Abstract
Epithelial breast malignancies are a group of several disease entities that vary in their biology and response to specific therapies. Historically, classification of different molecular types of breast cancer was done through the use of conventional methods such as tumor morphology, grade, and immunophenotyping for estrogen, progesterone, and HER-2/neu receptor expression. Such techniques, although helpful, are not sufficient to accurately predict biologic behavior of breast cancers. Over the last several years, much progress has been made in more precise identification of molecular breast cancer subtypes. Such advances hold a great promise in improving estimation of prognosis and assigning most appropriate therapies. Thanks to use of cDNA microarrays expression technology and quantitative reverse transcriptase polymerase chain reaction (RT-PCR), tumors with specific gene expression patterns can now be identified. This process is presently reshaping perceptions of how breast cancer should be classified and treated. Categorization of breast cancers by gene expression is only beginning to make its way into the daily clinical practice and likely will complement, but not replace, the conventional methods of classification.
Key words: Breast cancer, Classification, Gene expression profiling, Biologic behavior, Breast cancer subtypes
HISTORICAL PERSPECTIVE: BREAST CANCER IS NOT A SINGLE DISEASE ENTITY
Studies that employed use of endocrine therapies for breast cancer provided one of the first pieces of evidence that biology of breast malignancies is not all the same. In 1896 George Beatson demonstrated that performing bilateral oophorectomies in women with metastatic breast cancer led to regression of the malignancy (8). This finding revolutionized treatment of breast cancer and over the years resulted in development of various therapies that inhibit estrogen action in the breast tissue, resulting in regression of metastatic breast cancers or reduction of the recurrence risk in patients with operable disease (13). When studies of endocrine therapies were analyzed, it became apparent that patients with tumors expressing estrogen and/or progesterone receptors derived much greater degree of benefit from such therapy than those whose tumors have been estrogen and progesterone receptor negative (18). For example, probability of response to endocrine therapy in patients with estrogen and progesterone receptor-positive breast cancers was reported as 50–70% compared to 33% for patients with only estrogen or progesterone-positive disease and <10% in those with estrogen and progesterone-negative disease (46). In addition, hormone receptor expression could identify some patients with less rapid progression of the breast malignancy. Estrogen and progesterone receptor status became one of the most widely clinically used prognostic and predictive molecular markers.
However, not all women with hormone receptor-positive breast cancers derived benefit from endocrine therapy. Work by Slamon and colleagues identified expression of human epidermal receptor 2 (HER-2/neu) in about 30% of breast tumor specimens. HER-2/neu was classified as a member of the ERBB-like oncogene family, which was unique to epidermal growth factor receptor. Amplification of Her-2/neu gene identified a group of patients who had adverse prognosis regardless of hormone receptor expression. In a seminal study published in Nature in 1987, breast tumors of 189 patients were tested for amplification of HER-2/neu gene and correlated with disease-free and overall survival (53). The analysis revealed that patients with operable breast cancers that had 2- to >20-fold amplification of HER-2/neu exhibited overall survival of about 3 years compared to 6–7 years in patients with HER-2/neu nonamplified tumors. This difference persisted after adjustments were made for other known clinical prognostic factors. Additionally, amplification of HER-2/neu gene appeared to have greater prognostic value than other factors used at that time, such as hormonal receptor status and axillary lymph node involvement. This important discovery led to development of chimeric monoclonal antibody against HER-2/neu protein called trastuzumab as well as other small molecule inhibitors of HER-2/neu. Combining these agents with chemotherapy resulted in higher proportion of response and significantly longer time to progression in women with HER-2/neu amplified metastatic breast cancer (25,54). Trastuzumab, when added to adjuvant chemotherapy, also significantly improved disease-free and overall survival in women with operative HER-2/neu-overexpressing breast cancer (48,55). Importantly, in studies testing trastuzumab in the metastatic setting, only patients with breast cancers that had amplification of HER-2/neu gene responded to agents targeting HER-2/neu protein. Based on this and other evidence, amplification of HER-2/neu became a powerful biomarker for both disease prognosis and prediction of response to trastuzumab-containing therapies.
These findings established classification of breast cancers into three major molecular types: hormone receptor-positive group, HER-2/neu amplified group, and the triple negative group (i.e., tumors that did not express hormone receptors nor had amplification of HER-2/neu gene). This categorization relied on conventional immunohistochemistry (IHC) and fluorescence in-situ hybridization (FISH). Within each of these three groups of patients and with addition of other clinical and histologic factors, such as tumor size and grade, lymph node involvement, patients’ ages and menopausal status, prognosis and response to specific therapies could be estimated (52). However, there remained significant difference in the behavior of breast cancers even within each one of such classifications, making the prediction of responses to treatment and clinical outcomes rather challenging. For example, approximately 40–50% of patients with metastatic HER-2 amplified breast cancer did not respond to trastuzumab-containing therapies (54). This discordance in breast cancer biology suggested that the true heterogeneity of epithelial breast cancers is much more vast than initially suspected.
DEVELOPMENT OF cDNA MICROARRAY TECHNOLOGY AND DISCOVERY OF GENE EXPRESSION SIGNATURES IN BREAST CANCER
Discoveries in the Human Genome Project and emergence of hundreds of molecular markers made it possible to study expression of various genes as a tool to more precisely identify specific molecular subtypes of breast cancers, use them to better predict the biologic behavior of breast malignancies, and develop most effective therapeutic approaches (4,44). Initial research of clinically useful novel biomarker discovery involved analysis of expression of single genes. With the notable historical exception of hormone receptor and HER-2/neu expression, such studies for the most part could not reproducibly identify clinically important markers. This led to realization that expression pattern of multiple genes rather than any one of them alone may be more critical in accurate identification of specific molecular subtypes of breast cancer (30). Early work in patterns of gene expression relied on measurement of messenger ribonucleic acid (mRNA) within the tumor cells, by Northern blotting, and quantitative polymerase chain reaction (PCR). This was time consuming and limited the number of genes that could be studied (6). The biggest breakthrough came after development of complementary DNA (cDNA) microarray technology, which allowed efficient evaluation of expression of tens of thousands of genes in a single experiment (4). The two most commonly used methods of cDNA microarrays were oligonucleotide and spotted DNA microarrays (19,35,62). Spotted DNA microarrays quickly gained considerable popularity, as they employed two-color hybridization technique, one representing expressed genes in the tumor sample (often labeled red fluorescent dye such as Cy5) and the other representing expression of genes in a reference sample (often labeled by green dye, such as Cy3). This two-color approach allowed for determining the ratio of red to green fluorescence rather than absolute intensity increasing its reproducibility (4).
In a landmark publication cleverly entitled “Molecular portraits of human breast tumors,” Perou and colleagues reported one of the first characterizations of molecular subtypes of breast tumors based on gene expression signatures that utilized cDNA microarray technology and hierarchical clustering analysis (20,45). Sixty-five samples obtained from 42 patients with breast cancer were analyzed. The final results showed that tumors from each individual patient had a unique pattern of gene expression that was reproducible within the patient even when taken from different tumor sites, such as primary tumor and involved lymph node. Irrespective of this “uniqueness” the hierarchical clustering algorithm separated breast cancers into four molecular subtypes with relatively similar expression patterns. The luminal molecular subtype was characterized by high expression of genes characteristic of epithelial lining of the duct lumen such as cytokeratin (CK) 8 and 18. These tumors also demonstrated expression of estrogen receptor genes. In contrast, most basal-like breast cancers did not express hormone receptors but demonstrated high expression of genes typical for the basal epithelial cells such as CK5, CK7, and smooth muscle actin. These tumors also expressed genes responsible for proliferation and cell survival, such as epidermal growth factor receptor, insulin growth factor, hepatocyte growth factor, or c-Kit (57). Approximately 60–90% of basal-like breast cancers were found to lack hormone receptor or HER-2/neu expression (14). These cancers also are characterized by high degree of genetic instability and associated with low-level copy number gains of specific genes (15). High proportion of basal like breast tumors appear to have increased sensitivity to chemotherapy compared to other types (49). The third subclass was tumors with high expression of HER-2/neu and other closely related genes. Such cancers overexpress large numbers of genes with high number of amplifications of specific chromosomal loci (15). Finally, the fourth category consisted of normal-like breast tumors, which expressed markers of normal breast epithelium. This category is perceived by many as uncertain, since it is believed that this group was significantly contaminated by normal breast tissue (14).
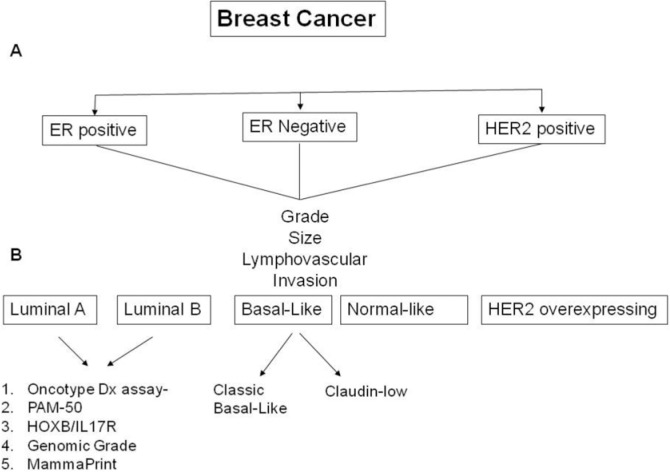
In a subsequent study, cDNA microarray experiments were performed to identify molecular subtype of 78 tumors obtained from breast cancer patients with known clinical follow-up (56). The study showed clinically significant difference in overall and relapse-free survival with the poorest outcome seen in patients with HER-2/neu-overexpressing and basal-like cancers. However, within the luminal type, a subset of patients with unique gene signature was identified whose outcomes were almost as poor as those with HER-2/neu-overexpresssing and basal-like subsets. Therefore, the luminal category was broken down into two subtypes: the more favorable luminal A (high estrogen receptor expression and low expression of proliferative markers such as Ki67) and luminal B (lower estrogen receptor expression and high level of expression of proliferation related genes) (36). Application of cDNA microarrays alongside the discoveries made by Perou et al. are already starting to have an impact on the way breast cancer is classified and treated (Fig. 1). For example, a novel class of agents called poly-adenosine ribose polymerase (PARP) inhibitors was found to be most active against cancers that lack BRCA1 protein function (5,10). Based on microarray expression experiments demonstrating low or no expression of BRCA1 gene in basal-like breast cancers, PARP inhibitors were predicted correctly to be most active in this tumor type.
Figure 1.
Schematic representation of classification of breast cancer. (A) Conventional classification. (B) Classification based on gene expression profiles.
TRANSLATING GENE EXPRESSION INTO CLINICAL PRACTICE: GENOMIC TESTS AS PROGNOSTIC AND PREDICTIVE TOOLS IN BREAST CANCER
Since the discovery of the intrinsic molecular breast cancer types by Perou et al., several gene microarrays were commercially developed as clinically useful prognostic and predictive tools. These genomic tests assess expression of different but sometimes overlapping sets of genes. Despite differences in candidate genes in each of the assays, most of them can quite reliably predict biology of tested tumors (58). In fact, when some of these tests were compared with each other, they were found to have quite similar ability to predict metastases-free and overall survival (23,29). In one study that compared five different prognostic signatures, surprisingly high correlation was found even among tests utilizing expression of very few genes in common. One important finding from analyses of various genomic tests is the fact that they assign almost all patients with hormone receptor-negative disease as high risk. For that reason, most of these tests are more applicable to patients with estrogen receptor-positive cancers who are a more heterogeneous group in terms of prognosis and probability of response to chemotherapy and are harder to categorize based on conventional clinical and pathologic criteria. The molecular signatures assign up to 93% of the patients with luminal B tumors to high-risk category, which correlates with known fact that such cancers are associated with outcome almost as poor as basal-like or HER-2-overexpressing tumors (23,36). Given this distinction, utility of these tests in practice will still depend on clinical and histologic assessment to identify specific patients who would then be appropriate for additional testing with gene expression signatures. A well-known example is the most widely used test, Oncotype Dx. The test was found to be most useful in women with smaller, hormone receptor-positive breast cancers without axillary lymph node involvement. Nevertheless, applications in other clinical scenarios are emerging (see below) (58). The most common genomic tests are summarized below.
MammaPrint
The investigators in the Netherlands Cancer Institute used tumor specimen from 78 patients that were younger than 55 years of age with primary node negative breast cancers smaller than 5 cm in size (61). Of those patients, 34 developed distant metastases and 44 were disease free at 5 years. mRNA was extracted from the tumors to reverse transcribe into cDNA, which was then tested on microarray that contained 25,000 human genes. Using unsupervised and supervised as well as cross-validation analysis, 70 genes that had the strongest association with outcome and accurately predicted good and poor risk disease were selected. The candidate genes that predicted unfavorable outcome were involved in regulating cell cycle, invasion, metastasis, and angiogenesis (e.g., cyclin E2, MCM6, MMP9 and MP1, RAB6B, PK428, ESM1, and FLT1). In the validation experiments on the set of tumor samples obtained from 19 young women with lymph node-negative breast cancer (7 remained metastases free and 12 developed distant recurrence), the test predicted outcome in all but 2 of these patients. Subsequently, two confirmatory retrospective studies validated accuracy of the assay in identifying patients with poor outcome (12,60). One of these studies also demonstrated superiority of the genomic test over online prognostic tool, called Adjuvant Online, which uses conventional clinical and pathologic factors. In addition, a prospective, community-based clinical feasibility study called Micro-arRAy PrognoSTics in Breast CancER (RASTER) that enrolled 812 women younger than 61 years of age with node-negative, operable breast cancer showed that the genomic expression assay (now called Mam-maPrint) result altered decisions regarding adjuvant chemotherapy in 26% of patients (11). Overall, adjuvant systemic treatment was advised less often based on MammaPrint result when compared to other conventional tools, such as Dutch CBO guidelines (49% vs. 62%, respectively) or “Adjuvant Online” (69%). MammaPrint has been approved by the Food and Drug Administration as a tool to predict prognosis in women with lymph node-negative breast cancer who are younger than 61 years of age and have tumors smaller than 5 cm. The major disadvantages of the assay are the fact that it requires fresh frozen tissue specimen and has not yet been sufficiently evaluated as a predictive tool. A prospective trial called Min-dAct, studying MammaPrint, is presently ongoing (2).
Oncotype Dx
The 21 gene expression assay called Oncotype Dx, which was developed by Genomic Health, is perhaps the most widely used prognostic and predictive geno-mic tool for women with hormone receptor-positive, node-negative breast cancer in the US. Since cDNA microarrays usually require use of fresh or snap frozen tissue due to the requirement for intact mRNA molecules, the investigators involved in development of Oncotype Dx employed quantitative RT-PCR technology that utilizes short and homogeneous amplicons. This method accurately measures gene expression even in the presence of mRNA fragmentation that occurs in archived formalin-fixed, paraffin-embedded (FFPE) tissues (17). The assay was developed through the expression analysis of 250 candidate genes that were selected based their significance in breast cancer biology and possible prognostic/predictive value. Expression of these genes was subsequently tested in FFPE tumor samples from 447 breast cancer patients participating in three clinical trials (16,22,40). Based on results of this analysis, 16 most suitable cancer-specific candidate genes and 5 reference genes were selected. The cancer-specific genes included those that were associated with proliferation (Ki67, STK15, Survivin, CCNB1, MYBL2), invasion (MMP11, CTSL2), HER-2/neu function (HER-2, GRB7), estrogen receptor function (ER, PGR, BCL2, SCUBE2), and others (GSTM1, CD68, BAG1). The measurement of expression of 16 cancer-specific genes was combined, normalized, and converted into a quantitative, clinically useful unit called “recurrence score” (41).
Multiple retrospective validation studies in various clinical settings established prognostic and predictive accuracy of Oncotype Dx assay. The first report analyzed tumor specimen from NSABP B14 trial, which tested use of adjuvant tamoxifen in women with estrogen receptor-positive, node-negative breast cancer. The 10-year recurrence rates of 7%, 14%, and 30% were seen in patients with low, intermediate, and high recurrence scores, respectively. In multivariate analysis, recurrence score was independent of age and tumor size. Another study confirmed the prognostic accuracy of Oncotype Dx regardless of whether or not adjuvant tamoxifen was used (28). Oncotype Dx has also been compared to conventional prognostic tools in clinical use and was found to be more accurate (27). Another analysis of tumor specimen from a randomized trial of patients with operable, hormone receptor-positive, node-negative breast cancer assigned to receive adjuvant tamoxifen with or without chemotherapy found that Oncotype Dx was also able to predict response to chemotherapy (42). Significant effect of chemotherapy on distant recurrence was seen in patients whose tumors had high recurrence score (relative risk for recurrence 0.22, 95% CI 0.13–0.53). Chemotherapy had no effect on distant recurrence in the low recurrence score group (relative risk for recurrence 1.31, 95% CI 0.46–3.78). The effect of chemotherapy on reducing risk of distant metastases in the intermediate group was unclear (relative risk 0.61, 95% CI 0.24–1.59). One study also demonstrated that Oncotype Dx can predict benefit of adjuvant chemotherapy in node-positive women (1). Two large randomized clinical trials TAI-LORx (in women with node-negative disease) and RxPonder (in women with breast cancer involving 1–3 axillary nodes), testing Oncotype Dx use to guide treatment decisions, are under way (2). Two additional prospective phase II trials that use recurrence score to determine neoadjuvant therapy are also ongoing (2). Oncotype Dx is presently endorsed by the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) as prognostic and predictive test for cases of hormone receptor-positive, lymph node-negative breast tumors that are greater than 1 cm in size. One recent analysis that looked at combining clinical factors and recurrence score to make treatment decisions found that confidence of recommending treatment increased by 60% after addition of oncotype Dx test (3).
HOXB13/IL17BR
Another assay measuring a ratio of Homeobox 13 (HOXB13) to interleukin 17B receptor (IL-17BR) in addition to choline dehydrogenase (CHDH) and hormone receptor expression on FFPE tissues can identify patients with high risk for relapse if treated with or without tamoxifen, independent of tumor grade (26,33,37). The development of this assay included quantitative analysis of HOXB13, IL-17BR, CHDH, estrogen receptor, and progesterone receptor by realtime polymerase chain reaction in 852 formalin-fixed, paraffin-embedded primary breast cancers from 566 untreated and 286 tamoxifen-treated breast cancer patients. The study identified that expression of HOXB13 was associated with shorter relapse-free survival (RFS) (p = 0.008) and expression of IL-17BR and CHDH was associated with longer RFS. The study established that HOXB13/IL-17BR index predicted clinical outcome especially in node-negative patients independently of estrogen receptor status, tumor size, S phase fraction, and treatment. Such an index is one of the simplest gene expression signatures that can reliably predict a patient’s outcome. Similar to Oncotype Dx, it can conveniently be performed on archived FFPE breast tumor samples. In one retrospective study, combining HOXB13/IL-17BR expression with the molecular tumor grade index (MGI) that measured expression of five cell cycle-related genes was shown to have improved accuracy of predicting adverse outcome of patients with early stage estrogen receptor-positive breast cancer (38).
PAM50
A 50 gene expression assay based on microarray and quantitative RT-PCR called PAM50 was developed by Parker and colleagues by analyzing 189 FFPE breast tumor samples to separate them into four known intrinsic molecular breast cancer subtypes (basal-like, HER-2/neu positive, luminal A, and luminal B). In the validation study, tumor analysis was performed on 761 samples from patients with early stage, node-negative breast cancer not treated with chemotherapy and 133 samples that came from patients who received taxane and anthracycline containing neoadjuvant chemotherapy. Patients with luminal A subtype as assessed by PAM50 had better prognosis in contrast to the other three intrinsic types. Two “risk of relapse” (ROR) scores were created, one using subtype correlation only (ROR-S) and the other one using subtype correlation in addition to tumor size (ROS-C). In multivariate analysis that controlled for known clinical risk factors, these scores correlated well with good prognosis. The ROR-S had sensitivity of 94% and negative predictive value of 97% for identifying nonresponders to chemotherapy. The tumors with high-risk scores had higher probability of complete pathologic response (pCR), in contrast to those within the low-risk group. This is consistent with prior studies showing that indolent ER-positive tumors that likely represented majority of tumors with low ROS (luminal A) are less responsive to chemotherapy but respond better to endocrine therapy. However, in the group of tumors with highest ROR score, a plateau pCR rate was reached. The investigators proposed that this was likely due to the fact that significant chemotherapy resistance can be present among the highest risk tumors (43).
Other Genomic Tests
A multitude of other genomic tests exist. Some, similar to MammaPrint, such as genomic grade signature (97 gene expression microarray) and 76 Gene Expression Grade Index (GGI) provide information to help characterize intermediate grade cancers (e.g., grade 2 tumors without axillary lymph node involvement) and assist in making decision regarding use of adjuvant chemotherapy (29). Other gene expression tests looking at gene signatures of stromal factors, embryonic or stem cell-like genes, and others correlating them with clinical outcome of patients with breast cancers are also in development (9,24,34).
GENE EXPRESSION SIGNATURES AS RESISTANCE TO CHEMOTHERAPY
Identifying breast cancer patients who may be resistant to specific therapies by analysis of genetic expression patters could have important applications in clinical practice. Such strategy would help provide additional predictive information and individualize treatment. Several microarray assays are being developed for this purpose. These assays were constructed and studied mainly through analysis of tumor specimen in neoadjuvant chemotherapy trials. In such trials, pathologic response at the time of definitive surgery is a powerful surrogate for long-term outcome, such as overall or disease-free survival, especially in patients with high grade, hormone receptor-negative tumors (51). In one published study, prospectively collected pretreatment core biopsies of tumors from 42 patients with stage I–III breast cancer treated with neoadjuvant chemotherapy consisting of 12 treatments of weekly paclitaxel followed by four courses of 5-fluorouracil, doxorubicin, and cyclophosphamide (T/FAC regimen) was analyzed for gene expression patterns (7). The tumor was analyzed for 30,721 gene sequences using cDNA microarray technology. Twenty-four patients were used for discovery of gene expression associated with achieving pCR at the time of the surgery. A set of 72 genes was found to correlate with pCR (p ≤ 0.09). The set was subsequently used on the remaining 18 patients to validate its predictive accuracy, which was established at 78% (14 out of 16 patients). The sensitivity of the assay was 43% and specificity was 100%.
Another study used oligonucleotide cDNA micro-arrays and identified expression of 30 genes in pre-treatment tumor samples of 133 patients with stage I–III breast cancer that predicted pCR to neoadjuvant T/FAC regimen (31). The gene signature had 76% accuracy (sensitivity of 96%, specificity 74%, positive predictive value of 52%, and negative predictive value 96%). Perhaps the largest trial to date that attempted to develop genetic signatures for patients with hormone receptor-positive, HER-2/neu-negative breast cancer utilized a statistical method called gene set analyses. This method uses sets of genes that share common biological function, chromosomal location, or regulation. The tested genes are ranked on the list based on strength of their association with outcome (59). Initially, 2,331 functionally annotated gene sets were assembled from Ingenuity Pathway Analysis and Gene Ontology databases. These sets corresponded to almost all known biological processes. The sets were applied to the gene expression of three cohorts of patients (N = 234, 170, and 175) with early stage, HER-2/neu-negative, node-negative breast cancer who did not receive adjuvant chemotherapy to identify specific sets associated with outcome. Another group of patients with stage I–III breast cancer who received various regimens of neo-adjuvant chemotherapy (N = 198, 85, and 62) were analyzed to identify gene sets that were associated with pCR. In the pooled analysis of the untreated groups, 131 and 14 gene sets were found to be associated with adverse prognosis in patients with estrogen receptor-positive and -negative cancers, respectively. The analysis of the preoperative chemotherapy group identified 69 and 23 sets that were significantly associated with achieving complete pathologic response in ER-positive and ER-negative groups, respectively. Of note, different gene sets had prognostic or predictive ability in estrogen-positive and -negative cancers, signifying that tumors identified on the clinical and histologic basis depend on different molecular pathways. It also suggests that different biologic processes may have impact on prognosis and resistance to chemotherapy depending on estrogen status of breast cancer. For example, proliferation-related sets of genes were associated with increased chemotherapy sensitivity in ER-positive breast cancers but not in ER-negative breast cancers (32). This study also elegantly utilized our understanding about important pathways involved in breast cancer biology and attempted to apply such knowledge to develop clinically useful tools.
Prospective studies to confirm the ability of these gene signatures to predict resistance to specific chemotherapy regimens are needed and will provide critical data on their clinical utility. They may find its use initially in clinical trials that can classify patients as chemotherapy resistant based on those assays and randomize them to alternative chemotherapies. Comparison of genomic test PAM50 to other clinical and histologic tumor characteristics have been utilized in phase II clinical trial testing use of various neoadjuvant endocrine therapies (21). For additional information, please refer to separate articles on role of micro-RNAs in drug-resistant breast cancer in the same issue of the journal.
CONCLUSION: WHERE DO WE GO FROM HERE?
The discovery of the molecular breast cancer subtypes by identifying unique expression signature based on cDNA microarray technology confirmed that breast malignancy is not one disease but rather heterogeneous group of cancers that morphologically may look alike but appear to differ widely with respect to their origin within the breast tissue, their biology, and response to different therapeutic strategies.
With more advanced technologies and taking into consideration expanding knowledge from a multitude of existing research, more subtypes of breast cancers are likely to be teased out from the known groups. Differentiating molecular subtypes into cancers with more uniform gene expression patterns holds great promise in improving assessment of prognosis and use of more effective chemotherapy. More recently, for instance, a subset of triple negative, non-basal-like breast cancers that was identified based on particular gene signature was discovered and named “claudin low” due to low expression of epithelial cell–cell adhesion genes such as Claudin 3, 4, 7 and E-cadherin as well as differentiated luminal cell surface markers such as EpCAM and MUC1 (47). This expression signature has been associated with epithelial mesenchymal transition (EMT). EMT normally occurs in embryogenesis and is characterized by increased expression of a mesenchymal marker vimen-tin (50). In addition, the claudin-low type has been found to have greater proportion of tumor cells that are CD44+/CD24−, high ALDH1A1, thought to be associated with stem cell or tumor-initiating cell phenotype. These cells appear to be slower growing than basal-like type. Claudin-low breast cancers were shown to be associated with poorer prognosis and greater degree of chemotherapy resistance.
Increasing appreciation of DNA regions responsible elements other than genes, such as those coding for microRNAs (miRNAs) may also provide future insights on biology and molecular subtypes of breast cancer. miRNAs are a class of short RNAs found in plants and animals, well conserved. They often inhibit gene expression posttranscriptionally and are increasingly implicated in regulation of expression genes that are crucial for cancer biology. The ability and ease of measuring (miRNAs) in FFPE tissue does make it a desirable tool for further development in correlating gene expression profiles with clinical outcomes. Recently, two groups have reported on the expression of miRNA221/222 in estrogen receptor-positive tumors as predictors of tamoxifen resistance (39,63). This is an early work and further efforts are needed to standardize the techniques and to confirm that miRNA expression profiles can provide independent prognostic or predictive information.
It is important to note that even with improvement in technology and progress towards standardization of methods of data analysis, gene expression assays are still rather time consuming and expensive for practical, everyday use. Hence, it is not likely that they will replace conventional clinical and pathologic evaluation to categorize breast cancers but rather serve as an adjunct to known clinical methods. As with Oncotype Dx, these genomic tests will likely be applied to specific clinical scenarios to fine tune the understanding of the tumor behavior and select the most appropriate management options. The genomic tests may become useful in developing novel therapies, especially in view of the fact that specific patterns of gene expression can further be explored to identify biologic processes that are driven by such genes. This can lead to new ideas about ways to inhibit tumor growth, minimize metastatic potential of breast cancer, or prevent occurrence of breast cancer in high-risk subjects.
In conclusion, the development and improvement of gene expression assays has led to major breakthroughs in the breast cancer field, which is already influencing the way clinicians treat patients. Application of this powerful tool will surely play an important role in shaping the future of the breast cancer field and other areas of oncology.
REFERENCES
- 1. Albain K. S.; Barlow W. E.; Shak S.; Hortobagyi G. N.; Livingston R. B.; Yeh I. T.; Ravdin P.; Bugarini R.; Baehner F. L.; Davidson N. E.; Sledge G. W.; Winer E. P.; Hudis C.; Ingle J. N.; Perez E. A.; Pritchard K. I.; Shepherd L.; Gralow J. R.; Yoshizawa C.; Allred D. C.; Osborne C. K.; Hayes D. F. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 11:55–65; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albain K. S.; Carey L.; Gradishar W. J.; Gralow J. R.; Lipton A.; Rugo H.; Tripathy D.; Peck S.; Abair T.; Pegram M. Proceedings of the First Global Workshop on Breast Cancer: Pathways to the evaluation and clinical development of novel agents for breast cancer. Clin. Breast Cancer 10:421–439; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Albanell J.; González A.; Ruiz-Borrego M.; Alba E.; García-Saenz J. A.; Corominas J. M.; Burgues O.; Furio V.; Rojo A.; Palacios J.; Bermejo B.; Martínez-García M.; Limon M. L.; Muñoz A. S.; Martín M.; Tusquets I.; Rojo F.; Colomer R.; Faull I.; Lluch A. Prospective transGEICAM study of the impact of the 21-gene recurrence score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann. Oncol. in press; 2011. [DOI] [PubMed] [Google Scholar]
- 4. Alizadeh A. A.; Ross D. T.; Perou C. M.; van de Rijn M. Towards a novel classification of human malignancies based on gene expression patterns. J. Pathol. 195:41–52; 2001. [DOI] [PubMed] [Google Scholar]
- 5. Alli E.; Sharma V. B.; Sunderesakumar P.; Ford J. M. Defective repair of oxidative DNA damage in triple-negative breast cancer confers sensitivity to inhibition of poly(ADP-ribose) polymerase. Cancer Res. 69:3589–3596; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alwine J. C.; Kemp D. J.; Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 74:5350–5354; 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayers M.; Symmans W. F.; Stec J.; Damokosh A. I.; Clark E.; Hess K.; Lecocke M.; Metivier J.; Booser D.; Ibrahim N.; Valero V.; Royce M.; Arun B.; Whitman G.; Ross J.; Sneige N.; Hortobagyi G. N.; Pusztai L. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J. Clin. Oncol. 15;22:2284–2293; 2004. [DOI] [PubMed] [Google Scholar]
- 8. Beatson G. T. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Lancet 2:104–107; 1896. [PMC free article] [PubMed] [Google Scholar]
- 9. Ben-Porath I.; Thomson M. W.; Carey V. J.; Ge R.; Bell G. W.; Regev A.; Weinberg R. A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40:499–507; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brody L. C. Treating cancer by targeting a weakness. N. Engl. J. Med. 353:949–950; 2005. [DOI] [PubMed] [Google Scholar]
- 11. Bueno-de-Mesquita J. M.; van Harten W. H.; Retel V. P.; van’t Veer L. J.; van Dam F. S.; Karsenberg K.; Douma K. F.; van Tinteren H.; Peterse J. L.; Wesseling J.; Wu T. S.; Atsma D.; Rutgers E. J.; Brink G.; Floore A. N.; Glas A. M.; Roumen R. M.; Bellot F. E.; van Krimpen C.; Rodenhuis S.; van de Vijver M. J.; Linn S. C. Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: A prospective community-based feasibility study (RASTER). Lancet Oncol. 8:1079–1087; 2007. [DOI] [PubMed] [Google Scholar]
- 12. Buyse M.; Loi S.; van’t Veer L.; Viale G.; Delorenzi M.; Glas A. M.; d’Assignies M. S.; Bergh J.; Lidereau R.; Ellis P.; Harris A.; Bogaerts J.; Therasse P.; Floore A.; Amakrane M.; Piette F.; Rutgers E.; Sotiriou C.; Cardoso F.; Piccart M. J. TRANS-BIG Consortium. Validation and clinical utility of a 70-gene prognostic signature for women with node negative breast cancer. J. Natl. Cancer Inst. 98:1183–1192; 2006. [DOI] [PubMed] [Google Scholar]
- 13. Buzdar A. U. Advances in endocrine treatments for post-menopausal women with metastatic and early breast cancer. Oncologist 8:335–341; 2003. [DOI] [PubMed] [Google Scholar]
- 14. Carey L.; Winer E.; Viale G.; Cameron D.; Gianni L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 7:683–692; 2010. [DOI] [PubMed] [Google Scholar]
- 15. Chin K.; DeVries S.; Fridlyand J.; Spellman P. T.; Roydasgupta R.; Kuo W. L.; Lapuk A.; Neve R. M.; Qian Z.; Ryder T.; Chen F.; Feiler H.; Tokuyasu T.; Kingsley C.; Dairkee S.; Meng Z.; Chew K.; Pinkel D.; Jain A.; Ljung B. M.; Esserman L.; Albertson D. G.; Waldman F. M.; Gray J. W. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 10:529–541; 2006. [DOI] [PubMed] [Google Scholar]
- 16. Cobleigh M. A.; Tabesh B.; Bitterman P.; Baker J.; Cronin M.; Liu M. L.; Borchik R.; Mosquera J. M.; Walker M. G.; Shak S. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin. Cancer Res. 11:8623–8631; 2005. [DOI] [PubMed] [Google Scholar]
- 17. Cronin M.; Pho M.; Dutta D.; Stephans J. C.; Shak S.; Kiefer M. C.; Esteban J. M.; Baker J. B. Measurement of gene expression in archival paraffin-embedded tissues: Development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am. J. Pathol. 164:35–42; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687–1717; 2005. [DOI] [PubMed] [Google Scholar]
- 19. Eisen M. B.; Brown P. O. DNA arrays for analysis of gene expression. Methods Enzymol. 303:179–205; 1999. [DOI] [PubMed] [Google Scholar]
- 20. Eisen M. B.; Spellman P. T.; Brown P. O.; Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863–14868; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellis M. J.; Suman V. J.; Hoog J.; Lin L.; Snider J.; Prat A.; Parker J. S.; Luo J.; Deschryver K.; Allred D. C.; Esserman L. J.; Unzeitig G. W.; Margenthaler J.; Babiera G. V.; Marcom P. K.; Guenther J. M.; Watson M. A.; Leitch M.; Hunt K.; Olson J. A. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 29:2342–2349; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esteban J.; Baker J.; Cronin M.; Liu M. L.; Llamas M. G.; Walker M. G.; Mena R.; Shak S. Tumor gene expression and prognosis in breast cancer: Multi-gene RT-PCR assay of paraffin-embedded tissue. Prog. Proc. Am. Soc. Clin. Oncol. 22:850; 2003. [Google Scholar]
- 23. Fan C.; Oh D. S.; Wessels L.; Weigelt B.; Nuyten D. S.; Nobel A. B.; van’t Veer L. J.; Perou C. M. Concordance among gene-expression-based predictors for breast cancer. N. Engl. J. Med. 355:560–569; 2006. [DOI] [PubMed] [Google Scholar]
- 24. Finak G.; Bertos N.; Pepin F.; Sadekova S.; Souleimanova M.; Zhao H.; Chen H.; Omeroglu G.; Meterissian S.; Omeroglu A.; Hallett M.; Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14:518–527; 2008. [DOI] [PubMed] [Google Scholar]
- 25. Geyer C. E.; Forster J.; Lindquist D.; Chan S.; Romieu C. G.; Pienkowski T.; Jagiello-Gruszfeld A.; Crown J.; Chan A.; Kaufman B.; Skarlos D.; Campone M.; Davidson N.; Berger M.; Oliva C.; Rubin S. D.; Stein S.; Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355:2733–2743; 2006. [DOI] [PubMed] [Google Scholar]
- 26. Goetz M. P.; Suman V. J.; Ingle J. N.; Nibbe A. M.; Visscher D. W.; Reynolds C. A.; Lingle W. L.; Erlander M.; Ma X. J.; Sgroi D. C.; Perez E. A.; Couch F. J. A two-gene expression ratio of homeobox 13 and interleukin-17B receptor for prediction of recurrence and survival in women receiving adjuvant tamoxifen. Clin. Cancer Res. 12:2080–2087; 2006. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein L. J.; Gray R.; Badve S.; Childs B. H.; Yoshizawa C.; Rowley S.; Shak S.; Baehner F. L.; Ravdin P. M.; Davidson N. E.; Sledge G. W. Jr.; Perez E. A.; Shulman L. N.; Martino S.; Sparano J. A. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J. Clin. Oncol. 26:4063–4071; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Habel L. A.; Shak S.; Jacobs M. K.; Capra A.; Alexander C.; Pho M.; Baker J.; Walker M.; Watson D.; Hackett J.; Blick N. T.; Greenberg D.; Fehrenbacher L.; Langholz B.; Quesenberry C. P. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 8:R25; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haibe-Kains B.; Desmedt C.; Piette F.; Buyse M.; Cardoso F.; Van’t Veer L.; Piccart M.; Bontempi G.; Sotiriou C. Comparison of prognostic gene expression signatures for breast cancer. BMC Genomics 9:394; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris L.; Fritsche H.; Mennel R.; Norton L.; Ravdin P.; Taube S.; Somerfield M. R.; Hayes D. F.; Bast R. C. Jr. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 25:5287–5312; 2007. [DOI] [PubMed] [Google Scholar]
- 31. Hess K. R.; Anderson K.; Symmans W. F.; Valero V.; Ibrahim N.; Mejia J. A.; Booser D.; Theriault R. L.; Buzdar A. U.; Dempsey P. J.; Rouzier R.; Sneige N.; Ross J. S.; Vidaurre T.; Gómez H. L.; Hortobagyi G. N.; Pusztai L. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J. Clin. Oncol. 24:4236–4244; 2006. [DOI] [PubMed] [Google Scholar]
- 32. Iwamoto T.; Bianchini G.; Booser D.; Qi Y.; Coutant C.; Shiang C. Y.; Santarpia L.; Matsuoka J.; Hortobagyi G. N.; Symmans W. F.; Holmes F. A.; O’Shaughnessy J.; Hellerstedt B.; Pippen J.; Andre F.; Simon R.; Pusztai L. Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer. J. Natl. Cancer Inst. 103:264–272; 2011. [DOI] [PubMed] [Google Scholar]
- 33. Jansen M. P.; Sieuwerts A. M.; Look M. P.; Ritstier K.; Meijer-van Gelder M. E.; van Staveren I. L.; Klijn J. G.; Foekens J. A.; Berns E. M. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: A retrospective study. J. Clin. Oncol. 25:662–668; 2007. [DOI] [PubMed] [Google Scholar]
- 34. Liu R.; Wang X.; Chen G. Y.; Dalerba P.; Gurney A.; Hoey T.; Sherlock G.; Lewicki J.; Shedden K.; Clarke M. F. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N. Engl. J. Med. 356:217–226; 2007. [DOI] [PubMed] [Google Scholar]
- 35. Lockhart D. J.; Dong H.; Byrne M. C.; Follettie M. T.; Gallo M. V.; Chee M. S.; Mittmann M.; Wang C.; Kobayashi M.; Horton H.; Brown E. L. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nature Biotechnol. 14:1675–1680; 1996. [DOI] [PubMed] [Google Scholar]
- 36. Loi S.; Haibe-Kains B.; Desmedt C.; Lallemand F.; Tutt A. M.; Gillet C.; Ellis P.; Harris A.; Bergh J.; Foekens J. A.; Klijn J. G.; Larsimont D.; Buyse M.; Bontempi G.; Delorenzi M.; Piccart M. J.; Sotiriou C. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J. Clin. Oncol. 25:1239–1246; 2007. [DOI] [PubMed] [Google Scholar]
- 37. Ma X. J.; Hilsenbeck S. G.; Wang W.; Ding L.; Sgroi D. C.; Bender R. A.; Osborne C. K.; Allred D. C.; Erlander M. G. The HOXB13:IL17BR expression index is a prognostic factor in early-stage breast cancer. J. Clin. Oncol. 24:4611–4619; 2006. [DOI] [PubMed] [Google Scholar]
- 38. Ma X. J.; Salunga R.; Dahiya S.; Wang W.; Carney E.; Durbecq V.; Harris A.; Goss P.; Sotiriou C.; Erlander M.; Sgroi D. A five-gene molecular grade index and HOXB13:IL17BR are complementary prognostic factors in early stage breast cancer. Clin. Cancer Res. 14:2601–2608; 2008. [DOI] [PubMed] [Google Scholar]
- 39. Miller T. E.; Ghoshal K.; Ramaswamy B.; Roy S.; Datta J.; Shapiro C. L.; Jacob S.; Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 283:29897–29903; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paik S.; Shak S.; Tang G. Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients—NSABP studies B-20 and B-14. Breast Cancer Res. Treat. 82:A16; 2003. [Google Scholar]
- 41. Paik S.; Shak S.; Tang G.; Kim C.; Baker J.; Cronin M.; Baehner F. L.; Walker M. G.; Watson D.; Park T.; Hiller W.; Fisher E. R.; Wickerham D. L.; Bryant J.; Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351:2817–2826; 2004. [DOI] [PubMed] [Google Scholar]
- 42. Paik S.; Tang G.; Shak S.; Kim C.; Baker J.; Kim W.; Cronin M.; Baehner F. L.; Watson D.; Bryant J.; Costantino J. P.; Geyer C. E.; Wickerham D. L.; Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24:3726–3734; 2006. [DOI] [PubMed] [Google Scholar]
- 43. Parker J. S.; Mullins M.; Cheang M. C.; Leung S.; Voduc D.; Vickery T.; Davies S.; Fauron C.; He X.; Hu Z.; Quackenbush J. F.; Stijleman I. J.; Palazzo J.; Marron J. S.; Nobel A. B.; Mardis E.; Nielsen T. O.; Ellis M. J.; Perou C. M.; Bernard P. S. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27:1160–1167; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perou C. M.; Jeffrey S. S.; van de Rijn M.; Rees C. A.; Eisen M. B.; Ross D. T.; Pergamenschikov A.; Williams C. F.; Zhu S. X.; Lee J. C.; Lashkari D.; Shalon D.; Brown P. O.; Botstein D. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA 96:9212–9217; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perou C. M.; Sørlie T.; Eisen M. B.; van de Rijn M.; Jeffrey S. S.; Rees C. A.; Pollack J. R.; Ross D. T.; Johnsen H.; Akslen L. A.; Fluge O.; Pergamenschikov A.; Williams C.; Zhu S. X.; Lønning P. E.; Børresen-Dale A. L.; Brown P. O.; Botstein D. Molecular portraits of human breast tumors. Nature 406:747–752; 2000. [DOI] [PubMed] [Google Scholar]
- 46. Pinder M. C.; Buzdar A. U. Endocrine therapy for breast cancer. In Hunt K. K.; Robb G. L.; Strom E. A.; Ueno N. T., eds. Breast cancer, 2nd ed. (M.D. Anderson Cancer Care Series) New York: Springer; 2008:411–434. [Google Scholar]
- 47. Prat A.; Parker J. S.; Karginova O.; Fan C.; Livasy C.; Herschkowitz J. I.; He X.; Perou C. M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12:R68; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romond E. H.; Perez E. A.; Bryant J.; Suman V. J.; Geyer C. E.; Davidson N. E.; Tan-Chiu E.; Martino S.; Paik S.; Kaufman P. A.; Swain S. M.; Pisansky T. M.; Fehrenbacher L.; Kutteh L. A.; Vogel V. G.; Visscher D. W.; Yothers G.; Jenkins R. B.; Brown A. M.; Dakhil S. R.; Mamounas E. P.; Lingle W. L.; Klein P. M.; Ingle J. N.; Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353:1673–1684; 2005. [DOI] [PubMed] [Google Scholar]
- 49. Rouzier R.; Perou C. M.; Symmans W. F.; Ibrahim N.; Cristofanilli M.; Anderson K.; Hess K. R.; Stec J.; Ayers M.; Wagner P.; Morandi P.; Fan C.; Rabiul I.; Ross J. S.; Hortobagyi G. N.; Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 11:5678–5685; 2005. [DOI] [PubMed] [Google Scholar]
- 50. Sarrió D.; Rodriguez-Pinilla S. M.; Hardisson D.; Cano A.; Moreno-Bueno G.; Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 68:989–997; 2008. [DOI] [PubMed] [Google Scholar]
- 51. Scholl S. M.; Pierga J. Y.; Asselain B. Breast tumours response to primary chemotherapy predicts local and distant control as well as survival. Eur. J. Cancer 31A:1969–1995; 1995. [DOI] [PubMed] [Google Scholar]
- 52. Simpson P. T.; Reis-Filho J. S.; Gale T.; Lakhani S. R. Molecular evolution of breast cancer. J. Pathol. 205:248–254; 2005. [DOI] [PubMed] [Google Scholar]
- 53. Slamon D. J.; Clark G. M.; Wong S. G.; Levin W. J.; Ullrich A.; McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182; 1987. [DOI] [PubMed] [Google Scholar]
- 54. Slamon D. J.; Leyland-Jones B.; Shak S.; Fuchs H.; Paton V.; Bajamonde A.; Fleming T.; Eiermann W.; Wolter J.; Pegram M.; Baselga J.; Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344:783–792; 2001. [DOI] [PubMed] [Google Scholar]
- 55. Smith I.; Procter M.; Gelber R. D.; Guillaume S.; Feyereislova A.; Dowsett M.; Goldhirsch A.; Untch M.; Mariani G.; Baselga J.; Kaufmann M.; Cameron D.; Bell R.; Bergh J.; Coleman R.; Wardley A.; Harbeck N.; Lopez R. I.; Mallmann P.; Gelmon K.; Wilcken N.; Wist E.; Sánchez Rovira P.; Piccart-Gebhart M. J.; HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet 369:29–36; 2007. [DOI] [PubMed] [Google Scholar]
- 56. Sørlie T.; Perou C. M.; Tibshirani R.; Aas T.; Geisler S.; Johnsen H.; Hastie T.; Eisen M. B.; van de Rijn M.; Jeffrey S. S.; Thorsen T.; Quist H.; Matese J. C.; Brown P. O.; Botstein D.; Eystein Lønning P.; Børresen-Dale A. L. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98:10869–10874; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sorlie T.; Tibshirani R.; Parker J.; Hastie T.; Marron J. S.; Nobel A.; Deng S.; Johnsen H.; Pesich R.; Geisler S.; Demeter J.; Perou C. M.; Lønning P. E.; Brown P. O.; Børresen-Dale A. L.; Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 100:8418–8423; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sotiriou C.; Pusztai L.; Gene-expression signatures in breast cancer. N. Engl. J. Med. 360:790–800; 2009. [DOI] [PubMed] [Google Scholar]
- 59. Subramanian A.; Tamayo P.; Mootha V. K.; Mukherjee S.; Ebert B. L.; Gillette M. A.; Paulovich A.; Pomeroy S. L.; Golub T. R.; Lander E. S.; Mesirov J. P. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102:15545–15550; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van de Vijver M. J.; He Y. D.; van’t Veer L. J.; Dai H.; Hart A. A.; Voskuil D. W.; Schreiber G. J.; Peterse J. L.; Roberts C.; Marton M. J.; Parrish M.; Atsma D.; Witteveen A.; Glas A.; Delahaye L.; van der Velde T.; Bartelink H.; Rodenhuis S.; Rutgers E. T.; Friend S. H.; Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347:1999–2009; 2002. [DOI] [PubMed] [Google Scholar]
- 61. van’t Veer L. J.; Dai H.; van de Vijver M. J.; He Y. D.; Hart A. A.; Mao M.; Peterse H. L.; van der Kooy K.; Marton M. J.; Witteveen A. T.; Schreiber G. J.; Kerkhoven R. M.; Roberts C.; Linsley P. S.; Bernards R.; Friend S. H. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536; 2002. [DOI] [PubMed] [Google Scholar]
- 62. Young R. A. Biomedical discovery with DNA arrays. Cell 102:9–15; 2000. [DOI] [PubMed] [Google Scholar]
- 63. Zhao J. J.; Lin J.; Yang H.; Kong W.; He L.; Ma X.; Coppola D.; Cheng J. Q. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 283:31079–31086; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]