Abstract
Background: The risk of type 1 diabetes (T1DM), infections, cancer, schizophrenia and multiple sclerosis (MS) has been associated with environmental factors including vitamin D status.
Materials and Methods: Data were obtained from all children born in Denmark in 1940 (n = 72,839), 1977 (n = 89,570), and 1996 (n = 74,015). Information on contacts to hospitals (1977–2009) was obtained from the National Hospital Discharge Register. The main exposure variable was season of birth as a proxy variable for vitamin D status (summer: April–September and winter: October–March).
Results: No associations between season of birth and risk of MS were seen in the 1940 cohort or the 1996 cohort. In the 1977 cohort, there was a borderline statistically significant decreased risk of MS in those born during wintertime compared with those born during summertime (HR = 0.70, 95% CI: 0.47–1.04, p = 0.07). There were no significant differences within the groups regarding season and risk of T1DM at any age, T1DM before 10 y, infection, any type of cancer, schizophrenia and myocardial infarction. In the 1977 cohort the risk of pneumonia was significantly lower among those born in the summer compared with the winter at any age (HR 0.91, 95% CI 0.85–0.97, p < 0.01) and at age < 10 y (HR 0.90, 95% CI 0.84–0.97, p < 0.01).
Conclusion: MS and pneumonia in young subjects may be related to season of birth and thus maternal vitamin D exposure. Low sunlight exposure in the winter time leading to low vitamin D levels during pregnancy may be a potential explanation.
Keywords: Vitamin D and Multiple Sclerosis, type 1 Diabetes, cancer, schizophrenia, pneumonia, myocardial infarction
Introduction
Clinical evidence suggests that maternal and thus infant vitamin D insufficiency can modulate the future risk of developing chronic disorders among the children.1-3 Vitamin D insufficiency and latitude have been related to the occurrence of disorders besides rickets, osteomalacia and osteoporosis. Such disorders include multiple sclerosis (MS),4 type 1 diabetes (T1DM),5 several types of cancer6 and infections.7 Other studies have addressed seasonal variation in cancer caused by season of birth, and in general found little variation.8-13 It should be noted that most studies have addressed breast cancer, while little attention has been paid to other cancer types besides those potentially linked to infection.14-18 These papers showed no evidence between cancer and infections. Besides sunlight and vitamin D,19,20 cold weather and humidity21,22 may affect the risk of pneumonia, and other infectious diseases such as influenza.23-25 However, no studies on the effect of sunshine and vitamin D on pneumonia in Denmark are available. Furthermore melatonin may perhaps play a role for the incidence of breast cancer.26 Regarding seasonality of detection and survival in cancer, studies have shown that high calcitriol at the cancer diagnosis, may improve the survival of the prognosis of breast-, colon- and prostate cancer.27,28 Also for heart disease some authors have suggested a role of vitamin D status,29 although a meta-analysis found no definite evidence for an association.30 For some of these diseases epidemiological studies have shown that the risk depends on season of birth. Being born in the spring may be associated with an increased risk of MS,4 and being born in winter and spring with an increased risk of T1DM.5 However, no studies with hard end-points conclusively lend support to this hypothesis. It has been suggested that low maternal vitamin D levels in prenatal or early neonatal life can have effects in later life through the epigenetic process of genomic imprinting.31 Imprinting is independent of the classical Mendelian inheritance and occurs only in a small proportion of genes through DNA methylation and histone modifications. The imprinted maternal or paternal alleles are silenced so that the expressed allele depends on its parental origin. The importance of imprinting may be that the process entails a possibility to modify genes to produce the best possible phenotype to survive in the actual environment.1 Plasma 25OHD, which reflects vitamin D status, shows seasonal variations with zenith in late summer (August to October) and nadir in early spring (February to April).32 Vitamin D and its metabolite 25OHD are believed to cross placenta as early as 4 weeks after gestation via megalin, cubilin and vitamin D receptor (VDR) mediated processes.1 In accordance, cord plasma concentrations of these compounds have been reported to reach 71–103% of Caucasian maternal levels at term.33 In a large randomized trial by Hollis et al.34 studying a Caucasian, Afro-American and Hispanic population, the infant levels of plasma 25OHD were 58% of maternal levels in a group of mothers supplemented with 400 IU/day of vitamin D3 group, 58% in a group supplemented with 2000 IU/day, and 60% in a group supplemented with 2400 IU/day. This may signal that mothers deficient in vitamin D during the pregnancy may give birth to children with very low 25OHD, thus conditioning the children for vitamin D deficiency related disorders. The active vitamin D metabolite, 1.25(OH)2D, which increases during pregnancy in the mother, does not seem to cross the placenta readily.35 As a consequence of these mechanisms, fetal vitamin D status may show seasonal changes parallel to those observed in their mothers. In this context it is of importance that maternal vitamin D insufficiency is widespread in all age groups, including younger women with childbearing potential.36 In Denmark and the UK, dietary intake of vitamin D is low (3.7 μg daily for UK men and 2.8 μg for UK women37 vs. 2.25 μg daily in Danish women).38 Regarding supplements, Danish women used around 5 μg daily,38 while in the UK intake from supplements contributed 0.5–0.9 μg per day.37 The total daily intake of vitamin D may thus be higher in Denmark than in the UK. In spite of these enhanced intakes from supplements, serum levels of plasma 25OHD show large seasonal variations39 that covariate with seasonal variations in UV exposure with a delay in peak plasma 25OHD concentration of around 6 weeks compared with maximal UV exposure.39 These findings confirm that stratification by season in Denmark reflect variations in vitamin D status as measured by plasma 25OHD. The National Board of Health in Denmark recommend a daily intake of vitamin D of 10 μg to pregnant and children up to 2 y old, and 20 μg to elderly from the age of 70 y.40 Prior recommendations have been in place as early as the 19-forties on a Nordic Scale,41 recommending intakes from around 10–20 μg per day. There are evidence supporting that 25OHD should be around 100–150 nmol/l during pregnancy, to attain that circulation level, a daily intake of 100 μg (4,000 IU) vitamin D3 is required.34,42 Current evidence supports the concept that circulating 25-hydroxyvitamin D should be 40–60 ng/ml (100–150 nmol) during pregnancy and a daily intake of 4000 IU vitamin D3 is required to attain that circulating level.34
Our working hypotheses were that season of birth affected the risk of contracting MS, T1DM, and T1DM in childhood (before age 10), infections, cancers, acute myocardial infarction and schizophrenia. To test these hypotheses we studied three birth cohorts in Denmark, which is a country with a high occurrence of MS,43 and also a country with large seasonal variations in sunshine hours.44 The birth cohorts were chosen from very different time periods to allow analysis of temporal variations.
Results
Multiple sclerosis (MS)
Figure 1 shows the MS free survival by cohort and season of birth. The 1940 cohort showed no significant difference in risk of MS between those born in winter and summer (n = 154 vs. n = 152 cases, HR = 1.04, 95% CI: 0.83–1.30, p = 0.73). In the 1977 cohort we were able to follow all patients from the time of birth to the onset of MS. There was a borderline statistically significant association between MS and season of birth, as 60.2% (n = 62) with MS was born in the summer compared with 39.8% (n = 41) in wintertime (HR = 0.70, 95% CI: 0.47–1.04, p = 0.07). In the 1996 cohort no difference in risk of MS was present, but few cases were observed (1 MS case among those born in the winter, 0 cases among those born in the summer). It did not alter the results to analyze by month of birth or quarter of birth.
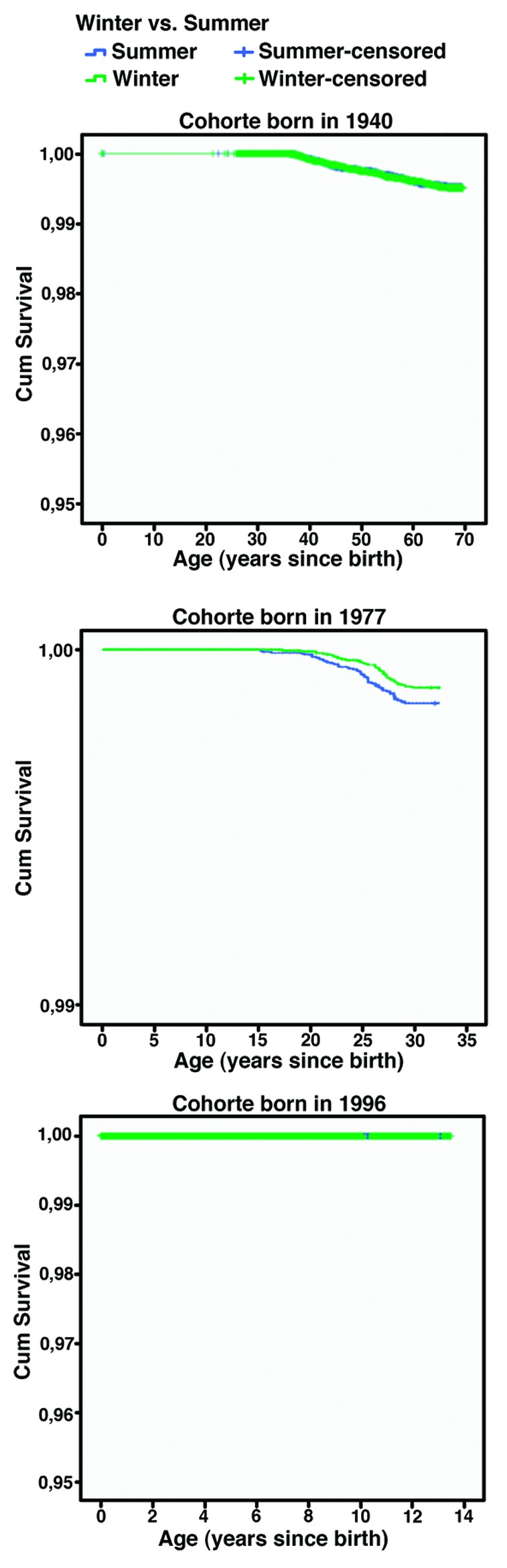
Figure 1. Shows the disease free survival in Kaplan-Meier survival curve, for multiple sclerosis from three cohorts where all were born in 1940, 1977 and 1996, respectively.
Type 1 Diabetes (T1DM): Any age
Table 1 shows the cumulated risk of T1DM at any age. In all three cohorts, the risk of being diagnosed with T1DM did not differ according season of birth.
Table 1. Shows the occurrence of T1D at any age, when subjects were compared within the three cohorts whether they were born during summertime or during wintertime (winter as reference).
| Birth cohort | Season of birth | N (%) Non cases | N (%) Cases | HR (95% CI) (p) |
|---|---|---|---|---|
| 1940 |
Summer |
36111 (98.0) |
752 (2.0) |
0.92 (0.83–1.02) (p = 0.11) |
| |
Winter |
35182 (97.8) |
794 (2.2) |
|
| 1977 |
Summer |
45826 (99.6) |
204 (0.4) |
0.98 (0.80–1.19) (p = 0.80) |
| |
Winter |
43342 (99.5) |
198 (0.5) |
|
| 1996 |
Summer |
37530 (99.8) |
73 (0.2) |
0.96 (0.69–1.32) (p = 0.78) |
| Winter | 36338 (99.8) | 74 (0.2) |
HR: Hazard rate (Cox proportional hazard regression) and 95% confidence intervals (CI).
T1DM before age 10
Table 2 shows the cumulated risk of T1DM before age 10. In the 1940 cohort there were no data for onset before age 10. Neither in the 1977 nor the 1996 cohort did the risk of T1DM before the age of 10 y depend on season of birth.
Table 2. Shows the occurrence of T1D before the age of 10 y, when subjects were compared within the three cohorts whether they were born during wintertime or during summertime (winter as reference).
| Birth cohort | Season of birth | N (%) Non cases | N (%) Cases | HR (95% C) (p) |
|---|---|---|---|---|
| 1940 |
Summer |
NA |
NA |
NA |
| |
Winter |
NA |
NA |
|
| 1977 |
Summer |
46020 (100) |
10 (0.0) |
0.95 (0.39–2.27) (p = 0.90) |
| |
Winter |
43530 (100) |
10 (0.0) |
|
| 1996 |
Summer |
37533 (99.8) |
70 (0.2) |
1.01 (0.72–1.42) (p = 0.94) |
| Winter | 36345 (99.8) | 67 (0.2) |
HR: Hazard rate (Cox proportional hazard regression) and 95% confidence intervals (CI).
Infections
Table 3 shows the risk of being hospitalized with any infection. There were no significant differences within the groups regarding season of birth and later risk of any infection. Limiting the analyses to pneumonia among subjects in the 1977 cohort, we found a significantly reduction in risk of pneumonia among those born in the summer compared with winter. The 1996 cohort displayed no significant excess risk of pneumonia among those born in summer compared with winter. For pneumonia before age 10 only data from the 1977 and 1996 cohorts could be analyzed. In both cohorts we saw the same trends as in the whole cohort (Table 3).
Table 3. Shows the occurrence of all types of infection and pneumonia caused by infection, when subjects were compared within the three cohorts on whether they were born during wintertime or during summertime (winter as reference).
| Infection Type | Birth cohort | Season of birth | N (%) Non cases | N (%) cases | HR (95% CI) (p) |
|---|---|---|---|---|---|
| all type |
1940 |
Summer |
34782 (94.4) |
2081 (5.6) |
1.02 (0.96–1.08) (p = 0.61) |
| |
Winter |
33975 (94.4) |
2001 (5.6) |
||
| 1977 |
Summer |
41653 (90.5) |
4377 (9.5) |
1.02 (0.98–1.07) (p = 0.33) |
|
| |
Winter |
39497 (90.7) |
4043 (9.3) |
||
| 1996 |
Summer |
33878 (90.1) |
3725 (9.9) |
1.01 (0.97–1.06) (p = 0.66) |
|
| |
Winter |
32802 (90.1) |
3610 (9.9) |
||
| Pneumonia |
1940 |
Summer |
35979 (97.6) |
884 (2.4) |
0.93 (0.85–1.02) (p = 0.11) |
| |
Winter |
35048 (97.4) |
928 (2.6) |
||
| 1977 |
Summer |
44464 (96.6) |
1566 (3.4) |
0.91 (0.85–0.97)* (p < 0.01) |
|
| |
Winter |
41913 (96.3) |
1627 (3.7) |
||
| 1996 |
Summer |
37326 (99.3) |
277 (0.7) |
0.87 (0.74–1.03) (p = 0.10) |
|
| |
Winter |
36105 (99.2) |
307 (0.8) |
||
| Pneumonia age < 10 |
1940 |
Summer |
36863 (100) |
NA |
NA |
| |
Winter |
35976 (100) |
NA |
||
| 1977 |
Summer |
44613 (96.9) |
1417 (3.1%) |
0.90 (0.84–0.97)* (p < 0.01) |
|
| |
Winter |
42052 (96.6) |
1488 (3.4%) |
||
| 1996 |
Summer |
37332 (99.3) |
271 (0.7%) |
0.86 (0.73–1.01) (p = 0.07) |
|
| |
Winter |
36107 (99.2) |
305 (0.8%) |
||
| Pneumonia age < 1 |
1940 |
Summer |
36863 (100) |
NA |
NA |
| |
Winter |
35976 (100) |
NA |
||
| 1977 |
Summer |
45386 (98.6) |
644 (1.4%) |
0.94 (0.84–1.05) (p = 0.27) |
|
| |
Winter |
42894 (98.5) |
646 (1.5%) |
||
| 1996 |
Summer |
37516 (99.8) |
87 (0.2%) |
0.84 (0.63–1.12) (p = 0.24) |
|
| |
Winter |
36312 (99.7) |
100 (0.3%) |
||
| Pneumonia age > 10 | 1940 |
Summer |
35979 (97.6) |
884 (2.4%) |
0.93 (0.85–1.02) (p = 0.11) |
| |
Winter |
35048 (97.4) |
928 (2.6%) |
||
| 1977 |
Summer |
45881 (99.7) |
149 (0.3%) |
1.01 (0.80–1.27) (p = 0.931) |
|
| |
Winter |
43401 (99.7) |
139 (0.3%) |
||
| 1996 |
Summer |
37597 (99.98) |
6 (0.02%) |
2.90 (0.59–14.4) (p = 0.19) |
|
| Winter | 36410 (99.99) | 2 (0.01%) |
HR: Hazard rate (Cox proportional hazard regression) and 95% confidence intervals (CI).
Cancer
Table 4 shows the cumulated risk of developing cancer. In none of the three birth cohorts, the risk of being diagnosed with cancer differed according season of birth. We also studied risk of developing certain types of common solid cancers in term of breast-, colon- or prostate-cancer. In none of the birth cohorts, the risk of being diagnosed with one of the three types of cancers differed between individuals born in summer- or winter-time.
Table 4. Shows the occurrence of all types of cancer, furthermore breast-, colon and prostate cancer, when subjects were compared within the three cohorts whether they were born during wintertime or during summertime (winter as reference).
| Cancer Type | Birth cohort | Season of birth | N (%) non cases | N (%) cases | HR (95% CI) |
|---|---|---|---|---|---|
| all type |
1940 |
Summer |
31908 (86.6) |
4955 (13.4) |
0.98 (0.95–1.02) (p = 0.43) |
| |
|
Winter |
31073 (86.4) |
4903 (13.6) |
|
| |
1977 |
Summer |
45688 (99.3) |
342 (0.7) |
0.92 (0.79–1.07) (p = 0.27) |
| |
|
Winter |
43188 (99.2) |
352 (0.8) |
|
| |
1996 |
Summer |
37540 (99.8) |
63 (0.2) |
1.00 (0.70–1.42) (p = 1.00) |
| |
|
Winter |
36351 (99.8) |
61 (0.2) |
|
| Breast |
1940 |
Summer |
35780 (97.1) |
1083 (2.9) |
0.97 (0.89–1.06) (p = 0.48) |
| |
|
Winter |
34888 (97.0) |
1088 (3.0) |
|
| |
1977 |
Summer |
46020 (100) |
10 (0.0) |
0.68 (0.30–1.52) (p = 0.35) |
| |
|
Winter |
43526 (100) |
14 (0.0) |
|
| |
1996 |
Summer |
37596 (100) |
7 (0.0) |
0.48 (0.20–1.20) (p = 0.12) |
| |
|
Winter |
36398 (100) |
14 (0.0) |
|
| Colon |
1940 |
Summer |
36432 (98.8) |
431 (1.2) |
0.92 (0.81–1.05) (p = 0.22) |
| |
|
Winter |
35520 (98.7) |
456 (1.3) |
|
| |
1977 |
Summer |
46022 (100) |
8 (0.0) |
0.84 (0.33–2.18) (p = 0.72) |
| |
|
Winter |
43531 (100) |
9 (0.0) |
|
| |
1996 |
Summer |
37598 (100) |
5 (0.0) |
0.69 (0.22–2.18) (p = 0.53) |
| |
|
Winter |
36405 (100) |
7 (0.0) |
|
| Prostate |
1940 |
Summer |
36589 (99.3) |
274 (0.7) |
0.89 (0.76–1.05) (p = 0.17) |
| |
|
Winter |
35676 (99.2) |
300 (0.8) |
|
| |
1977 |
Summer |
46030 (100) |
0 (0.0) |
NA |
| |
|
Winter |
43539 (100) |
1 (0.0) |
|
| |
1996 |
Summer |
37603 (100) |
0 (0.0) |
NA |
| Winter | 36412 (100) | 0 (0.0) |
HR: Hazard rate (Cox proportional hazard regression) and 95% confidence intervals (CI).
Schizophrenia
Table 5 shows the risk of schizophrenia. In the youngest 1996 cohort no cases were observed. In the 1940 and 1977 cohorts no significant trends related to season of birth were present.
Table 5. Shows the occurrence of schizophrenia when subjects were compared within the three cohorts whether they were born during wintertime or during summertime (winter as reference).
| Schizophrenia | Birth cohort | Season of birth | N (%) non cases | N (%) cases | HR (95% CI) |
|---|---|---|---|---|---|
| |
1940 |
Summer |
36777 (99.8%) |
86 (0.2%) |
1.02 (0.76–1.38) (p = 0.89) |
| |
|
Winter |
36894 (99.8%) |
82 (0.2%) |
|
| |
1977 |
Summer |
45976 (99.9%) |
54 (0.1%) |
1.11 (0.75–1.65) (p = 0.60) |
| |
|
Winter |
43494 (99.9%) |
46 (0.1%) |
|
| |
1996 |
Summer |
37603 (100%) |
NA |
NA |
| Winter | 36412 (100%) | NA |
HR: Hazard rate (Cox proportional hazard regression) and 95% confidence intervals (CI).
Myocardial infarction
We found no trends for acute myocardial infarction (data not shown).
Discussion
In this large-scale population and register based cohort study we did not find any consistent associations between season of birth and risk of being diagnosed with MS, T1DM, and T1DM at early age, all infections, any type of cancer, schizophrenia or acute myocardial infarct. However, in the 1977 cohort, risk of developing MS was non-statistically significantly higher among individuals born in summer compared with winter. Moreover, being born in winter was associated with a higher risk of later being hospitalized with pneumonia in all three birth cohorts.
In MS several limitations may restrict the interpretation of outcome. In the 1996 cohort, the risk of MS had not reached its zenith, i.e., few cases of MS were present for analysis. In the 1940 cohort the results may be flawed because the Central Personal Register and the National Hospital Discharge Register first started to include data from 1977. At that time people in the 1940 cohort were 37 y old, and MS had already had its onset. The difference regarding season of birth observed in the 1977 cohort with a borderline statistical significant higher risk of MS with summer births are in accordance with prior results from Denmark,45 Sweden,46 the Netherlands47 and Canada48 with peak incidence in MS in those born between March through June. It is also supported by a study from Italy49 where an excess of patients were born between June and November. In relation to vitamin D status, the findings may be explained in two ways. Those born during summer (May, June and July) must have been conceived during winter (November, December and January). I.e., they were conceived and in the embryonic or fetal state during a period of time where serum levels of 25OHD were low and born at a time where serum 25OHD levels were higher.39 Hence, the effect of imprinting on risk of MS could in theory either be caused by low vitamin D status during early pregnancy or by low vitamin D status in late pregnancy or early after birth. However, the opposite could also be possible, i.e., that low vitamin D in the early pregnancy may be protective. Against this hypothesis may point that most studies have indicated a protective effect of higher vitamin D levels.
If the increased risk of MS was founded early in life e.g., through vitamin D insufficiency, it could explain the fact that the occurrence of MS changes little in subjects migration to another country after childhood.50 A geographical variation in the risk of MS is well-established with an increase in incidence with latitude on the Northern hemisphere.31,51 This may be explained by the decrease in exposure to sunlight especially during winter at higher latitudes. Actually it has been demonstrated that as much as 73–84% of the variation in MS may be linked to geographical variation and thus potentially the sunlight exposure.52 However, recent data have suggested that this geographical difference in occurrence of MS has disappeared.53 The variation in MS according to season of birth has to be conceptually separated from the known seasonal variations in diagnosis and exacerbations with a zenith in spring and a nadir in winter.54 However, the latter observation support that some of the pathogenesis or patophysiology of MS may be linked to the effect of recent alterations in vitamin D status on e.g., the immune system.55 However, the seasonal occurrence of MS may beside variations in vitamin D also be explained by variations in the occurrence of infections.31,51,55 The low variation in MS with season of birth in our study may reflect the findings that the geographical differences previously described seem to be diminishing.53 However, not all studies agree with Koch-Henriksen.56 By the time T1DM is diagnosed, about 80% of the β-cells have been destroyed.57 The incidence peaks around puberty, and the disease is usually diagnosed before age 30.58 There is a marked geographic variation in incidence, with a child in Finland being about 350 times more likely than a child in Venezuela to acquire diabetes.59 People with affected first-degree relatives are a lot more likely to develop T1DM than the population in general—this points to an important genetic influence.60 However, in a Finnish study from 1992 the authors showed low concordance among identical twins and that many children with a genetic predisposition to the disease did not develop the disease, suggesting that it could have an important environmental factor.61 One of the environmental factors thought to be protective against development of T1DM, is early supplementation with vitamin D.60 This is supported by previous studies from Finland62 Norway63 and other European countries60 showing that a high vitamin D intake, including intake of cod liver oil during pregnancy or in early life may reduce the risk of T1DM for up to 31 y.62,63 However, in our study we did not show any significant effect of season of birth on the occurrence of T1DM.
The risk of respiratory infection may depend on the present vitamin D status. In this way the risk of developing respiratory infection in children is increased by coexisting nutritional rickets.64 An acute severe lower respiratory tract infection in non-rachitic children also has been associated with sub-clinical vitamin D deficiency.65 This could imply that vitamin D deficiency is involved in the pathogenesis of respiratory infections. However, this should be separated from the effect of season and vitamin D status at birth and future disease. In our study we failed to find any association between season of birth and the overall occurrence of any infection. This may support, that other mechanisms influence the seasonal variation in the occurrence of respiratory infections. However, restricting the analyses to risk of pneumonia showed an increased future risk in individuals born at wintertime, but this may by itself not prove an effect of vitamin D. Due to the nature of our study, we are not able to conclude whether this is due to an effect of vitamin D status or to other potential mechanisms related to risk of infections. Further studies are needed to elucidate this hypothesis.
The risk of cancer may be related to recent or average adult vitamin D status and solar exposure.66 However, in our study we failed to find any association between season of birth and the overall later risk of any cancer, breast-, colon- or prostate cancer suggesting that genetic imprinting caused by vitamin D probably is of minor or no importance.
Previous data have suggested that winter births are associated with an increased incidence of later schizophrenia.67 However, we observed no effect of season of birth on risk of schizophrenia. The absence of cases in the youngest group is in accordance with the fact that schizophrenia does not present itself before the age of 13 y.
In this study we did not find that season of birth had major long-term effects on hard endpoints related to vitamin D status except for a potential effect on MS. A short-term effect on pneumonia risk may be possible.
We conclude that the risk of later MS varied with season of birth being highest with summer births but only in the age group 15–35 y. Neither the risk of T1DM, cancer, all types of infections or schizophrenia varied with season of birth. However, winter birth was associated with a future increased risk of pneumonia. Accordingly, our results do not exclude an effect of time of year of birth on subsequent risk of certain diseases, and further studies should aim to elucidate whether this may be due to vitamin D induced genetic imprinting.
Materials and Methods
The subjects consisted of three Danish birth cohorts. The first cohort comprised all Danish citizens born in 1940, the second all born in 1977 and the third all subjects born in 1996. All three cohorts were followed for the occurrence of a diagnosis of MS and T1DM, cancer ischemic heart disease and schizophrenia, and for hospitalizations for infections.
The codes for the occurrence of MS were ICD-8 [340 and ICD-10 (G35)]. The codes of the occurrence of T1DM were ICD8 (249) and ICD-10 (E10). Infection was defined as any infection [bacterial (incl. tuberculosis), virus, parasites and fungi] and we counted all hospital contacts as outcome, furthermore we studied separately pneumonia caused by infections. The codes of the occurrence of infection were ICD-8 (000–130) and ICD10 (A00-B90) and the codes for pneumonia were ICD-8 (48099–48101, 48108, 48109, 48209, 48219, 48290, 48299, 48300, 48308, 48309, 48499, 48599, 48699) and ICD-10 (J13, J14, J15). Cancer was subdivided into all types, breast, prostate and colon cancer using the codes ICD-8 (140–200.9) and ICD10 (C00-D00). When only appraising colon cancer, the codes were ICD-8 (153) and ICD-10 (C18). The ICD-8 codes for breast cancer were (174–17402, 17408, 17409) and for ICD10 (C50). ICD-8 codes for prostate cancer were (18599) and for ICD-10 (DC61). ICD-8 codes for schizophrenia were (29509, 29519, 29529, 29539, 29549, 29559, 29569, 29579, 29589 and 29599) and for ICD-10 (F20). For acute myocardial infarction, the codes were 41009, 41099, and I211-I219.
For the cancers breast cancer, colon cancer, and prostate cancer were chosen as candidates for further study, as these cancers are frequent (many end-points could be expected, especially in the older age strata). The occurrence of these end-points between January 1, 1977 and December 31 2009 was tracked through the National Hospital Discharge Register.68 This register was founded in 1977 and has national coverage with an almost 100% capture of diagnoses.68 From 1968 all Danish citizens were assigned a unique identification code (the Central Personal Register number; CPR number), which allows tracking of all inhabitants with regard to day of birth, gender, death and emigration. The CPR number to some extent is similar to the American Social Security Number. By combining the CPR number and The National Hospital Discharge Register it is possible to link information about gender and month of birth to the occurrence of later disease. The Danish health system is financed through taxes and is free of charge to use. The public hospitals, which are covered by the National Hospital Discharge Register, provide almost 100% of the health services in the country. Data were obtained from: (1) all born in 1940 (n = 72,839, and still alive as of January 1, 1977 upon start of the register); (2) all born in 1977 (n = 89,570, all included); and (3) all born in 1996 (n = 74,015, all included). We retrieved all contacts to hospitals from 1977 to 2009 from the National Hospital Discharge Register. The main exposure variable was season of birth (subdivided into: summer, i.e., April–September and winter, i.e., October–March) and the main outcome variable was occurrence of MS, T1DM, T1DM before the age of 10, hospitalization due to infections, hospitalization due to pneumonia, all cases of cancer, colon cancer, breast cancer and prostate cancer, schizophrenia and acute myocardial infarction. We re-analyzed the results by individual birth month, quarter of birth, and by changing the definition of winter/summer by moving this across a number of alternatives. This did not change the results (data not shown). The study was performed according to the Helsinki Declaration II. The study was notified to the Danish Data Protection Agency (journal no. 2008–41–2185) and approved by the The Central Region Committee on Biomedical Research Ethics, Aarhus County (protocol No. M-2007–0255).
Statistics
Cumulated incidence proportions were calculated for all three cohorts by month of birth and stratified by season. Statistical analyses were done using one-sided Fisher’s exact test and Kaplan-Meier survival plots using IBM SPSS version 19, and calculating hazard ratios (HR) with 95% CI from a Cox proportional hazard model using time from birth to event and season of birth. We tested the proportional hazard assumption by checking the log-minus-log plots.
Strengths and limitations
The major advantage of the study is its size and that it is population based and uses information sampled without specific reference to birth season. This has reduced selection and information bias. In general the quality of the Danish registries is considered to be high.68 The high number of comparisons may increase the likelihood of type 1 errors due to by chance findings. However, even though we performed many separate analyses the number of significant findings was limited, which may speak against major influence from type 1 error from multiple testing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22779
References
- 1.Kaludjerovic J, Vieth R. Relationship between vitamin D during perinatal development and health. J Midwifery Womens Health. 2010;55:550–60. doi: 10.1016/j.jmwh.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Disanto G, Chaplin G, Morahan JM, Giovannoni G, Hypponen E, Ebers GC, et al. Month of birth, vitamin D and risk of immune mediated disease: a case control study. BMC Med. 2012;10:69. doi: 10.1186/1741-7015-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonal birth patterns of neurological disorders. Neuroepidemiology. 2000;19:177–85. doi: 10.1159/000026253. [DOI] [PubMed] [Google Scholar]
- 4.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC, Canadian Collaborative Study Group Timing of birth and risk of multiple sclerosis: population based study. BMJ. 2005;330:120. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223:230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 6.Grant WB. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012;32:223–36. [PubMed] [Google Scholar]
- 7.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65:106R–13R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristoffersen S, Hartveit F. Is a woman’s date of birth related to her risk of developing breast cancer? Oncol Rep. 2000;7:245–7. doi: 10.3892/or.7.2.245. [DOI] [PubMed] [Google Scholar]
- 9.Hu YH, Kuroishi T, Matsushita Y, Nagata C, Shimizu H. Birth season and breast cancer risk in Japan. Breast Cancer Res Treat. 1996;39:315–9. doi: 10.1007/BF01806159. [DOI] [PubMed] [Google Scholar]
- 10.Nakao H. Season of birth of breast cancer patients and its relation to patients’ reproductive history in Tokyo, Japan. Acta Med Okayama. 1988;42:231–41. doi: 10.18926/AMO/30994. [DOI] [PubMed] [Google Scholar]
- 11.Severson RK, Davis S. Breast cancer incidence and month of birth: evidence against an etiologic association. Eur J Cancer Clin Oncol. 1987;23:1067–70. doi: 10.1016/0277-5379(87)90360-9. [DOI] [PubMed] [Google Scholar]
- 12.Vassilaros S, Tsiliakos S, Adamopoulos D, Papadiamantis J, Varveris H, Sakelaris J, et al. Seasonal variations in the frequency distribution of breast cancer in Greek women according to the month of their birth. J Cancer Res Clin Oncol. 1985;110:79–81. doi: 10.1007/BF00402507. [DOI] [PubMed] [Google Scholar]
- 13.Jansson B, Malahy MA. Cancer risk, age at diagnosis, and age at death as functions of season of birth. Cancer Detect Prev. 1981;4:291–4. [PubMed] [Google Scholar]
- 14.Basta NO, James PW, Craft AW, McNally RJ. Season of birth and diagnosis for childhood cancer in Northern England, 1968-2005. Paediatr Perinat Epidemiol. 2010;24:309–18. doi: 10.1111/j.1365-3016.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 15.Basta NO, James PW, Craft AW, McNally RJ. Seasonality in the incidence of cervical carcinoma in teenagers and young adults in Northern England, 1968-2005. Chronobiol Int. 2011;28:819–24. doi: 10.3109/07420528.2011.611602. [DOI] [PubMed] [Google Scholar]
- 16.Feltbower RG, Pearce MS, Dickinson HO, Parker L, McKinney PA. Seasonality of birth for cancer in Northern England, UK. Paediatr Perinat Epidemiol. 2001;15:338–45. doi: 10.1046/j.1365-3016.2001.00377.x. [DOI] [PubMed] [Google Scholar]
- 17.Langagergaard V, Nørgård B, Mellemkjaer L, Pedersen L, Rothman KJ, Sørensen HT. Seasonal variation in month of birth and diagnosis in children and adolescents with Hodgkin disease and non-Hodgkin lymphoma. J Pediatr Hematol Oncol. 2003;25:534–8. doi: 10.1097/00043426-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt LS, Grell K, Frederiksen K, Johansen C, Schmiegelow K, Schüz J. Seasonality of birth in children with central nervous system tumours in Denmark, 1970-2003. Br J Cancer. 2009;100:185–7. doi: 10.1038/sj.bjc.6604813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuster SP, Tuite AR, Kwong JC, McGeer A, Fisman DN, Toronto Invasive Bacterial Diseases Network Investigators Evaluation of coseasonality of influenza and invasive pneumococcal disease: results from prospective surveillance. PLoS Med. 2011;8:e1001042. doi: 10.1371/journal.pmed.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White AN, Ng V, Spain CV, Johnson CC, Kinlin LM, Fisman DN. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis. 2009;9:196. doi: 10.1186/1471-2334-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaman J, Goldstein E, Lipsitch M. Absolute humidity and pandemic versus epidemic influenza. Am J Epidemiol. 2011;173:127–35. doi: 10.1093/aje/kwq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaman J, Jeon CY, Giovannucci E, Lipsitch M. Shortcomings of vitamin D-based model simulations of seasonal influenza. PLoS ONE. 2011;6:e20743. doi: 10.1371/journal.pone.0020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918-1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1:215–9. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moan J, Dahlback A, Ma L, Juzeniene A. Influenza, solar radiation and vitamin D. Dermatoendocrinol. 2009;1:307–9. doi: 10.4161/derm.1.6.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 26.Oh EY, Ansell C, Nawaz H, Yang CH, Wood PA, Hrushesky WJ. Global breast cancer seasonality. Breast Cancer Res Treat. 2010;123:233–43. doi: 10.1007/s10549-009-0676-7. [DOI] [PubMed] [Google Scholar]
- 27.Porojnicu A, Robsahm TE, Berg JP, Moan J. Season of diagnosis is a predictor of cancer survival. Sun-induced vitamin D may be involved: a possible role of sun-induced Vitamin D. J Steroid Biochem Mol Biol. 2007;103:675–8. doi: 10.1016/j.jsbmb.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15:149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 30.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 31.McGrath J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med Hypotheses. 2001;56:367–71. doi: 10.1054/mehy.2000.1226. [DOI] [PubMed] [Google Scholar]
- 32.Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L. Determinants of plasma PTH and their implication for defining a reference interval. Clin Endocrinol (Oxf) 2011;74:37–43. doi: 10.1111/j.1365-2265.2010.03894.x. [DOI] [PubMed] [Google Scholar]
- 33.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 34.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosking DJ. Calcium homeostasis in pregnancy. Clin Endocrinol (Oxf) 1996;45:1–6. doi: 10.1111/j.1365-2265.1996.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 36.Møller UK, Ramlau-Hansen CH, Rejnmark L, Heickendorff L, Henriksen TB, Mosekilde L. Postpartum vitamin D insufficiency and secondary hyperparathyroidism in healthy Danish women. Eur J Clin Nutr. 2006;60:1214–21. doi: 10.1038/sj.ejcn.1602440. [DOI] [PubMed] [Google Scholar]
- 37.Henderson L, Irving K, Gregory J, Bates CJ, Prentice A, Perks J, et al. The National Diet & Nutrition Survey: adults aged 19 to 64 years. Vitamin and mineral intake and urinary analytes. Food Standards Agency and theDepartments of Health by the Social Survey Division of the Office for National Statisticsand Medical Research Council Human Nutrition Research 2003 [cited 2012 Sep 2];(1):1-168. Available from: http://www.food.gov.uk/multimedia/pdfs/ndnsv3.pdf
- 38.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–8. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 39.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/BJN2001345. [DOI] [PubMed] [Google Scholar]
- 40.Sundhedsstyrelsen. D-vitamin - Forebyggelse af d-vitaminmangel. National Board of Health 2010 May 25 [cited 2011 Oct 6];Available from: http://www.sst.dk/~/media/Sundhed%20og%20forebyggelse/Ernaering/D-vitamin/Kilder%20til%20D-vitamin/Oversigt_D-vitaminanbefalinger270510.ashx
- 41.Nordisk lærebog i pædiatri. 3 ed. Copenhagen: Munksgaard; 1952. [Google Scholar]
- 42.Hollis BW, Wagner CL. Vitamin D requirements and supplementation during pregnancy. Curr Opin Endocrinol Diabetes Obes. 2011;18:371–5. doi: 10.1097/MED.0b013e32834b0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bentzen J, Flachs EM, Stenager E, Brønnum-Hansen H, Koch-Henriksen N. Prevalence of multiple sclerosis in Denmark 1950--2005. Mult Scler. 2010;16:520–5. doi: 10.1177/1352458510364197. [DOI] [PubMed] [Google Scholar]
- 44.Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol (Oxf) 2005;63:506–13. doi: 10.1111/j.1365-2265.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- 45.Templer DI, Trent NH, Spencer DA, Trent A, Corgiat MD, Mortensen PB, et al. Season of birth in multiple sclerosis. Acta Neurol Scand. 1992;85:107–9. doi: 10.1111/j.1600-0404.1992.tb04007.x. [DOI] [PubMed] [Google Scholar]
- 46.Wiberg M, Templer DI. Season of Birth in Multiple Sclerosis in Sweden: Replication of Denmark Findings. J Orthomol Med. 1994;•••:71–4. [Google Scholar]
- 47.Tromp SW. Month of disease and proneness. Prog Biometeorol. 1977;•••:164–6. [Google Scholar]
- 48.Sadovnick AD, Yee IM. Season of birth in multiple sclerosis. Acta Neurol Scand. 1994;89:190–1. doi: 10.1111/j.1600-0404.1994.tb01659.x. [DOI] [PubMed] [Google Scholar]
- 49.Salemi G, Ragonese P, Aridon P, Reggio A, Nicoletti A, Buffa D, et al. Is season of birth associated with multiple sclerosis? Acta Neurol Scand. 2000;101:381–3. doi: 10.1034/j.1600-0404.2000.90336.x. [DOI] [PubMed] [Google Scholar]
- 50.Elian M, Nightingale S, Dean G. Multiple sclerosis among United Kingdom-born children of immigrants from the Indian subcontinent, Africa and the West Indies. J Neurol Neurosurg Psychiatry. 1990;53:906–11. doi: 10.1136/jnnp.53.10.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brody JA. Epidemiology of multiple sclerosis and a possible virus aetiology. Lancet. 1972;2:173–6. doi: 10.1016/S0140-6736(72)91340-2. [DOI] [PubMed] [Google Scholar]
- 52.Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol. 2004;55:65–71. doi: 10.1002/ana.10788. [DOI] [PubMed] [Google Scholar]
- 53.Koch-Henriksen N, Sorensen PS. Why does the north-south gradient of incidence of multiple sclerosis seem to have disappeared on the northern hemisphere? J Neurol Sci. 2011;311:58–63. doi: 10.1016/j.jns.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Goodkin DE, Hertsgaard D. Seasonal variation of multiple sclerosis exacerbations in North Dakota. Arch Neurol. 1989;46:1015–8. doi: 10.1001/archneur.1989.00520450085025. [DOI] [PubMed] [Google Scholar]
- 55.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–72. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 56.Grant WB, Mascitelli L. Evidence that the north-south gradient of multiple sclerosis may not have disappeared. J Neurol Sci. 2012;315:178–9. doi: 10.1016/j.jns.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Kukreja A, Maclaren NK. Autoimmunity and diabetes. J Clin Endocrinol Metab. 1999;84:4371–8. doi: 10.1210/jc.84.12.4371. [DOI] [PubMed] [Google Scholar]
- 58.Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr. 2005;135:323–5. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 59.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–26. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 60.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 61.Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35:1060–7. doi: 10.1007/BF02221682. [DOI] [PubMed] [Google Scholar]
- 62.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 63.Stene LC, Joner G, Norwegian Childhood Diabetes Study Group Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003;78:1128–34. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 64.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50:364–8. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 65.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 66.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 67.Morgan VA, Jablensky AV, Castle DJ. Season of birth in schizophrenia and affective psychoses in Western Australia 1916-61. Acta Psychiatr Scand. 2001;104:138–47. doi: 10.1034/j.1600-0447.2001.00188.x. [DOI] [PubMed] [Google Scholar]
- 68.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–8. [PubMed] [Google Scholar]


