Abstract
The active form of vitamin D [1,25-dihydroxycholecalciferol, 1,25(OH)2D3] and the vitamin D receptor (VDR) regulate susceptibility to experimental colitis. The effect of the bacterial microflora on the susceptibility of C57BL/6 mice to dextran sodium sulfate–induced colitis was determined. Mice that cannot produce 1,25(OH)2D3 [Cyp27b1 (Cyp) knockout (KO)], VDR KO as well as their wild-type littermates were used. Cyp KO and VDR KO mice had more bacteria from the Bacteroidetes and Proteobacteria phyla and fewer bacteria from the Firmicutes and Deferribacteres phyla in the feces compared with wild-type. In particular, there were more beneficial bacteria, including the Lactobacillaceae and Lachnospiraceae families, in feces from Cyp KO and VDR KO mice than in feces from wild-type. Helicobacteraceae family member numbers were elevated in Cyp KO compared with wild-type mice. Depletion of the gut bacterial flora using antibiotics protected mice from colitis. 1,25(OH)2D3 treatment (1.25 μg/100 g diet) of Cyp KO mice decreased colitis severity and reduced the numbers of Helicobacteraceae in the feces compared with the numbers in the feces of untreated Cyp KO mice. The mechanisms by which the dysbiosis occurs in VDR KO and Cyp KO mice included lower expression of E-cadherin on gut epithelial and immune cells and fewer tolerogenic dendritic cells that resulted in more gut inflammation in VDR and Cyp KO mice compared with wild-type mice. Increased host inflammation has been shown to provide pathogens with substrates to out-compete more beneficial bacterial species. Our data demonstrate that vitamin D regulates the gut microbiome and that 1,25(OH)2D3 or VDR deficiency results in dysbiosis, leading to greater susceptibility to injury in the gut.
Introduction
Vitamin D is a member of the steroid/thyroid superfamily of hormones. The vitamin D receptor (VDR)9 is a nuclear receptor that in the presence of ligand [1,25-dihydroxycholecalciferol, 1,25(OH)2D3] regulates genes via transcription (1). The active form of vitamin D, 1,25(OH)2D3, is produced from the hydroxylation of the precursor (25-hydroxycholecalciferol) by the enzyme Cyp27B1 (Cyp) found primarily in the kidney. VDRs have been identified in cells of the immune system and in particular vitamin D and 1,25(OH)2D3 regulate gastrointestinal homeostasis and several different experimental models of inflammatory bowel disease (IBD) (2). The targets of vitamin D include macrophages, dendritic cells (DCs), epithelial cells, and T-cells. The described effects of vitamin D in experimental IBD models include the inhibition of Th1/Th17 cells and the induction of several regulatory T-cells (3, 4). The effects of vitamin D on T-cells are both direct and indirect through effects on antigen-presenting cells, including both DCs and macrophages (5, 6). In the presence of 1,25(OH)2D3, DCs do not mature following activation and macrophages produce fewer of several inflammatory cytokines, including TNFα and IL-12 (7, 8). 1,25(OH)2D3-treated DCs are tolerogenic and fail to induce T-cell activation in vivo (7). In addition, vitamin D deficiency is common in patients with Crohn’s disease (9–11) and a couple of small vitamin D interventions suggest that supplementing Crohn’s patients with vitamin D might be beneficial (12, 13). Vitamin D regulates experimental IBD and may be useful in patients with Crohn’s disease.
IBD in both mice and humans develops because of a combination of genetic and environmental factors. Evidence for strong environmental influences on IBD development includes the relatively low concordance rates between identical twins for ulcerative colitis (14–19%) and Crohn’s (20–50%) disease (14). The composition of the gastrointestinal microflora is one of the environmental factors that has been demonstrated to affect the development of IBD. Disruption of the gut microflora or the overgrowth of potentially pathogenic bacteria (dysbiosis) has been demonstrated in patients with IBD (15). Increased numbers of the Proteobacteria and Actinobacteria phyla and decreased Lachnospiraceae from the Firmicutes phylum were present in the intestine of IBD patients compared with healthy controls (15). In addition, treatments with antibiotics (ABX) or probiotics have been shown to improve the symptoms of IBD in some patients (16–19), suggesting the association between intestinal microbiota and IBD pathogenesis. In mice, the development of acute colitis [induced by dextran sodium sulfate (DSS)] as well as chronic colitis [IL-10 knockout (KO)] all depend on the composition of the bacterial microflora (20–22). Interestingly, the IL-10 KO mouse does not develop IBD in the germ-free state, whereas the colitis is more severe in the germ-free mice in the DSS model (20, 22). The bacterial flora can either contribute to intestinal disease (IL-10 KO) or protect from disease (DSS colitis). The relative numbers and types of bacteria present in the gastrointestinal tract are the critical determinants that control the development of colitis.
Here, we determined whether vitamin D regulated experimental IBD by controlling the composition of the gastrointestinal microflora. The composition of the gastrointestinal microflora was evaluated in mice that could not produce active 1,25(OH)2D3 (Cyp KO) and their wild-type (WT) littermates and in mice that cannot respond to vitamin D (VDR KO) and their WT littermates. Our data suggest that vitamin D regulates the composition of the gut bacterial microflora and that deficiency of 1,25(OH)2D3 or VDR caused impaired epithelial integrity, dysbiosis, increased inflammation, and more severe experimental colitis.
Materials and Methods
Mice and diets.
Cyp KO breeders were a gift from Dr. Hector DeLuca (University of Wisconsin). VDR KO breeders were purchased from Jackson Laboratories. Age- and sex-matched C57BL/6 mice were produced and housed at the Pennsylvania State University. Cyp or VDR heterozygote breeders were used to generate WT and KO mice from the same breeders and litters for all experiments. Mice were fed purified diets complete for all nutrients as previously described (23, 24). For some experiments, Cyp KO mice were supplemented daily with 50 ng of 1,25(OH)2D3 (SAFC) in the purified diet (1.25 μg/100 g diet) as previously described (23). The 1,25(OH)2D3 treatment was started 2 wk prior to the induction of colitis and continued throughout the experiment. For some experiments, broad-spectrum ABX were administered as follows (1 g/L neomycin, 1 g/L metronidazole, 0.5 g/L vancomycin, and 1 g/L ampicillin) in the drinking water 2 wk prior to induction of colitis and throughout the experiment (21). To monitor possible effects of ABX on dehydration of the treated mice (25), Cyp KO, WT, ABX-treated Cyp KO and ABX-treated WT mice were weighed throughout the ABX treatment and prior to DSS treatments. All of the experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University.
Denaturing gradient gel electrophoresis.
Mice were placed in a clean cage without bedding to collect feces left in the cages. Feces were collected in all instances just prior to the induction of colitis in the mice. Total DNA was isolated from fecal samples using a QIAamp DNA stool minikit (Qiagen). Extracted fecal DNA was amplified with universal 16S rDNA primers (26). Denaturing gradient gel electrophoresis (DGGE) was performed with a DCode, Universal Mutation Detection System (Bio-Rad) and as described (27). DGGE fingerprints were analyzed by GelCompar II software (Applied Maths). The effects of genotype, sex, and litter were determined by comparing the percent similarity (number of bands, location, and density) from DGGE banding patterns derived from mice of different genotypes, sex, and litter. Samples were clustered based on the degree of similarity in the DGGE banding patterns.
Metagenomic analaysis.
Breeder- and sex-matched WT and KO littermates were cohoused and used for pyrosequencing. Fecal DNA was isolated from 4 Cyp WT, 4 Cyp KO littermates, 2 VDR WT, and 2 VDR KO littermates and each individual fecal DNA sample was sequenced on a 454 Titanium sequencer at The Pennsylvania State University. The low numbers of samples sequenced was due in part to the high cost needed for sequencing. The goal of the metagenomic sequencing was to confirm the DGGE analysis that showed a change in the microflora in Cyp KO/VDR KO and their WT littermates and to give new information on the possible changes in phyla and class that occurred. The sequences obtained were analyzed using the MOTHUR software (28) and all analysis scripts and datasets are available on The Penn State Bioinformatics Consulting Center Web site (29). The sequencing reads have been filtered to remove reads that had an average read quality of <20. This initial filtering removed ~20% of the reads and then reads from the same genotype were merged. Two additional read quality filtering steps were done. First potential chimeric reads were removed (ChimeraSlayer developed by Broad Institute) and a preclustering step was applied that merged reads due to pyrosequencing errors. At the end of the quality filtering steps, the final reads were 315,803 reads for the Cyp KO and WT mice and 191,684 reads for the VDR KO and WT mice. The read counts for each group spanned from 91,927 to 158,558 reads. Taxonomical classification was performed with the rdp_multiclassfier (30) using the RDP taxonomy. A statistical model based on Pearson chi-square goodness-of-fit test was then applied for statistical analysis. Because of the small sample sizes, the cutoff value for significance was more stringent and P < 0.001 was used. Thus, only large effects with small variance would be important in our model.
DSS colitis.
Colitis was induced with DSS as previously described (31). There are no sex differences in the susceptibility of WT mice to DSS colitis and therefore both male and female mice were used in the experiments (data not shown). Briefly, mice consumed ad libitum 3.5% DSS (molecular weight = 40 kDa; ICN Biomedicals) in drinking water for 5 d and resumed with water. Body weight (BW) change, colonic length, and blood scores (0–3) were measured exactly as described (31). The distal colon was fixed and scored on a scale from 0 to 11 (31).
Quantitative real-time PCR.
For total bacterial numbers, fecal DNA was amplified with 16S rDNA primers using an ABI 7500 Fast RT PCR machine (Applied Biosystems). Relative 16S rDNA copies were calculated using the ΔΔCt method [2^ (Ct sample − Ct ctrl)] and then converted to the total relative copies in the amount of DNA isolated per gram feces. To measure bacteria in the small intestine (SI), 2 cm of terminal SI was excised and flushed. DNA was then isolated and relative segmented filamentous bacteria copies were calculated using the ΔΔCt method. Helicobacteraceae copy numbers in fecal DNA from WT, Cyp KO, and 1,25(OH)2D3 or ABX-treated Cyp KO mice were determined by absolute quantification using standard curves generated with serial dilutions of DNA using a known number of bacteria as the starting material. The limits of detection were <30 copies. The primer sequences are listed in the Supplemental Table 1.
Total RNA was isolated from colon tissues of mice following the manufacturer’s instructions (Qiagen). cDNA was synthesized with the TaqMan RT reagents kit (Applied Biosystems) and amplified for cathelicidin-related antimicrobial peptide (Cramp), β-defensin 3, angiogenin 4, RegIII-γ, Mucin 1–4, trefoil factor family 3, toll-like receptor (Tlr)2, Tlr4, and nucleotide-binding oligomerization domain 2. Expression levels were calculated using the ΔΔCt method and normalized with 18S rRNA. The primer sequences are listed in Supplemental Table 1.
Cell isolation.
Isolation of intestinal epithelial cells (IECs) and lamina propria (LP) lymphocytes was performed as previously described (32, 33). IEC and LP samples were layered onto 40%/80% Percoll gradients (Sigma-Aldrich) to collect the mononuclear cells at the interface. Cells were stained with fluorescein isothiocyanate (FITC) CD11b, FITC B220, FITC E-cadherin, APC CD45, PE CD103, PECy5 TCRβ (BD Biosciences), PE TCRγδ (GL-3), PECy5 CD3, and PECy7 CD11c (eBiosciences). For intracellular IgA staining, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich), permeabilized with 0.1% saponin (Sigma-Aldrich), and stained with PE IgA (Southern Biotech) or PE IgG1κ isotype control (eBiosciences) to set appropriate gating. Cells were analyzed on a FC500 bench top cytometer (Beckman Coulter) and further evaluated with Flowjo 7.6.1 software (Tree Star).
FITC dextran permeability assay.
A FITC dextran permeability assay was performed as described (34). Briefly, mice were removed from feed and water for 4 h and then gavaged with 0.4 mg FITC dextran (4000 Da, Sigma) per gram BW. Blood was collected 4 h post inoculation and FITC dextran in the serum was measured by fluorometer at 525 nm. A standard curve with serial dilutions of FITC dextran in PBS was measured to determine the concentration in the serum.
Statistical analysis.
Statistical analysis was performed via GraphPad Prism software (GraphPad). Two-tailed Student’s t tests were used to calculate differences between genotype (WT vs. KO) or treatment (VDR KO vs. antibiotic-treated VDR KO) groups. One-way ANOVA with Bonferroni post-hoc tests were used to compare the outcome of multiple experimental groups for histological scores and Helicobacteraceae expression. Two-way ANOVA with Bonferroni post-hoc tests were used to calculate the effects of experimental groups, time, and their interaction or the effects of genotype, treatment, and their interaction. The variances were unequal for BW. These data were transformed (square root transformation) to eliminate unequal variances, followed by a repeated-measures (mixed model) 2-way ANOVA to examine the effect of treatment by time on BW. The blood scores and FITC dextran permeability scores had a non-Gaussian distribution. These data were log-transformed to normalize the distribution of the data, followed by a repeated-measures (mixed model) 2-way ANOVA to examine the effect of treatment × time on blood scores and FITC dextran permeability scores. Prior to transformation, a value of 0.5 was added to each blood score to allow transformation of the data. Using the square root or the log-transformed data and the untransformed data resulted in qualitatively similar results, where genotype and time had a strong interactive effect on BW and genotype and time had independent effects on FTIC dextran permeability. Therefore, the untransformed data are presented. The Pearson chi-square goodness-of-fit test was applied for the metagenomic analysis using P < 0.0001 as the cut-off for significance. For all other analyses, P ≤ 0.05 was used as the limit for significance. Values are presented as means ± SEMs of one of 2–3 independent experiments.
Results
Cyp KO mice are more susceptible to DSS colitis.
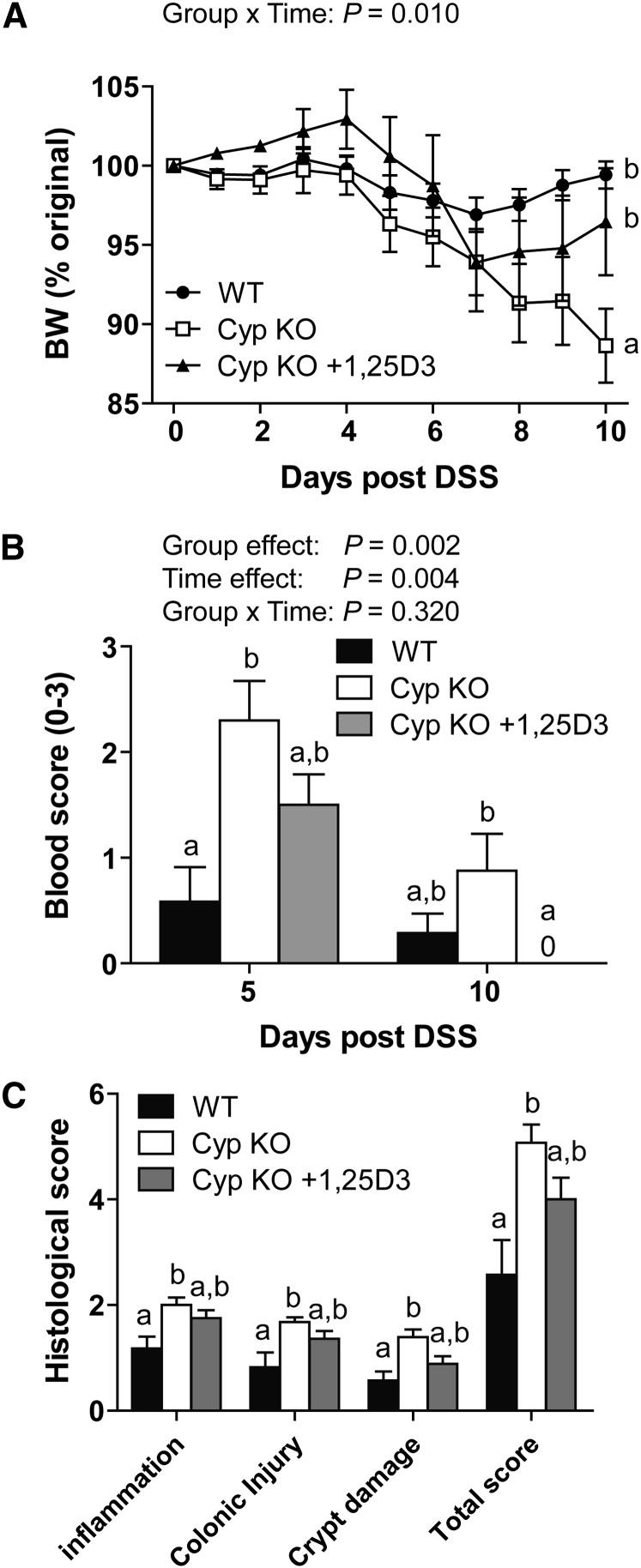
WT mice lost <5% of their starting BW following induction of DSS colits (Fig. 1A). 1,25(OH)2D3-deficient Cyp KO mice developed severe symptoms of DSS colitis that resulted in a substantial loss of their original BW (Fig. 1A). 1,25(OH)2D3 feeding of the Cyp KO mice (Cyp KO + 1,25D3) partially protected the Cyp KO mice from weight loss (Fig. 1A). In addition, the colonic blood scores at d 5 were most severe in the Cyp KO mice, intermediate in the 1,25(OH)2D3-treated Cyp KO mice, and least severe in the WT mice (Fig. 1B). 1,25(OH)2D3-treated Cyp KO mice had no blood evident in the colon at d 10 and the 1,25(OH)2D3-treated Cyp KO mouse blood scores were substantially less than the blood scores in the Cyp KO mice at d 10 (Fig. 1B). Histopathology sections of the colon at d10 post-DSS showed that Cyp KO mice had the most damage, with more lymphocyte infiltration and injury in both the mucosa and submucosa that resulted in substantially higher histopathology scores than in WT mice (Fig. 1C). Colitis severity at d 10 did not differ between WT and 1,25(OH)2D3-treated Cyp KO mice (Fig. 1C). Cyp KO mice are more susceptible to DSS colitis and 1,25(OH)2D3 treatment prevented weight loss (Fig. 1A) and reduced the blood scores (Fig. 1B) of Cyp KO mice following DSS induction.
FIGURE 1.
Symptoms of DSS-induced colitis in WT, Cyp KO, and CypKO+1,25D3-treated mice. (A) Percent original BW change following induction of DSS colitis. Groups without a common letter differed in the BW change. (B) Colonic blood scores at d 5 and 10 post-DSS. Means without a common letter differ at the indicated time point, P < 0.05. (C) Histological scores of the distal colon of mice at d 10 post-DSS. Means without a common letter substantially differ, P < 0.05. Values are means ± SEMs, n = 7–9/group. Data shown are from 1 of 3 independent experiments. BW, body weight; Cyp, Cyp27b1; DSS, dextran sodium sulfate; KO, knockout; WT, wild-type; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol.
Changes in the microbiome in Cyp KO and VDR KO mice.
The microbial composition in the feces from Cyp KO, Cyp WT, VDR KO, and VDR WT mice was determined. DGGE analysis showed considerable heterogeneity in the composition of the fecal microbial flora from mouse to mouse that was demonstrated by the different bands across the gels from mice of the same genotype (Supplemental Fig. 1A,B). Cluster analyses of the DGGE banding patterns showed that the banding pattern of the Cyp KO and Cyp WT mice were more similar within genotypes (Cyp KO: 86–90%; Cyp WT: 83–84%) than between genotypes (74% similarity) (Supplemental Fig. 1C). With the exception of VDR WT mouse no. 4, most of the VDR WT samples (mouse nos. 1–3) had DGGE banding patterns with high similarity (94–98%) and only 80% similarity to the VDR KO mice (Supplemental Fig. 1D). VDR WT female mice (nos. 1–3) were from the same breeder and were more similar (94–98%) to each other than to the VDR WT female no. 4 mouse from a different breeder (80% similar to nos. 1–3) (Supplemental Fig. 1D). Male Cyp KO and WT mice DGGE banding patterns were more similar to the other male samples than to the one female sample (Supplemental Fig. 1C). The data show that litter and sex can affect the composition of the bacterial microflora.
To exclude the factors that influence the microbial composition, DNA samples of cohoused WT and KO littermates from the same breeders and genders were used for pyrosequencing. Bacterial DNA from the feces of VDR KO/WT female mice and Cyp KO/WT male mice were sequenced. Similarity measurements (Jaccard and Bray-Curtis) for bacterial community membership and structure showed that there were higher similarities between the bacteria in the feces of Cyp WT mice and their Cyp KO littermates than between Cyp WT and VDR WT mice (data not shown). In addition, VDR WT mice had bacterial communities that were more similar to their VDR KO littermates than to the bacterial communities found in the Cyp WT mice (Jaccard and Bray-Curtis similarity measures; data not shown). There was an effect of both sex and litter on the bacterial DNA identified in the feces of the mice.
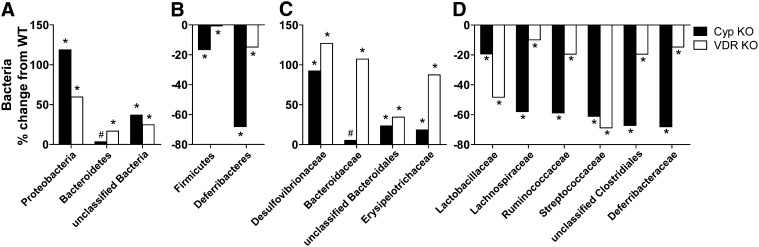
DSS susceptibility did not differ for male and female mice or between mice from different litters, such that microbial differences observed between strains and as a result of sex or litter do not account for the increased susceptibility of the VDR KO and Cyp KO mice to DSS colitis. Firmicutes and Bacteroidetes were the dominant phyla in all of the mice (Supplemental Fig. 2). Bacteroidetes, Proteobacteria, and unclassified bacteria were over-represented in the fecal microflora of Cyp KO and VDR KO mice relative to their WT counterparts (Fig. 2A). Conversely, the Cyp KO and VDR KO mice had fewer bacteria from the Firmicutes and Deferribacteres phyla compared with their respective WT littermates (Fig. 2B). There were higher numbers of bacteria from the Desulfovibrionaceae, Bacteroidaceae, and unclassified Bacteroidales that are family members from the Proteobacteria and Bacteroidetes phyla in the KO mouse feces as compared with WT mice (Fig. 2C). Within the Firmicutes phylum, there were substantially more Erysipelotrichaceae family members (Fig. 2C) and fewer Ruminococcaceae, Lachnospiraceae, Lactobacillaceae, and Streptococcaceae (Fig. 2D) in the Cyp KO and VDR KO feces compared with feces of WT mice. In addition, the KO mice had fewer Deferribacteraceae family members from the Deferribacteres phylum than did their respective WT littermates (Fig. 2D).
FIGURE 2.
Bacterial metagenomic analysis of the phylum and family level differences evident in the DNA isolated from the feces of Cyp WT, Cyp KO, VDR WT, and VDR KO mice. Relative change in the bacterial phyla enriched (A) or depleted (B) in the feces from Cyp KO mice compared with WT littermates and VDR KO mice compared with WT littermates. Relative change in the bacterial families enriched (C) or depleted (D) in feces from Cyp KO mice compared with WT littermates and VDR KO mice compared with WT littermates. Bacterial changes in KO mice were calculated by subtracting WT values from KO values and dividing the differences by WT values [(KO − WT)/WT × 100]. n = 4 males each for WT and Cyp KO and n = 2 female each for WT and VDR KO. Means of KO mice in A–D differ from means of their respective WT littermates: #P < 0.05, *P < 0.0001. Cyp, Cyp27b1; KO, knockout; VDR, vitamin D receptor; WT, wild-type.
ABX protect mice from DSS colitis.
There was no effect of ABX treatment on the BW of the mice prior to DSS, which suggests that ABX treatment did not result in dehydration (Supplemental Fig. 3A). The ABX treatment resulted in a 50–75% reduction in the amount of bacterial 16S rDNA found in the feces of the ABX-treated mice compared with the untreated controls (Supplemental Fig. 3B). ABX treatment also resulted in fewer DGGE bands, which suggests a decline in the overall bacterial diversity in the mice (Supplemental Fig. 1). The ABX treatment resulted in a shift in the composition of the bacteria found in the feces, because the similarity in DGGE banding patterns before (0 wk) ABX treatment was higher than after (2 wk) the treatment (Supplemental Fig. 1C,D). For example, VDR WT females nos. 2–3 were 95% similar before ABX and only 88% similar afterwards (Supplemental Fig. 1D). The lower similarity in the DGGE banding patterns as a result of ABX was true of Cyp WT, Cyp KO, VDR WT, and VDR KO mice (Supplemental Fig. 1C,D).
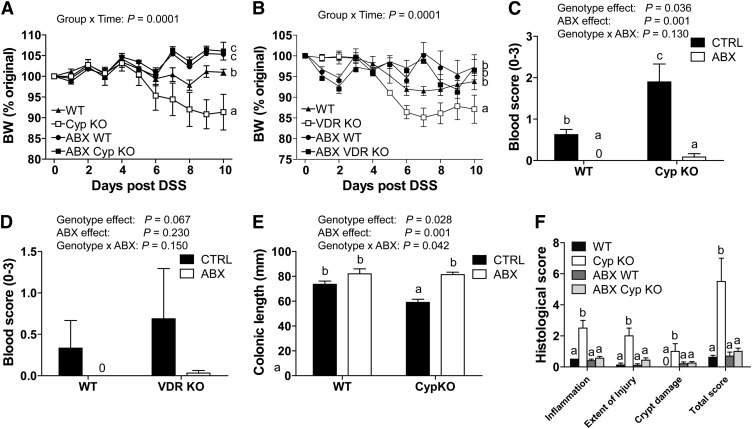
ABX treatment of WT mice completely prevented the weight loss induced by DSS compared with untreated WT mice (Fig. 3A,B). ABX treatment was also effective at preventing weight loss in the Cyp KO and VDR KO mice following DSS treatment (Fig. 3A,B). Other symptoms of DSS colitis, including blood scores (Fig. 3C,D), colonic shortening (Fig. 3E; Supplemental Fig. 3C), and histopathology scores (Fig. 3F; Supplemental Fig. 3D) were either reduced or eliminated by the ABX treatment. ABX treatment reduced bacterial numbers and protected mice from DSS-induced colitis.
FIGURE 3.
Symptoms of DSS-induced colitis in WT, Cyp KO, VDR KO, ABX WT, ABX Cyp KO, and ABX VDR KO mice. Percent original BW change in Cyp KO and WT littermates (A) and VDR KO and WT littermates (B) treated or not with ABX and induced to develop DSS colitis. Groups without a common letter differed in the BW change. Colonic blood scores at d 5 post-DSS in Cyp KO and WT littermates (C) and VDR KO and WT littermates (D) with and without ABX treatment. (E) Colonic lengths at d 10 post-DSS in Cyp KO and WT littermates with and without ABX treatment. (F) Histological scores of the distal colon of Cyp KO and WT littermates at d 10 post-DSS. Values are means ± SEMs, n = 3–9 mice/group and time point. When labeled, means without a common letter differ, P < 0.05. Data shown is 1 representative experiment of 3 independent experiments. ABX, antibiotics; ABX Cyp KO, antibiotic-treated Cyp27b1 knockout; ABX VDR KO, antibiotic-treated vitamin D receptor knockout; ABX WT, antibiotic-treated WT; BW, body weight; CTRL, control no antibiotics; Cyp, Cyp27b1; DSS, dextran sodium sulfate; KO, knockout; VDR, vitamin D receptor; WT, wild-type.
Helicobacteraceae numbers are affected by 1,25(OH)2D3 and ABX.
The number of total bacteria found in the feces of WT and Cyp KO mice was not different either with or without ABX treatment (Supplemental Fig. 3B; Fig. 4A). Segmented filamentous bacteria were not found in the SI of either WT or Cyp KO mice (Fig. 4A) or from other mice in our colony (data not shown). Cyp KO mice had substantially more Helicobacteraceae family members from the Proteobacteria phylum compared with their WT littermates (Fig. 4B). 1,25(OH)2D3 treatment of the Cyp KO mice reduced the numbers of Helicobacteraceae, although the numbers of Helicobacteraceae did not differ between the Cyp KO and 1,25(OH)2D3-treated Cyp KO feces (P = 0.43) (Fig. 4B). The numbers of Helicobacteraceae in the feces of WT and 1,25(OH)2D3-treated Cyp KO mice also did not differ from one another (P = 0.47) (Fig. 4B). Conversely, the ABX treatment eliminated all Helicobacteraceae in the mice [ABX-treated Cyp KO (Fig. 4B; ABX-treated WT (data not shown)].
FIGURE 4.
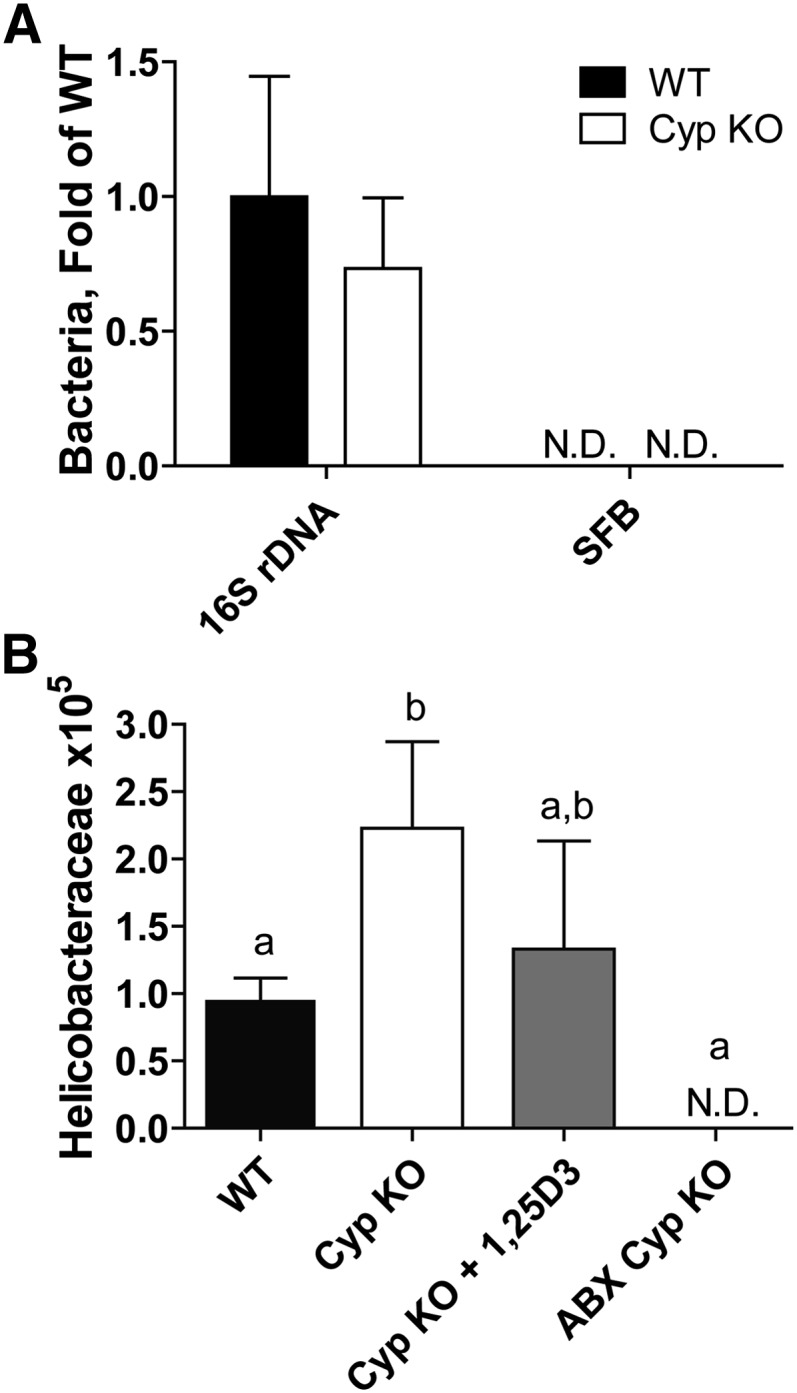
Relative amount of bacterial 16S rDNA and SFB in WT and Cyp KO mice and numbers of Helicobacteraceae in WT, Cyp KO, Cyp KO+1,25(OH)2D3, and ABX Cyp KO mice. (A) Relative fold change of fecal 16S rDNA and SFB-specific DNA in the SI of Cyp KO and WT mice. (B) Helicobacteraceae copy numbers in fecal DNA from WT and Cyp KO mice with or without 1,25(OH)2D3 or ABX treatment. Values are means ± SEMs, n = 3–9 mice/group. Means without a common letter differ, P < 0.05. Data shown is 1 representative experiment of 2 independent experiments. ABX, antibiotics; ABX Cyp KO, antibiotic-treated Cyp27b1 knockout; Cyp, Cyp27b1; KO, knockout; ND, not detectable (<30 copies); rDNA, ribosomal DNA; SFB, segmented filamentous bacteria; SI, small intestine; WT, wild-type; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol.
Cyp KO mice express lower E-cadherin.
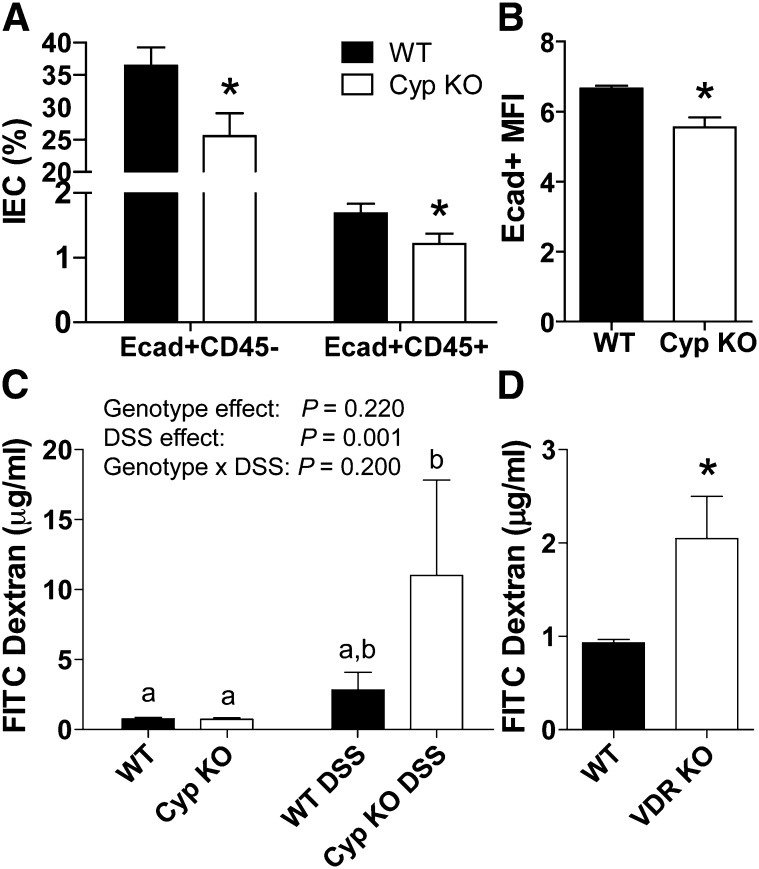
The total numbers of cells isolated from the IEC did not differ between WT (6.0 ± 0.1 × 105) and Cyp KO mice (7.0 ± 0.1 × 105). Colonic IECs contained mostly epithelial cells (75% of CD45−). Both the CD45+ and CD45− cells from the IEC expressed E-cadherin (Fig. 5A). Cyp KO mice had substantially fewer epithelial and immune cells that were E-cadherin positive than the WT controls (Fig. 5A). In addition, the amount of E-cadherin expressed on the WT cells (mean fluorescence intensity) was substantially higher than on the Cyp KO cells (Fig. 5B). Equal amounts of FITC dextran were recovered in the serum following gavage of Cyp KO and WT mice (Fig. 5C). Inflammation caused an insignificant increase in the amount of FITC dextran recovered in the serum of DSS-treated WT mice at d 5 compared with the untreated WT mice (P = 0.47) (Fig. 5C). Substantially more FITC dextran was detected in the serum of DSS-treated Cyp KO mice following DSS treatment compared with the untreated WT or Cyp KO mice (Fig. 5C). FITC dextran recovery in the serum was substantially higher in untreated VDR KO mice compared with their WT counterparts (Fig. 5D).
FIGURE 5.
Expression of Ecad in the gut of WT and Cyp KO mice and gastrointestinal leakage in untreated and DSS treated VDR KO, Cyp KO and WT mice. (A) Frequency of Ecad+CD45− and Ecad+CD45+ cells in the IECs. (B) MFI of Ecad positive cells in the IECs. Values are means ± SEMs, n = 3 mice/group. (C) FITC dextran recovered from the serum of untreated and d 5 DSS-treated Cyp WT and KO mice. Values are means ± SEMs, n = 6–8 mice/group. Means without a common letter differ, P < 0.01. (D) FITC dextran from untreated VDR WT and KO mice. Values are means ± SEMs, n = 5 mice/group. Data shown is 1 representative experiment of 2 independent experiments. *Different from WT, P < 0.05. Cyp, Cyp27b1; DC, dendritic cell; DSS, dextran sodium sulfate; Ecad, E-cadherin; FITC, fluorescein isothiocyanate; IEC, intestinal epithelial cell; KO, knockout; MFI, mean fluorescence intensity; VDR, vitamin D receptor; WT, wild-type.
Decreased tolerogenic DCs in Cyp KO mice.
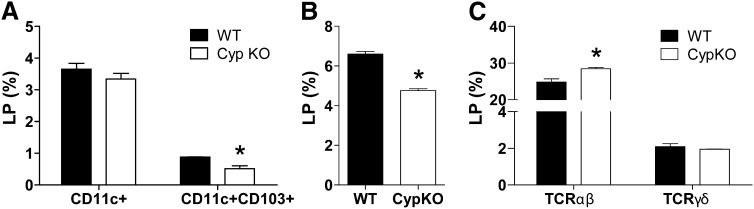
The frequencies of total DCs (CD11c+ cells) and CD11c/CD103+ tolerogenic DCs in the IECs from both WT and Cyp KO mice were low and not different from one another (data not shown). The LP also contained DCs. Although there were no differences in the frequencies of CD11c+ DC, there were substantially lower frequencies of tolerogenic DC that were CD11c/CD103+ in the Cyp KO LP compared with WT mice (Fig. 6A). Equal numbers of LP lymphocytes were isolated from WT [(2.8 ± 0.1) × 106] and Cyp KO [(3.9 ± 0.6) × 106] mice. The frequencies of IgA-positive plasma cells were the same in the Cyp KO and WT LP (Supplemental Fig. 4A). There were lower frequencies of CD11b+ myeloid cells (Fig. 6B) and higher frequencies of TCR αβ in the Cyp KO LP compared with WT LP (Fig. 6C). The frequencies of TCR γδ T-cells did not differ between Cyp KO and WT LP (Fig. 6C). The amount of mRNA for Cramp, mouse β-defensin 3, angiogenin 4, Reg III γ, mucins, and trefoil factor family 3 did not differ in the colons of Cyp KO and WT mice (Supplemental Fig. 4). Tlr and nucleotide-binding oligomerization domain 2 expression were also similar in the colons of Cyp KO and WT mice (Supplemental Fig. 4).
FIGURE 6.
Frequencies of immune cell subsets in the LP of WT and Cyp KO mice. Frequencies of CD11c+ and CD11c/CD103+ cells (A) CD11b+ cells (B) and TCRαβ and TCRγδ cells (C) in the LP of WT and Cyp KO mice. Values are means ± SEMs, n = 3 mice/group. Data shown is 1 representative of 2 independent experiments. *Different from WT, P < 0.05. Cyp, Cyp27b1; KO, knockout; LP, lamina propria; WT, wild-type.
Discussion
The data support a role for vitamin D in controlling the microbiome. Cyp KO and VDR KO mice are extremely susceptible to DSS colitis and the susceptibility of the mice corresponded with greater numbers of bacteria from the Proteobacteria phylum and lower numbers of Lachnospiraceae from the Firmicutes phylum. Similar changes have been described in the colon of IBD patients compared with healthy controls (15). Specifically, the data show that 1,25(OH)2D3 treatments reduced the Helicobacteraceae numbers and suppressed the symptoms of DSS colitis. Prokaryotes do not express the VDR and therefore the effects of vitamin D and/or 1,25(OH)2D3 on the microbiome are likely indirect effects through the regulation of the host immune response. Salmonella is a member of the Proteobacteria phylum that has been shown to use the host inflammatory response to provide a substrate that allows Salmonella to out-compete the commensal microflora (35). Other members of the Proteobacteria phylum also express tetrathionate reductase, and it has been suggested that tetrathionate respiration might contribute to changes in the microbiome during inflammation in the gut (35). Bacteria that express tetrathionate reductase include Desulfovibrio species from Desulfovibrionaceae family members (36), which were enriched in both Cyp KO and VDR KO compared with their respective WT littermates. VDR KO mice are hyper-responsive to LPS challenge and overproduce TNFα, IL-1β, and several other inflammatory cytokines (31, 37). In addition, VDR and vitamin D deficiency results in the overproduction of Th1 and Th17 immune responses (6, 38) and a reduction in the overall number of tolerogenic DCs and regulatory T-cells (3). Vitamin D and the VDR inhibit Th1, Th17, and the production of inflammatory cytokines in the gastrointestinal tract that serve to reduce inflammation, shift the microbiome, and maintain tolerance in the gut.
Contrary to what was previously published, our data did not show increased dehydration and weight loss following broad-spectrum ABX usage in mice (25). The synthetic diets used for these experiments included gelatin that likely increased the moisture content of the food to eliminate the ABX-mediated dehydration observed in chow-fed mice (25). In addition, our data contradict previous data from C57BL/6 WT mice that showed an increase in severity of DSS colitis with the same broad-spectrum ABX cocktail (21). There are several experimental details that might explain the contradictory data, but the most important differences are likely to be the microbial flora at the 2 different institutes and our synthetic experimental diets. It should be noted that DSS has also been reported to be more severe in germ-free mice albeit in a different strain of mouse (IQI/Jic) (20). It is well known that diet affects the composition of the microbiome and the severity of experimental IBD. Whether there is an independent host-mediated effect of diet on inflammation and DSS-induced injury would be of interest.
Others have previously published that DSS-treated VDR KO mice had leaky gastrointestinal tracts, demonstrating that vitamin D regulates gut epithelial integrity (39). We show here that Cyp KO mice also had greater gastrointestinal permeability but only following DSS treatment. Gut permeability has been reported in patients with IBD (40), indicating that dysfunction of the epithelial barrier may contribute to IBD. In colonic cell cultures, it has been shown that 1,25(OH)2D3 induced expression of E-cadherin and improved epithelial cell junctions (39, 41). Intestinal epithelial integrity is critical for the host defense against pathogen infection (42). The colonic antibacterial responses did not differ in either the VDR- or the 1,25(OH)2D3-deficient mice compared with WT mice. It has been shown in vitro that 1,25(OH)2D3 and vitamin D increase the production of the antibacterial peptide cathelicidin in human macrophage (43). Mouse cells do not express the same cathelicidin gene as humans and it has been shown that murine cathelicidin (Cramp) is not regulated by 1,25(OH)2D3, because the vitamin D response element is missing in the murine promoter (44). In the colon, Cramp expression was not different in the Cyp KO mice compared with WT mice. Decreased expression of angiogenin-4 mRNA and protein has been reported in vitamin D-deficient mice (45). There were no differences in several of the antibacterial peptides, mucins, etc. in the colon of the Cyp KO mice. The data support an important function of vitamin D in maintaining barrier function of the host gastrointestinal tract, but in this model there was no vitamin D effect on the expression of several innate immune responses in the colon.
It has been well described that vitamin D regulates gastrointestinal innate and adaptive immune responses. We show here important effects of vitamin D on the bacterial microbiome and that the alterations in the bacterial flora underlie the effectiveness of vitamin D to inhibit DSS colitis development. Specifically, the data suggest that in the absence of the VDR or the ability to produce 1,25(OH)2D3, unregulated inflammation of the gut results in an environment that supports the expansion of bacteria in the Proteobacteria phylum. The expansion of the Proteobacteria phylum (including the Helicobacteraceae family members) out-compete beneficial members of the Firmicutes and Deferribacteres phyla. The result of the shift in the composition of the bacterial microflora is an amplified response of the host to injury. The VDR and 1,25(OH)2D3 indirectly regulate the microbiome to maintain tolerance in the gastrointestinal tract. Vitamin D supplementation might be a way to manipulate the composition of the bacterial microbiome and protect against gastrointestinal injury.
Supplementary Material
Acknowledgments
We thank Dr. Hector DeLuca for the Cyp KO mice and Dr. Istvan Albert for help with the metagenomic analyses. J.H.O. and M.T.C. designed research; J.H.O. conducted research; J.H.O., Y.L., C.J.R., and M.T.C. analyzed research; J.H.O. and M.T.C. wrote the paper; and M.T.C. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABX, antibiotics; BW, body weight; Cramp, cathelicidin-related antimicrobial peptide; Cyp, Cyp27b1; DC, dendritic cell; DGGE, denaturing gradient gel electrophoresis; DSS, dextran sodium sulfate; FITC, fluorescein isothiocyanate; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; KO, knockout; LP, lamina propria; SI, small intestine; Tlr, toll-like receptor; VDR, vitamin D receptor; WT, wild-type; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol.
Literature Cited
- 1.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. [DOI] [PubMed] [Google Scholar]
- 2.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. 2012;523:103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantorna MT. Why do T cells express the vitamin D receptor? Ann N Y Acad Sci. 2011;1217:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantorna MT, Zhao J, Yang L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc Nutr Soc. 2012;71:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc. 2010;69:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D(3) curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217:1292–300. [DOI] [PubMed] [Google Scholar]
- 9.Ardizzone S, Cassinotti A, Bevilacqua M, Clerici M, Porro GB. Vitamin D and inflammatory bowel disease. Vitam Horm. 2011;86:367–77. [DOI] [PubMed] [Google Scholar]
- 10.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229:1136–42. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn's disease: a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT. Therapeutic effect of vitamin D supplementation in a pilot study of Crohn's patients. Clin Transl Gastroenterol. 2013;4:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M, et al. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991;32:1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. [DOI] [PubMed] [Google Scholar]
- 18.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs K, Herfarth H. Role of probiotic therapy in IBD. Inflamm Bowel Dis. 2008;14:1597–605. [DOI] [PubMed] [Google Scholar]
- 20. Kitajima S, Morimoto M, Sagara E, Shimizu C, Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50:387–95. [DOI] [PubMed]
- 21.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. [DOI] [PubMed] [Google Scholar]
- 22.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. [DOI] [PubMed] [Google Scholar]
- 24.Cantorna MT, Humpal-Winter J, DeLuca HF. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr. 1999;129:1966–71. [DOI] [PubMed] [Google Scholar]
- 25.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varshney J, Ooi JH, Jayarao BM, Albert I, Fisher J, Smith RL, Patterson AD, Cantorna MT. White button mushrooms increase microbial diversity and accelerate the resolution of Citrobacter rodentium infection in mice. J Nutr. 2013;143:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bioinformatics Consulting Center at Penn State [homepage on the internet]. University Park (PA): Bioinformatics Consulting Center at Penn State; c2010 [cited 2012 Oct 12]: http://bcc.bx.psu.edu.
- 30.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–11. [DOI] [PubMed] [Google Scholar]
- 33. Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyeraposs patch, and lamina propria cells. Curr Protoc Immunol. 2001:19. [DOI] [PubMed]
- 34.Brandl K, Rutschmann S, Li X, Du X, Xiao N, Schnabl B, Brenner DA, Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci USA. 2009;106:3300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter SE, Baumler AJ. A breathtaking feat: to compete with the gut microbiota, Salmonella drives its host to provide a respiratory electron acceptor. Gut Microbes. 2011;2:58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett EL, Clark MA. Tetrathionate reduction and production of hydrogen sulfide from thiosulfate. Microbiol Rev. 1987;51:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–4. [DOI] [PubMed] [Google Scholar]
- 39.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. [DOI] [PubMed] [Google Scholar]
- 40.Welcker K, Martin A, Kolle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9:456–60. [PubMed] [Google Scholar]
- 41.Pálmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trulzsch K, Muller-Hocker J, Vogelmann R, Allgauer M, Gerhard M, Steininger S, et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS ONE. 2010;5:e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20:371–6. [DOI] [PubMed] [Google Scholar]
- 44.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. [DOI] [PubMed] [Google Scholar]
- 45.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.