Abstract
Nrf2:INrf2 (Keap1) are cellular sensors of oxidative and electrophilic stress. Nrf2 is a nuclear factor that controls the expression and coordinated induction of a battery of genes which encode detoxifying enzymes, drug transporters (MRPs), anti-apoptotic proteins and proteasomes. In the basal state, Nrf2 is constantly degraded in the cytoplasm by its inhibitor, INrf2. INrf2 functions as an adapter for Cul3/Rbx1 E3 ubiquitin ligase mediated degradation of Nrf2. Chemicals including antioxidants, tocopherols including α-tocopherol (vitamin E), phytochemicals and radiations antagonize the Nrf2:INrf2 interaction and leads to the stabilization and activation of Nrf2. The signaling events involve pre-induction, induction and post-induction responses that tightly control Nrf2 activation and repression back to the basal state. Oxidative/electrophilic signals activate unknown tyrosine kinase(s) in a pre-induction response which phosphorylates specific residues on Nrf2 negative-regulators, INrf2, Fyn and Bach1, leading to their nuclear export, ubiquitination and degradation. This prepares nuclei for unhindered import of Nrf2. Oxidative/electrophilic modification of INrf2cysteine151 followed by PKC phosphorylation of Nrf2serine40 in the induction response results in the escape or release of Nrf2 from INrf2. Nrf2 is thus stabilized and translocates to the nucleus resulting in a coordinated activation of gene expression. This is followed by a post-induction response that controls the ‘switching off’ of Nrf2-activated gene expression. GSK3β under the control of AKT and PI3K, phosphorylates Fyn leading to Fyn nuclear localization. Fyn phosphorylates Nrf2Y568 resulting in nuclear export and degradation of Nrf2. The activation and repression of Nrf2 provides protection against oxidative/electrophilic stress and associated diseases, including cancer. However, deregulation of INrf2 and Nrf2 due to mutations may lead to nuclear accumulation of Nrf2 that reduces apoptosis and promotes oncogenesis and drug resistance.
Keywords: Nrf2, INrf2(Keap1), Antioxidants, Vitamins, Phytochemicals, ROS, Signaling, Regulation, Chemoprotection, Oncogenesis
Oxidative Stress
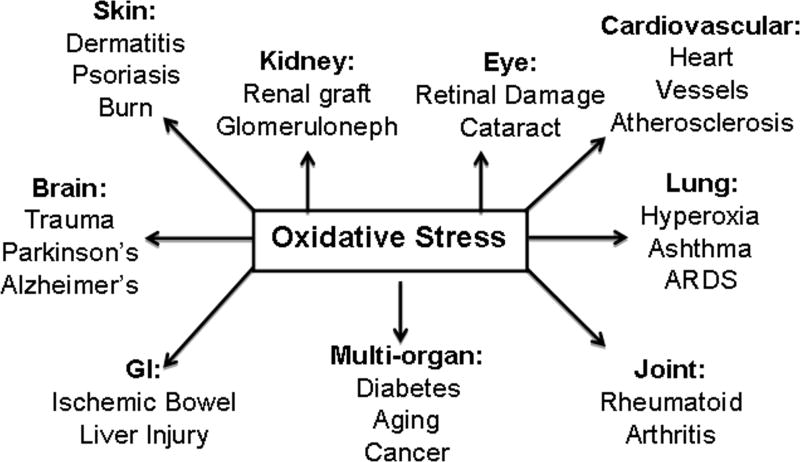
Cells are constantly challenged by environmental (xenobiotics, drugs and UV) and endogenous (reactive oxygen species, hydroperoxides and quinone) stressors [1–2]. If unchecked, these lead to oxidative stress and diseases of many organs (Fig. 1). Oxidative stress related diseases include skin (dermatitis, psoriasis and burn); kidney (renal graft and glomeruloneph); eye (Retinal damage and cataract); cardiovascular (heart and vessels diseases including atherosclerosis); lung [(hyperoxia, ashthma and acute respiratory distress syndrome (ARDS)]; Joints (rheumatoid arthritis); liver (injury and Ischemic bowel); Brain (Trauma, Parkinson’s and Alezheimer’s) and multi-organ diseases including diabetes, aging and cancer [Fig. 1, Ref. 1–2].
Fig. 1.
Oxidative stress related diseases.
Chemical Protection against Oxidative Stress
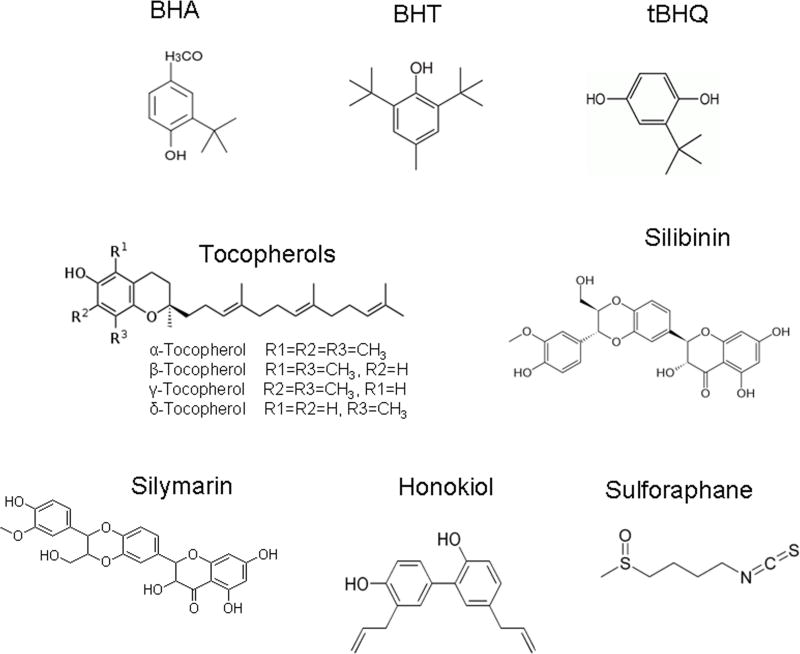
Phenolic antioxidants, vitamins and naturally occurring phytochemicals are known to reduce oxidative stress leading to protection of cells against its adverse effects [3–6]. Many of these compounds are well known drugs in prevention and cure of cancer. The chemical structures of a few representative phenolic antioxidants, vitamins and phytochemicals are shown (Fig. 2). The phenolic antioxidants BHA (tert-butyl-4-hydroxyanisole) and BHT [3,5-di-tert-butyl-4-hydroxytoluene] inhibit chemical carcinogenesis [7–8]. Vitamins are essential for the normal growth and development of organisms. Vitamin E among the various vitamins demonstrated antioxidant properties. Vitamin E is a group of tocopherols and tocotrienols. However, only α-tocopherol functions as the vitamin E in vivo. Each Tocopherol contains a chromanol ring system and a 16 carbons phytyl chain (Fig. 2). Depending on the position and number of methyl groups they exist as α, β, γ, δ-tocopherols (Fig. 2). All tocopherols are antioxidants. However, γ- and δ-tocopherols are stronger antioxidants than others because of unmethylated carbon 5 [9]. Epidemiological studies have shown cancer preventive activity of vitamin E (α-tocopherol) [4]. However, its role in cancer prevention is controversial. More recent studies suggest that γ- and δ-tocopherols are cancer preventive, whereas α-tocopherol is not [4]. Phytochemicals includes a large number of wide varieties of compounds produced from plants [10]. A few of phytochemicals are sulforaphane, silibinin, honokiol, (−)-epigallocatechin gallate (EGCG), and quercetin. Sulforaphane, a product from broccoli sprouts retarded prostate tumor growth in TRAMP mouse and suppressed the growth of prostate cancer PC-3 cells in nude mice [11–12]. Silymarin and its major constituent, silibinin, are extracts from the medicinal plant Silybum marianum (milk thistle) and have traditionally been used for the treatment of liver diseases [13]. Recently, these orally active, flavonoid agents have also been shown to exert significant anti-neoplastic effects in a variety of in vitro and in vivo cancer models, including skin, breast, lung, colon, bladder, prostate and kidney carcinomas [13]. Honokiol is a product of Mangnolia officinalis that restarted growth of PC-3 xenografts in nude mice [14]. EGCG reduced tumor size and completely abrogated tumors in both androgen repressed prostate cancer LNCaP and androgen-refractory the PC3 tumor xenograft in athymic nude mice [15]. Quercetin from vegetables and fruits suppressed development of preneoplastic lesions and proliferation of azoxymethane induced aberrant crypt foci [16]. Therefore, phenolic antioxidants, vitamins and naturally occurring phytochemicals have one thing in common that they prevent oxidative stress and other diseases.
Fig. 2.
Structures of a few representative antioxidants, tocopherols including α-tocopherol (vitamin E) and phytochemicals.
Cellular Protection against Oxidative Stress: Nrf2:INrf2 or Keap1 system
Cytoprotective proteins
Cells have evolved adaptive mechanisms to endure oxidative stress. These include a battery of cytoprotective/defensive proteins that protect cells against oxidative stress and promote cell survival. Included among the cytoprotective proteins are phase II defenses, such as those involved in biotransformation of xenobiotics and drugs (e.g. NAD(P)H:quinone oxidoreductase 1 (NQO1), NRH:quinone oxidoreductase 2 (NQO2), glutathione S-transferase (GST), and γ-glutamate-cysteine synthetase [GCS], and molecules such as reduced glutathione [GSH], and metallothioneins [17–18]. NQO1 and NQO2 catalyze detoxification of quinones, which prevent the generation of reactive semiquinones, O2- and H2O2 [19–21]. NQO2 is identified as melatonin binding site MT3 with functions in CNS [22–23]. NQO1−/− and NQO2−/− mice demonstrated myelogenous hyperplasia [24–28] and γ-radiation-induced myeloproliferative disease/B-cell lymphoma [27–28] and exhibited significantly increased sensitivity to chemical-induced skin carcinogenesis [29–31]. GST conjugates hydrophobic electrophiles, H2O2 and lipid hydroperoxides with glutathione, aiding in their excretion [32–33]. HO-1 catalyzes the rate-limiting step in heme catabolism [34]. γ-GCS play a role in glutathione metabolism [32–33, 35]. Glutathione [GSH], metallothioneins and ferritins scavenge ROS and metal ions. Therefore, GSH and proteins, like metallothioneins and ferritins, act as endogenous factors in protection against oxidative stress and associated diseases including cancer. The battery of cytoprotective proteins also include drug transporters that play important role in drugs intake and efflux [36–40]; anti-apoptotic proteins Bcl-2 and Bcl-xL that prevent apoptotic cell death and promote cell survival [41–43]; and proteasomes that remove oxidized/damaged proteins [44].
NQO1, NQO2 and other cytoprotective genes are ubiquitously expressed and induced in response to xenobiotics, antioxidants, oxidants, heavy metals and UV light [17, 43–48]. Interestingly, the induction of these genes is part of an oxidative/electrophilic stress induced defense mechanism that includes the coordinated induction of two hundred plus genes [17, 43–48]. Both constitutive and inducible expression of defense genes is regulated by antioxidant response element (ARE/Core sequence (TGA****GC) that is present in the upstream regions of such genes [49–51].
NF-E2 Related Factors and INrf2 (Keap1)
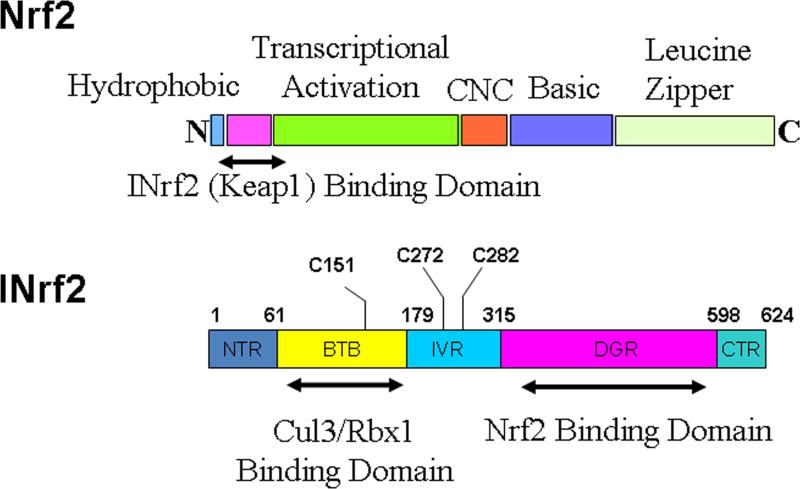
Coordinated induction of cytoprotective gene transcription through the ARE is essential for cellular protection against oxidative stress and related disorders [17, 45–48]. Induction is controlled by cap ‘n’ collar (CNC) family of factors that comprises four members namely Nrf1, Nrf2, Nrf3 and, p45 NF-E2 [17, 43–48, 52–54]. Nrf1 and Nrf2 are ubiquitously expressed, whereas the expression of Nrf3 is restricted to placenta and liver and NF-E2 restricted to erythrocytes [55, 54]. Knockout studies reveal that Nrf1 and Nrf2 have distinct phenotypes and different roles [56–57]. The Nrf1 gene is essential for embryonic development and liver-specific Nrf1 knockout mice develop non-alcoholic steatohepatitis [58–59]. In contrast, Nrf2 knockout mice are viable and exhibit no obvious phenotypic defects, but are nevertheless sensitive to oxidative stress [60–63]. Nrf2 reportedly is the main mediator of cellular adaptation to redox stress [17, 45–48]. Nrf2 is a leucine zipper/CNC protein which when present in the nucleus functions as transcription factor that regulates coordinated activation of a battery of cytoprotective genes that include biotransformation enzymes, antioxidant proteins, drug transporters, anti-apoptotic proteins and proteasomes [17, 45–48]. The various domains of Nrf2 are depicted in Fig. 3. It contains an N-terminal hydrophobic domain, followed by INrf2 (Keap1) binding domain, transcriptional activation domain, CNC domain, basic and leucine zipper domain. Nrf2 through its leucine zipper domain heterodimerizes with small Maf or Jun proteins and bind to ARE [17, 45–48]. INrf2 (Inhibitor of Nrf2) or KEAP1 (Kelch-like ECH-associated protein1), a homodimeric protein, retains Nrf2 in the cytoplasm [64–66]. INrf2 functions as an adapter for Cul3/Rbx1 mediated degradation of Nrf2 [67–71]. INrf2 with its N-terminal BTB domain binds to Rbx1 bound Cul3 and with C-terminal DGR domain binds to Nrf2. This leads to ubiquitination and degradation of Nrf2.
Fig. 3.
Protein domain structures of Nrf2 and INrf2 (Keap1).
Antioxidant induction of Nrf2/ARE-mediated cytoprotective gene expression as mechanism of antioxidant protection
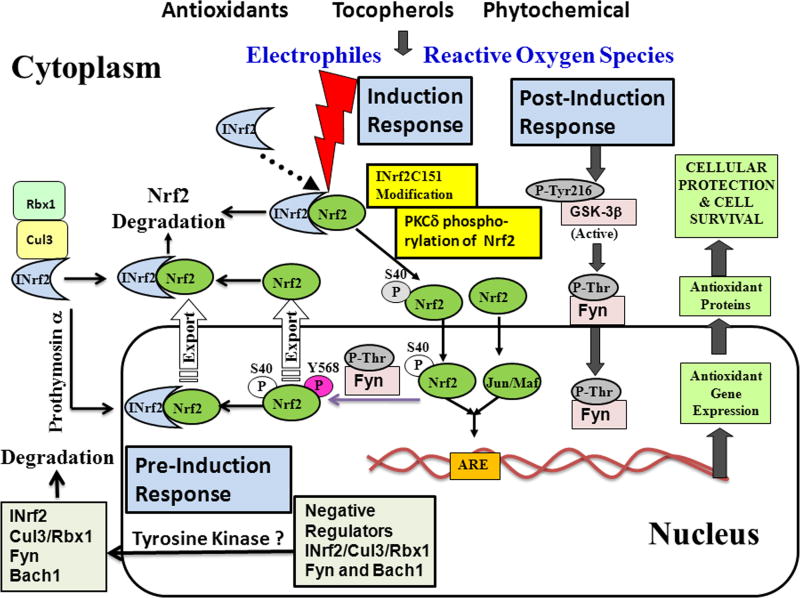
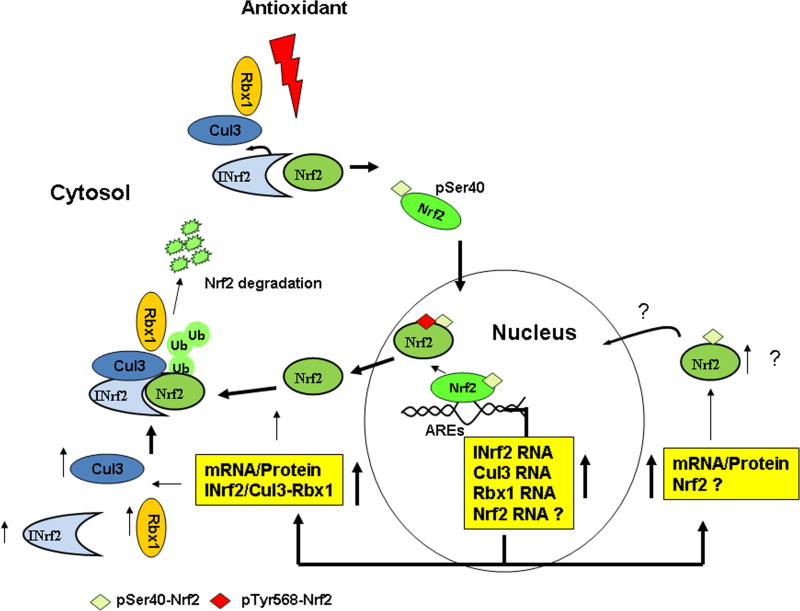
Nrf2:INrf2 complex serves as a sensor of chemical and radiation-induced oxidative stress in the cells. Antioxidants are strong activators of Nrf2 since these after metabolism produce small amount of oxidative stress that signals Nrf2 activation. Antioxidants have been frequently used to study the mechanism of signal transduction from antioxidant to the coordinated induction of cytoprotective gene expression that is essential for cytoprotection and cell survival. The mechanism of signal transduction from antioxidant to Nrf2 is complex and involves basal, pre-induction, induction and post-induction phases (Fig. 4).
Fig. 4.
Mechanism of signal transduction from antioxidants, tocopherols including α-topherol (vitamin E) and phytochemicals to Nrf2.
Under physiological/basal conditions, INrf2/Cul3-RBX1 complex is present in the cytosol constantly degrading Nrf2 [67–70; Fig. 4]. While the INrf2-mediated ubiquitination and degradation occurs primarily in the cytosol, INrf2/Cul3-RBX1 complex is also present in the nucleus and degrades Nrf2 under basal conditions [72]. Along with INrf2/Cul3-RBX1 complex additional negative regulators of Nrf2 are present in the nucleus. These include Src subfamily A members Fyn, Src, Yes and Fgr that phosphorylate Nrf2Tyr568 leading to nuclear export and degradation of Nrf2 [73] and Bach1 that competes with Nrf2 for binding to ARE resulting in suppression of ARE-mediated gene expression [74]. It is noteworthy that active GSK3β phosphorylates Src A sub-family members including Fyn that enter in the nucleus leading to phosphorylation of Nrf2Tyr568, nuclear export and degradation of Nrf2 [75–76, 73]. More recently, β-TrCP present in the cytosol is also shown to degrade GSK3β phosphorylated Nrf2 [77]. Therefore, two independent mechanisms degrade Nrf2 under basal conditions.
Cellular exposure to antioxidant leads to pre-induction response in which negative regulators of Nrf2 are exported out of nucleus (Fig. 4). It is reported that unknown tyrosine kinase(s) phosphorylate INrf2Tyr85, FynTyr213 and Bach1 Tyr486 within 0.5–1 hour of antioxidant exposure leading to nuclear export, ubiquitination and degradation of INrf2, Fyn and Bach1 [78–80]. The Nrf2 ubiquitin factors Cul3-Rbx1 are also exported out of nucleus through its interaction with INrf2 [78]. It is suggested that nuclear export and degradation of Nrf2 negative regulators allows for unhindered nuclear import of Nrf2 and efficient induction of cytoprotective gene expression [78–80].
The induction phase presumably runs in parallel to pre-induction phase upon exposure to antioxidant. As the Nrf2 negative regulators are going out of nucleus, Nrf2 is imported in the nucleus to activate ARE-mediated cytoprotective gene expression. Antioxidant modification of INrf2Cys151 followed by PKCδ phosphorylation of Nrf2Ser40 results in the escape or release of Nrf2 from INrf2 [70–71]. Nrf2 is stabilized, translocate to the nucleus, heterodimerize with small Maf or c-Jun protein, and binds with ARE that leads to coordinated activation of cytoprotective gene expression [17, 81].
Induction phase is followed by post-induction phase that switches ‘OFF’ Nrf2 activation. Activation of GSK3β phosphorylates Fyn leading to nuclear localization of Fyn [76]. Fyn phosphorylates Nrf2Tyr568 resulting in nuclear export of Nrf2, binding with INrf2, and degradation of Nrf2 [75]. By this time the negative regulators of Nrf2 including INrf2/Cul3-RBX1, Fyn and Bach1 are de novo synthesized and imported in the nucleus. INrf2 through ubiquitination and degradation of Nrf2; Fyn through phosphorylation of Nrf2Tyr568 followed by ubiquitination and degradation of Nrf2 and Bach1 through competition with Nrf2 for binding to ARE leads to switching ‘OFF’ of Nrf2 and suppression of cytoprotective gene expression to basal level. Bach1 is known to form heterodimers with small Maf proteins and Bach1:Maf dimers compete with Nrf2 for binding to ARE [74].
Autoregulatory loop that controls cellular abundance of Nrf2 and INrf2/Cul3-Rbx1
Recently, we have shown that a feedback auto-regulatory loop between Nrf2 and INrf2 exist that controls cellular abundance of Nrf2 and INrf2 [Ref. 82; Fig. 5]. Nrf2 regulated transcription of INrf2 and INrf2 degraded Nrf2. More recently, we found that another autoregulatory loop exists between Nrf2 and Cul3-Rbx1 [83]. Nrf2 controls transcription of Cul3 and Rbx1 and Cul3-Rbx1 complex ubiquitinate and degrade Nrf2. In other words, there exist cellular homeostasis between Nrf2 and INrf2/Cul3-RBX1 complex. The autoregulatory loop between Nrf2 and INrf2/Cul3-Rbx1 is deregulated in cells carrying mutations in one of the component of the autoregulatory loop. Many cancer cells have loss of function mutations in INrf2 resulting in nuclear accumulation of Nrf2 and persistent activation of cytoprotective proteins [84–85]. Similarly, mutations are also known in Nrf2 that leads to abrogation of the interaction between Nrf2 and INrf2 and nuclear accumulation of Nrf2 [86].
Fig. 5.
Autoregulatory loop between Nrf2 and INrf2/Cul3-Rbx1.
Proteins that regulate Nrf2 and INrf2
Protein-protein and protein-DNA interactions are known that affect Nrf2 and/or INrf2 stabilization and/or expression with implications in cytoprotective gene expression/induction. Nrf2 interaction with INrf2 and PKCδ are described above. The role of Cul3-Rbx1, Bach1, Src subfamily members, GSK3β and β-TrCP all are also described above. In addition, p21 through its KRR motif is known to directly interact with the DLG and ETGE motifs in Nrf2 resulting in Nrf2 stabilization and accumulation [87]. Autophagy related p62 protein sequesters INrf2 into aggregates resulting in stabilization and activation of Nrf2 [88–90]. PALB2 through its ETGE motif interacts directly with Kelch domain of INrf2 that leads to stabilization of Nrf2 [91]. K-Ras, B-Raf and Myc all increase Nrf2 transcription and increase basal Nrf2 expression [92]. Casein kinase II phosphorylates INrf2Thre55 leading to INrf2 interaction with HSP90 and stabilization of INrf2 [93]. Prothymosin-α interaction with INrf2 is required for nuclear import of INrf2 [72].
Role of Nrf2:INrf2 system in tocopherols including α-tocopherol (vitamin E) and Phytochemicals protection of cells against adverse effects of oxidative stress
Epidemiological studies suggested a role of tocopherols in cancer prevention [94]. However, the chemoprevention studies with α-tocopherols were disappointing [95–96]. Recently, several reports have suggested a role of γ and not α-tocopherol in cancer prevention [4]. Dietary tocopherols especially γ-tocopherol inhibits cell proliferation and decrease serum inflammatory markers during development of mammary hyperplasia [97]. In addition, γ-tocopherol enriched diet was reported to inhibit prostate carcinogenesis in TRAMP mice [98]. The role of Nrf2 in tocopherols-mediated protection is also controversial. Nrf2 is shown to mediate tocopherol including α-tocopherol (vitamin E) protection against acrolein-induced oxidative stress and mitochondrial dysfunction [99]; allergens-induced alveolar macrophages in vivo [100]; development of mammary hyperplasia [97; and prostate tumors in TRAMP mice [101]. On contrary, it is also reported that the antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice [102]. Further studies are required to determine the relative role of Nrf2 in different tocopherols-mediated protection against oxidative stress and associated diseases.
Phytochemicals are promising chemopreventive agents that can prevent macromolecular damages including mutations and block carcinogenesis [6]. Several different mechanisms involving direct reaction with carcinogens and/or modulation of phase I enzymes and/or alterations in phase II enzymes contribute to the mode of action of phytochemicals. Phytochemicals are also known to protect against neurodegenerative, cardiovascular and renal diseases [6]. We will discuss here phytochemicals activation of phase II enzymes as mechanism of its activity as cytoprotective agent. Sulforaphane is a potent Nrf2 dependent inducer of phase II gene expression that leads to protection against oxidative stress and associated adverse effects [103]. Sulforaphane stabilizes Nrf2 through the modification of INrf2 (Keap1) and release of Nrf2 as described above [104]. Curcumin and (−)-epigallo-catechin-3-gallate are also known to activate Nrf2 for its antioxidant function [105–106]. Similarly, the flavanol (−)-epicatechin prevents stroke damage through activation of Nrf2/HO-1 pathway [107]. In addition, food polyphenols activated Nrf2 protect against neurodegenerative disorders [108]. In summary, many phytochemicals activate Nrf2 to coordinately increase phase II detoxifying enzymes and other cytoprotective proteins that play significant role not only in chemoprotection but also prevention of neurodegenerative, cardiovascular and renal diseases.
Nrf2 in cancer prevention, oncogenesis and drug resistance
The precise role of Nrf2 in cancer prevention and cancer remains unknown. It is considered to be both tumor suppressor, as well as tumor promoter [81]. The property of Nrf2 to coordinately activate cytoprotective proteins including detoxifying enzymes, drug transporters, antioxidants and anti-inflammatory proteins as described above plays significant role in reducing electrophiles and ROS, decrease genomic instability and mutations that leads to chemoprotection and tumor suppression. Tumor suppressor function of Nrf2 is also supported by in vivo studies using Nrf2-null mice. Nrf2−/− mice are prone to acute damages induced by acetaminophen, ovalbumin, pentachlorophenol and 4-vinylcyclohexene diepoxide [109–113]. Nrf2−/− mice showed increased pulmonary DNA adducts and bladder tumors when exposed to diesel exhaust and N-nitrosobutyl (4-hydroxybutyl) amine, respectively [114–116]. Moreover, loss of Nrf2 in lung cancer cells has been associated with providing a microenvironment that favors metastasis [117]. Nrf2 and downstream protein level declines with age that could also contribute to tumorigenesis [118]. Another line of evidence supporting Nrf2 as tumor suppressor is that one of the important property of chemopreventive compounds, such as curcumin, sulforaphane, isothiocynates, green and black tea, and others, is their ability to activate Nrf2 [45, 116]. Tumor suppressor function of Nrf2 is further supported by observations that tumor suppressors target Nrf2 stabilization and oncoproteins degrade Nrf2. Tumor suppressors p21 and BRCA2 (PALB2) are known to stabilize Nrf2 by preventing INrf2 interaction with Nrf2 [87, 91]. In addition, oncoproteins Src subfamily A members Fyn and Src and SCFβ-TrCP degrade Nrf2 [73, 77]. It is noteworthy that SCFβ-TrCP degradation of Nrf2 is independent of INrf2 (Keap1). Collectively, the above observations suggest that Nrf2 is chemoprotector and a tumor suppressor.
On contrary to above, Nrf2 is also considered as tumor promoter that promotes oncogenesis. Several reports support a role of Nrf2 in oncogenesis. First, a report showed that oncogenes K-Ras, B-Raf and Myc targeted the transcription and amplification of Nrf2 in cancer cells [92]. Overexpression of these oncogenes in mice led to increased Nrf2 transcription, increased basal expression of Nrf2, and decreased ROS leading to oncogenesis. In addition, genetic knock down of Nrf2 led to decreased ability of K-Ras to induce oncogenesis. These results supported a role of Nrf2 in oncogenesis due to escaping of cancer cell death because of lower ROS. Second, recently a paper showed that higher Nrf2 in tumor cells up-regulates many of the enzymes of glucose metabolism towards glycolysis that redirects cells in anabolic mode, which promotes nucleotide synthesis and cell proliferation [119]. This also supported a role of Nrf2 in oncogenesis. Third, more recently, we have shown that Nrf2 up-regulates transcription of anti-apoptotic genes encoding Bcl-2 and Bcl-xL [42, 120]. This leads to increased anti-apoptotic proteins Bcl-2 and Bcl-xL that reduces apoptosis. Therefore, Nrf2-mediated decreased apoptosis contributes to increased cancer cell survival and oncogenesis. Fourth, many cancers show up-regulation of Nrf2 because of mutations in INrf2 and Nrf2 that abolishes INrf2 and Nrf2 interaction and degradation of Nrf2 [84–86]. Mutations in INrf2 resulting in loss of expression and function and hypermethylation of INrf2 leading to decreased INrf2 expression are known in many cancers including that of lung, breast and prostate cancer [84–85]. The loss of INrf2 because of mutations leads to stabilization and nuclear accumulation of Nrf2 and increased expression of cytoprotective proteins and cell survival. Mutations are also known in the region of Nrf2 that interacts with INrf2 leading to stabilization of Nrf2, nuclear accumulation of Nrf2, activation of cytoprotective proteins, reduced apoptosis and increased cell survival [86]. The above evidences suggest that Nrf2 is an oncoprotein that reduces apoptosis and promotes cell survival and oncogenesis.
The persistent activation of Nrf2 due to mutations or deregulation of factors controlling Nrf2 also leads to drug resistance [121–123]. Nuclear accumulation of Nrf2 leads to higher levels of cytoprotective proteins including detoxifying/biotransformation enzymes, drug transporters, antioxidants and anti-apoptotic proteins. This leads to decreased apoptosis, increased cell survival and drug resistance.
The dual function of Nrf2 under different circumstances suggests that Nrf2 is a protooncogene [81]. Nrf2 when expressed at normal level and is properly regulated by positive and negative factors is essential for cellular protection against chemical and radiation stressors, normal growth and survival of cells. However, deregulation of Nrf2 either due to gain of function mutations or altered because of regulatory factors becomes oncogenic.
Future Perspectives
Future investigations are required to understand a complete mechanism of signal transduction from antioxidant, tocopherols including α-tocopherol (vitamin E) and phytochemicals to Nrf2 leading to coordinated activation of Nrf2 downstream gene expression. In addition, in vivo role of Nrf2 and INrf2 in apoptosis and protection against chemical and radiation induced neurodegeneration, cardiovascular diseases and cancer also requires further investigation. INrf2 is mutated in many cancers including that of lung, breast and prostate. However, the precise role of Nrf2 and/or INrf2 in oncogenesis remains obscure and warrants further studies. Similarly, the role of Nrf2 in cancer metastasis is another area that requires investigation. Development of natural activators of Nrf2 as effective agents of chemoprotection is also warranted. Similarly, development of inhibitors of Nrf2 is expected to help reduce drug resistance and improve therapy.
Highlights.
Nrf2:INrf2 system protects cells against chemical and radiation stress.
Antioxidants, tocopherols and phytochemicals are activators of Nrf2.
Positive and negative factors regulate activation and suppression of Nrf2.
Nrf2 if properly regulated protects against inflammation and cancer.
Nuclear accumulation of Nrf2 promotes oncogenesis and drug resistance.
Nrf2 may be a proto-oncogene?
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grants RO1GM047466 and RO1ES012265.
Abbreviations
- Nrf2
NF-E2 Related Factor 2
- INrf2
Inhibitor of Nrf2 also known as Keap1
- ARE
Antioxidant Response Element
- ROS
Reactive Oxygen Species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klauning JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Path. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 2.Tudek B, Winczura A, Janik J, Siomek A, Foksinski M, Olinski R. Involvement of oxidatively damaged DNA and repair in cancer development and aging. Am J Transl Res. 2010;2:254–284. [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Rad Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 4.Yang CS, Suh N, Kong AN. Does vitamin E prevent or promote cancer. Cancer Prev Res. 2012;5:701–705. doi: 10.1158/1940-6207.CAPR-12-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair S, Li W, Kong A. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sinica. 2007;28:459–472. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 6.Shu L, Cheung K, Khor TO, Chen C, Kong A. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 7.Frankfurt OS, Lipchina LP, Bunto TV, Emanuel NM. Effect of 4-methyl-2,6-di-tert-butylphenol (Ionol) on induction of liver tumors in rats. Biull Eksp Biol Med USSR. 1967;64:86–88. [PubMed] [Google Scholar]
- 8.Wattenberg LW, Jerina DM, Lam LK, Yagi H. Neoplastic effects of oral administration of (+/−)trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene and their inhibition by butylated hydroxyanisole. J Natl Cancer Inst. 1979;62:1103–1106. [PubMed] [Google Scholar]
- 9.Traber MG. Vitamin E. In: Bowman BA, Russell RM, editors. Present knowledge in nutrition. 9. Washington DC: ILSI Press; 2006. pp. 211–219. [Google Scholar]
- 10.Johnson SM, Wang X, Mark Evers B. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J Surg Res. 2011;168:197–205. doi: 10.1016/j.jss.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keum MC, Khor TO, Lin W, Shen G, Kwon KH, Barve A, Li W, Kong AN. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 12.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232:227–234. [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin—a promising new treatment for cancer. Anticancer Agents Med Chem. 2010;10:186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- 14.Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14:1248–1257. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- 15.Liao S, Hiipakka RA. Selective inhibition ofsteroid 5 alpha-reductase isozymes by tea epicatechin-3-gallate and epigallocatechin-3-gallate. Biochem Biophys Res Commun. 1995;214:833–838. doi: 10.1006/bbrc.1995.2362. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S, Yasui Y, Ohigashi H, Tanaka T, Murakami A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem Biol Interact. 2010;183:276–283. doi: 10.1016/j.cbi.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Rad Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 19.Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev. 2004;36:639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 20.Vasiliou V, Ross D, Nebert DW. Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family. Hum Genomics. 2006;2:329–335. doi: 10.1186/1479-7364-2-5-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaikwad A, LongII DJ, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem. 2001;276:22559–22564. doi: 10.1074/jbc.M101053200. [DOI] [PubMed] [Google Scholar]
- 22.Nosjean O, Ferro M, Coge F, Beauverger P, Henlin J, Lefoulon F, Fauchere J, Delagrange P, Canet E, Boutin JA. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 23.Mailliet F, Ferry G, Vella F, Thiam K, Delagrange P, Boutin JA. Organs from mice deleted for NRH:quinone oxidoreductase 2 are deprived of the melatonin binding site MT3. FEBS Lett. 2004;578:116–120. doi: 10.1016/j.febslet.2004.10.083. [DOI] [PubMed] [Google Scholar]
- 24.Radjendirane V, Joseph P, Lee H, Kimura S, Klein-Szanto AJP, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem. 1998;273:7382–7389. doi: 10.1074/jbc.273.13.7382. [DOI] [PubMed] [Google Scholar]
- 25.Long DJ, Iskander K, Gaikwad A, Arin M, Roop DR, Knox R, Barrios R, Jaiswal AK. Disruption of dihydronicotinamide riboside:quinone oxidoreductase 2 (NQO2) leads to myeloid hyperplasia of bone marrow and decreased sensitivity to menadione toxicity. J Biol Chem. 2002;277:46131–46139. doi: 10.1074/jbc.M208675200. [DOI] [PubMed] [Google Scholar]
- 26.LongII DJ, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, Jaiswal AK. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002;62:3030–3036. [PubMed] [Google Scholar]
- 27.Iskander K, Barrios RJ, Jaiswal AK. Disrution of NAD(P)H:quinone oxidoreductase 1 gene in mice leads to radiation-induced myeloproliferative disease. Cancer Res. 2008;68:7915–7922. doi: 10.1158/0008-5472.CAN-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iskander K, Barrios R, Jaiswal AK. NRH:Quinone Oxidoreductase 2 Deficient Mice are Highly Susceptible to Radiation-Induced B-Cell Lymphomas. Clin Cancer Res. 2009;15:1534–1542. doi: 10.1158/1078-0432.CCR-08-1783. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.LongII DJ, Waikel RL, Wang X, Perlaky L, Roop DR, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 deficiency increases susceptibility to benzo(a)pyrene-induced mouse skin carcinogenesis. Cancer Res. 2000;60:5913–5915. [PubMed] [Google Scholar]
- 30.Iskander K, Paquet M, Brayton C, Jaiswal AK. Deficiency of NRH:quinone oxidoreductase 2 increases susceptibility to 7,12-dimethybenz(a)anthracene and benzo(a)pyrene-induced skin carcinogenesis. Cancer Res. 2004;64:5925–5928. doi: 10.1158/0008-5472.CAN-04-0763. [DOI] [PubMed] [Google Scholar]
- 31.Long DJ, Waikel RL, Wang XJ, Roop DR, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 deficiency and increased susceptibility to 7,12-dimethylbenz[a]-anthracene-induced carcinogenesis in mouse skin. J Natl Cancer Inst. 2001;93:1166–1170. doi: 10.1093/jnci/93.15.1166. [DOI] [PubMed] [Google Scholar]
- 32.Pickett CB, Lu AYH. Glutathione S-transferases: gene structure, regulation and biological function. Ann Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar M, Reinhardt D, Schlechtweg J, Machnik G, Klinger W, Schirrmeister W. Glutathione homeostasis in rats chronically treated with ethanol. Evidence for an increased hepatic GSH export in vivo. Exp Toxicol Pathol. 1992;44:344–348. doi: 10.1016/S0940-2993(11)80225-3. [DOI] [PubMed] [Google Scholar]
- 34.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchida S, Sato K. Glutathione transferases and cancer. Crit Rev Biochemist Mole Biol. 1992;27:337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 37.Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos. 2005;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- 38.Slitt AL, Cherrington NJ, Dieter MZ, Aleksunes LM, Scheffer GL, Huang W, Moore DD, Klaassen CD. trans-Stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors. Mol Pharmacol. 2006;69:1554–1563. doi: 10.1124/mol.105.014571. [DOI] [PubMed] [Google Scholar]
- 39.Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, Dalton TP, Yamamoto M, Klaassen CD. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 40.Maher J, Aleksunes L, Dieter M, Tanaka Y, Peters J, Manautou J, Klaassen C. Nrf2 and PPAR{alpha}-Mediated Regulation of Hepatic Mrp Transporters after Exposure to Perfluorooctanoic Acid and Perfluorodecanoic Acid. Toxicol Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niture SK, Jaiswal AK. INrf2 (Keap1) targets Bcl2 degradation and controls cellular apoptosis. Cell Death Differ. 2011;18:439–451. doi: 10.1038/cdd.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niture SK, Jaiswal AK. Inhibitor of Nrf2 (INrf2 or Keap1) protein degrades Bcl-xL via phosphoglycerate mutase 5 and controls cellular apoptosis. J Biol Chem. 2011;286:44542–4456. doi: 10.1074/jbc.M111.275073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 46.Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Path. 2007;35:459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 47.Hayes JD, McMahon M. Nrf2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Kwak MK, Cho JM, Huang B, Shin S, Kensler TW. Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic Biol Med. 2007;43:809–817. doi: 10.1016/j.freeradbiomed.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene: role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 50.Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci USA. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasserman W, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan JY, Cheung MC, Moi P, Chan K, Kan YW. Chromosomal localization of the human NF-E2 family of bZIP transcription factors by fluorescence in situ hybridization. Hum Genet. 1995;95:265–269. doi: 10.1007/BF00225191. [DOI] [PubMed] [Google Scholar]
- 53.Blank V. Small Maf proteins in mammalian gene control: Mere dimerization partners or dynamic transcriptional regulators? J Mol Biol. 2008;376:913–925. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 54.Motohashi H, o’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 55.Sankaranarayanan K, Jaiswal AK. Nrf3 negatively regulates antioxidant-response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. J Biol Chem. 2004;279:50810–50817. doi: 10.1074/jbc.M404984200. [DOI] [PubMed] [Google Scholar]
- 56.Leug L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 57.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TS, Kan YW. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu ZR, Chen LY, Leung L, Yen TSB, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci USA. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan K, Lu R, Chang JC, Kan YT. Nrf2, a member of the NF-E2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 62.Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, Yamamoto M, Kensler TW. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res. 2001;480–481:305–315. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2001;20:3906–3917. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- 65.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niture SK, Jain AK, Jaiswal AK. Antioxidant induced modification of INrf2 cysteine151 and PKCδ-mediated phosphorylation of Nrf2 serine40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J Cell Science. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niture SK, Jaiswal AK. Prothymosin-alpha mediates nuclear import of the INrf2/Cul3 Rbx1 complex to degrade nuclear Nrf2. J Biol Chem. 2009;284:13856–13868. doi: 10.1074/jbc.M808084200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Niture SK, Jain AK, Shelton P, Jaiswal AK. Src subfamily kinases regulate nuclear export and degradation of transcription factor Nrf2 to switch off Nrf2-mediated antioxidant activation of cytoprotective gene expression. J Biol Chem. 2011;286:28821–28832. doi: 10.1074/jbc.M111.255042. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 75.Jain AK, Jaiswal AK. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J Biol Chem. 2006;281:12132–12142. doi: 10.1074/jbc.M511198200. [DOI] [PubMed] [Google Scholar]
- 76.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 77.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaspar JW, Niture SK, Jaiswal AK. Antioxidant-induced INrf2 (Keap1) tyrosine 85 phosphorylation controls the nuclear export and degradation of the INrf2-Cul3-Rbx1 complex to allow normal Nrf2 activation and repression. J Cell Sci. 2012;125:1027–1038. doi: 10.1242/jcs.097295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Kaspar JW, Jaiswal AK. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. The FASEB Journal. 2011;25:1076–1087. doi: 10.1096/fj.10-171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaspar J, Jaiswal AK. Antioxidant induced phosphorylation of tyrosine486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to ARE and activate defensive genes expression. J Biol Chem. 2010;285:153–162. doi: 10.1074/jbc.M109.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shelton P, Jaiswal AK. The transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene? The FASEB J. 2012 doi: 10.1096/fj.12-217257. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007;282:36412–36420. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- 83.Kaspar JW, Jaiswal AK. An autoregulatory loop between Nrf2 and Cul3-Rbx1 controls their cellular abundance. J Biol Chem. 2010;285:21349–21358. doi: 10.1074/jbc.M110.121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional Keap1-Nrf2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3: 1865–1876. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in Nrf2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 89.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A Noncanonical Mechanism of Nrf2 Activation by Autophagy Deficiency: Direct Interaction between Keap1 and p62. Mole and Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. The J of Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma J, Cai H, Wu T, Sobhian B, Huo Y, Alcivar A, Mehta M, Cheung KL, Ganesan S, Kong AN, Zhang DD, Xia B. PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol Cell Biol. 2012;32:1506–1517. doi: 10.1128/MCB.06271-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niture SK, Jaiswal AK. Hsp90 interaction with INrf2(Keap1) mediates stress-induced Nrf2 activation. J Biol Chem. 2010;285:36865–36875. doi: 10.1074/jbc.M110.175802. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS. Cancer preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E and the risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smolarek AK, So J, Thomas PE, Lee HJ, Paul S, Dombrowski A, Wang CX, Saw CL, Khor TO, Kong AN, Reuhl K, Lee MJ, Yang CS, Suh N. Dietary tocopherols inhibit cell proliferation, regulate expression of ERα, PPARg, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol Carcinog. 2012 doi: 10.1002/mc.21886. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, Newark H, Kong AN. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng Z, Liu Z, Li X, Jia H, Sun L, Tian C, Jia L, Liu J. Alpha-tocopherol is an effective phase II enzyme inducer: protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal epithelial cells. Epithelial cells. J Nutr Bioche. 2010;21:1222–1231. doi: 10.1016/j.jnutbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Dworski R, Han W, Blackwell TS, Hoskins A, Freeman ML. Vitamin E prevents Nrf2 suppression by allergens in esthmatic alveolar macrophase in vivo. Free Radic Biol Med. 2011;51:516–521. doi: 10.1016/j.freeradbiomed.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang Y, Khor TO, Shu L, Saw CL, Wu TY, Suh N, Yang CS, Kong AN. A gamma-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J Nutr. 2012;142:818–823. doi: 10.3945/jn.111.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li G, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, Yang CS. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free Rad Biol Med. 2012;52: 1151–1158. doi: 10.1016/j.freeradbiomed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Cheung KL, Khor TO, Kong AN. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharmaceut Res. 2009;26:224–231. doi: 10.1007/s11095-008-9734-9. [DOI] [PubMed] [Google Scholar]
- 104.Hong F, Freeman MI, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 105.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476:171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dors S. The flavonol (−) – epicathechin prevents stress damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modification of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 110.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iizuka T, Ishii Y, Ito K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2 deficient mice are highly susceptible to cigarette smoke-induced emphyseema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 113.Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated ovarian failure induced by 4-Vinyl Cyclohexane diepoxide in Nrf2-null mice. Mol Cell Biol. 2006;26:940–954. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharm. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 115.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme induccers is lost in Nrf2 transcription factor deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 117.Satoh H, Moriguchi T, Taguchi K, Takai J, Maher J, Suzuki T, Winnard PT, Raman V, Ebina M, Nukiwa T, Yamamoto M. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31: 1833–1843. doi: 10.1093/carcin/bgq105. [DOI] [PubMed] [Google Scholar]
- 118.Sykiotis GP, Bohmann D. Stress-activated cap ‘n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 120.Niture S, Jaiswal AK. Nrf2-induced anti-apoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Rad Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.12.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vollrath V, Wielandt AM, Iruretagoyena M, Chianale M. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 123.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the Keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]