Abstract
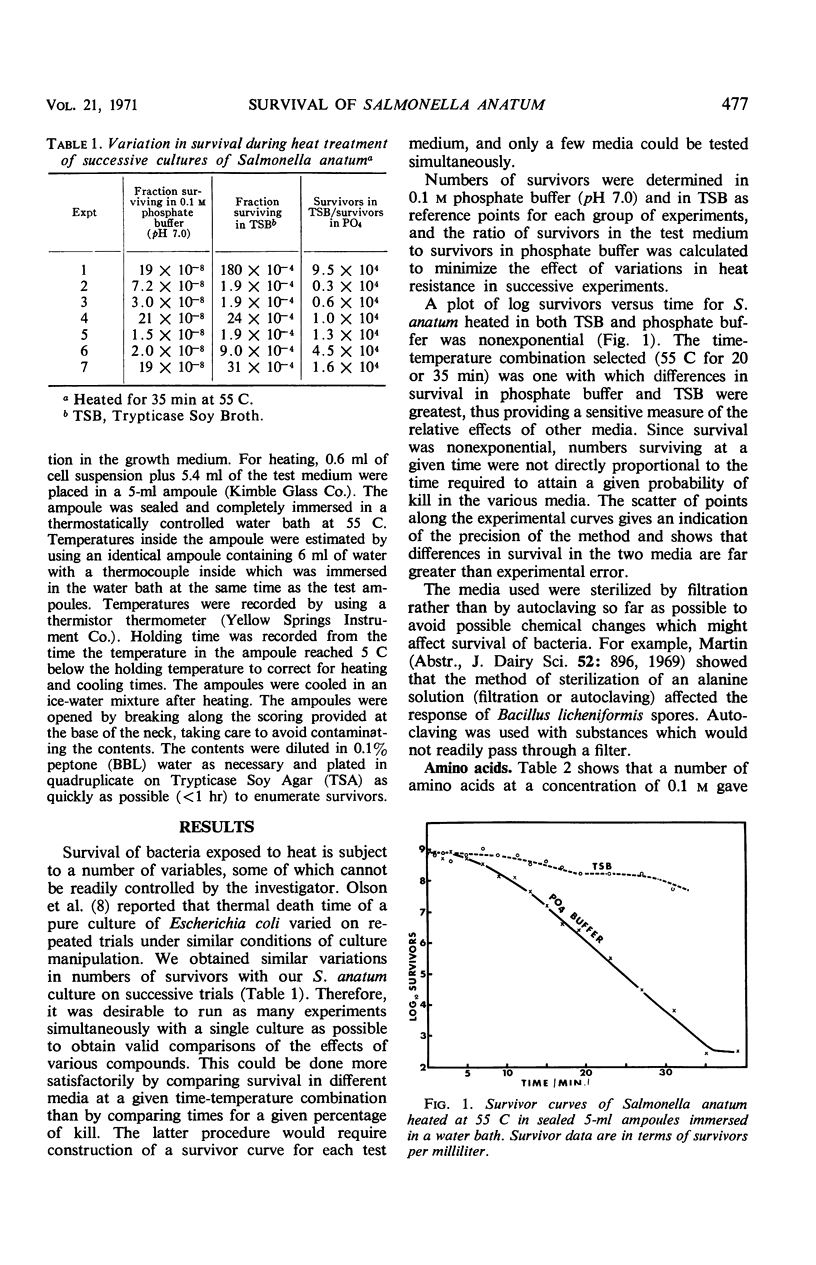
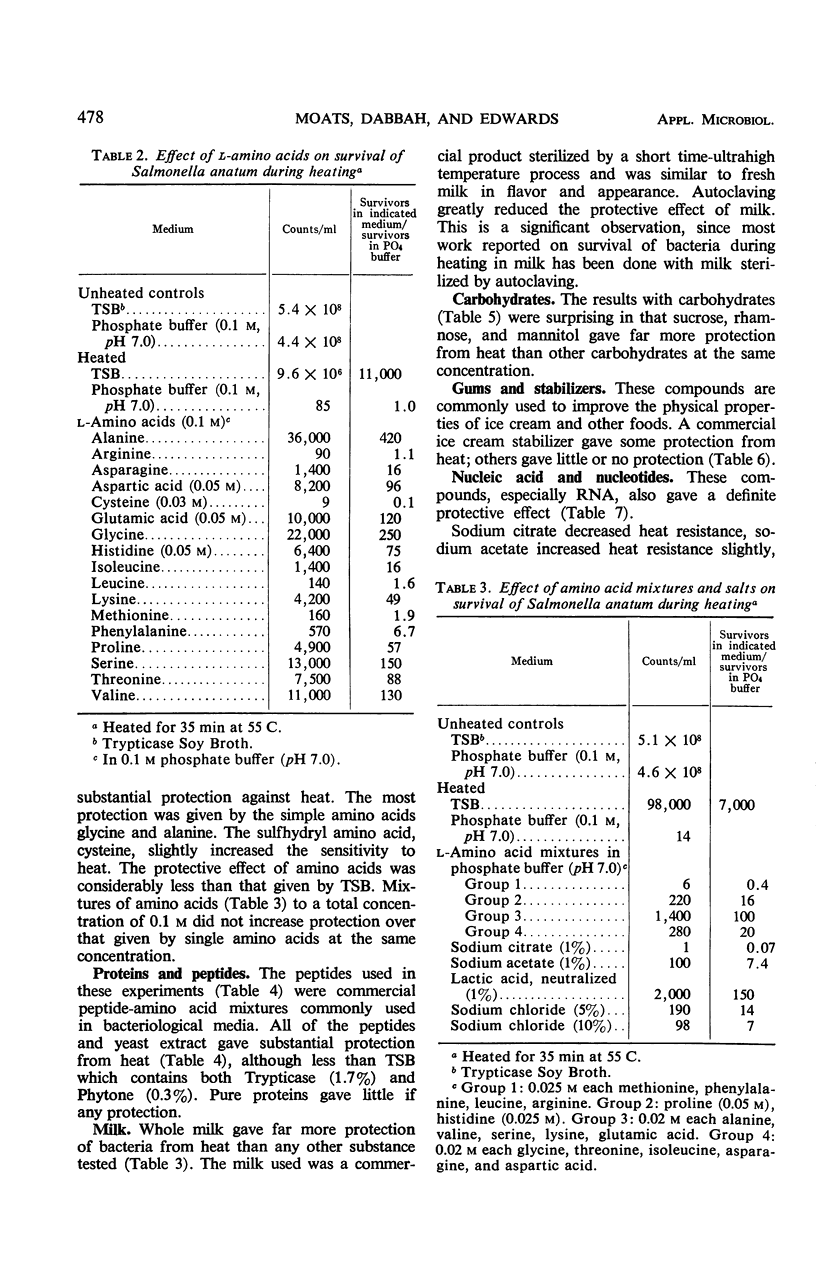
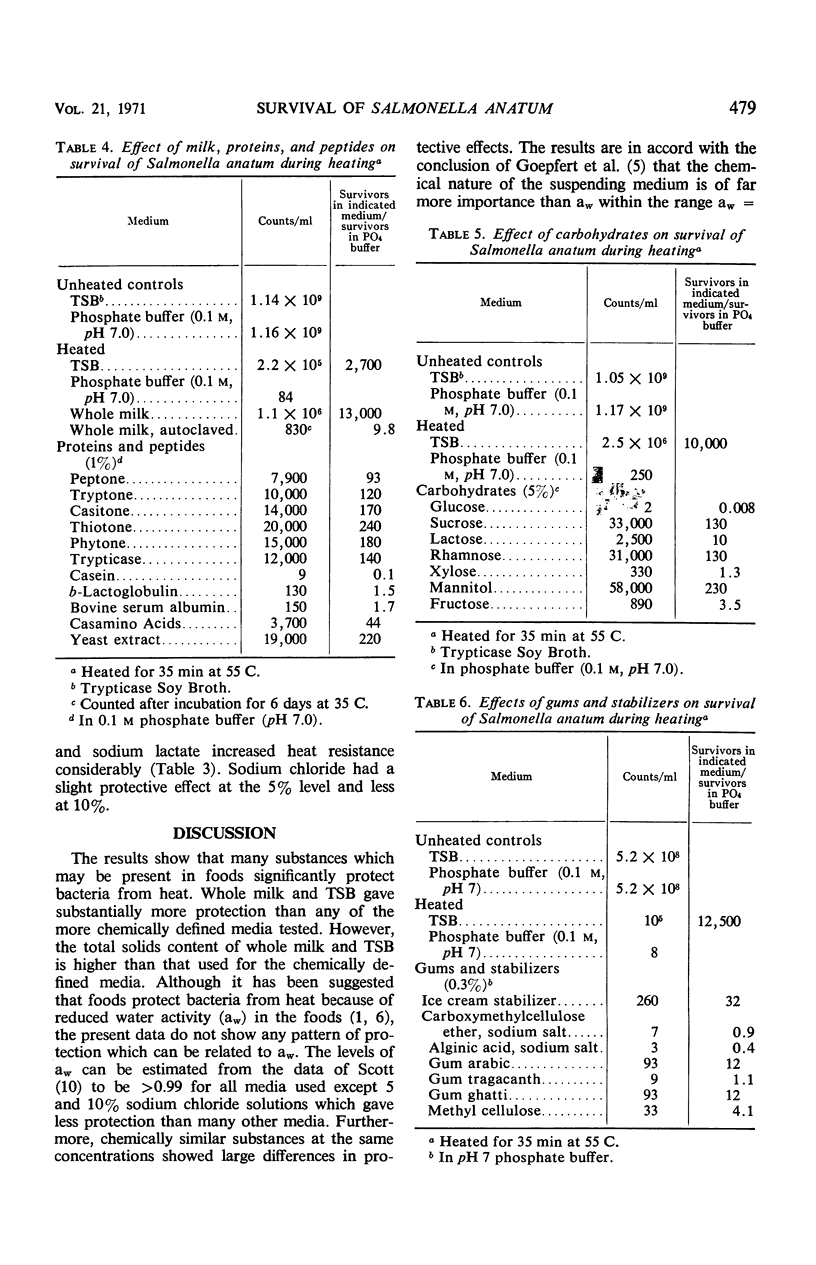
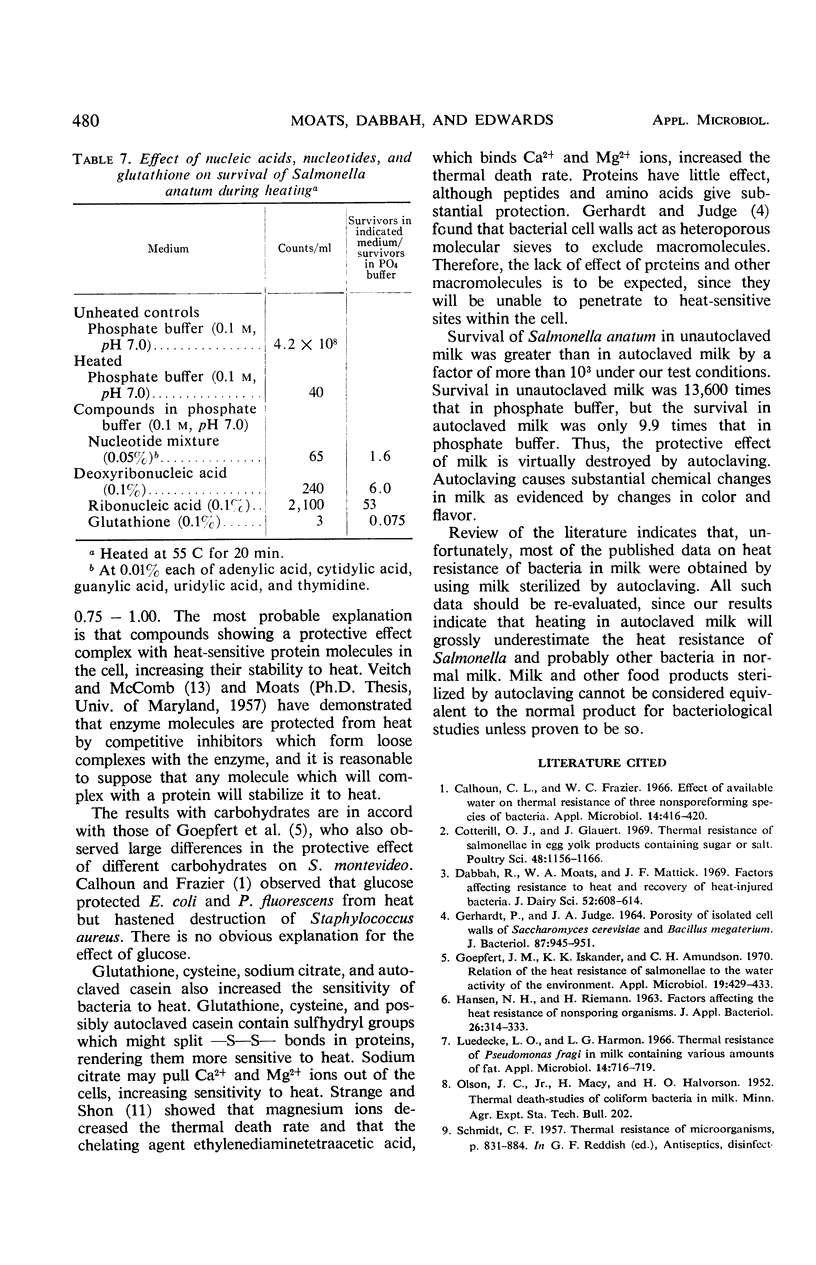
Survival of Salmonella anatum heated at 55 C for 35 min was determined in solutions of various chemical constituents of foods including salts, carbohydrates, amino acids, peptides, nucleic acids, gums, and stabilizers and compared with survival in 0.1 m phosphate buffer (pH 7.0). Commercially sterilized whole milk gave the most protection against heat. Trypticase Soy Broth, various peptide mixtures, and some amino acids gave substantial protection. Results with carbohydrates were variable, with mannitol, sucrose, and rhamnose providing substantial protection and glucose decreasing heat resistance. A few other substances, cysteine, glutathione, and sodium citrate, also decreased heat resistance. Pure proteins had little effect. Results show that water activity is of little significance under the test conditions. Protection from heat probably results from complexing of substances with heat-sensitive proteins in the cells. Autoclaved milk should not be considered equivalent to raw milk for studies of survival of bacteria during heating.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calhoun C. L., Frazier W. C. Effect of available water on thermal resistance of three nonsporeforming species of bacteria. Appl Microbiol. 1966 May;14(3):416–420. doi: 10.1128/am.14.3.416-420.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterill O. J., Glauert J. Thermal resistance of salmonellae in egg yolk products containing sugar or salt. Poult Sci. 1969 Jul;48(4):1156–1166. doi: 10.3382/ps.0481156. [DOI] [PubMed] [Google Scholar]
- Dabbah R., Moats W. A., Mattick J. F. Factors affecting resistance to heat and recovery of heat-injuried bacteria. J Dairy Sci. 1969 May;52(5):608–614. doi: 10.3168/jds.s0022-0302(69)86615-4. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., JUDGE J. A. POROSITY OF ISOLATED CELL WALLS OF SACCHAROMYCES CEREVISIAE AND BACILLUS MEGATERIUM. J Bacteriol. 1964 Apr;87:945–951. doi: 10.1128/jb.87.4.945-951.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert J. M., Iskander I. K., Amundson C. H. Relation of the heat resistance of salmonellae to the water activity of the environment. Appl Microbiol. 1970 Mar;19(3):429–433. doi: 10.1128/am.19.3.429-433.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedecke L. O., Harmon L. G. Thermal resistance of pseudomonas fragi in milk containing various amounts of fat. Appl Microbiol. 1966 Sep;14(5):716–719. doi: 10.1128/am.14.5.716-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., SHON M. EFFECTS OF THERMAL STRESS ON VIABILITY AND RIBONUCLEIC ACID OF AEROBACTER AEROGENES IN AQUEOUS SUSPENSION. J Gen Microbiol. 1964 Jan;34:99–114. doi: 10.1099/00221287-34-1-99. [DOI] [PubMed] [Google Scholar]


