Abstract
Bio-electrospraying (BES) is a technique used for the processing of cells and can be applied to tissue engineering. The association of BES with scaffold production techniques has been shown to be an interesting strategy for the production of biomaterials with cells homogeneously distributed in the entire structure. Various studies have evaluated the effects of BES on different cell types. However, until the present moment, no studies have evaluated the impact of BES time on mesenchymal stem cells (MSC). Therefore, the aim of this work was to standardise the different parameters of BES (voltage, flow rate, and distance of the needle from the collecting plate) in relation to cell viability and then to evaluate the impact of BES time in relation to viability, proliferation, DNA damage, maintenance of plasticity and the immunophenotypic profile of MSC. Using 15 kV voltage, 0.46 ml/h flow rate and 4 cm distance, it was possible to form a stable and continuous jet of BES without causing a significant reduction in cell viability. Time periods between 15 and 60 min of BES did not cause alterations of viability, proliferation, plasticity, and immunophenotypic profile of the MSC. Time periods above 30 min of BES resulted in DNA damage; however, the DNA was able to repair itself within five hours. These results indicate that bio-electrospraying is an adequate technique for processing MSC which can be safely applied to tissue engineering and regenerative medicine.
INTRODUCTION
Tissue engineering, through the association of cells and scaffolds, is a promising tool for the regeneration of damaged organs and tissue. One of the main challenges of the area is the development of an efficient technique of seeding cells, which ensures a uniform occupation of the cells on the scaffold structures and better interaction between the two components to provide fast and complete tissue regeneration.1 Bio-electrospraying (BES) is a technique in which a cellular suspension is submitted to an electric field of high intensity and which, after passing through a fine needle, is fragmented, creating micrometric drops containing cells.2, 3, 4 This technique can be applied to tissue engineering for the production of scaffolds already seeded with cells within them. Through the association of BES with scaffold production techniques such as electrospinning, cells can be seeded at the same time that the scaffolds are being formed, ensuring uniform cell colonisation on the entire structure of the biomaterial.5, 6
Several studies have provided evidence that different cell types can be processed using BES. It was observed that the method does not cause a significant reduction in mesenchymal stem cell viability, significant chromosomal alterations in mononuclear cells, alterations in the pluripotency of the embryonic stem cells or genetic and physical damage that can affect the development of multicellular organism.7, 8, 9, 10 Although these studies show that BES is a safe technique for cell processing, until now, no study has investigated if the time necessary for the realization of BES can have a negative effect on the cells. Regarding the association of BES with scaffold production techniques, the time parameter becomes an important factor for observation. The techniques must be combined for adequate and controlled time periods in order that, at the end of the procedure, scaffolds with a resistant structure suitable for manipulation and tissue engineering are produced and, at the same time do not create cellular damage. Therefore, the aim of this study was to standardize the parameters, especially the time of BES technique for human mesenchymal stem cell processing.
MATERIALS AND METHODS
Isolation and cultivation of mesenchymal stem cells
Primary mesenchymal stem cells (MSC) were isolated from human deciduous teeth pulp (n = 5) as described by Bernardi and colleagues,11 after approval by the Ethics Committee of the Federal University of Rio Grande do Sul.
The cells were cultivated in Dulbecco's Modified Eagle's Medium (DMEM) containing 2.5 g/l of Hepes (free-acid) (Sigma-Aldrich) supplemented with 10% bovine fetal serum (BFS) (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco) and 0.45 μg/ml gentamicin, and maintained in a humid atmosphere of 5% CO2, at 37 °C. The culture medium was changed every 3 or 4 days. When the cell culture reached 90% confluence, a passage using 0.5% trypsin-EDTA solution (Sigma-Aldrich) was carried out. The cells at the sixth passage were used in all the experiments.
Standardization of BES of MSC
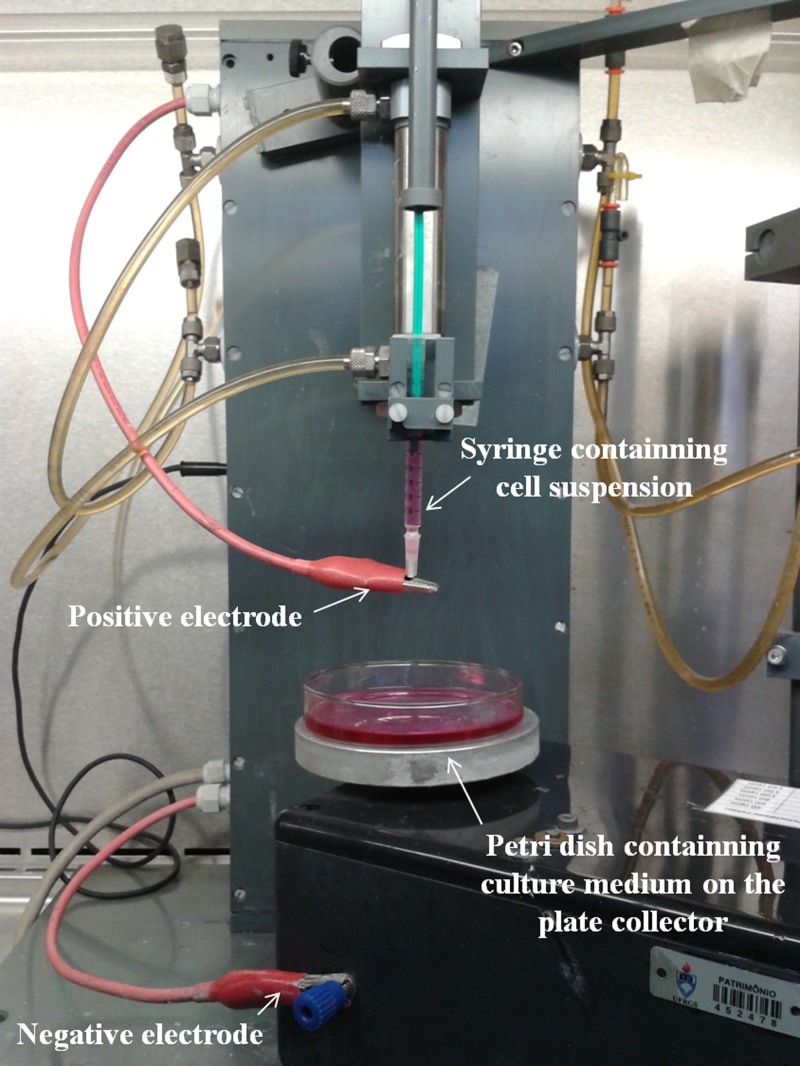
To evaluate the biological effects of BES on MSC, this study was divided into two steps. First, the BES parameters were standardized in relation to cellular viability. Second, after the definition of the most suitable parameters to maintain maximum cell viability, the impact of BES time on the behavior of the MSC was evaluated. Figure 1 shows the BES equipment used for all subsequent experiments.
Figure 1.
Bio-electrospraying equipment.
The BES parameters were compared in terms of their effects on cellular viability. Suspension of MSC (3 × 106 cells/ml) was electrosprayed for 15 min, using a voltage ranging from 15 to 30 kV, a flow rate ranging from 0.28 to 2.60 ml h−1 and a distance of the needle to the collecting plate varying from 4 to 8 cm. Each parameter was evaluated individually, e.g., while a parameter was tested, the others were kept constant. The electrosprayed cells were collected in Petri dishes containing DMEM culture medium, containing 2.5 g/l of Hepes (free-acid), supplemented with 10% BFS, 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco) and 0.45 μg/ml gentamicin at room temperature. Immediately after the end of the BES, the medium was centrifuged at 300xg for 5 min. The viability of MSC was assessed before and after BES procedure by trypan blue dye exclusion test. Besides cell viability, the proportion of bioelectrosprayed cells that could be recovered from the collecting Petri dishes was also determined. The number of electrosprayed cells was calculated from the volume and concentration of the cellular suspension used for the BES process. The cells collected in the Petri dishes after BES were counted by a hemocytometer. The environmental temperature during the BES procedures was maintained at about 23 °C and relative humidity at about 30%.
Evaluation of the impact of BES time on MSC
The time period which BES can be applied for the manipulation of MSC without the latter suffering any kind of damage, was studied in terms of viability, proliferation, maintenance of plasticity, immunophenotypic profile, as well as possible DNA cell damage. For these evaluations, a MSC suspension (3 × 106 cells/ml) was electrosprayed at 15 kV of voltage, 0.46 ml h−1 of flow rate and 4 cm of distance from the needle to the collector. Three time periods of duration of BES were evaluated: 15, 30, and 60 min. After each BES time, the number of recuperated cells was counted in hemocytometer. The cells were then re-suspended in culture medium and seeded in 24 well plates. For the cell proliferation assay and the evaluation of plasticity, the cells were seeded at a density of 5000 cells/cm2. To evaluate possible DNA damage, a density of 22 000 cells/cm2 was used. For the immunophenotyping, the cells were seeded in 75 cm2 bottles at a minimum density of 5000 cells/cm2. Cells which were not submitted to BES were seeded at the same densities for each experiment and used as control groups.
Evaluation of cellular metabolism/proliferation after bio-electrospraying
The metabolism/proliferation of the cells submitted to the different BES time periods was evaluated by the colorimetric method of tetrazolium salt (MTT) (n = 5). After 1, 7, and 15 days of cultivation, the culture medium was removed and the cells were incubated with 0.25 μg/ml MTT in CMF buffer for 2 h at 37 °C. The MTT solution was then removed and the formed crystals were solubilized in 400 μl of dimethylsulfoxide (DMSO). The obtained absorbance was read by spectrophotometer (Wallac EnVision–Perkin Elmer) in the wave lengths of 560 nm and 630 nm. The results were calculated by the difference between the two readings (560 nm−630 nm).
Evaluation of cellular plasticity after bio-electrospraying
When the cultures reached 70% confluence, the cells were induced to chondrogenic, adipogenic and osteogenic differentiation, as described by Nardi and Meirelles.12 Cells not submitted to BES were similarly induced to differentiate in the same three lines (positive control) or kept in conventional DMEM/Hepes medium (negative control) (n = 3).
Chondrogenic differentiation
The cells were cultivated in DMEM/Hepes medium supplemented with 6.25 μg/ml bovine insulin, 10 ng/ml transforming growth factor-beta 1 (TGF-β1), and 50 nM ascorbic acid 2-phosphate. The medium was changed every 3–4 days. After approximately 21 days, the cells were fixed with 4% paraformaldehyde for 20 min and stained with 1% Alcian Blue solution.
Adipogenic differentiation
The cells were cultivated for 3 weeks in Iscove's Modified Dulbecco's Media (IMDM) supplemented with 20% human plasma, 10−7 M dexamethasone, 2.5 μg/ml bovine insulin, 5 μM indomethacin, 5 μM rosiglitazone, and 10 IU/ml of sodium heparin. The cells were fixed with 4% paraformaldehyde for 40 min and adipocytes were identified by staining the lipid vacuoles with Oil Red.
Osteogenic differentiation
The cells were kept for 3 to 4 weeks in DMEM/Hepes medium supplemented with 10% bovine fetal serum, 10−8 M dexamethasone, 5 μg/ml ascorbic acid 2-phosphate and 10 mM β-glycerophosphate. Differentiation detection was performed using Alizarin Red, which stains the phosphate crystals in the calcified extracellular matrix.
Evaluation of the immunophenotypic profile after bio-electrospraying
After BES, the cells were cultivated during 24 h and their immunophenotypic profile was analysed by flow cytometry. The following conjugated antibodies against human cell surface molecules were used: CD14/FITC, CD29/PE, CD34/PE, CD44/FITC, CD45/FITC, CD73/PE, CD90/FITC, CD184/PE, HLADR/FITC and Stro1/PE (Pharmingen, Becton Dickinson, San Jose, CA). The cells were trypsinized and 100 μl cellular suspension containing 105 cells/ml was incubated with 5 μl of each antibody for 30 min at 4 °C, protected from light. The cells were then washed and re-suspended in 1 ml of PBS. The marker 7AAD (7-Amino Actinomycin D) was used for cellular viability evaluation. Living cells were analysed using FACSAria III (Becton Dickinson) flow cytometer. Fluorescence adjustment was made with the aid of the PE and FITC isotypic controls. Approximately 10 000 events were acquired for each sample. The analysis of the data was performed using the FACSDiva software, version 6.0 (Becton Dickinson).
Evaluation of DNA damage after bio-electrospraying
To evaluate possible DNA damage, the alkaline comet assay (n = 3) was performed, as described by Singh et al.,13 with minor modifications.14, 15 After BES, the cells were incubated at 37 °C and 5% CO2 for 1, 3, and 5 h. After each time point, the cells were then trypsinized and re-suspended in culture medium. Following this, 20 μl of cell suspension was mixed with 0.75% low-melting point agarose and immediately applied onto a glass slide, pre-coated with a layer of 1% normal melting point agarose. Ice-cold lysis buffer (2.5 M NaCl, 100 mM EDTA, and 10 mM Tris, pH 10.0, with 1% Triton X-100 and 10% DMSO) was added to the cells at 4 °C for at least 1 h in order to remove cellular proteins and membranes, leaving the DNA nucleoids. The slides were then placed in a horizontal electrophoresis unit and incubated with a buffer solution (300 mM NaOH and 1 mM EDTA, pH 13.0) at 4 °C for 15 min for the unwinding of the DNA, and the expression of alkali-labile sites. Electrophoresis was conducted for 20 min at 25 V (94 V/cm). All the above stages were carried out under yellow lighting or in the dark to avoid additional DNA damage. The slides were then neutralized (Tris 0.4 M, pH 7.5) and stained, using silver staining in accordance with the protocol described by Nadin et al.16 After the staining stage, the slides were dried at room temperature and analysed in a light microscope. One hundred cells were analysed (50 cells from each of two replicate slide for each sample) in relation to DNA migration. These cells were classified according to the tail length into five classes: class 0: no damage, without tail; class 1: shorter tail with the same diameter as the head of the nucleus; class 2: tail with length 1 or 2 times greater than the diameter of the head; class 3: tail 2 times greater than the diameter of the head; class 4: comets without heads. The DNA damage index (DI) was established for each sample. The DI is an arbitrary score rating based on the number of cells with different categories of damage. The DI scale varies from 0 (cells without damage: 100 × 0) to 400 (cells with maximum migration: 100 × 4).14, 17 When selected, the cells which were like air bubbles, were disregarded.
Statistical analysis
The results were analysed by mean ± standard error and the symmetry study of the distributions was made using the Shapiro-Wilk test. The different groups were compared by one-way analysis of variance (ANOVA) following the Tukey post test (in cell viability tests and comet assay) or Kruskal-Wallis, followed by Dunn’s post test (in immunophenotypic profile analysis). Multiple groups were compared by two-way ANOVA in metabolism/proliferation assays (MTT). Differences between the groups were considered significant when P < 0.05.
RESULTS AND DISCUSSION
Standardization of BES
For the BES, a cellular suspension with concentration of 3 × 106 cells/ml was electrosprayed. This cellular concentration provided an adequate and sufficient number of recovered cells in the Petri dish, making further cell analysis possible. After evaluation of the variation of each BES parameter, the cell viability was evaluated. Parameters that were able to form a stable bio-electrospraying jet and at the same time did not provoke cell damage were chosen to carry out the BES in all subsequent studies.
Voltages between 15 and 30 kV (using −1 kV in the contra-electrode) were examined. When voltages below 15 kV were applied, unstable or no spray was formed. The use of voltage between 15 and 20 kV did not reduce MSC survival. However, when the applied voltage was increased to 25 and 30 kV, cell viability was significantly reduced in relation to initial viability (p < 0.05 e p < 0.001 respectively) (Figure 2a). Similar results were reported by Sahoo et al. where the application of 30 kV voltage BES provoked a reduction in the viability and in the proliferation of bone marrow MSC.7 The use of strong voltage during the BES can induce the formation of pores and cause damage to the cellular membrane, similar to electroporation technique.7 The mechanisms related to this cellular damage involve electric and thermal phenomena. It is known that high fields strength provokes a supra-physiological potential though the cellular membrane, causing an increase in the permeabilization of the membrane and disfunction of the membrane proteins, especially electrogenic pumps and voltage-gated ion channels.18 The increased permeability of the membrane for prolonged periods causes the loss of potassium and other metabolites and the entrance of sodium and water, causing a cellular osmotic imbalance.18 These effects lead to a loss of cellular homeostasis and subsequent cell death.7 When 15 kV voltage was applied, a stable BES jet was formed and cell viability was not altered. This voltage was applied for all further experiments.
Figure 2.
Influence of bio-electrospraying voltage (a), flow rate (b) and distance (c) on viability of MSC. Number of recovered MSC after BES (d) (n = 4). (*P < 0.05 and ***P < 0.001 using ANOVA followed by Tukey post-test)
When the BES flow rate was evaluated, cell viability reduction was observed with flows 0.28 and 0.65 ml h−1 (p < 0.001), though this did not occur with flows of 0.46 and 2.60 ml h−1 (Figure 2b). It is therefore believed that there is no direct relationship between cell survival and the BES flow rate. Although a reduction in the two flow rate values has been observed, it should be emphasized that cell viability remained high, above 93%, for all the tested groups. Although a significant reduction in the two flow rate values has been observed, there appears to be no impact in physiological terms. It should be emphasized that cell viability remained high, above 93%, for all the tested groups. The flow rate of 0.46 ml h−1 formed a continuous jet and was used for the other experiments. This intermediate flow rate was chosen because it provides a sufficient concentration of recovered cells in the Petri dish, avoiding an unnecessary expense of cells.
Cell viability was also maintained with the increase of the distance between the needle and the collection plate (Figure 2c). Paletta and colleagues showed that survival of the cells is significantly reduced when longer distances are used for BES. In their study, when the distance between the needle and the collection plate was increased from 6 to 11 cm, cell viability showed a reduction of approximately 10%.19 Under the conditions in which the present work was realized, distances of up to 8 cm were favourable for BES, which did not cause a reduction in cell survival (p > 0.05). However, the distance between the tip of the needle to the collecting plate had effects on the efficiency of the BES process. When the collected cells in the Petri dish were counted (recovered cells), it was observed that a greater number of cells was recovered when a distance of 4 cm was used (Figure 2d). The increase of distance caused a great loss of cells during the BES process. It is probable that the short distance (4 cm) created a more direct cell jet directed to the Petri dish than the larger distances (6 and 8 cm), resulting in a low cellular loss and subsequently better efficiency of the BES process. This, therefore, was the distance chosen for the ongoing work.
Impact of BES time on MSC
The BES technique is interesting for tissue engineering because of the possibility to associate it with techniques to produce scaffolds. This way, scaffolds with cells already incorporated in their structure can be obtained. The choice of BES time is of great importance. A suitable time is necessary in which scaffolds can be produced with an adequate structure for their manipulation and for sustenance of the cells for use in regenerative medicine. At the same time, the technique and the conditions in which the cells are submitted cannot cause damage to the latter.
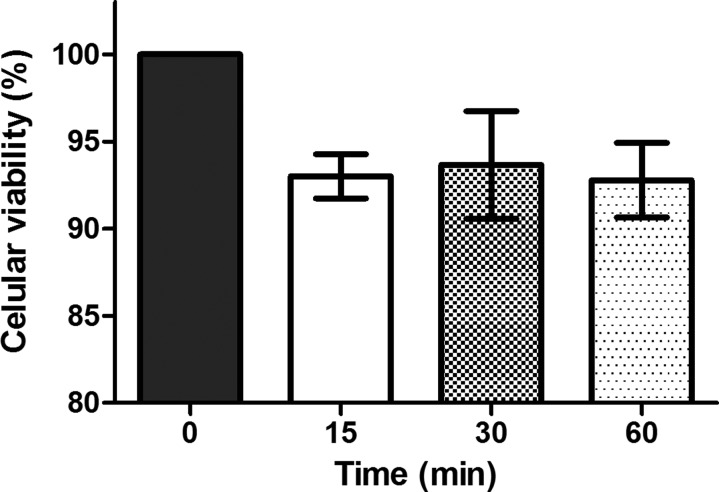
As can be seen in Figure 3, when the cells were submitted to a longer time period of BES (in previously established conditions) there was no reduction in cell survival, and no difference to initial cell viability. Although the evaluation with Trypan blue demonstrated that the increase in BES time did not alter MSC viability, a wider investigation was made in relation to the effects of this parameter on MSC because of the lack of information in the literature on this subject.
Figure 3.
Influence of bio-electrospraying time on viability of cells. (n = 4) No significant differences were observed using Anova followed by Tukey post test.
After being submitted to different BES times, the MSC sustained the capacity of adherence to the polystyrene plates, which was observed immediately after 1 h of the procedure. All the cell groups submitted to BES presented normal mesenchymal morphology and they became confluent during 15 days of cultivation, exhibiting similar behaviour to the control group (Figure 4). In a study in which astrocytoma cells were used, it was shown that BES, despite being applied for a period less than for those tested in the present study, did not cause alterations in the morphology, neither in the capacity for adherence and proliferation of this type of cell which corroborates with the encountered results.20
Figure 4.
Morphology of bio-electrosprayed mesenchymal stem cells after 1 day of culture: control group (a) and 15 min (b), 30 min (c), and 60 min (d) of BES. Scale bars represent 1 μm. The images were taken using 200× magnification.
Evaluation of cellular metabolism/proliferation after bio-electrospraying
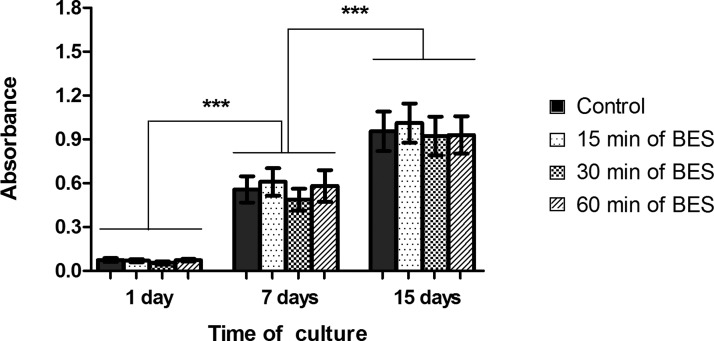
The MTT test showed that the cells submitted to different BES times remained viable during the 15 days of cultivation, presenting gradual and significant proliferation between 1, 7, and 15 days. As can be seen in Figure 5, within each period of evaluated time, no metabolic/proliferation differences were found among the cells submitted to the different BES times and between these and the control group.
Figure 5.
MTT assay showing no differences in the metabolism/proliferation between the control group and the bio-electrosprayed MSC after 1, 7, and 15 days in culture. The bio-electrosprayed MSC showed a gradual and significant increase of viable cells over 15 days. (n = 5) (***P < 0.001 using two-way ANOVA)
These results show that the cells do not show alterations in their proliferation capacity even after being exposed to BES for prolonged periods of time.
Evaluation of cellular plasticity after bio-electrospraying
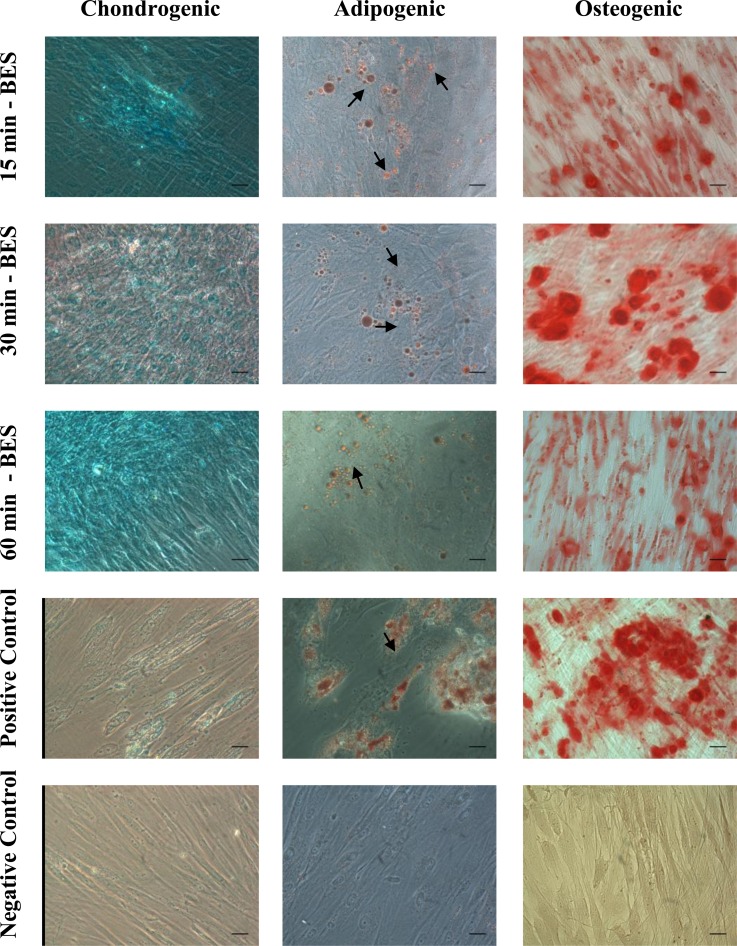
After cultivation in induction medium, the cells which were submitted to different BES times maintained their plasticity and were able to differentiate into adipocytes, osteoblasts, and chondrocytes at the same time as the cells which were not submitted to BES (positive control) (Figure 6). These results are in accordance with a previous study that demonstrated no differences in the differentiation capacity of mesenchymal stem cells in the same lineages after being submitted to BES.7
Figure 6.
Chondrogenic, adipogenic and osteogenic differentiation of MSC after staining with specific dyes for each differentiation protocol. The results showed no differences in the plasticity of bio-electrosprayed MSC at any time during analysis, compared to the positive control group (n = 3). Black arrows indicate fat deposits. Scale bars represent 1 μm. The pictures were taken using 400× of magnification.
Immunophenotypic profile of MSC after bio-electrospraying
The cells were phenotypically characterized before and after the BES process by expressing a set of cell surface markers and the absence of others. A list of markers has been described to define the MSC population. For characterization as MSC, the cells should express mesenchymal stem cell markers and should not express hemopoietic and endothelial cell markers. The cells isolated from deciduous teeth pulp were phenotypically characterized as MSC. The cells expressed MSC markers CD29, CD44, CD73, CD90 and not hemopoietic or endothelial markers CD14, CD34, CD45, CD184, and HLA-DR. The STRO-1 is a mesenchymal stem cell marker that showed low expression in this study. This value is in accordance with other works. However, as shown in a previous study of this research group, there is the possibility of the STRO-1 being internalized in these cells.11
The flow cytometry analysis showed that the cells submitted to BES maintained the same characteristics of size and complexity, forming a homogenous cellular population (data not shown). In corroboration with the in vitro differentiation, the flow cytometry results showed that electrosprayed MSC were positive for typical markers of the mesenchymal stem cells and showed low expression for non-mesenchymal markers, with no difference to the control group (p > 0.05) (Table TABLE I.). Even after longer BES time periods, there is no alteration in the immunophenotypic profile of MSC, indicating that the multipotency of these cells was preserved after BES. This result was also observed by Abeyewickreme et al., who demonstrated that after BES, murine embryonic stem cells continue to express the genes Oct4, Sox2 and Nanog, maintaining their pluripotency. However, it should be highlighted that the processing time of these cells by BES was not cited by the authors.9
TABLE I.
Immunophenotypic profile of bio-electrosprayed MSC (n = 3). Results less than 0.1% were considered 0%.
| Time of BES | ||||
|---|---|---|---|---|
| Control (0 min) | 15 min | 30 min | 60 min | |
| CD29 | 99.6 ± 2.0% | 98.9 ± 1.2% | 98.6 ± 2% | 98.4 ± 2% |
| CD44 | 74.0 ± 28% | 74.6 ± 23% | 71.4 ± 32% | 69.8 ± 38% |
| CD73 | 99.5 ± 0.5% | 99.3 ± 0.7% | 99.0 ± 1% | 99 ± 1% |
| CD90 | 99.6 ± 2.3% | 99.3 ± 0.7% | 98.7 ± 2.1% | 99.1 ± 1.4% |
| CD14 | 0.1 ± 0.1% | 0.2 ± 0.3% | 0.1 ± 0.2% | 0.1 ± 0.06% |
| CD34 | 0% | 0% | 0% | 0% |
| CD45 | 0.1 ± 0.2% | 0.1 ± 0.1% | 0% | 0% |
| CD184 | 0% | 0% | 0% | 0% |
| HLA-DR | 0% | 0.1 ± 0.1% | 0.1 ± 0.2% | 0% |
| STRO-1 | 0.1 ± 0.1% | 0.1 ± 0.1% | 0.1 ± 0.2% | 0.1 ± 0.1% |
Evaluation of DNA damage after bio-electrospraying
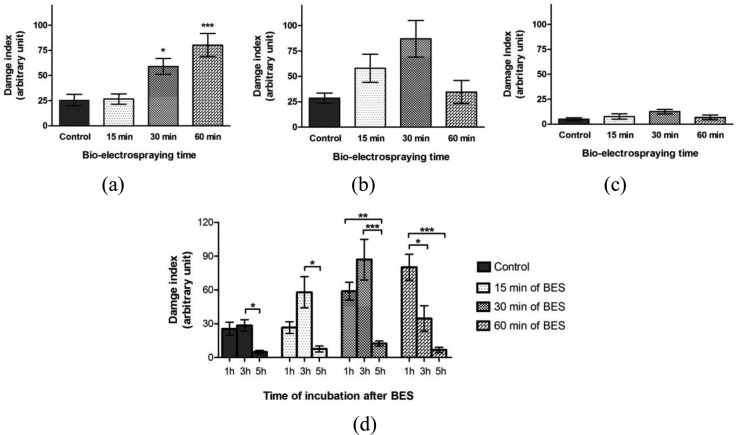
The comet assay is widely used for the evaluation of DNA damage and repair in eukaryotic cells. Performing the comet assay in alkaline conditions affords the detection of single and double strand breaks and alkaline label sites in the DNA macromolecule.21 Therefore, the comet assay was performed with the aim of verifying if BES produces genotoxicity for MSC. MSC were submitted to 15, 30, and 60 min of BES. After performing BES, the cells were incubated for 3 different time periods (1, 3, and 5 h) and the comet assay was realized.
After 1 h of incubation, an increase of DI was detected in the cells submitted to 30 and 60 min BES (Figure 7a), indicating the existence of breaks in the DNA. These results suggest that the submission of cells to BES for more prolonged periods of time provokes cellular genotoxicity. The effects of this technique on cell DNA have not been greatly studied. Many works have been published regarding the genotoxic effects of electromagnetic fields; however, these results have been both conflicting and controversial.8 In the studies that indicate an increased genomic instability, it is suggested that DNA single and double breaks are one of the factors for the increase of genotoxicity, though the exact mechanism of action has not been demonstrated.22
Figure 7.
DNA damage evaluation in MSC after 15, 30, and 60 min of BES (n = 3). Cultured cells for 1 h before performing the comet assay (a), cultured cells for 3 h before performing the comet assay (b) and cultured cells for 5 h before performing the comet assay (c). Comparison between time of culture before performing the comet assay (d). The DI reduction suggests DNA repair. (P < 0,05, **P < 0,01 and ***P < 0,001 using ANOVA followed by Tukey post-test)
Another factor that may have caused the breakdown of cellular DNA was the fact that the cells are not in a completely adequate environment during the BES process. The cells were electrosprayed under different conditions from the ideal culture conditions (different temperature of 37 °C and different atmosphere of 5% CO2). These parameters may have caused or contributed to DNA damage.
In order to evaluate the profile of these breakages over time, evaluations were also carried out after 3 and 5 h BES. In these evaluations (3 and 5 h) no significant difference was observed between the tested groups and the control (Figures 7b, 7c). These results indicate that the cells incubated for more time, before performing the comet assay, have time to carry out repairs of potential alterations to their DNA. To evaluate the possibility of DNA repair, the DI in the same BES times was compared to the different incubation periods (inter-groupal analysis). After 3 and 5 h incubation, it was verified that the DI in the group of 60 min of BES was significantly reduced in comparison with 1 h incubation, corroborating with the fact that there is probably a DNA repair mechanism (Figure 7d).
Although not significant, an increase in the DI was observed between 1 and 3 h incubation for the group of cells submitted to 15 and 30 min of BES (Figure 7d). Some types of DNA damage, such as oxidation, depurination and deamination of bases, which can be related to heat and reactive oxygen species (ROS) induced by BES, did not directly cause macromolecule breaks and therefore they are not detected by the comet assay. However, during the process of DNA repair, the excision of the altered bases occurs, causing breaks in the DNA macromolecules.23 During this stage, an increase in DNA migration can be evaluated by comet assay. This increase in the damage index which occurs after 3 h of cell recovery could be associated with the beginning of the repair process of the bases.
It was observed that in the control group, the cells incubated for 1 and 3 h exhibited a higher DI than the cells incubated for 5 h. The DI value of the control group in the three evaluated incubation times (1, 3, and 5 h) is in accordance with the history of Laboratory of Genetic Toxicology for negative controls. The recommendations set out in the comet guidelines have been followed. In these situations, there is no DNA damage induction and the damage observed is derived from the cellular basal metabolism.17, 24 When incubated for 5 h, the control cells showed lower DI value. This event was probably observed as a result of the time available for cellular DNA repair. The DI values found in the control group are also in accordance with other works that showed a similar range of basal DI values.25 In the study of Ghaderi and colleagues, mesenchymal stem cells used as a control group of comet assay exhibited a higher damage index from cell basal metabolism (until almost 40, arbitrary index) than the DI found in the present study.26 The variation of DI values found in the control group also occurred in other studies.27 In the work of Hackenberg and colleagues, the authors also show a variation of DI values in the control groups using mesenchymal stem cells.28 This data suggest that the significant difference observed in the DI values between the control groups (1 h and 5 h cells incubation, 3 h and 5 h cells incubation) are not physiologically significant.
The comet assay showed important results about the MSC processing by bio-electrospraying. However, the question remains about what causes cellular DNA damage: the BES process, the inadequate environmental conditions during the BES process or both. In the future, more studies about cell DNA damage will be carried out using a control group for each MSC processing by BES (15, 30, and 60 min). Although the cause of cell DNA damage has not been elucidated, this work brings substantial results about the use of BES to process MSC. The study shows that the MSC from deciduous teeth can be safely electrosprayed until 15 min, without have any type of damage. The results obtained after BES for cellular viability, proliferation, plasticity and immunophenotypic profile does not seems to be a peculiarity of MSC. Different types of cells have already been submitted to BES and had no change in their biological characteristics.7, 8, 29 However, if different conditions or BES parameters are used, different results may be found and then, new experiments can be performed.
CONCLUSION
The study demonstrated that BES, using a 15 kV voltage and 15 min of time period, can be applied to process MSC, without causing alterations to the cell viability, proliferation capacity, plasticity, immunophenotypic profile and DNA damage. When the BES time was increased, it was observed that BES technique caused damage to cell DNA. Nevertheless, the cells were capable of repairing this damage after 5 h of cultivation. The results presented in this study demonstrated that BES can be safely used as a seeding technique of human MSC in scaffolds and therefore can be applied to tissue engineering. The combination of BES and electrospinning techniques affords the development of scaffolds with cells homogeneously distributed along their structures. This ensures the formation of a true 3-D system, favouring correct remodelling and tissue formation.
ACKNOWLEDGMENTS
The authors wish to thank CNPq, Capes and Stem Cell Research Institute which funded the project. The authors deny any conflicts of interest related to this study.
References
- Adebiyi A. A., Taslim M. E., and Crawford K. D., Biomaterials 32, 8753 (2011). 10.1016/j.biomaterials.2011.08.028 [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N., Eagles P. A., and Qureshi A. N., Biotechnol. J. 1, 86 (2006). 10.1002/biot.200500025 [DOI] [PubMed] [Google Scholar]
- Mongkoldhumrongkul N., Flanagan J. M., and Jayasinghe S. N., Biomed. Mater. 4, 015018 (2009). 10.1088/1748-6041/4/1/015018 [DOI] [PubMed] [Google Scholar]
- Eagles P. A. M., Qureshi A. N., and Jayasinghe S. N., Biochem. J. 394, 375 (2006). 10.1042/BJ20051838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankus J. J., Guan J., Fujimoto K., and Wagner W. R., Biomaterials 27, 735 (2006). 10.1016/j.biomaterials.2005.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankus J. J., Soletti L., Fujimoto K., Hong Y., Vorp D. A., and Wagner W. R., Biomaterials 28, 2738 (2007). 10.1016/j.biomaterials.2007.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S., Lee W. C., Goh J. C., and Toh S. L., Biotechnol. Bioeng. 106, 690 (2010). 10.1002/bit.22734 [DOI] [PubMed] [Google Scholar]
- Hall R. P., Ogilvie C. M., Aarons E., and Jayasinghe S. N., Analyst 133, 1347 (2008). 10.1039/b806901h [DOI] [PubMed] [Google Scholar]
- Abeyewickreme A., Kwok A., McEwan J. R., and Jayasinghe S. N., Integr. Biol. (Camb) 1, 260 (2009). 10.1039/b819889f [DOI] [PubMed] [Google Scholar]
- Joly P., Jennings B. H., and Jayasinghe S. N., Biomicrofluidics 3, 044107 (2009). 10.1063/1.3267044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L., Luisi S. B., Fernandes R. et al. , J. Endod. 37, 973 (2011). 10.1016/j.joen.2011.04.010 [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L., Chagastelles P. C., and Nardi N. B., J. Cell Sci. 119, 2204 (2006). 10.1242/jcs.02932 [DOI] [PubMed] [Google Scholar]
- Singh N. P., McCoy M. T., Tice R. R., and Schneider E. L., Exp. Cell Res. 175, 184 (1988). 10.1016/0014-4827(88)90265-0 [DOI] [PubMed] [Google Scholar]
- Collins A. R., Mol. Biotechnol. 26, 249 (2004). 10.1385/MB:26:3:249 [DOI] [PubMed] [Google Scholar]
- Hartmann A. and Speit G., Toxicol. Lett. 90, 183 (1997). 10.1016/S0378-4274(96)03847-7 [DOI] [PubMed] [Google Scholar]
- Nadin S. B., Vargas-Roig L. M., and Ciocca D. R., J. Histochem. Cytochem. 49, 1183 (2001). 10.1177/002215540104900912 [DOI] [PubMed] [Google Scholar]
- Tice R. R., Agurell E., Anderson D. et al. , Environ. Mol. Mutagen 35, 206 (2000). [DOI] [PubMed] [Google Scholar]
- Chen W., Han Y., Chen Y., and Xie J. T., Bioelectrochem. Bioenerg. 47, 237 (1998). 10.1016/S0302-4598(98)00194-9 [DOI] [Google Scholar]
- Paletta J. R. J., Mack F., Schenderlein H. et al. , Eur. Cell Mater. 21, 384–395 (2011), available at http://www.ecmjournal.org/journal/papers/vol021/vol021a29.php. [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N. and Townsend-Nicholson A., Lab Chip 6, 1086 (2006). 10.1039/b606508m [DOI] [PubMed] [Google Scholar]
- Collins A. R., Oscoz A. A., Brunborg G. et al. , Mutagenesis 23, 143 (2008). 10.1093/mutage/gem051 [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi O. G., Bioelectromagnetics 26, 412 (2005). 10.1002/bem.20111 [DOI] [PubMed] [Google Scholar]
- Paz-Elizur T., Sevilya Z., Leitner-Dagan Y., Elinger D., Roisman L. C., and Livneh Z., Cancer Lett. 266, 60 (2008). 10.1016/j.canlet.2008.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey-Martin V. J., Green M. H., Schmezer P., Pool-Zobel B. L., De Meo M. P., and Collins A., Mutat. Res. 288, 47 (1993). 10.1016/0027-5107(93)90207-V [DOI] [PubMed] [Google Scholar]
- Fuchs R., Stelzer I., Drees C. M. et al. , Cell Biol. Int. 36, 113 (2012). 10.1042/CBI20110251 [DOI] [PubMed] [Google Scholar]
- Ghaderi M., Allameh A., Soleimani M., Rastegar H., and Ahmadi-Ashtiani H. R., Mutat. Res. 719, 14 (2011). 10.1016/j.mrgentox.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Lambert A. P. F., Moura D., Zandonai A. F. et al. , J. Tissue Sci. Eng. 2, 109 (2011). 10.4172/2157-7552.1000109 [DOI] [Google Scholar]
- Hackenberg S., Scherzed A., Kessler M. et al. , Toxicol. Lett. 201, 27 (2011). 10.1016/j.toxlet.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Eddaoudi A., Townsend-Nicholson A., Timms J. F., Schorge S., and Jayasinghe S. N., Analyst 135, 2600 (2010). 10.1039/c0an00213e [DOI] [PubMed] [Google Scholar]