Abstract
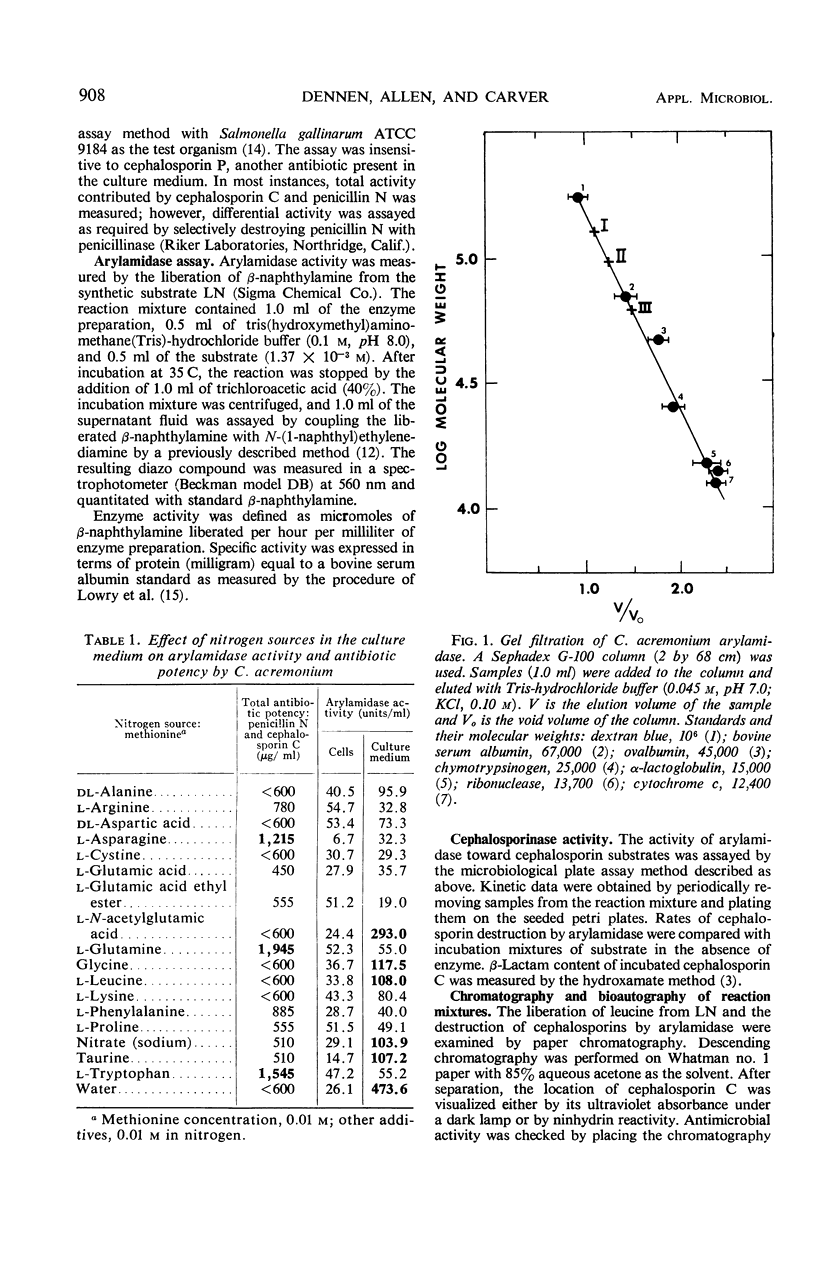
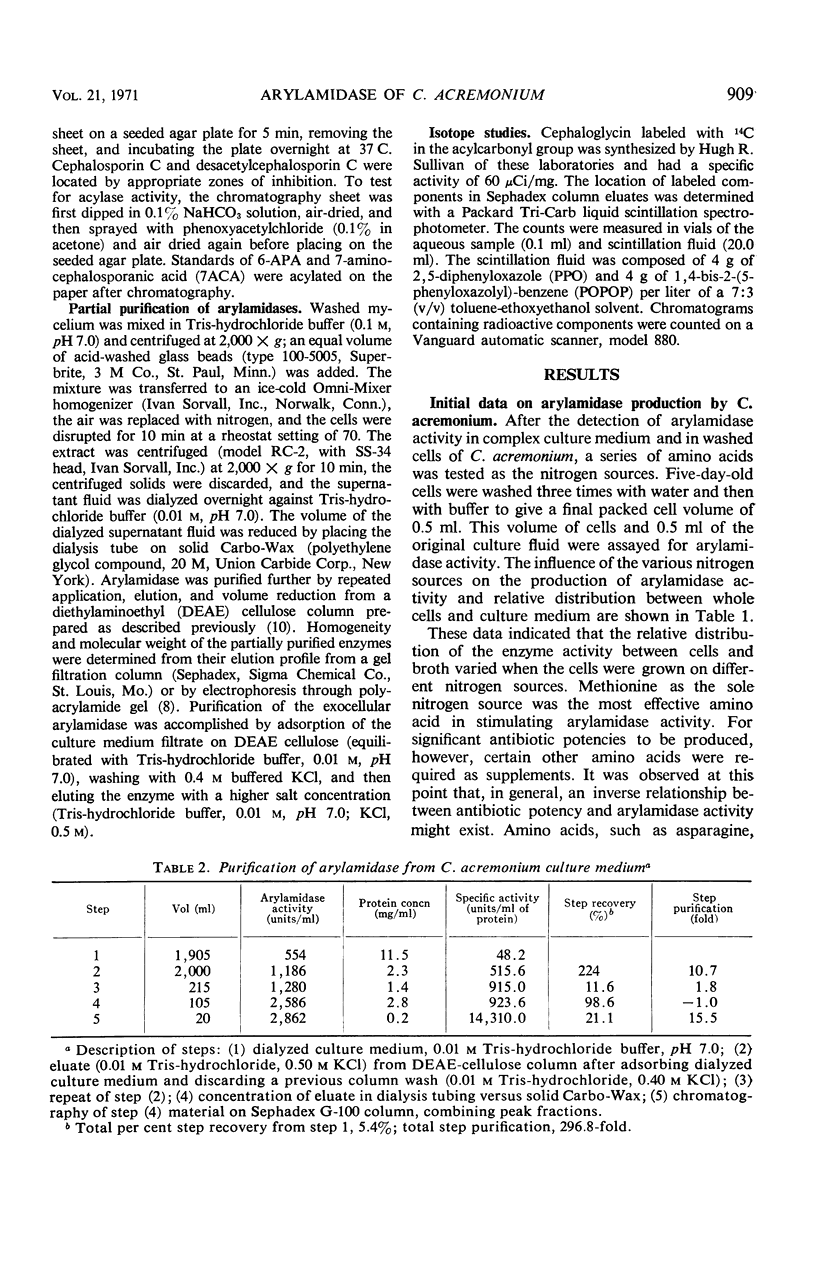
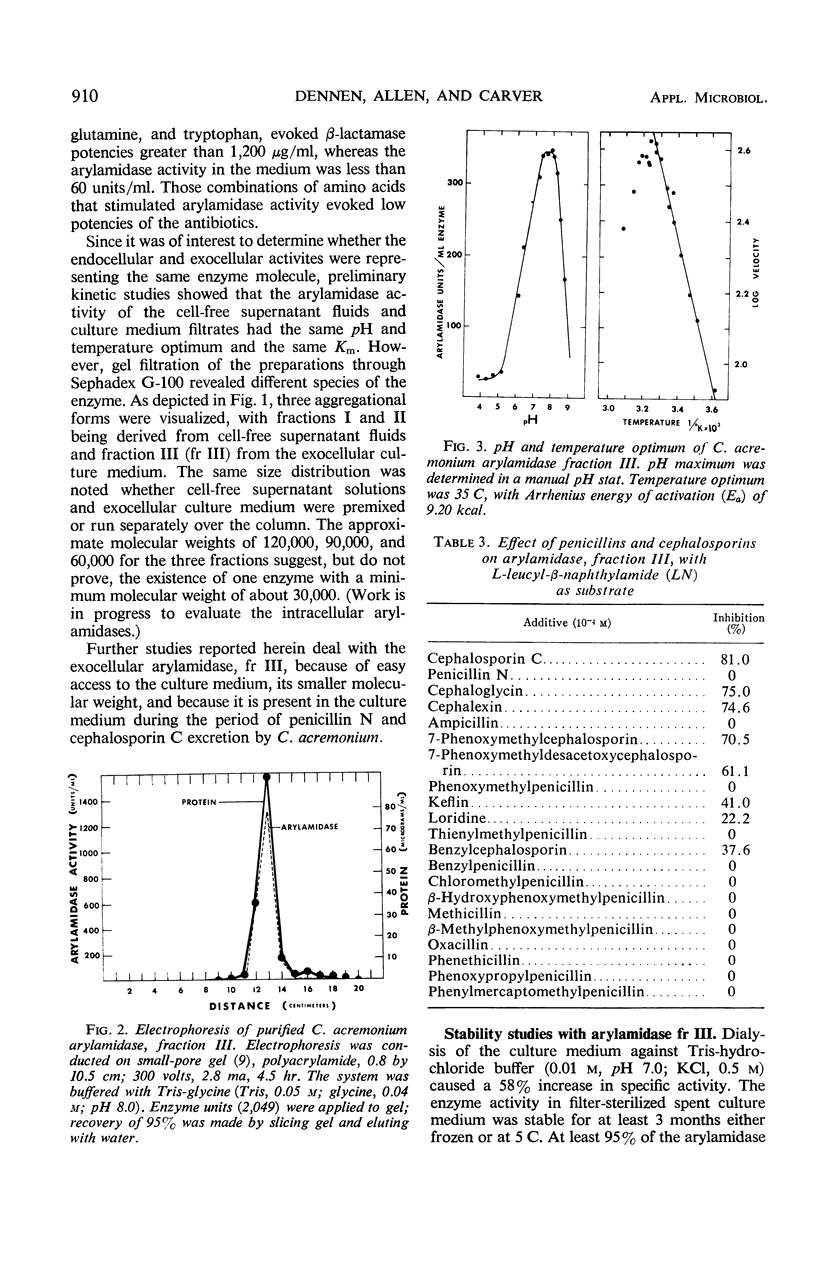
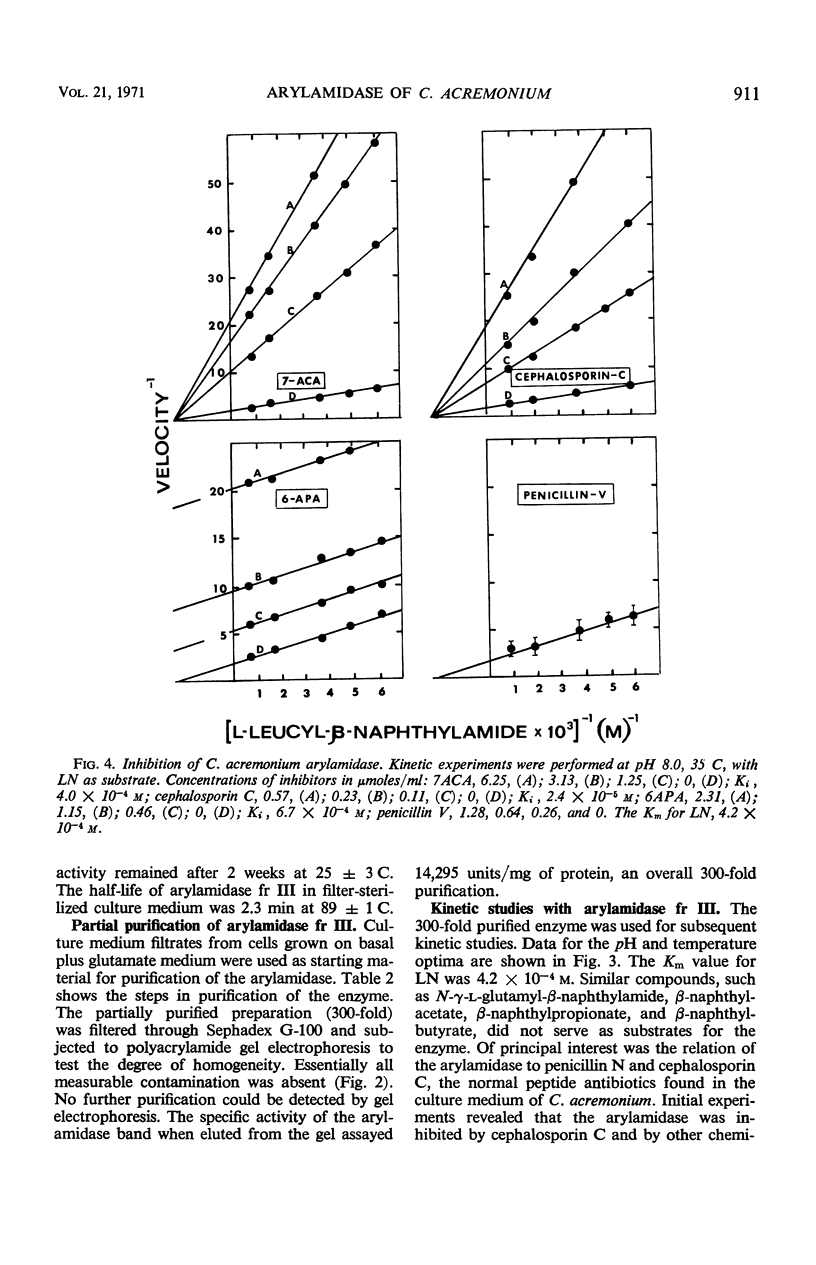
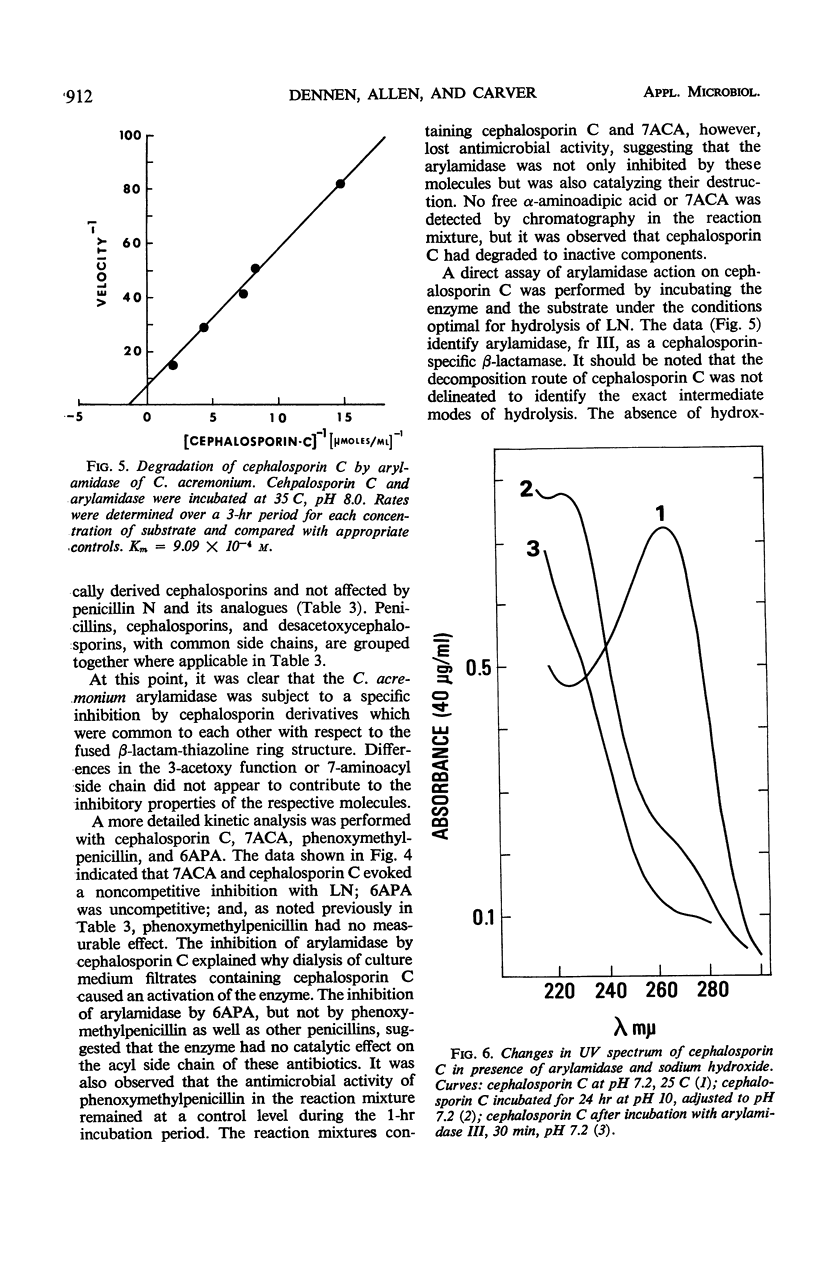
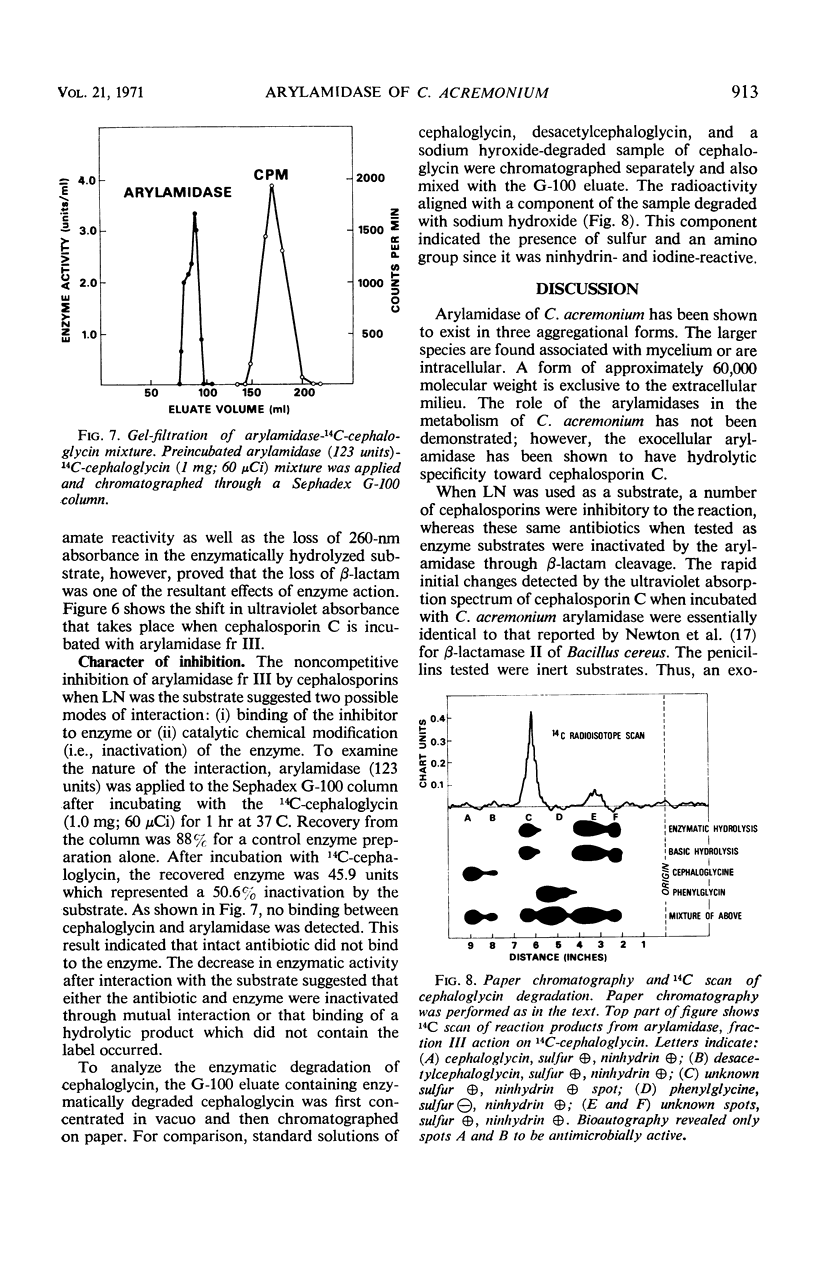
Three aggregational forms of arylamidase are produced by Cephalosporium acremonium. The exocellular enzyme, with an approximate molecular weight of 60,000, was purified 300-fold by diethylaminoethyl cellulose chromatography, gel filtration, and gel electrophoresis. With l-leucyl-β-naphthylamide as the substrate, the Km is 4.2 × 10−4m; the optimum pH, 7.7; and the temperature optimum, 35 C. The enzymatic hydrolysis of l-leucyl-β-naphthylamide is inhibited by a number of cephalosporins, whereas a variety of penicillins show no effect. Alternatively, the enzyme specifically catalyzes the β-lactam hydrolysis of a number of cephalosporins; a number of penicillins are resistant. The Km for cephalosporin C is 9.09 × 10−4m.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behal F. J., Cox S. T. Arylamidase of Neisseria catarrhalis. J Bacteriol. 1968 Oct;96(4):1240–1248. doi: 10.1128/jb.96.4.1240-1248.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARIDGE C. A., LUTTINGER J. R., LEIN J. SPECIFICITY OF PENICILLIN AMIDASES. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:1008–1012. doi: 10.3181/00379727-113-28559. [DOI] [PubMed] [Google Scholar]
- Caltrider P. G., Niss H. F. Role of methionine in cephalosporin synthesis. Appl Microbiol. 1966 Sep;14(5):746–753. doi: 10.1128/am.14.5.746-753.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C., Bennett R. E. Purification and properties of penicillin amidase from Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):302–308. doi: 10.1128/jb.93.1.302-308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. Formation of 6-aminopenicillanic acid, penicillins, and penicillin acylase by various fungi. Appl Microbiol. 1966 Jan;14(1):98–104. doi: 10.1128/am.14.1.98-104.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dennen D. W., Carver D. D. Sulfatase regulation and antibiotic synthesis in Cephalosporium acremonium. Can J Microbiol. 1969 Feb;15(2):175–181. doi: 10.1139/m69-029. [DOI] [PubMed] [Google Scholar]
- Dennen D. W., Niederpruem D. J. Regulation of glutamate dehydrogenases during morphogenesis of Schizophyllum commune. J Bacteriol. 1967 Mar;93(3):904–913. doi: 10.1128/jb.93.3.904-913.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S., Nuenke J. M. Dipeptidyl arylamidase III of the pituitary. Purification and characterization. J Biol Chem. 1967 Oct 25;242(20):4623–4629. [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- JEFFERY J. D., ABRAHAM E. P., NEWTON G. G. Further degradation products of cephalosporin C. Isolation and synthesis of 2-(4-amino-4-carboxybutyl)thiazole-4-carboxylic acid. Biochem J. 1960 May;75:216–223. doi: 10.1042/bj0750216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahadevan S., Tappel A. L. Arylamidases of rat liver and kidney. J Biol Chem. 1967 May 25;242(10):2369–2374. [PubMed] [Google Scholar]
- PATTERSON E. K., HSIAO S. H., KEPPEL A. STUDIES ON DIPEPTIDASES AND AMINOPEPTIDASES. I. DISTINCTION BETWEEN LEUCINE AMINOPEPTIDASE AND ENZYMES THAT HYDROLYZE L-LEUCYL-BETA-NAPHTHYLAMIDE. J Biol Chem. 1963 Nov;238:3611–3620. [PubMed] [Google Scholar]
- Tjeder A. The occurrence of amino acid naphthylamidase in baker's yeast. Acta Chem Scand. 1966;20(5):1442–1444. doi: 10.3891/acta.chem.scand.20-1442. [DOI] [PubMed] [Google Scholar]
- Westley J. W., Anderson P. J., Close V. A., Halpern B., Lederberg E. M. Aminopeptidase profiles of various bacteria. Appl Microbiol. 1967 Jul;15(4):822–825. doi: 10.1128/am.15.4.822-825.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


