To the editor:
Hepcidin impedes iron absorption and is suppressed when erythropoietic iron requirements are increased. Recent studies show that during acute exposure to high-altitude hypoxia, plasma hepcidin concentrations drop when iron demands for erythropoiesis and hemoglobin synthesis are sharply increased.1,2 However, the effects of chronic exposure to high-altitude hypoxia with stable erythropoietic iron requirements have not been examined. We hypothesized that plasma hepcidin would not be suppressed in iron-replete individuals chronically adapted to high altitude.
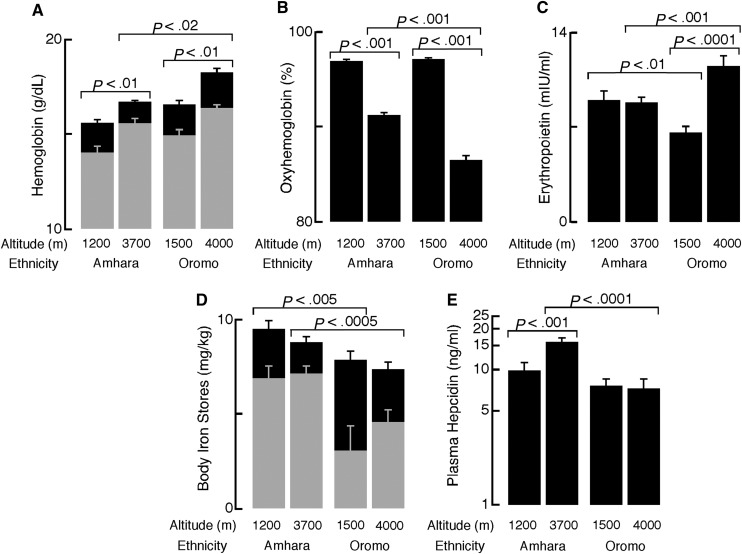
People of Amhara and Oromo ethnicity have been living at high altitude in Ethiopia for more than 5000 years and about 500 years, respectively, and have been shown to differ from one another in hemoglobin and oxyhemoglobin percentage.3 Healthy volunteers from 3700 to 4000 m (high altitude) and 1200 to 1500 m (low altitude) were recruited, and they provided blood samples for analyses (see the supplemental Video(s)/Data Set(s) link at the top of the online article for genetic analysis methodology). The sample reported here had normal calculated body iron stores4 (Figure 1D) and did not have infection or inflammation, assessed with C-reactive protein levels and malarial plasmodium DNA.
Figure 1.
Oxyhemoglobin percentage, hemoglobin, ferritin, hepcidin, and erythropoeitin of Ethiopians differ by ethnicity at high and low altitudes. A total of 116 high-altitude Amhara (27 females, age 32 ± 0.8 years), 49 low-altitude Amhara (9 females, 34 ± 1.4 years), 75 high-altitude Oromo (27 females, 28 ± 1.0 years), and 39 low-altitude Oromo (6 females, 25 ± 0.9 years) were included in the analyses. Data were analyzed using the JMP 9 statistical software (SAS Institute, Cary, NC). Data are reported as mean and standard error of the mean. (A) Hemoglobin was higher in men (black) compared with women (gray) in all populations (all P < .005). Hemoglobin was increased at high altitudes in both ethnic groups, but Oromo had higher hemoglobin than Amhara at high altitudes. Hemoglobin concentration was measured using the cyanmethemoglobin technique (Hemocue, Sweden) immediately after drawing venipuncture samples. (B) Oxyhemoglobin percentage was significantly lower among Ethiopians at high altitude compared with low altitudes. At high altitude (3700-4000 m), Oromo had significantly lower saturations than Amhara. (C) Amhara had no altitude differences in erythropoietin, whereas Oromo had significantly higher erythropoietin at high vs low altitude. Compared with the Oromo, Amhara had lower erythropoietin at high altitude and higher erythropoietin at similar low altitudes. Erythropoietin was measured in serum by ELISA (R&D Systems, Minneapolis, MN). Extreme outliers (EPO > 40 mIU/mL) were excluded in advance of statistical testing. (D) Body iron stores were significantly lower among women (gray bars) compared with men (black bars) except among low-altitude Amhara (all others, P < .01). Neither Amhara nor Oromo had altitude differences in body iron stores. At high altitudes, Amhara had higher body iron stores than Oromo. Body iron stores were calculated as previously described4 from serum transferrin receptor and ferritin concentrations, measured by enzyme immunoassay and radioimmunoassay (Ramco, Houston, TX), respectively. (E) Amhara highlanders had higher hepcidin than Amhara lowlanders and the Oromo at either altitude. Hepcidin was measured using enzyme-linked immunosorbant assay (ELISA, Bachem, UK) in heparinized plasma. Hepcidin was natural log transformed for statistical testing.
In contrast to acutely exposed Europeans2 (see also the online supplement), high-altitude Amhara had higher plasma hepcidin, and high-altitude Oromo had similar hepcidin, compared with their respective lowland counterparts (Figure 1E). Furthermore, Amhara had higher plasma hepcidin and oxyhemoglobin percentage as well as lower hemoglobin and erythropoietin than Oromo at high altitudes (Figure 1A-C,E). Within Ethiopian subsamples, age, sex, BMI, erythropoietin, hemoglobin, oxyhemoglobin percentage, and transferrin receptor were not correlated with hepcidin (natural-log transformed for normality). Like Europeans at low altitude,5 serum ferritin (r = 0.35 to 0.77) and body iron stores (r = 0.39 to 0.85) correlated with ln(hepcidin) (all P < .05, except in the small sample of female low-altitude Oromo [n = 6]). An intronic SNP in GRAMD3 was associated with plasma hepcidin among Amhara at genome-wide significance (P = 4.94*10−8), accounting for 22% of the variation in covariate-adjusted hepcidin level. Allele A of rs7700582 increased hepcidin levels by 8.1 ng/mL only among Amhara, although allele frequency was similar in all 4 subsamples.
Hepcidin was not suppressed in Amhara or Oromo highland samples under steady-state hypoxia, likely because erythropoietic drive was stable.6 It is interesting to speculate that the higher plasma hepcidin of highlander Amhara, compared with Oromo, is due to lower iron demand indicated by lower hemoglobin and erythropoietin concentrations and higher body iron stores. Variants in GRAMD3 are associated with macular degeneration, a retinal disease that has been related to abnormalities in hepcidin and iron accumulation.7,8 Another variant near GRAMD3 (rs1366100) has been associated with erythrocyte counts,9 consistent with the idea that this region plays a role in iron metabolism. Thus, the genetic results also support the idea that iron stores are primary regulators of hepcidin levels in hypoxic populations without increased erythropoietic drive. Previous work has shown that various highlander populations demonstrate different responses to hypoxia,10 which may also be the case with iron regulation.
Authorship
Acknowledgments: We thank the Ethiopian park, university, and government officials for permission to conduct the research, our excellent research assistants, and generous study volunteers for their participation and hospitality during our fieldwork. The Cleveland Clinic IRB, the Case Western Reserve University IRB, and the Ethiopian Research Council provided human subjects and research approval for this study.
This work was supported in part by the generous assistance of Cleveland Clinic Research Programs Council, The National Institutes of Health (HL60917) (S.C.E.), (GM79558 and GM79558-S1) (A.D.), and The National Science Foundation (BCS-0452326) (C.M.B.).
Contribution: E.L.L. performed research and statistical analysis, interpreted data, and wrote the manuscript. A.J.J. and C.D.K. performed research, collected data, and analyzed and interpreted data. A.G. designed the research and interpreted data. A.D., G.A.-A., and G.M.B. analyzed and interpreted data. S.C.E. designed research, analyzed and interpreted data, and wrote the manuscript. C.M.B. designed and performed the research, collected data, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cynthia M. Beall, 238 Mather Memorial Building, 11220 Bellflower Rd, Cleveland, OH 44106-7125; e-mail: cmb2@case.edu.
References
- 1.Piperno A, Galimberti S, Mariani R, et al. HIGHCARE investigators. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood. 2011;117(10):2953–2959. doi: 10.1182/blood-2010-08-299859. [DOI] [PubMed] [Google Scholar]
- 2.Talbot NP, Lakhal S, Smith TG, et al. Regulation of hepcidin expression at high altitude. Blood. 2012;119(3):857–860. doi: 10.1182/blood-2011-03-341776. [DOI] [PubMed] [Google Scholar]
- 3.Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK, Di Rienzo A. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet. 2012;8(12):e1003110. doi: 10.1371/journal.pgen.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 5.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117(25):e218–e225. doi: 10.1182/blood-2011-02-337907. [DOI] [PubMed] [Google Scholar]
- 6.Winslow RM, Chapman KW, Gibson CC, et al. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol. 1989;66(4):1561–1569. doi: 10.1152/jappl.1989.66.4.1561. [DOI] [PubMed] [Google Scholar]
- 7.Hadziahmetovic M, Song Y, Ponnuru P, et al. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci. 2011;52(1):109–118. doi: 10.1167/iovs.10-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strunnikova NV, Maminishkis A, Barb JJ, et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet. 2010;19(12):2468–2486. doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q, Kathiresan S, Lin J-P, Tofler GH, O’Donnell CJ. Genome-wide association and linkage analyses of hemostatic factors and hematological phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S12. doi: 10.1186/1471-2350-8-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol. 2006;46(1):18–24. doi: 10.1093/icb/icj004. [DOI] [PubMed] [Google Scholar]