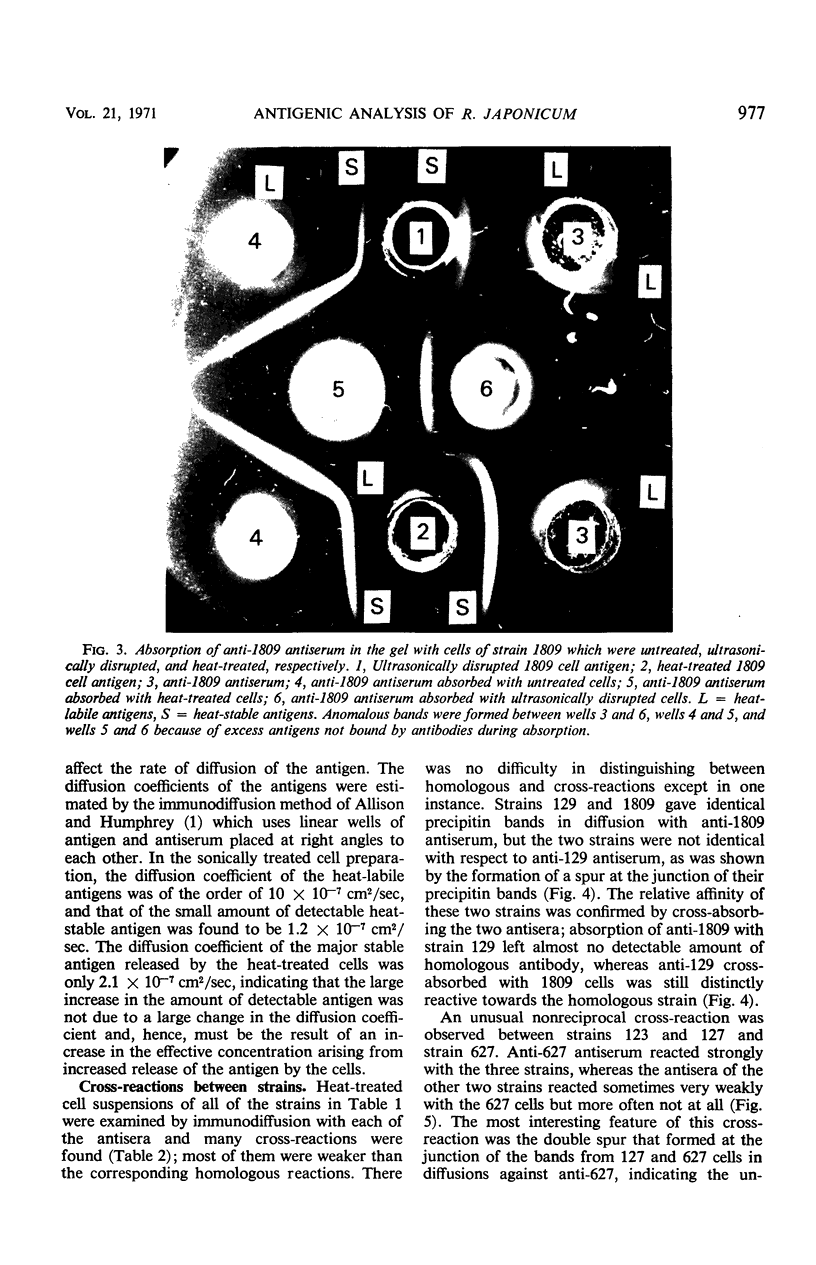
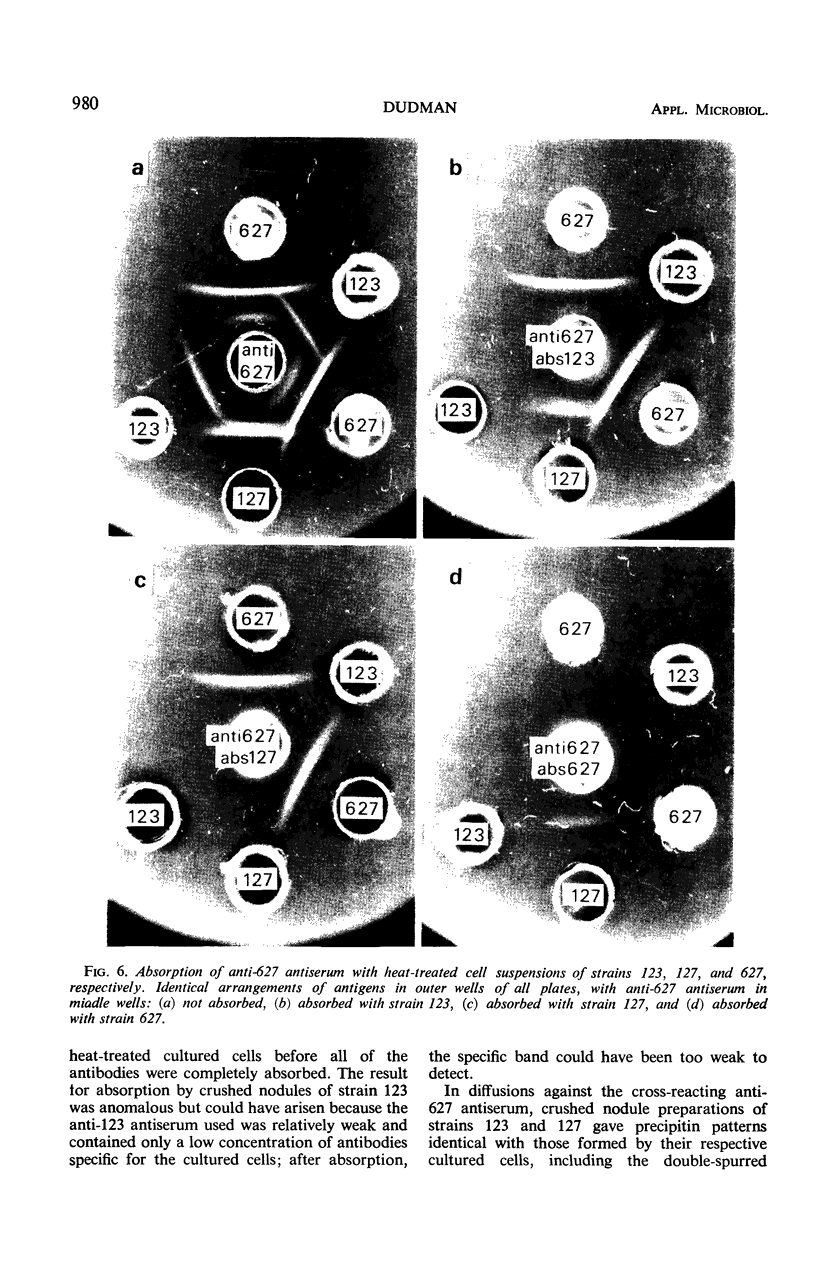
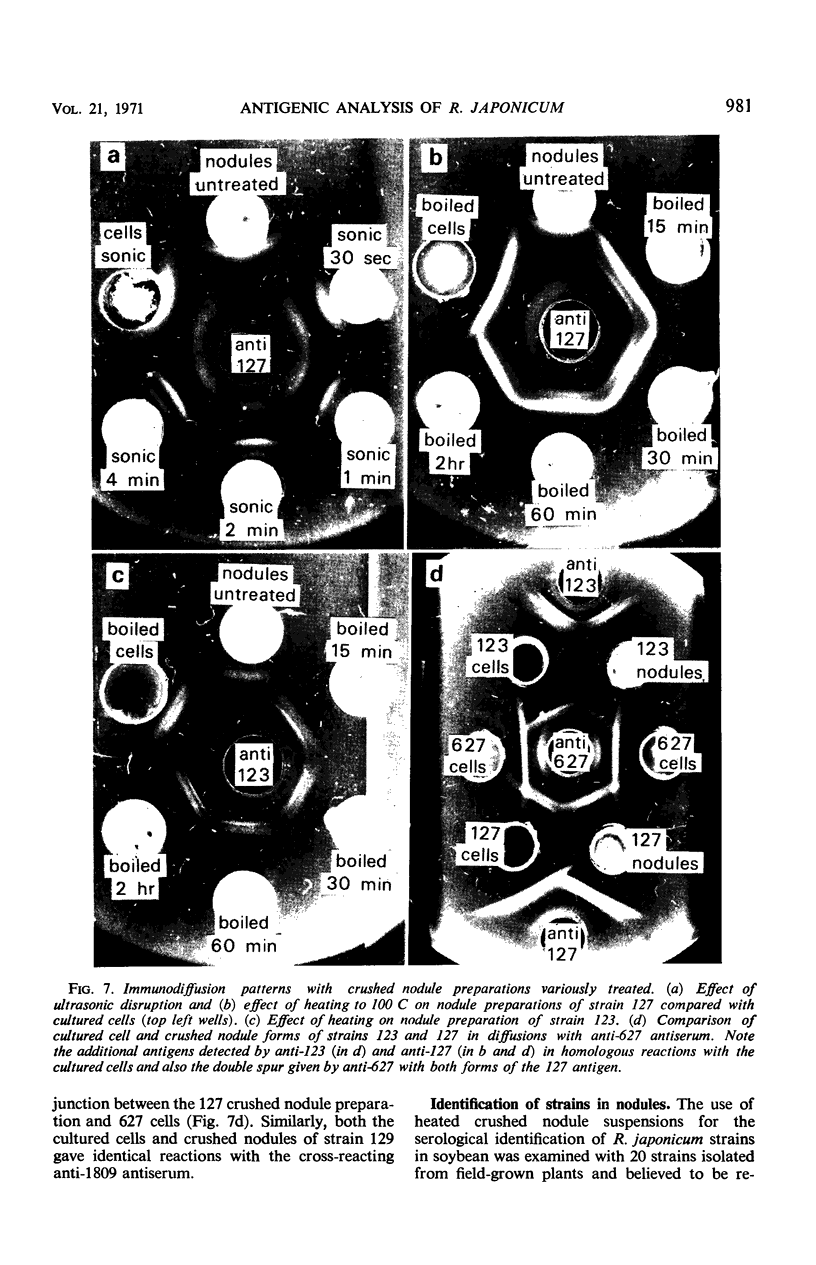
Abstract
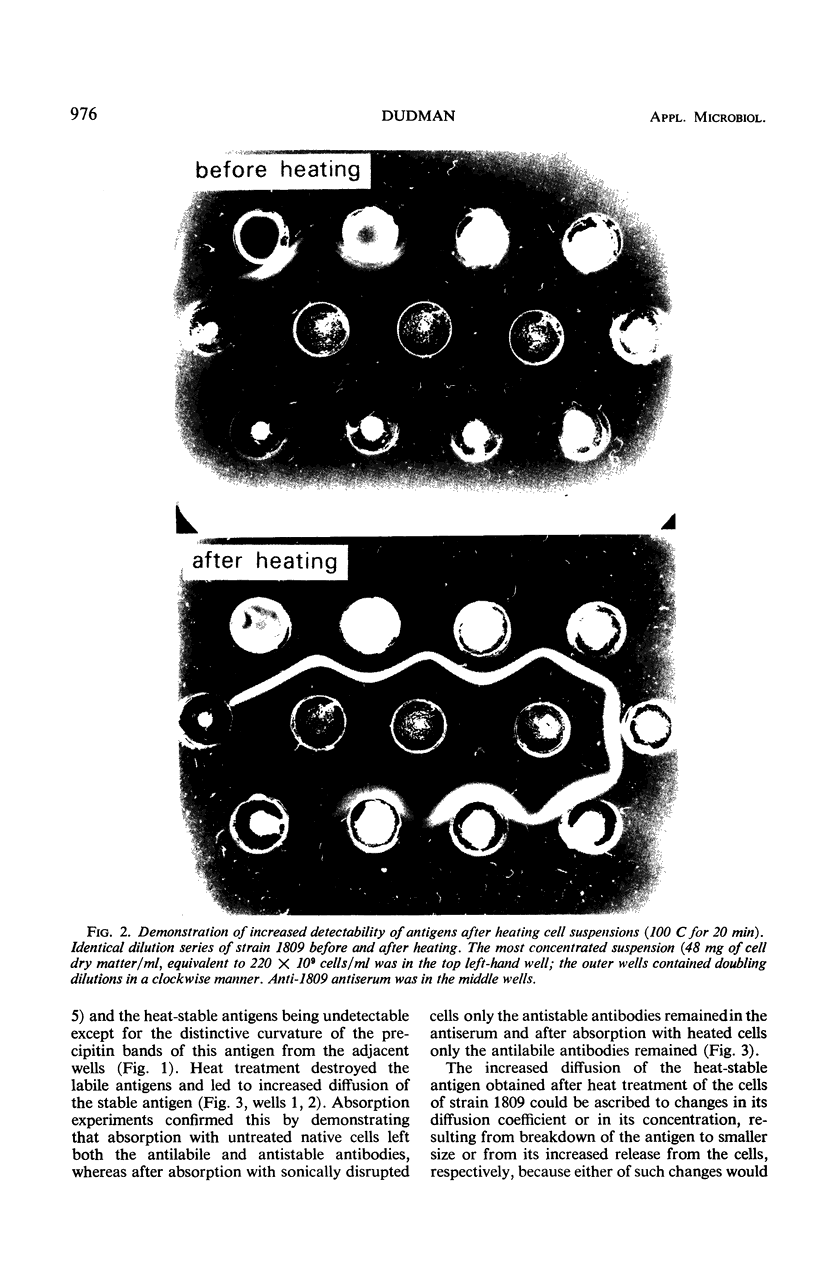
Immunodiffusion reactions were studied with seven strains of Rhizobium japonicum and three strains of the cowpea miscellany by using antisera against eight of the strains. Most strains yielded only weak precipitin bands when untreated cell suspensions were used as antigens in the diffusions. Ultrasonic disruption or heat treatment of the cells led to stronger bands, and immersion in boiling water for 20 min was used as the standard procedure for preparing these bacteria for immunodiffusion analysis. Heat-labile antigens were detected in only a few strains; the major antigens of all of the strains appeared to be heat-stable. Many of the strains cross-reacted, sometimes in a nonreciprocal manner; unheated cell suspensions cross-reacted more widely but more weakly than the heated suspensions. Heat-treated crushed nodule preparations reacted well in immunodiffusions. The antigens of cultured cell and nodule extract (bacteroid) forms of three strains were compared. In one of these strains, an antigen present in the cultured cells was absent from the bacteroids. Unknown strains present in soybean root nodules were readily identified by immunodiffusion.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., HUMPHREY J. H. A theoretical and experimental analysis of double diffusion precipitin reactions in gels, and its application to characterization of antigens. Immunology. 1960 Jan;3:95–106. [PMC free article] [PubMed] [Google Scholar]
- Bushnell O. A., Sarles W. B. Investigations upon the Antigenic Relationships among the Root-Nodule Bacteria of the Soybean, Cowpea, and Lupine Cross-Inoculation Groups. J Bacteriol. 1939 Oct;38(4):401–410. doi: 10.1128/jb.38.4.401-410.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATE R. A., DECKER A. M. MINIMAL ANTIGENIC CONSTITUTION OF 28 STRAINS OF RHIZOBIUM JAPONICUM. Can J Microbiol. 1965 Feb;11:1–8. doi: 10.1139/m65-001. [DOI] [PubMed] [Google Scholar]
- DUDMAN W. F. IMMUNE DIFFUSION ANALYSIS OF THE EXTRACELLULAR SOLUBLE ANTIGENS OF TWO STRAINS OF RHIZOBIUM MELILOTI. J Bacteriol. 1964 Sep;88:782–794. doi: 10.1128/jb.88.3.782-794.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman W. F. Capsultation in Rhizobium species. J Bacteriol. 1968 Mar;95(3):1200–1201. doi: 10.1128/jb.95.3.1200-1201.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM P. H. ANTIGENIC AFFINITIES OF THE ROOT-NODULE BACTERIA OF LEGUMES. Antonie Van Leeuwenhoek. 1963;29:281–291. doi: 10.1007/BF02046070. [DOI] [PubMed] [Google Scholar]
- Gibbins L. N. The preparation of antigens of Rhizobium meliloti by ultrasonic disruption: an anomaly. Can J Microbiol. 1967 Oct;13(10):1375–1378. doi: 10.1139/m67-184. [DOI] [PubMed] [Google Scholar]
- Holland A. A. Serologic characteristics of certain root-nodule bacteria of legumes. Antonie Van Leeuwenhoek. 1966;32(4):410–418. doi: 10.1007/BF02097492. [DOI] [PubMed] [Google Scholar]
- Humphrey B. A., Vincent J. M. The effect of calcium nutrition on the production of diffusible antigens by Rhizobium trifolii. J Gen Microbiol. 1965 Oct;41(1):109–118. doi: 10.1099/00221287-41-1-109. [DOI] [PubMed] [Google Scholar]
- MEANS U. M., JOHNSON H. W., DATE R. A. QUICK SEROLOGICAL METHOD OF CLASSIFYING STRAINS OF RHIZOBIUM JAPONICUM IN NODULES. J Bacteriol. 1964 Mar;87:547–553. doi: 10.1128/jb.87.3.547-553.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means U. M., Johnson H. W. Thermostability of antigens associated with serotype of Rhizobium japonicum. Appl Microbiol. 1968 Feb;16(2):203–206. doi: 10.1128/am.16.2.203-206.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrdleta V. Application of immunoprecipitation in agar gel for the serological typing of soybean root-nodules. Folia Microbiol (Praha) 1969;14(1):32–35. doi: 10.1007/BF02869395. [DOI] [PubMed] [Google Scholar]
- Skrdleta V. Determination of the soybean root-nodule origin by the Ouchterlony method. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1969;123(2):111–115. [PubMed] [Google Scholar]
- Skrdleta V., Karimová J. Competition between two somatic serotypes of Rhizobium japonicum used as double-strain inocula in varying proportions. Arch Mikrobiol. 1969;66(1):25–28. doi: 10.1007/BF00414659. [DOI] [PubMed] [Google Scholar]
- Skrdleta V. Serological analysis of eleven strains of Rhizobium japonicum. Antonie Van Leeuwenhoek. 1969;35(1):77–83. doi: 10.1007/BF02219118. [DOI] [PubMed] [Google Scholar]
- Young J. D., Leung C. Y. Immunochemical studies on lysozyme and carboxymethylated lysozyme. Biochemistry. 1970 Jul 7;9(14):2755–2762. doi: 10.1021/bi00816a001. [DOI] [PubMed] [Google Scholar]