 10.1023/a:1006998801135. [DOI] [PubMed] [Google Scholar]
- 250.Stjärne L. Novel dual ‘small’ vesicle model of ATP- and noradrenaline-mediated sympathetic neuromuscular transmission. Auton Neurosci. 2001;87:16–36. doi: 10.1016/S1566-0702(00)00246-0. [DOI] [PubMed] [Google Scholar]
- 251.Storkebaum E, Carmeliet P. Paracrine control of vascular innervation in health and disease. Acta Physiol (Oxf) 2011;203:61–86. doi: 10.1111/j.1748-1716.2011.02333.x. [DOI] [PubMed] [Google Scholar]
- 252.Straub AC, Johnstone SR, Heberlein KR, Rizzo MJ, Best AK, Boitano S, Isakson BE. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res. 2010;47:277–286. doi: 10.1159/000265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Straub SV, Wagner LE, Bruce JI, Yule DI. Modulation of cytosolic calcium signaling by protein kinase A-mediated phosphorylation of inositol 1,4,5-trisphosphate receptors. Biol Res. 2004;37:593–602. doi: 10.4067/s0716-97602004000400013. [DOI] [PubMed] [Google Scholar]
- 255.Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1–11. doi: 10.1152/ajpregu.00269.2007. [DOI] [PubMed] [Google Scholar]
- 256.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 257.Toda M, Okamura T, Azuma I, Toda N. Modulation by neurogenic acetylcholine of nitroxidergic nerve function in porcine ciliary arteries. Invest Ophthalmol Vis Sci. 1997;38:2261–2269. [PubMed] [Google Scholar]
- 258.Toda N, Ayajiki K, Okamura T. Inhibition of nitroxidergic nerve function by neurogenic acetylcholine in monkey cerebral arteries. J Physiol. 1997;498 ( Pt 2):453–461. doi: 10.1113/jphysiol.1997.sp021871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Toyoshima H, Nasa Y, Hashizume Y, Koseki Y, Isayama Y, Kohsaka Y, Yamada T, Takeo S. Modulation of cAMP-mediated vasorelaxation by endothelial nitric oxide and basal cGMP in vascular smooth muscle. J Cardiovasc Pharmacol. 1998;32:543–551. doi: 10.1097/00005344-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 260.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302:C1226–1242. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tran CH, Welsh DG. Current perspective on differential communication in small resistance arteries. Can J Physiol Pharmacol. 2009;87:21–28. doi: 10.1139/Y08-104. [DOI] [PubMed] [Google Scholar]
- 262.Tsuruta T, Masuko S, Watanabe H. Immunohistochemical study of the sympathetic and sensory innervation to the blood vessels of the dog forepaw. Tohoku J Exp Med. 1992;168:549–560. doi: 10.1620/tjem.168.549. [DOI] [PubMed] [Google Scholar]
- 263.Tuttle JL, Falcone JC. Nitric oxide release during alpha1-adrenoceptor-mediated constriction of arterioles. Am J Physiol Heart Circ Physiol. 2001;281:H873–881. doi: 10.1152/ajpheart.2001.281.2.H873. [DOI] [PubMed] [Google Scholar]
- 264.Uvnäs B. Cholinergic vasodilator nerves. Fed Proc. 1966;25:1618–1622. [PubMed] [Google Scholar]
- 265.van Zwieten PA, Doods HN. Muscarinic receptors and drugs in cardiovascular medicine. Cardiovasc Drugs Ther. 1995;9:159–167. doi: 10.1007/BF00877757. [DOI] [PubMed] [Google Scholar]
- 266.Vanhoutte PM, Verbeuren TJ, Webb RC. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981;61:151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]
- 267.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Vass Z, Dai CF, Steyger PS, Jancsó G, Trune DR, Nuttall AL. Co-localization of the vanilloid capsaicin receptor and substance P in sensory nerve fibers innervating cochlear and vertebro-basilar arteries. Neuroscience. 2004;124:919–927. doi: 10.1016/j.neuroscience.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vedernikov YP, Fulep EE, Saade GR, Garfield RE. Calcitonin gene-related peptide dilates the pregnant rat uterine vascular bed via guanylate cyclase, ATP-and Ca-sensitive potassium channels and gap junctions. Curr Med Res Opin. 2002;18:465–470. doi: 10.1185/030079902125001001. [DOI] [PubMed] [Google Scholar]
- 270.Wahlestedt C, Edvinsson L, Ekblad E, Håkanson R. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J Pharmacol Exp Ther. 1985;234:735–741. [PubMed] [Google Scholar]
- 271.Wang L, Karlsson L, Moses S, Hultgårdh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 272.Wang LH, Luo M, Wang Y, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. J Hypertens. 2006;24:2399–2408. doi: 10.1097/01.hjh.0000251900.78051.56. [DOI] [PubMed] [Google Scholar]
- 273.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507 ( Pt 1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol. 1997;273:H156–163. doi: 10.1152/ajpheart.1997.273.1.H156. [DOI] [PubMed] [Google Scholar]
- 275.Westcott E, Segal S. Ageing alters perivascular nerve function of mouse mesenteric arteries in vivo. J Physiol. 2013 doi: 10.1113/jphysiol.2012.244483. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.White JD, Stewart KD, Krause JE, McKelvy JF. Biochemistry of peptide-secreting neurons. Physiol Rev. 1985;65:553–606. doi: 10.1152/physrev.1985.65.3.553. [DOI] [PubMed] [Google Scholar]
- 277.Whittle BJ, Lopez-Belmonte J, Rees DD. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989;98:646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wier WG, Zang WJ, Lamont C, Raina H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol. 2009;94:31–37. doi: 10.1113/expphysiol.2008.043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wisskirchen FM, Burt RP, Marshall I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br J Pharmacol. 1998;123:1673–1683. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol. 2004;555:545–563. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Xiong Z, Sperelakis N. Regulation of L-type calcium channels of vascular smooth muscle cells. J Mol Cell Cardiol. 1995;27:75–91. doi: 10.1016/s0022-2828(08)80009-0. [DOI] [PubMed] [Google Scholar]
- 282.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 283.Yang XP, Chiba S. Neuropeptide Y inhibits double peaked vasoconstrictor responses to periarterial nerve stimulation primarily through prejunctional Y2 receptor subtype in canine splenic arteries. Auton Autacoid Pharmacol. 2002;22:119–126. doi: 10.1046/j.1474-8673.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- 284.Yaprak M. The Axon Reflex. Neuroanatomy. 2008;7:17–19. [Google Scholar]
- 285.Yashiro Y, Duling BR. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- 286.Yoshida K, Okamura T, Kimura H, Bredt DS, Snyder SH, Toda N. Nitric oxide synthase-immunoreactive nerve fibers in dog cerebral and peripheral arteries. Brain Res. 1993;629:67–72. doi: 10.1016/0006-8993(93)90482-3. [DOI] [PubMed] [Google Scholar]
- 287.Yu JG, Kimura T, Chang XF, Lee TJ. Segregation of VIPergic-nitric oxidergic and cholinergic-nitric oxidergic innervation in porcine middle cerebral arteries. Brain Res. 1998;801:78–87. doi: 10.1016/s0006-8993(98)00548-4. [DOI] [PubMed] [Google Scholar]
- 288.Zacharia J, Hillier C, MacDonald A. Alpha1-adrenoceptor subtypes involved in vasoconstrictor responses to exogenous and neurally released noradrenaline in rat femoral resistance arteries. Br J Pharmacol. 2004;141:915–924. doi: 10.1038/sj.bjp.0705690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zang WJ, Zacharia J, Lamont C, Wier WG. Sympathetically evoked Ca2+ signaling in arterial smooth muscle. Acta Pharmacol Sin. 2006;27:1515–1525. doi: 10.1111/j.1745-7254.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 290.Zhu BS, Blessing WW, Gibbins IL. Parasympathetic innervation of cephalic arteries in rabbits: comparison with sympathetic and sensory innervation. J Comp Neurol. 1997;389:484–495. doi: 10.1002/(sici)1096-9861(19971222)389:3<484::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 291.Zhu W, Zhang Y, Han C. Characterization of subtype of alpha1-adrenoceptor mediating vasoconstriction in perfused rat hind limb. Eur J Pharmacol. 1997;329:55–61. doi: 10.1016/s0014-2999(97)10104-2. [DOI] [PubMed] [Google Scholar]
- 292.Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–278. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]