Abstract
Study Objectives:
Chronic intermittent hypoxia (IH) acts as a stimulator of mesenchymal stem cell (MSC) mobilization, intensifying osteoblast formation in animal models. The recurrence of apnea and oxygen desaturation in obstructive sleep apnea (OSA) may mimic experimental models of IH. We hypothesized that in elderly with OSA, apnea-related IH may mobilize MSCs and thereby prevent the age-related decline in osteogenesis. This study explored the relationship between OSA and bone mineral density (BMD), and the effect of IH on BMD, in a large sample of elderly subjects.
Participants:
There were 833 volunteers age 68.6 ± 0.8 y (59% women).
Intervention:
Each participant underwent evaluation of BMD at lumbar spine and femoral sites by dual-energy x-ray absorptiometry (DEXA) as well as clinical and polygraphic examinations. OSA was diagnosed on the basis of an apnea-hypopnea index (AHI) ≥ 15.
Measurements and Results:
There were 55% of the participants who presented with OSA, and these subjects were predominantly male and overweight. Compared with subjects without OSA, those with OSA had a higher femoral and spinal BMD (P < 0.001). Body mass index (BMI), AHI, and oxygen desaturation index (ODI) (P < 0.01) were significantly related to BMD. After adjustment for sex, BMI, metabolic values, and hypertension, multiple regression analysis showed a significant association between femoral and lumbar T scores and both daily energy expenditure (P < 0.001) and ODI (P = 0.007).
Conclusions:
In elderly subjects, the presence of obstructive sleep apnea is associated with higher bone mineral density, with oxygen desaturation index being a significant determinant of bone metabolism. These results suggest that apnea-related intermittent hypoxia may stimulate the bone remodeling process in older population.
Clinical Trial Registration:
NCT 00759304 and NCT 00766584.
Citation:
Sforza E; Thomas T; Barthélémy JC; Collet P; Roche F. Obstructive sleep apnea is associated with preserved bone mineral density in healthy elderly subjects. SLEEP 2013;36(10):1509-1515.
Keywords: Bone mineral density, intermittent hypoxia, obstructive sleep apnea, osteogenesis, osteoporosis
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep disorder characterized by sleep disruption, recurrent apnea, and intermittent hypoxia. The resulting oxidative stress, endothelial dysfunction, inflammation, and enhanced sympathetic activity1,2 are considered to be the key factors determining the consequences of OSA.
At the bone level, experimental models suggest that chronic exposure to anoxia down-regulates osteogenic differentiation and stimulates osteoclast formation and thus bone resorption.3,4 This response to hypoxia may explain the physiological changes occurring with aging and the age-related risk of osteoporosis. In animal models of intermittent hypoxia, i.e., 5 h of intermittent hypoxia, promoted early mobilization of mesenchymal stem cells (MSC) from the bone marrow into the blood circulation.5–7 The release of MSC may be considered a reparative mechanism, activated by tissue lesions and allowing differentiation of MSC into various types of cells such as osteoblasts8 and endothelial progenitor cells.9 These experimental models of intermittent hypoxia may be applicable to OSA, in which cyclical arterial oxygen desaturation occurs during apnea.10
It is well known that elderly populations are at increased risk of OSA11,12 due to changes in the anatomy or function of the upper airways13 and the frequent coexistence of other medical disorders, e.g., diabetes, hypertension, and cardiovascular disease. At the same time, bone mineral density (BMD) gradually declines with aging.14 The increased prevalence of OSA in the elderly may therefore be considered as an additional risk factor for accelerated bone loss leading to osteoporosis and risk of fractures.
To the best of our knowledge, only two studies have evaluated the association between bone metabolism and OSA. Tomiyama et al.15 studied bone metabolism abnormalities in 50 patients with OSA and 15 controls, examining the link between OSA and abnormal bone metabolism by means of serum/urinary bone resorption markers. They found that the levels of these bone metabolism markers were significantly higher in patients with OSA compared with controls. In addition, a significant decrease in the concentrations of these markers was seen after 3 months of continuous positive airway pressure (CPAP) therapy, suggesting a reduced level of bone turnover after treatment. More recently, Uzkeser and co-workers16 found an increased risk of osteoporosis in 21 patients with OSA compared with control patients. The authors of these two studies postulated that bone metabolism is impaired in patients with OSA, as in individuals with lung disease,17–19 with increased bone resorption and relatively suppressed bone formation. However, both of these studies have limitations in terms of the small numbers of patients included and the lack of elderly patients.
The aim of our study was twofold: first, to analyze the potential association between OSA and BMD levels and the presence of osteoporosis in a large sample of healthy elderly; second, to determine whether, as in experimental models, intermittent apnea-related hypoxia acts as a protective mechanism counteracting the age-related decline in bone density.
METHODS
Population
The study sample was recruited from the population-based cohort of 1,011 volunteers age 65 y, residents in the city of Saint-Etienne (France), and enrolled in the PROOF (PROgnostic indicator OF cardiovascular and cerebrovascular events) survey,20 a longitudinal long-term follow-up study to assess the influence of the autonomic nervous system activity on cardiovascular and cerebrovascular diseases. An ancillary study (the Synapse study) addressing the association between OSA (assessed by home-based polygraphy) and cardiovascular consequences, during a 7-y follow-up, was proposed to the members of this cohort. The criteria for inclusion in the Synapse study were: (1) absence of previous myocardial infarction, stroke, and atrial fibrillation; (2) prior treatment or diagnosis of OSA; and (3) willingness to undergo polygraphic assessments, 24-h blood pressure and heart rate measurements, and withdrawal of blood samples for analysis. On the basis of the inclusion and exclusion criteria, 854 patients (58.5% women) were eligible to participate in the Synapse study. All of these patients underwent a clinical assessment including a questionnaire on demographics, medical history, and medication. After exclusion of patients taking anti-osteoporosis medication or with self-reported osteoporosis, the study population comprised 832 patients, age 68.6 ± 0.03 y, who underwent dual-energy x-ray absorptiometry (DEXA) measurement of BMD. Some of the DEXA data reported here were also cited in a previous paper focusing on the effect of BMD on the sex effect in OSA.21
The study was approved by the local ethics committee (CPPRB Rhône-Alpes Loire), and written consent was obtained from all participants.
Anthropometric Measurements
Body mass index (BMI) was calculated as weight/height squared (kg/m2). Neck circumference (NC) was measured at the midpoint of the neck between the midcervical spine and the midanterior neck 0.5 cm below the laryngeal prominence. Waist circumference (WC) was measured midway between the lower rib margin and the iliac crest.
Serum concentrations of fasting glucose, total cholesterol, triglycerides and high-density lipoprotein cholesterol were measured using enzymatic kits (Roche Diagnostics, Switzerland).
Measurement of Bone Metabolism by DEXA
The patients were referred to the Rheumatologic Research Group of the University Hospital of Saint-Etienne (France) for the evaluation of bone metabolism. Bone metabolism was measured using a whole-body DEXA scanner (Hologic QDR-2000, software version V5.67A, Hologic Inc., Bedford, MA, USA). The standard procedures described in the literature for DEXA measurement were applied.22
BMD was measured in all patients at the proximal end of the femur and at the lumbar spine (L1-L4) with a coefficient of variation (CV) of 0.8% and < 1.2%, respectively. BMD was expressed in g/cm2 and as the difference, expressed as standard deviations (SD), from the normal average value of peak bone mass at 30 y (T score). According to their BMD results for the femoral neck and lumbar spine, patients were classified into three groups according to World Health Organization criteria: those with normal BMD (T score > -1.0 SD), osteopenia (T score -1.0 to -2.5 SD), and osteoporosis (T score < -2.5 SD).23
Daily Energy Expenditure
Daily energy expenditure (DEE) was assessed by a self-administered physical activity questionnaire concerning seven main dimensions of everyday life, with specific emphasis on autonomy and perceived exertion.24 This questionnaire has been evaluated over a wide range of activity levels, even in populations with very low levels of physical activity. It provides a quantitative picture of the individual's mean habitual activities, based on calculation of DEE (kJ.24h-1) = sum of intensity of activity (J.min-1.kg-1) and duration of specific activity (min.day-1) according to age, weight, severity of the condition considered, and autonomy.
Sleep Study
All patients underwent an unattended nocturnal sleep assessment at home using a polygraphic system (HypnoPTT, Tyco Healthcare, Puritan Bennett, California, US). The following parameters were measured: sound emission, electrocardiogram, pulse transit time, R-R timing, airflow measured by nasal pressure, respiratory efforts and body position. Oxygen saturation (SaO2) was measured by pulse oximetry. All recordings were visually validated and manually scored for respiratory events and nocturnal SaO2 according to standard criteria.25 Hypopnea was defined as a 50% or greater reduction in airflow from the baseline value lasting at least 10 sec and associated with at least 3% oxygen desaturation. Apnea was defined as the absence of airflow on the nasal cannula lasting for > 10 sec. The absence of rib cage movements associated with apnea defined the event as central, whereas a progressive increase in pulse transit time and respiratory efforts allowed definition of the event as obstructive. To minimize potential overestimation of sleep duration, patients completed a sleep diary to set the analysis between lights-off and lights-on. The apnea-hypopnea index (AHI) was defined as the ratio of the number of episodes of apnea or hypopnea per hour of reported sleep time. The indices of nocturnal hypoxemia were as follows: mean SaO2; percentage of recording time spent with an SaO2 below 90%; minimum SaO2 value recorded during sleep (minimum SaO2); and oxygen desaturation index (ODI), i.e., the number of episodes of oxyhemoglobin desaturation per hour of reported sleep time during which blood oxygen level fell by 3% or more. In accordance with recent data concerning the elderly,26 OSA was diagnosed on the basis of an AHI ≥ 15 with at least 85% of events scored as obstructive. An AHI ≥ 15 and < 30 indicated mild OSA, and an AHI ≥ 30 denoted severe OSA.
Statistical Analysis
Results are presented as means ± standard deviation (SD) for continuous variables and percentages for categorical variables.
The characteristics of the study population, as well as DEXA and anthropometric measurements, were compared between patients with and without OSA using a univariate analysis of variance or chi-square test as appropriate. The relationships between BMD measurements, anthropometric measurements, and polygraphic data were estimated using Pearson correlation coefficient. Multiple linear regression analysis was then performed to analyze the relationship between femoral and lumbar T scores (dependent variables) and sex, DEE, anthropometric data, hypertension, smoking status, alcohol consumption, AHI, and ODI.
Data were analyzed using the Statistical Package for the Social Sciences version 17 for Windows (SPSS Inc., Chicago, IL, USA) and Stata release 11 (Stat Corp, College Station, TX, USA). All reported P values are two-tailed, the threshold of statistical significance being set at P < 0.05.
RESULTS
Clinical, anthropometric, and polygraphic data for the entire study population and for men and women separately are summarized in Table 1. Of the total population, 58.9% were female and 41.1% were male. OSA was manifested by 55% of the participants, with 44.9% having an AHI < 15. Comparison between patients with and without OSA in the total sample revealed significant differences in anthropometric variables and prevalence of diabetes and hypertension, but no differences in smoking, alcohol consumption, or dyslipidemia. With regard to sex, we found that men, whether with or without OSA, had greater NC and WC than the corresponding groups of women, as well as higher values of AHI. Men with OSA had higher ODI (P < 0.001). Sex differences were also evident with respect to BMI, metabolic factors, and hypertension.
Table 1.
Anthropometric and polygraphic data for subjects with and without obstructive sleep apnea for the whole group and for women (n = 491) and men (n = 341) separately (mean ± standard deviation)
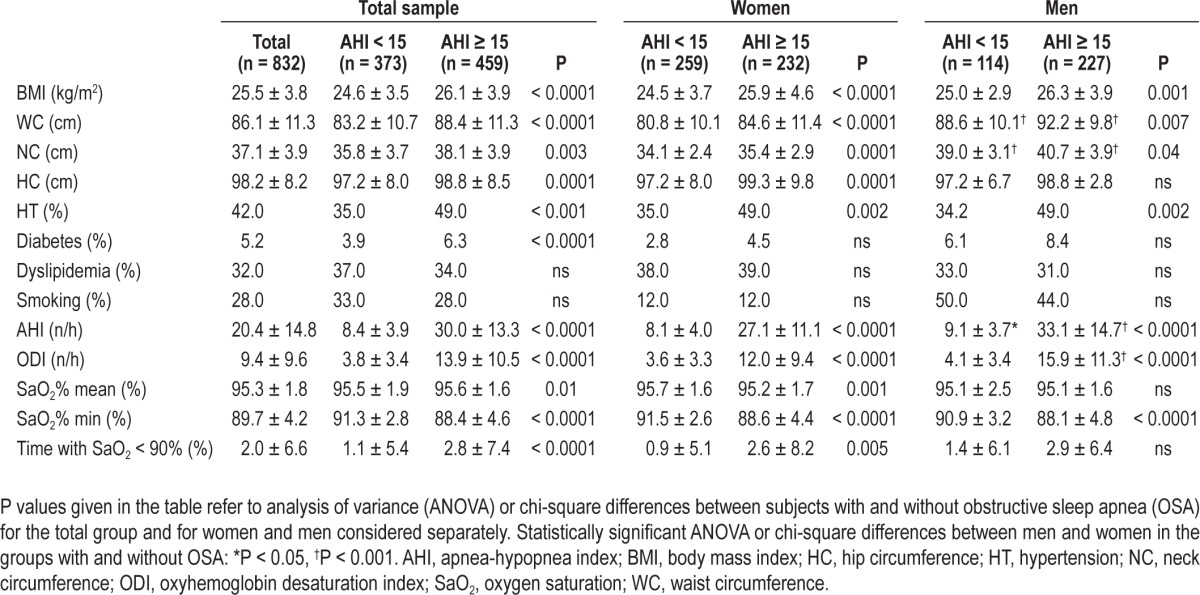
Table 2 shows the mean BMD and DEE values and the prevalence of osteoporosis/osteopenia in patients with and without OSA for the whole group and for men and women separately. Overall, patients with OSA had a higher BMD at both femoral and lumbar sites (P < 0.001) and a greater DEE. According to threshold values,18 femoral and lumbar spine osteopenia was found in 42% and 39%, respectively, of the total sample, with osteoporosis being present in 5% and 11%, respectively. No significant differences in the prevalence of osteopenia/osteoporosis were found between patients with and without OSA.
Table 2.
Comparison of bone mineral density at two anatomical sites between subjects with and without obstructive sleep apnea (mean ± standard deviation) in the total group and in women and men separately
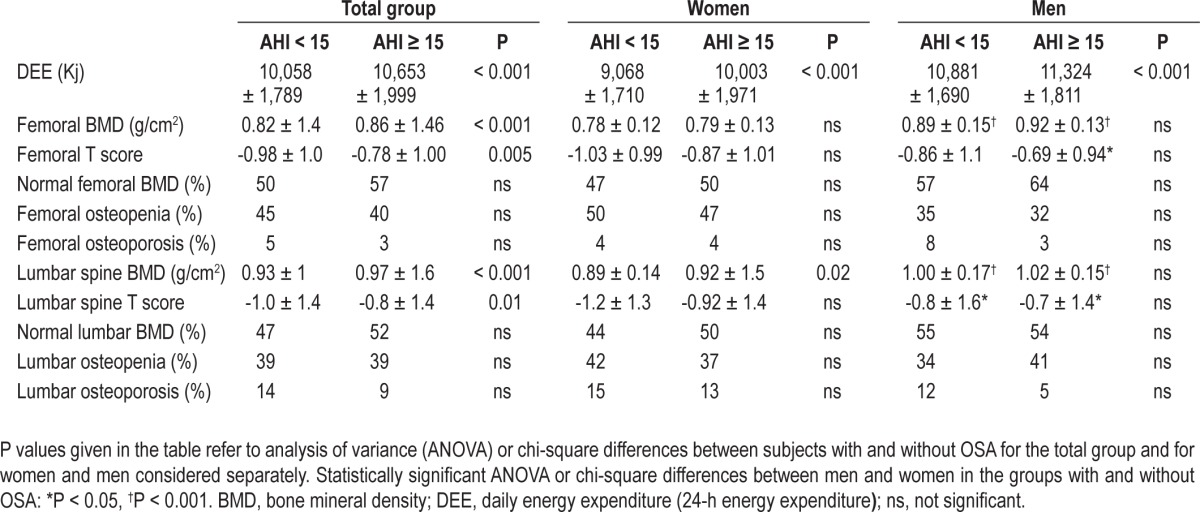
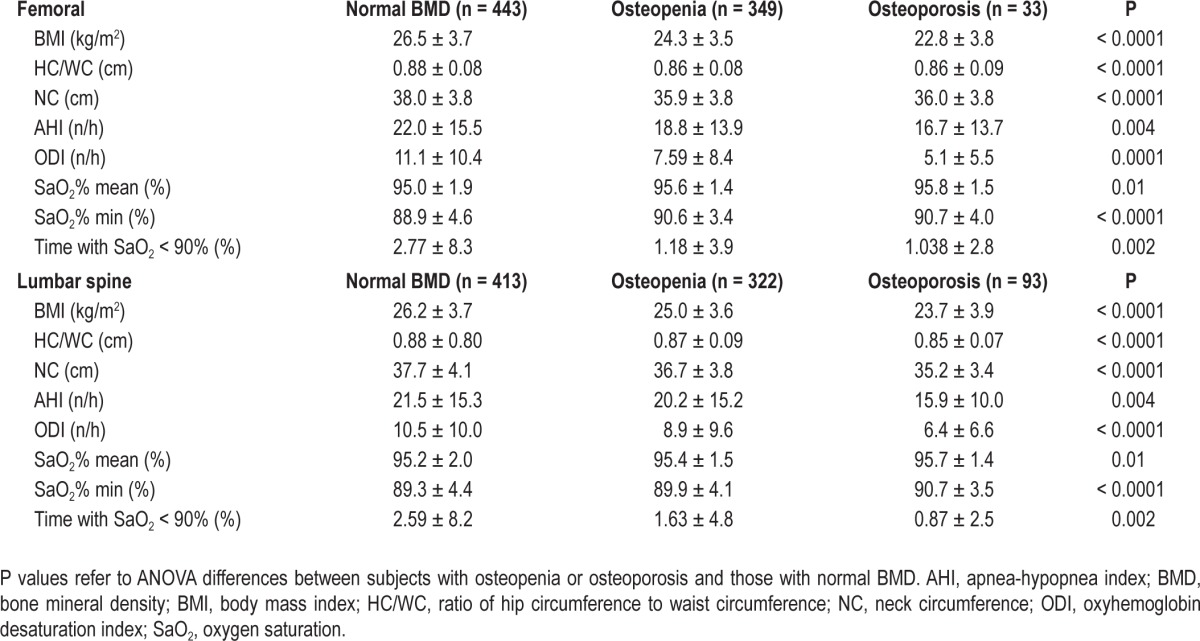
Considering men and women separately, stratified according to the absence or presence of OSA (Table 2), no significant differences in BMD or prevalence of osteopenia/osteoporosis were found between patients with and without OSA regardless of sex. In women, there was a trend toward greater osteopenia and lower femoral and lumbar BMD in patients without OSA, although these differences reached statistical significance only with respect to BMD. Table 3 reports the data for patients with and without osteopenia/osteoporosis at the femoral and lumbar sites. Compared to patients with normal BMD, patients with osteopenia/osteoporosis had lower values of AHI, ODI, and other hypoxemia variables, suggesting a protective role of nocturnal hypoxemia on bone density.
Table 3.
Anthropometric, biological, and polygraphic data of subjects with normal bone mineral density and subjects with osteopenia or osteoporosis at the femur and lumbar spine (mean ± standard deviation).
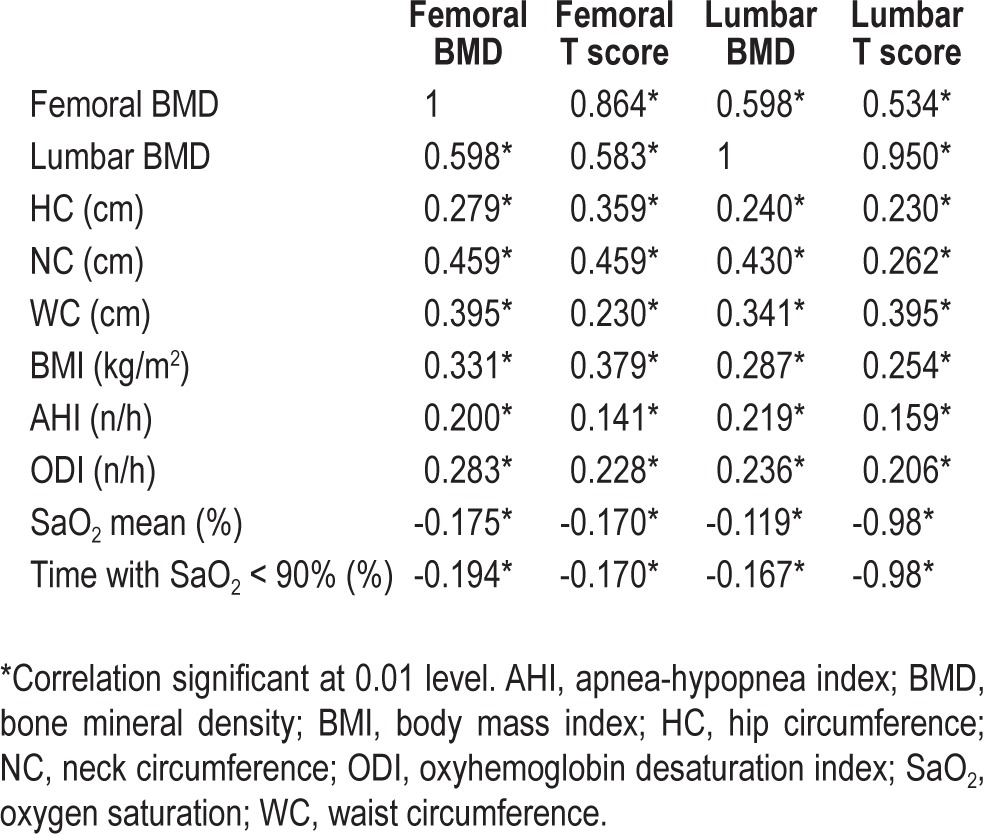
In the bivariate analysis (Table 4), femoral BMD correlated positively with all anthropometric variables, in particular NC and WC, as well as with AHI and ODI. Similarly, spinal BMD was correlated with BMI, NC and WC, and with AHI and ODI (P = 0.01). These correlations did not differ between men and women.
Table 4.
Pearson correlation coefficients of bone mineral density data and clinical and polygraphic data
A multiple regression analysis was performed to examine the independent relationship between T score at femoral and lumbar sites and sex, hypertension, glycemia, smoking habits, DEE, BMI, waist-to-hip ratio, AHI, and ODI. As shown in Tables 5 and 6, a significant relationship was found between femoral T score and DEE (P < 0.0001), BMI (P < 0.0001), ODI (P = 0.007) and, to a lesser extent, AHI (P = 0.04). Similarly, at the lumbar site, the T score was significantly associated with DEE (P = 0.009), BMI (P = 0.005), ODI (P = 0.001) and, more weakly, with AHI (P = 0.02). No statistically significant relationship was found between femoral and lumbar T scores and other variables of nocturnal hypoxemia, such as the mean and minimum SaO2 and the time spent with SaO2 below 90%.
Table 5.
Multiple regression analysis of the relationship between anthropometric and polygraphic variables and femoral T scores
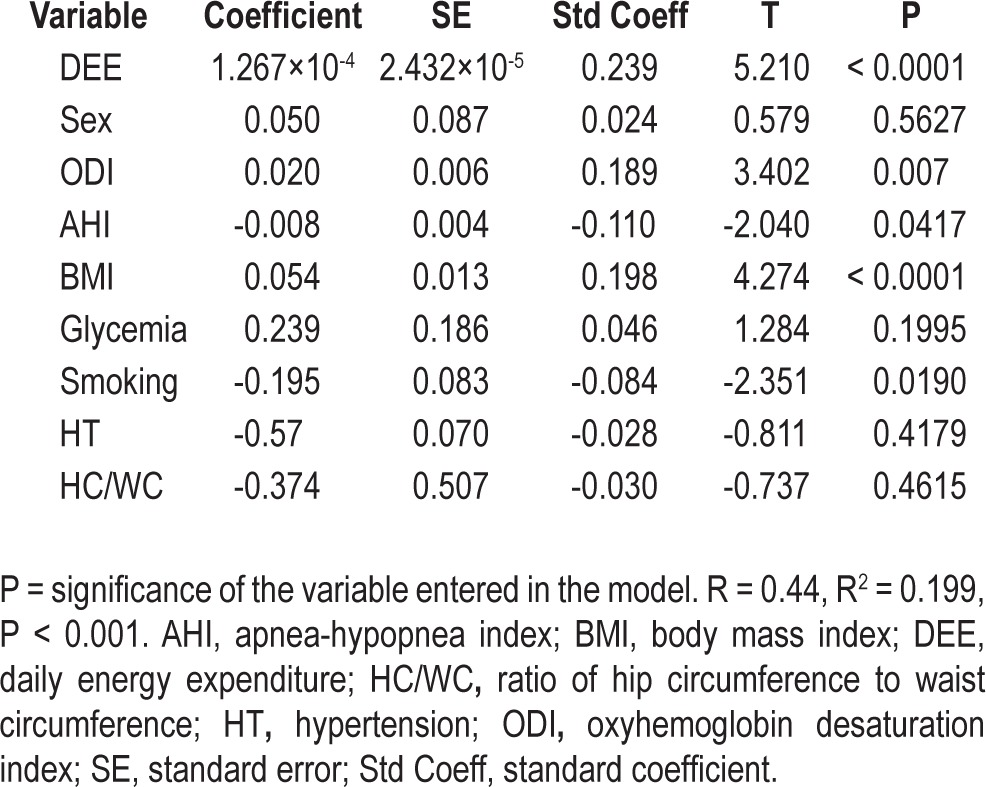
Table 6.
Multiple regression analysis of the relationship between anthropometric and polygraphic variables and lumbar spine T scores
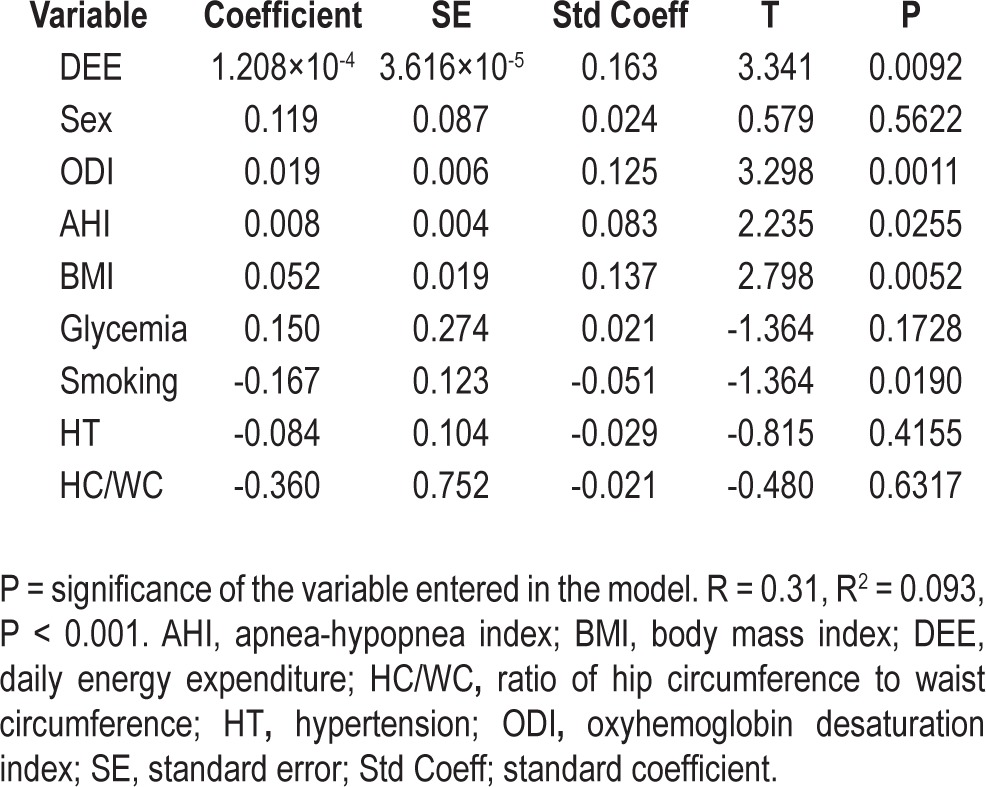
To assess whether the risk of osteoporosis was affected by the severity of OSA in terms of AHI, we calculated the odds ratio (OR) for osteoporosis at the lumbar and femoral sites in patients with mild and severe OSA after adjustment for DEE, BMI, and ODI. At the lumbar site, the OR was 1.09 (95% confidence interval, CI [0.66-1.81]; P = 0.739) in patients with mild OSA and 0.46 (95% CI [0.20-0.99]; P = 0.04) in those with severe OSA (AHI ≥ 30), suggesting a decreased risk of osteoporosis in the latter group. At the femoral site, the AHI played no role either in patients with mild OSA (≥ 15 AHI < 30) OR: 0.95, 95% CI (0.37-1.94), P = 0.69 or in those with severe OSA (AHI ≥ 30) OR: 0.85, 95% CI (0.28-2.3), P = 0.76).
DISCUSSION
The current study was designed to evaluate the association between OSA and BMD in a large number of healthy elderly as well as to assess the effect of recurrent apnea-related hypoxemia on BMD and risk of osteoporosis. The first interesting finding of our study is that in elderly subjects, the presence of OSA did not induce a decrease in BMD and did not increase the risk of osteoporosis compared to patients without OSA. Moreover, in contrast to middle-aged patients, the risk of osteopenia/osteoporosis was lower in patients with higher DEE, ODI, and BMI, suggesting that the apnea-related hypoxemia, higher activity, and higher BMI preserve bone mineral content in the elderly. Finally, the statistically significant effect of ODI on BMD, in the absence of any effect of AHI, mean oxygen saturation, or time spent with an oxygen saturation < 90%, appears to support the hypothesis that intermittent hypoxia plays a protective role with respect to bone metabolism in the elderly.
Little has been published about BMD and osteoporosis in individuals with OSA. Tomiyama and coworkers15 observed an elevation of bone resorption marker (urinary C-terminal telo-peptide of type I collagen (CTX) in 50 middle-aged patients with OSA, reversed by CPAP therapy in 21 of the 40 patients with moderate to severe OSA. Because the effect of CPAP treatment was evident in only 21 patients, the authors suggested that patients with OSA have abnormal bone metabolism characterized by increased bone resorption and suppressed bone formation. Similarly, Uzkeser and colleagues16 found decreased levels of BMD at femoral and lumbar sites in 21 patients with OSA, indicating a greater risk of osteoporosis in patients with this disorder. In contrast with these data, obtained in middle-aged patients, our study did not reveal any significant link between OSA severity, as defined by AHI, and increased osteoporosis. In our sample higher ODI and increased DEE are associated with higher BMD in the elderly. These conflicting results concerning the link between OSA and the risk of osteopenia/osteoporosis risk might be explained in part by the different selection criteria used in the previous studies, such as age, the small sample sizes, the inclusion of patients with comorbidities and different diagnostic methods. In our study, we followed strict rules for selection of the study population, excluding patients with confounding factors such as osteoporosis medication and pulmonary or coronary artery disease, which especially affect bone mass. In addition, we took into account DEE,27 anthropo-metric variables,28 and general factors that may have a beneficial or deleterious effect on BMD.
To explain the lack of BMD impairment in our elderly subjects with OSA, some pathophysiological data should be considered. We know that recurrent hypoxia is the major factor associated with OSA likely to affect bone cell function, either directly or by acting on the proinflammatory processes, oxidative stress, and endothelial dysfunction implicated in OSA.29–33 The increased levels of biochemical markers of physiological regeneration of tissues, e.g., mesenchymal cells, observed in rats after intermittent hypoxic exposure are in accordance with our cross-sectional observations in healthy elderly subjects with OSA. In our study population, there was a significant association between femoral and lumbar BMD and ODI, with no effect of AHI, mean oxygen saturation, or time spent at an SaO2 < 90%. These findings appear to support the hypothesis that, in contrast with chronic hypoxia, recurrent hypoxemia may prevent osteopenia/osteoporosis in elderly patients with OSA. Although speculative and based on experimental data, a hypothesis could be proposed to explain the protective effect of intermittent hypoxia on bone metabolism. As in animal models,5–8,10 the recurrent transient falls in oxygen saturation could mobilize MSCs capable of undergoing differentiation into osteocytes. The resulting promotion of osteoblastic activity might slow bone resorption and consequently the risk of osteopenia/osteoporosis related to the aging process.34
Although obesity, through mechanical load-bearing and production of cytokines and hormones, may reduce BMD,35,36 there is no clear consensus regarding the true effect of obesity on BMD. Obesity-related comorbidities, e.g., oxidative stress, systemic inflammation, and metabolic factors, more than BMI in itself may be implicated to a great extent in the association between obesity and osteoporosis.37,38 In our study we found that DEE and ODI and BMI were the most significant factors affecting BMD and the risk of osteoporosis. These preliminary data and the positive correlation between BMD and BMI deserve further study in large, prospective cohorts of obese patients with OSA in order to understand the true effect of obesity and BMI on bone structure.
Some strengths and limitations of our study should be highlighted. The strengths of this study include use of the current gold standard diagnostic method for the diagnosis of osteoporosis, namely DEXA, the collection of data from a large population sample, the inclusion of numerous untreated women and the objective documentation of breathing disorders by overnight polygraphy. Moreover, we examined the effect of other factors potentially implicated in abnormal bone metabolism, such as smoking habits, physical activity, anthropometric variables, hypertension, and diabetes, which all potentially play a role in the pathophysiological association between OSA and osteoporosis.
Our study has a number of limitations. First, due to its cross-sectional nature, implying analysis of a homogenous group of elderly subjects of similar age and with no previous clinically relevant disease, we cannot extrapolate our results to middle-aged or elderly patients, or assess the causative relationship between ODI and BMD. Second, we did not measure the serum and urinary levels of bone metabolism markers. However, as previously described, the values of certain variables did not differ between patients with OSA and controls15,16 at baseline or after CPAP treatment,15 suggesting a more complex dysfunction of bone metabolism in patients with OSA. Finally, we used ambulatory polygraphy, which does not allow assessment of sleep structure and sleep duration, two factors recently shown to be implicated in the risk of osteoporosis.39,40
In conclusion, this study is the first to evaluate the true association between BMD and OSA, taking into consideration the role of confounding factors, such as metabolic and vascular factors, on bone composition. In a large sample of healthy elderly subjects, we did not find any increased risk of osteoporosis among those with OSA, even in women. Interestingly, intermittent hypoxia, as assessed by ODI, seems to exert a protective role with regard to the age-related decline in BMD, reducing the prevalence of osteopenia/osteoporosis in our elderly. Larger clinical studies are needed to clarify whether or not OSA is an independent risk factor for osteoporosis and whether, paradoxically, recurrent hypoxia may act as a protective factor with respect to the risk of osteoporosis.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The PROOF study group thanks all the subjects included in the present study, as well as Mr. Olivier Grataloup, Ms. Delphine Maudoux, and Dr. Stéphane Chomienne (CHU Saint-Etienne, France) for their expert help in data acquisition.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMD
bone mineral density
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DEE
daily energy expenditure
- DEXA
dual-energy X-ray absorptiometry
- ESS
Epworth sleepiness scale
- IH
intermittent hypoxia
- ODI
oxygen desaturation index
- OR
odds ratio
- OSA
obstructive sleep apnea
- SaO2
oxygen saturation
REFERENCES
- 1.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010;503:103–9. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Arnett TR, Gibbons DC, Utting JC, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 5.Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep. 2009;32:117–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Carreras A, Rojas M, Tsapikouni T, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early activation of mesenchymal stem cells and enhancement of endothelial wound healing. Respiratory Research. 2010;11:91. doi: 10.1186/1465-9921-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almendros I, Carreras A, Montserrat J, Gozal D, Navajas D, Farré R. Potential role of adult stem cells in obsructive sleep apnea. Front Neur. 2012;3:1–8. doi: 10.3389/fneur.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharib SA, Dayyat EA, Khalifa A, et al. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic-like stem cells and activates developmental transcriptional programs in mice. Sleep. 2010;33:1439–46. doi: 10.1093/sleep/33.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger S, Lavie L. Endothelial progenitor cells in cardiovascular disease and hypoxia -potential implications to obstructive sleep apnea. Transl Res. 2011;158:1–13. doi: 10.1016/j.trsl.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Almendros I, Carreras A, Montserrat JM, Gozal D, Navajas D, Farré E. Potential role of adult stem cells in obstructive sleep apnea. Front Neurol. 2012;3:112. doi: 10.3389/fneur.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult-a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 13.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–9. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32:39–63. doi: 10.1016/s0889-8529(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 15.Tomiyama H, Okazaki R, Inoue D, et al. Link between obstructive sleep apnea and increased bone resorption in men. Osteoporos Int. 2008;19:1185–92. doi: 10.1007/s00198-007-0556-0. [DOI] [PubMed] [Google Scholar]
- 16.Uzkeser H, Yildirim K, Aktan B, et al. Bone mineral density in patients with obstructive sleep apnea syndrome. Sleep Breath. 2013;17:339–42. doi: 10.1007/s11325-012-0698-y. [DOI] [PubMed] [Google Scholar]
- 17.Papaioannou A, Parkinson W, Ferko N, et al. Prevalence of vertebral fractures among patients with chronic obstructive pulmonary disease in Canada. Osteoporos Int. 2003;14:913–7. doi: 10.1007/s00198-003-1449-5. [DOI] [PubMed] [Google Scholar]
- 18.Ionescu AA, Schoon E. Osteoporosis in chronic obstructive pulmonary disease. Eur Respir J. 2003;46:64s–75s. doi: 10.1183/09031936.03.00004609. [DOI] [PubMed] [Google Scholar]
- 19.Ionescu AA. When should we suspect osteoporosis in patients with chronic airway disease? Chron Respir Dis. 2005;2:1–2. doi: 10.1191/1479972305cd059ed. [DOI] [PubMed] [Google Scholar]
- 20.Barthélémy JC, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events. The PROOF Study. Neuroepidemiology. 2007;29:18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 21.Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J. 2011;37:1137–43. doi: 10.1183/09031936.00043210. [DOI] [PubMed] [Google Scholar]
- 22.Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol. 2000;89:345–52. doi: 10.1152/jappl.2000.89.1.345. [DOI] [PubMed] [Google Scholar]
- 23.Orimo H, Hayasashi Y, Fukunaga M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–7. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 24.Garet M, Barthélémy JC, Degache F, et al. A questionnaire-based assessment of daily physical activity in heart failure. Eur J Heart Fail. 2004;6:577–84. doi: 10.1016/j.ejheart.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Ruehland WR, Rochford PD, O' Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–8. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonaiuti D, Shea B, Iovine R, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;3:CD000333. doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 28.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–55. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 29.Farre R, Montserrat JM, Navajas D. Morbidity due to obstructive sleep apnea: insights from the animal study. Curr Opin Pulm Med. 2008;14:530–6. doi: 10.1097/mcp.0b013e328312ed76. [DOI] [PubMed] [Google Scholar]
- 30.Nacher M, Farre R, Montserrat JM, et al. Biological consequences of oygen desaturation and respiratory effort in an acute animal model of obstructive sleep apnea (OSA) Sleep Med. 2009;10:892–7. doi: 10.1016/j.sleep.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–6. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 33.Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosc. 2012;4:1391–403. doi: 10.2741/469. [DOI] [PubMed] [Google Scholar]
- 34.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao LJ, Liu PY, Hamilton J, Becker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–6. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao LJ, Jiang H, Papasian CJ, Drees B, Hamilton J, Deng HW. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubbins I, Schott AM, Azari R, Krommal R. Body mass index is not a good predictor of bone density: results from the WHI, CHS and EPIDOS. J Clinical Densitum. 2006;9:329–34. doi: 10.1016/j.jocd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Mariani S, Fiore D, Varone L, et al. Obstructive sleep apnea and bone mineral density in obese patients. Diabetes Metab Syndr Obes. 2012;5:395–401. doi: 10.2147/DMSO.S37761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between osteoporosis and sleep duration in healthy middle-aged and elderly adults: a large-scale, cross-sectional study in Japan. Sleep Breath. 2012;16:579–83. doi: 10.1007/s11325-011-0545-6. [DOI] [PubMed] [Google Scholar]
- 40.Stone KL, Ewing SK, Lui LY, et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1177–83. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]


