Abstract
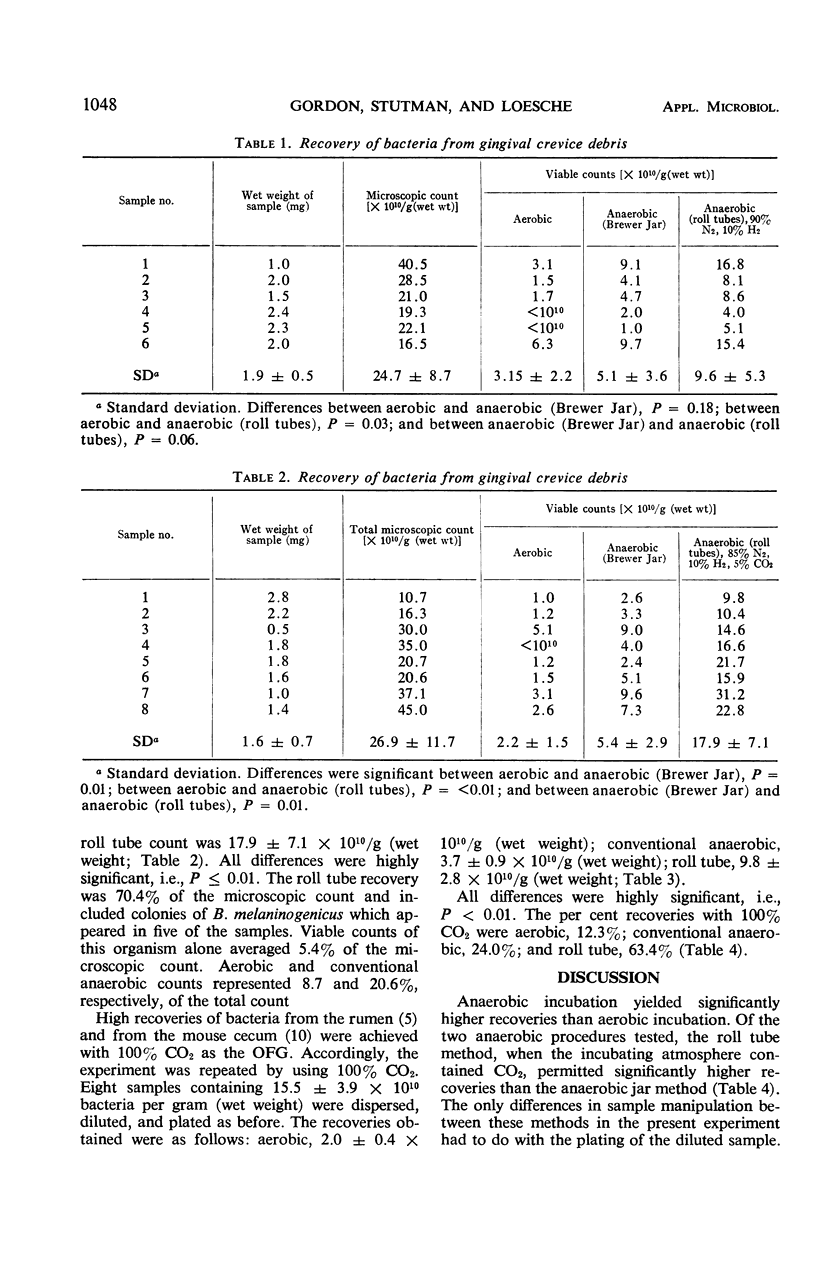
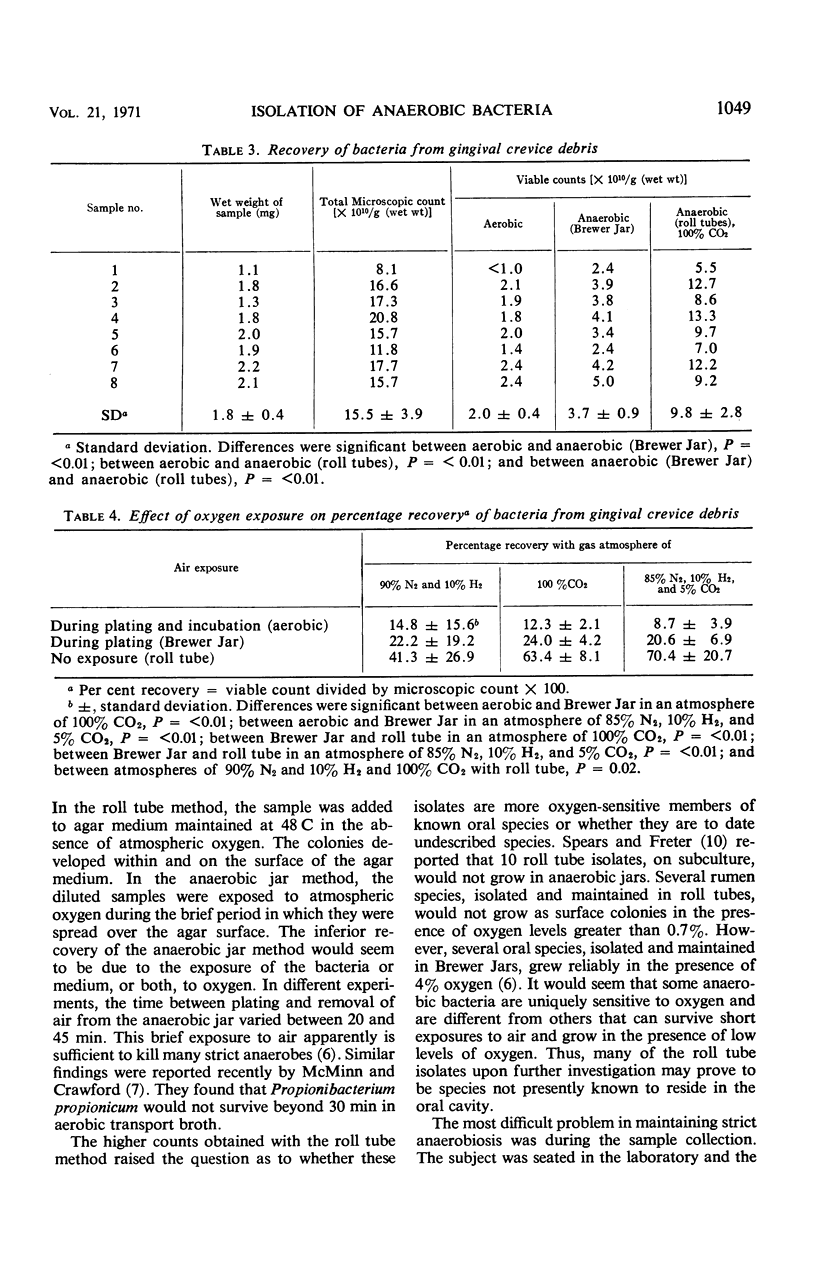
A roll tube technique (Hungate method) was employed in an attempt to cultivate a maximal portion of the organisms in the gingival crevice area of man. This technique achieves an anaerobic state by flushing the local environment with oxygen-free gas. Once collected, the crevicular debris was immediately placed into sterile oxygen-free test tubes which were flushed out by the oxygen-free gas. In this manner, the samples were weighed, dispersed, diluted, and cultured in roll tubes and plates. The medium for control (Brewer Jar technique) and Hungate techniques was Heart Infusion Agar fortified with 10% defibrinated horse blood. When the Hungate technique was used, the recovery of viable bacteria, as a percentage of the direct microscopic count, was significantly greater than plates incubated aerobically or utilizing the Brewer Anaerobic technique. Cultural counts by using the Hungate method averaged 41.3% for six samples when 90% nitrogen and 10% hydrogen were used, 70.4% for eight samples when 85% nitrogen, 10% hydrogen, and 5% carbon dioxide were used, and 63.4% for eight samples when 100% carbon dioxide was the gaseous atmosphere. At no time were cultural counts, by using anaerobic plates (Brewer Jar), more than 24% of the direct microscopic count. This suggests that exclusion of oxygen and the presence of carbon dioxide maximized recovery of gingival crevice bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M., Socransky S. S. Predominant cultivable micro-organisms inhabiting periodontal pockets. Br Dent J. 1968 Jun 18;124(12):560–564. [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., SAWYER S., KAPSIMALIS B., MACDONALD J. B. The microbiota of the gingival crevice area of man. II. The predominant cultivable organisms. Arch Oral Biol. 1963 May-Jun;8:281–289. doi: 10.1016/0003-9969(63)90020-7. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn M. T., Crawford J. J. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970 Feb;19(2):207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J., DALE A. C., BORTNICK L., ROSENTHAL E., MACDONALD J. B. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch Oral Biol. 1963 May-Jun;8:275–280. doi: 10.1016/0003-9969(63)90019-0. [DOI] [PubMed] [Google Scholar]
- Spears R. W., Freter R. Improved isolation of anaerobic bacteria from the mouse cecum by maintaining continuous strict anaerobiosis. Proc Soc Exp Biol Med. 1967 Mar;124(3):903–909. doi: 10.3181/00379727-124-31882. [DOI] [PubMed] [Google Scholar]


