Robotic sacrocolpopexy may increase insufflation complications including massive subcutaneous emphysema.
Keywords: Subcutaneous emphysema, Sacrocolpopexy, Robotic sacrocolpopexy, Sacral colpopexy, Laparoscopy, Complications
Abstract
The advent of robotic surgery has increased the popularity of laparoscopic sacrocolpopexy. Carbon dioxide insufflation, an essential component of laparoscopy, may rarely cause massive subcutaneous emphysema, which may be coincident with life-threatening situations such as hypercarbia, pneumothorax, and pneumomediastinum. Although the literature contains several reports of massive subcutaneous emphysema after a variety of laparoscopic procedures, we were not able to identify any report of this complication associated with laparoscopic or robotic sacrocolpopexy. Massive subcutaneous emphysema occurred in 3 women after robotic sacrocolpopexy in our practice. The patients had remarkable but reversible physical deformities lasting up to 1 week. A valveless endoscopic dynamic pressure system was used in all 3 of our cases. Our objective is to define the risk of massive subcutaneous emphysema during robotic sacrocolpopexy in light of these cases and discuss probable predisposing factors including the use of valveless endoscopic dynamic pressure trocars.
INTRODUCTION
Abdominal sacrocolpopexy is an effective and durable procedure for the correction of apical vaginal prolapse.1 Robotic-assisted laparoscopic application of this procedure has been demonstrated to be a feasible minimally invasive alternative to the open abdominal technique. Although insufflation with carbon dioxide (CO2) for laparoscopic procedures is considered to be relatively safe, there exists a small but important risk of developing complications, including massive subcutaneous emphysema (SE), hypercarbia, pneumothorax, pneuomomediastinum, and even CO2 embolism.2
SE, a well-known complication of laparoscopic surgery, results from leakage of CO2 into the subcutaneous tissue.3 SE usually occurs on the chest, neck, and face and can spread along fascial planes. There is a characteristic crackling sensation palpable beneath the surface of the skin, namely, subcutaneous crepitation.3,4 Mild forms of SE are common, however; massive SE, which can lead to temporary physical deformity, is rare.3–5 To our knowledge, its occurrence during robotic-assisted laparoscopic sacrocolpopexy has not been the focus of any previous reports.
In our practice, we encountered 3 cases of massive SE after robot-assisted sacrocolpopexy. Interestingly, these were the only procedures in our practice for which a valveless endoscopic dynamic pressure system (Airseal, SurgiQuest, Orange, CT) was used. All cases were performed with the patient in a steep Trendelenburg position. A Veress needle was used for initial CO2 insufflation. Pneumoperitoneum was achieved with 3 L of CO2. A total of 5 trocars were placed—1 for an 8-mm robotic camera port, 3 for 8-mm robotic operating ports, and 1 for a 12-mm assistant port (a valveless trocar). Operating time, defined as time from induction of anesthesia to extubation, ranged from 160 to 275 minutes. Massive SE with significant physical deformities developed in 3 patients and lingered for approximately 1 week. Here we present these cases and discuss the possible causes, including use of the valveless continuous CO2 circulating system.
CASE STUDIES
Case 1
A 63-year-old woman (gravida 2, para 2, body mass index [BMI] 22 kg/m2) underwent a robotically assisted laparoscopic total hysterectomy, bilateral salpingo-oophorectomy, and sacrocolpopexy under general anesthesia for stage III pelvic organ prolapse. Her past medical history was uneventful. After a 160-minute uncomplicated procedure, grade III SE (according to the classification proposed by Sumpf et al.) was noted in her upper extremities, upper abdomen, chest, neck, and left side of the face (Figure 1).5 Her start and maximal end-tidal CO2 pressures (PET CO2) were 37 mm Hg and 39 mm Hg, respectively. There was no difficulty with extubation, and the patient was transferred to the postanesthesia recovery unit. She had little pain associated with her surgery, and her main complaint was her physical appearance. General surgery consultation was necessary to reassure her. Although her SE did not lead to any clinical sequelae, consequent physical deformation did not resolve until the seventh postoperative day.
Figure 1.
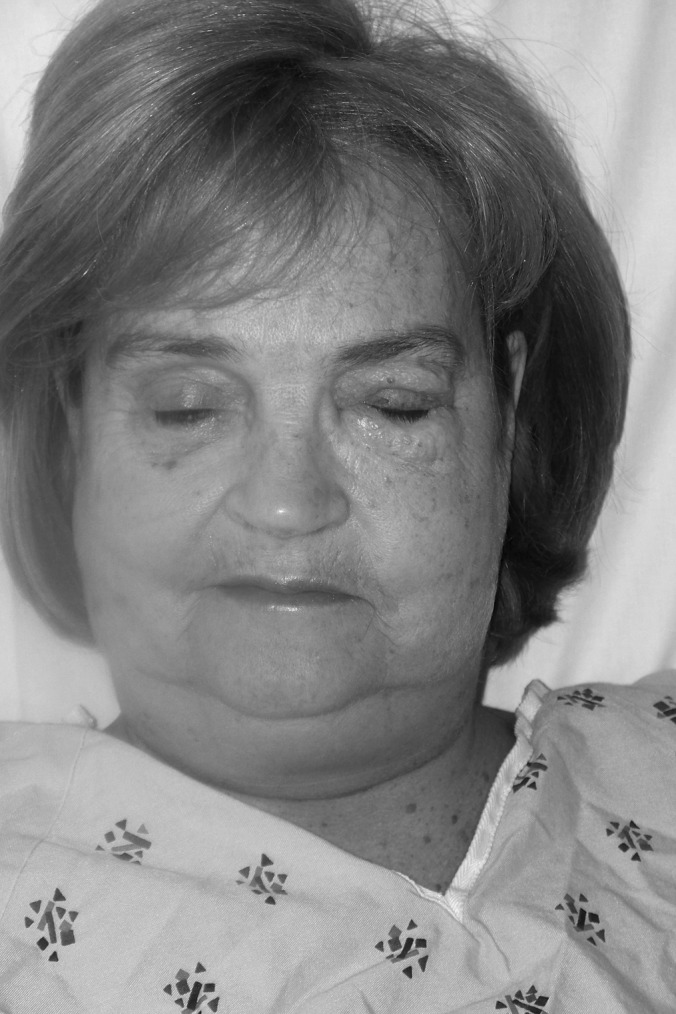
Massive emphysema extending to the face and neck. This woman has a BMI of 22 kg/m2.
Case 2
A 72-year-old woman (gravida 3, para 3, BMI 23 kg/m2) underwent a robotically assisted laparoscopic total hysterectomy, bilateral salpingo-oophorectomy, lysis of adhesions, sacrocolpopexy, and transobturator sling. Her comorbidities were depression, anxiety, hypothyroidism, osteopenia, and hyperlipidemia. Her past surgical history included a vaginal cystocele repair, appendectomy, tonsillectomy, and ventral herniorrhaphy. She was taking levothyroxin, sertraline, baby aspirin, simvastatin, and alendronate. During her procedure, diffuse adhesions from prior surgeries were released without complications. Operative time was 275 minutes. Her start and maximal PET CO2 were 32 mm Hg and 42 mm Hg, respectively. Grade III SE with crepitus was appreciated in her upper extremities, abdomen, neck, and face. There was no difficulty with extubation. She was discharged home on the following day, but on postoperative day 2, she was taken to an outside hospital for shortness of breath. Her chest radiograph was negative for pneumothorax, consolidation, atelectasis, and other findings. She was subsequently released with a presumable acute respiratory tract infection and anxiety. Her massive SE lasted little more than 1 week.
Case 3
A 65-year-old woman (gravida 2, para 2, BMI 25 kg/m2) underwent a robotically assisted laparoscopic sacrocolpopexy and cystoscopy. The procedure required approximately 30 minutes of extra time for lysis of anterior abdominal wall adhesions. Operative time was 290 minutes. Her start and maximal PET CO2 were 33 mm Hg and 51 mm Hg, respectively. Her past medical history was significant for hypothyroidism, diverticulosis, and narrow angle glaucoma. Prior surgical history included a total abdominal hysterectomy, bilateral salpingo-oophorectomy for cervical cancer, and inguinal herniorrhaphy. She was taking levothyroxin. Upon completion of the case, grade III SE was noted on the patient's left side including her face, upper chest, and arm. Her SE resolved completely within 1 week.
DISCUSSION
SE, in its mild form, is not uncommon after laparoscopic procedures. It generally resolves within 1 to 2 days, but its true incidence is underreported.5,6 Based on radiographic and computerized imaging, it has been reported to occur in as much as 99% of all laparoscopic cases.3,4 Massive SE, although rare, is clinically significant because of its potential to cause severe hypercarbia. The CO2 may track along the prefascial planes and cause life-threatening conditions such as pneumothorax, pneumomediastium, pneumopericardium, and the most devastating complication: gas embolism.2
The predisposing factors for SE include factors such as target insufflation pressure and flow rate, low BMI, higher older age, cardiopulmonary diseases, the use of 6 or more laparoscopic ports, and operative time >200 minutes.2,6,7 Gas leaks in or around laparoscopic ports because of improper trocar placement and preperitoneal insufflation may also play a role in the development of SE.8,9 A BMI <25 has been associated with development and persistence of SE.7 SE was shown to resolve within 24 hours in patients with a BMI >25 kg/m2 and in 20% of those with lower BMI. Procedures that require extensive retroperitoneal dissection or that are located near the diaphragm may also increase the risk of massive SE.6 Undoubtedly, sacrocolpopexy is in this class of procedures because it requires peritoneal dissection in the presacral, rectovaginal, and vesicovaginal spaces. In addition, older patient age, steep Trendelenburg positioning, and relatively longer operative time for robotic sacrocolpopexy may also be contributing factors for SE development in these cases.3–6
Massive SE occurred in 3 patients at the time of robotic sacrocolpopexy procedure at our institution. All cases completely resolved within 1 week without sequelae. Patients were satisfied overall with the surgical outcomes at their 3-month postoperative visits. However, dramatic physical changes that occurred because of massive SE prompted remarkable patient concern and anxiety. We decided to analyze these cases in detail to see if there was any preventable factor that incited this complication. Only the patient in case 3 was among the first 10 robotic cases performed by a surgeon. Besides the risk factors inherent to robotic sacrocolpopexy, our patients had a few of the factors that have been associated with massive SE: Although they were in relatively good cardiopulmonary health, all 3 were >60 years of age. Their BMIs were 25 kg/m2 or less. In 2 patients, hysterectomy was performed concomitant with sacrocolpopexy, which prolonged the operating time. Extensive adhesiolysis was needed in cases 2 and 3. An interesting finding was that, among 237 robotic sacrocolpopexy procedures performed at our institution over 3 years, all 3 cases of massive SE occurred within a period of 3 months. More importantly, the valveless endoscopic dynamic pressure trocar system was used in all of them on a trial basis. After our analysis, which increased our suspicion that the valveless system may be related to these incident cases, we decided to return to our standard trocar/insufflation system. We have since had no additional cases of massive SE.
A valveless endoscopic dynamic pressure system works by creating a dynamic equilibrium that uses pressure gradients to separate intra-abdominal gas from ambient air. A recent prospective study demonstrated that valveless trocars are associated with reduced CO2 use, absorption, and elimination.8 In a study by Herati et al, 2 cases of massive SE were reported among 26 laparoscopic renal procedures with this valveless trocar. They postulated that this occurred because of extraperitoneal displacement of the valveless trocar. They were able to prevent SE in later cases by anchoring the trocar to the skin with a suture.7,8 The manufacturer subsequently made some changes to the design of these trocars to avoid this problem.
An association between high positive PET CO2 and SE has previously been demonstrated.6,7 In the study by Murdock et al, PET CO2 pressures of ≥50 mm Hg were found to be a risk for SE. Punnet et al found that starting PET CO2 levels >30 mm Hg and peak PET CO2 exceeding 40 mm Hg were a risk for SE. A difference of >10 mm Hg between these two parameters has been suggested as a predisposing factor for massive SE.10Among our cases with this complication, only case 3 had PET CO2 values consistent with this risk factor.
Robotic sacrocolpopexy may increase insufflation complications including massive SE, possibly because of the extensive retroperitoneal dissection, operating time, and patient positioning. This may rarely be persistent for up to 1 week. Pelvic surgeons should be aware of this potential complication. It may be wise to mention this temporary and almost always benign condition to the patients preoperatively to prevent patient anxiety after the surgery. We hope further research will help determine whether valveless endoscopic dynamic pressure system increases insufflation complications in longer cases.
Contributor Information
Hatice Celik, Department of Obstetrics and Gynecology, Tufts University School of Medicine, Baystate Medical Center, Springfield, MA, USA..
Angela Cremins, Department of Obstetrics and Gynecology, Tufts University School of Medicine, Baystate Medical Center, Springfield, MA, USA..
Keisha A. Jones, Department of Obstetrics and Gynecology, Tufts University School of Medicine, Baystate Medical Center, Springfield, MA, USA..
Oz Harmanli, Department of Obstetrics and Gynecology, Tufts University School of Medicine, Baystate Medical Center, Springfield, MA, USA..
References:
- 1. Nygaard IE, McCreery R, Brubaker L, et al. ; Pelvic Disorder Network Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805–823 [DOI] [PubMed] [Google Scholar]
- 2. Lehmann LJ, Lewis MC, Goldman H, Marshall JR. Cardiopulmonary complications during laparoscopy: two case reports. South Med J. 1995;88(10):1072–1075 [DOI] [PubMed] [Google Scholar]
- 3. Worrell JB, Cleary DT. Massive subcutaneous emphysema and hypercarbia: complications of carbon dioxide absorption during extraperitoneal and intraperitoneal laparoscopic surgery—case studies. AANA J. 2002;70(6):456–462 [PubMed] [Google Scholar]
- 4. Saggar VR, Singhal A, Singh K, Sharma B, Sarangi R. Factors influencing development of subcutaneous carbon dioxide emphysema in laparoscopic totally extraperitoneal inguinal hernia repair. J Laparoendosc Adv Surg Tech A. 2008;18(2):213–216 [DOI] [PubMed] [Google Scholar]
- 5. Sumpf E, Crozier TA, Ahrens D, Brauer A, Neufang T, Braun U. Carbon dioxide absorption during extraperitoneal and transperitoneal endoscopic hernioplasty. Anesh Analg. 2000;91(3):89–95 [DOI] [PubMed] [Google Scholar]
- 6. Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000;95(5):704–709 [DOI] [PubMed] [Google Scholar]
- 7. Lee DW, Kim MJ, Lee YK, Lee HN. Does intraabdominal pressure affect development of subcutaneous emphysema at gynecologic laparoscopy? J Minim Invasive Gynecol. 2011;18(6):761–765 [DOI] [PubMed] [Google Scholar]
- 8. Herati AS, Andonian S, Rais-Bahrami S, et al. Use of the valveless trocar system reduces carbon dioxide absorption during laparoscopy when compared with standard trocars. J Urol. 2011;77:1126–1132 [DOI] [PubMed] [Google Scholar]
- 9. Herati AS, Atalia MA, Rais-Bahrami S, Andonian S, Vira M, Kavoussi LR. A new valve-less trocar for urologic laparoscopy: initial evaluation. J Endourol. 2009;23:1535–1539 [DOI] [PubMed] [Google Scholar]
- 10. Puneet, Mahalik SK, Gupta SK. Infective subcutaneous emphysema after laparoscopic rectopexy: a rare complication. Surg Laparosc Endosc Percutan Tech. 2008;18(3):308–309 [DOI] [PubMed] [Google Scholar]


