Abstract
Periodontal disease is highly prevalent, with 90% of the world population affected by either periodontitis or its preceding condition, gingivitis. These conditions are caused by bacterial biofilms on teeth, which stimulate a chronic inflammatory response that leads to loss of alveolar bone and, ultimately, the tooth. Current treatment methods for periodontitis address specific parts of the disease, with no individual treatment serving as a complete therapy. The present research sought to demonstrate development of a multiple drug delivery system for stepwise treatment of different stages of periodontal disease. More specifically, multilayered films were fabricated from an association polymer comprising cellulose acetate phthalate and Pluronic F-127 to achieve sequential release of drugs. The four types of drugs used were metronidazole, ketoprofen, doxycycline, and simvastatin to eliminate infection, inhibit inflammation, prevent tissue destruction, and aid bone regeneration, respectively. Different erosion times and adjustable sequential release profiles were achieved by modifying the number of layers or by inclusion of a slower-eroding polymer layer. Analysis of antibiotic and anti-inflammatory bioactivity showed that drugs released from the devices retained 100% bioactivity. The multilayered CAPP delivery system offers a versatile approach for releasing different drugs based on the pathogenesis of periodontitis and other conditions.
Keywords: Controlled drug release, Drug delivery, Drug release, Periodontium, Sequential drug release
INTRODUCTION
Periodontitis is one of the most common inflammatory diseases and is a leading cause of tooth loss in adults [1, 2]. It is also related to systemic disorders, such as coronary artery disease, stroke, and diabetes [3, 4]. In the initial stages of periodontitis, the onset of bacterial infection is followed by the host response of active and progressive inflammation, leading to resorption and loss of tissue [5]. When periodontitis is well-established, effective therapeutic and surgical intervention is required for the removal of bacterial plaque, control of inflammation, and inhibition of progressive bone loss with subsequent complete repair and regeneration of functional periodontium [3]. One of the most common methods for treating chronic periodontitis involves mechanical debridement of periodontal pockets by scaling and root planning along with effective plaque control to eliminate bacterial infection [6]. Subsequent periodontal regenerative procedures are time-consuming and financially demanding [7], and currently there is no ideal therapeutic approach to completely cure periodontitis and achieve predictable tissue regeneration [3]. Because the progression of periodontitis involves a complex, sequential relationship between infection, inflammation, and tissue loss [8], treatment might be improved by controlled release of multiple biologically active agents in an appropriate sequence [9].
Vyas et al. reviewed controlled drug delivery systems that have been employed for treating periodontal diseases [10]. Some approaches involved localized delivery of antibiotics for elimination of bacterial infection [6, 11], while others have addressed inflammation [12, 13] or bone resorption [14]. Periodontal regeneration has also been attempted using local delivery of osteogenic agents [15-18]. None of these methods, however, addressed all aspects of the disease to achieve comprehensive treatment.
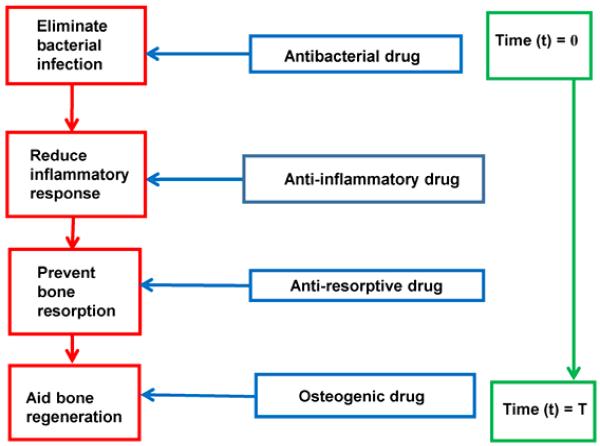
The present research was aimed at developing an “all-encompassing”, multiple drug delivery system capable of delivering antibacterial, anti-inflammatory, anti-resorptive, and osteogenic agents in the appropriate sequence for potential treatment of periodontitis. Figure 1 shows a schematic representation of the order in which the drugs will be delivered at different stages based on pathogenesis of the disease.
Figure 1.
Proposed sequential drug delivery based on the pathogenesis of periodontal disease.
MATERIALS AND METHODS
Fabrication of multilayered devices
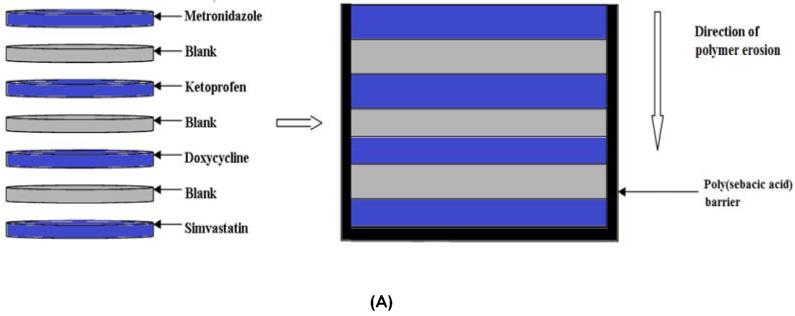
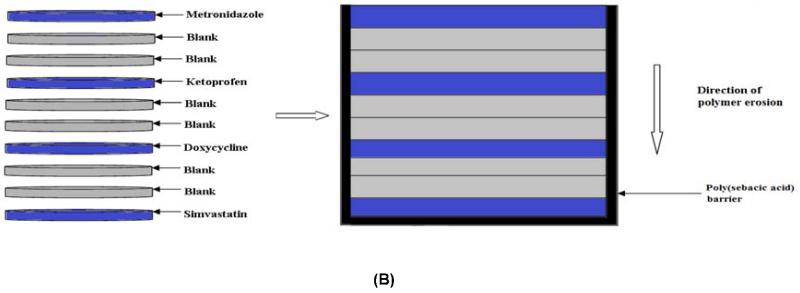
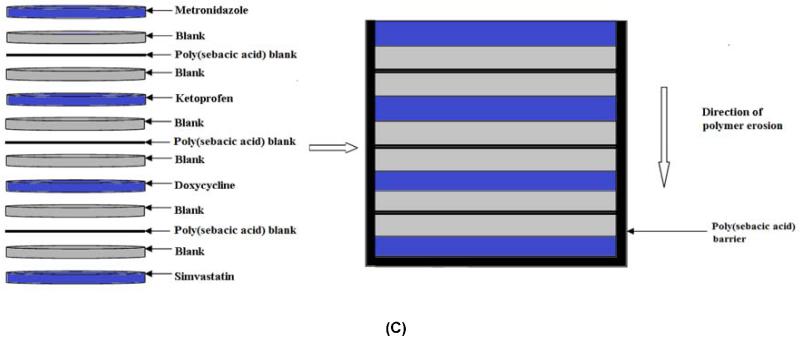
Devices were fabricated using a surface-eroding association polymer system (CAPP) comprising cellulose acetate phthalate (CAP) (Sigma-Aldrich, St. Louis, MO) and Pluronic F-127 (P) (Sigma-Aldrich) [19, 20]. CAPP films, prepared by a solvent evaporation technique, were used to fabricate the multilayer devices. CAP and Pluronic F-127 were mixed together in the weight ratio of 70:30, respectively, and dissolved in acetone to obtain an 8% polymer solution. The drug of interest (5 wt %) was added to the acetone-polymer solution and mixed thoroughly until the drug was completely dissolved. The drug-polymer solution was poured in a Teflon dish and stored at 4°C for 24 hours for slow evaporation of the solvent. Blank CAPP films were prepared in the same way but without the addition of drugs. For this study, CAPP films were loaded with metronidazole (Sigma-Aldrich), ketoprofen (Sigma-Aldrich), doxycycline (Sigma-Aldrich), or simvastatin (Haorui Pharma-Chem, Inc., Edison, NJ). Samples with diameter of around 6 mm and thickness of 0.5 mm were punched out of the CAPP films. The drug-loaded discs were arranged in the desired sequence with alternating layers of blank CAPP films (Figure 2). The stack of the CAPP films was bonded together by compressing them after acetone had been applied between the layers. The multilayered device was then coated with poly(sebacic acid) (diacetoxy-terminated; PSA; Sigma-Aldrich), which acted as a barrier to enable unidirectional erosion and drug release. Blank (drug-free) multilayered devices were used for comparison. Three different device designs were investigated for increasing the duration of erosion and release. In addition to the single CAPP blank layers, either two blank layers were used or a thin PSA layer was included between the blank layers.
Figure 2.
Schematic representation of how multilayered CAPP devices were fabricated. (A) 7-layer device with one blank layer between drug layers. (B) 10-layer device with two blank layers between drug layers. (C) 10-layer device with PSA layer between the blank layers. Note: Illustration is not to scale.
Mass loss and drug release
The multilayered devices were eroded in phosphate-buffered saline (PBS), pH 7.4, during incubation at 37°C with gentle shaking. After collecting the supernatants at regular time intervals, the samples were weighed and then fresh PBS was added. The measured mass of the samples was used to construct the mass loss profiles of the multilayered CAPP devices. Collected supernatants were used to determine the amount of metronidazole, ketoprofen, doxycycline, and simvastatin using high performance liquid chromatography (HPLC; Shimadzu Prominence). For measuring the concentration of ketoprofen, an isocratic mobile phase composed of acetonitrile (60%) and 0.1% trifluroacetic acid (TFA) in DI (deionized) water (40%) was used with UV detection at 260 nm, and for simvastatin, the isocratic mobile phase was acetonitrile (70%) and 0.1% TFA in DI water (30%) with UV detection at 240 nm. A gradient mobile phase with acetonitrile and 0.1% TFA in DI water was developed for measuring the concentration of metronidazole and doxycycline with UV detection at 318 and 350 nm, respectively.
Mathematical modeling
Profiles of drugs released from the multilayered CAPP device weres evaluated using Hopfenberg’s model for controlled release from erodible slabs (Eq. 1):
| (1) |
where Mt is the amount of drug released (mg) at time t (hours), M∞ the total amount of drug released from the device (mg), ko the erosion constant (mg/hr/mm2), Co initial concentration of the drug in the device (mg/mm3), a the half thickness of the slab, and n=1 for a slab [21]. Because only one side of the CAPP layer (slab) was exposed for polymer erosion and drug release due to the presence of the PSA barrier, the term a (half the thickness of the slab) was replaced with 2a (total thickness of the slab in mm) in equation (1). The release profiles predicted using this mathematical model were compared with the experimentally-obtained cumulative release profiles of the four drugs.
Bioactivity
Bioactivity of released metronidazole or ketoprofen was measured to assess effects of encapsulation and release. The Kirby-Bauer assay was performed to test the antibacterial activity of metronidazole. An aliquot of Porphyromonas gingivalis (FDC381) (P. gingivalis) culture was uniformly spread on blood agar plates using polystyrene beads. Release supernatant (7 μL) containing metronidazole was added to 7 mm diameter filter paper discs and placed on the P. gingivalis-inoculated plates. After 24 hour incubation under anaerobic conditions, the plates were imaged, and the area of inhibition (clear zone) around the filter papers was measured using ImageJ software. Results from the release supernatants were compared to the clear zones obtained using serial dilutions of fresh antibiotic to determine the percent bioactivity. A cyclooxygenase (COX) inhibitor assay kit (Cayman Chemical Company, Ann Arbor, MI) was used to determine the bioactivity of the ketoprofen released from the CAPP films. Activity against COX-1 enzyme was measured using the manufacturer’s protocol. As for metronidazole, COX inhibition by the release supernatants was compared with that of freshly prepared standard dilutions of ketoprofen to determine the percent bioactivity.
Statistical analysis
Mass loss and drug release profiles, both experimental and predicted from mathematical modeling, were analyzed by linear regression using GraphPad Prism software (La Jolla, CA). Statistically significant differences between the bioactivity of the “fresh” drugs and the those released from the CAPP films were determined using the Student’s t-test (InStat, GraphPad Software).
RESULTS
Mass loss profiles
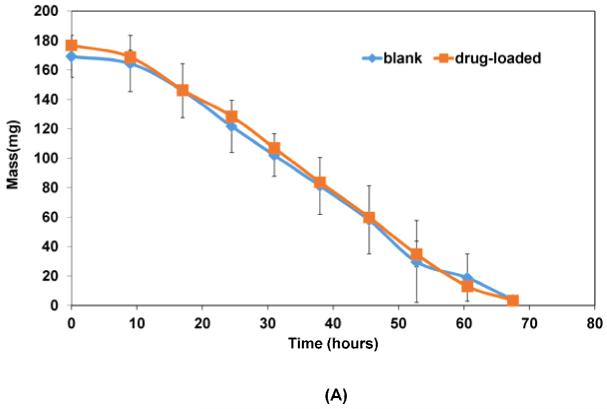
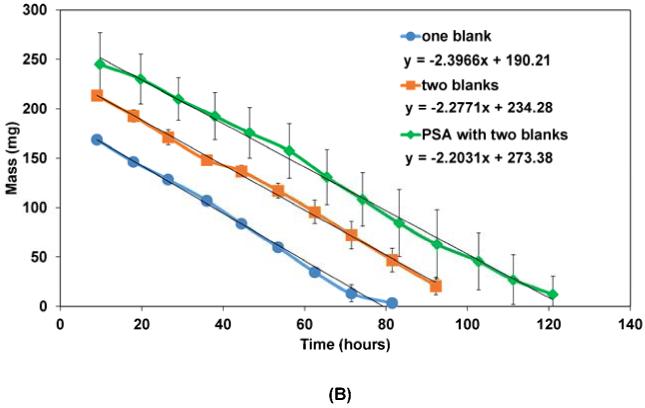
Figure 3 shows the mass loss profiles for the multilayered CAPP devices when eroded in PBS. Both the control (blank) devices without drug and the drug-loaded CAPP devices followed similar linear erosion profiles (Figure 3A). There was no statistically significant difference between the slopes of the mass loss curves during the course of the study. Mass loss profiles of the multilayered devices with single blank layers, two blank layers, and with PSA blank layers are shown in Figure 3B. Comparison of the slopes of the mass loss curves also showed that there were no statistically significant differences in the rate of erosion between the different types of devices. The y-intercept of the lines indicates the average initial mass of the different devices. On average, mass loss occurred at a rate of 0.08 mg/mm2/hour when sink conditions were maintained by replacing PBS at regular time intervals.
Figure 3.
Mass loss profiles for: (A) 7-layered blank and drug-loaded CAPP devices; (B) Drug-loaded devices with one blank layer, two blank layers, or two blank layers along with PSA between the drug layers. Data are mean ± standard deviation (n=3).
Drug release profiles
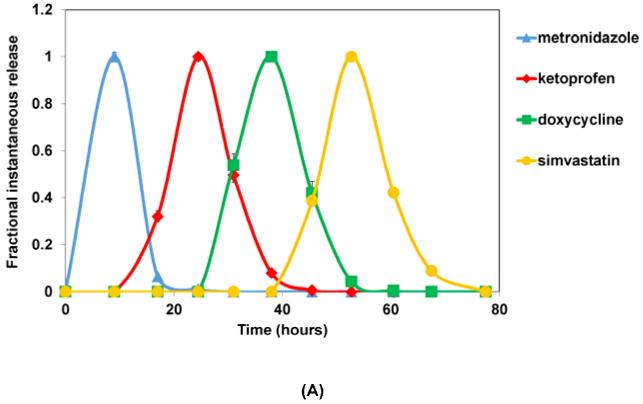
Figure 4A shows the instantaneous profiles for four drugs released from the multilayered delivery system. Because metronidazole was loaded in the layer initially exposed when erosion started, it was the first drug released within 15-20 hours of incubation in PBS. This was followed by release of the anti-inflammatory drug ketoprofen during the course of further device erosion. Ketoprofen release finished midway (around 40 hours) through the total device erosion time (70-80 hours). The third drug released from the system was doxycycline, which started around 25 hour and lasted through 50 hours. The last drug, simvastatin, was released during the final stages of device erosion (50-80 hours).
Figure 4.
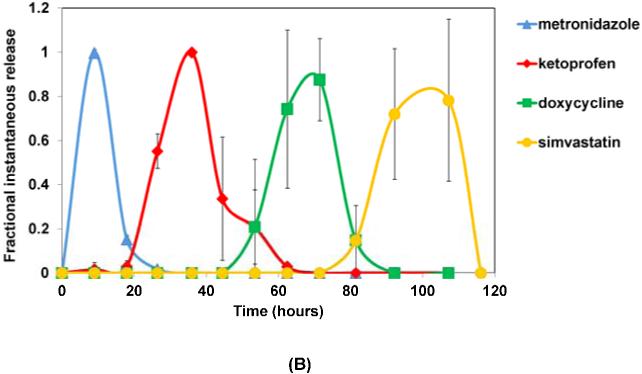
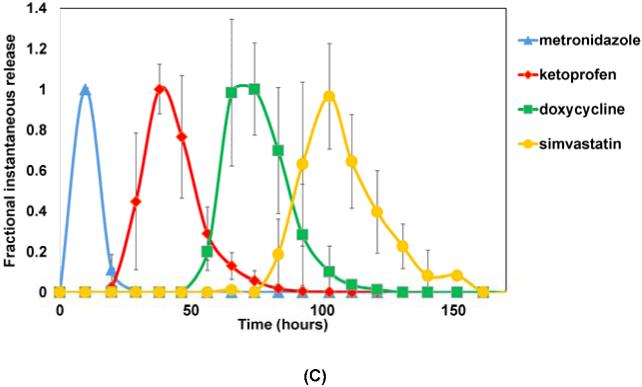
Fractional instantaneous release profiles of four drugs from: (A) 7-layer devices with single blank layers; (B) 10-layer devices with two blank layers; and (C) 10-layer devices with double blank layers plus PSA. Data are mean ± standard deviation (n=3).
The multilayered devices fabricated with two blank layers, instead of just one, between the drug-loaded layers showed similar sequential release of four drugs (Figure 4B). With two blank layers, however, the separation between the drug release peaks was more distinct, and the erosion time of the whole device was increased to 120 hours. In this case, the first drug, metronidazole, was released within the first 25 hours followed by the release of ketoprofen through 60 hours, which was half the erosion time of the device. Release of the third drug, doxycycline, started at around 40 hours and lasted until 100 hours, and this was followed by release of simvastatin through complete erosion of the devices at 120 hours.
The use of the PSA layers between the CAPP blank layers further increased the erosion time of the device to 160 hours. Release of metronidazole occurred within the first 25 hours, as in the case of the other two device types. But the presence of PSA layers before the other drug layers slowed erosion and extended the release periods of ketoprofen, doxycycline, and simvastatin to 20-80, 50-100, and 90-160 hours, respectively. The times at which the peaks were observed for all four drugs in the three different device types are given in Table 1.
Table 1.
Time of metronidazole, ketoprofen, doxycycline, and simvastatin peaks for the three types of devices fabricated and tested.
| Device type |
Metronidazole (hr) |
Ketoprofen (hr) |
Doxycycline (hr) |
Simvastatin (hr) |
Total erosion time (hr) |
|---|---|---|---|---|---|
| One blank | 9 | 24 | 38 | 53 | 78 |
| Two blanks | 9 | 36 | 70 | 100 | 116 |
| PSA with two blanks |
10 | 38 | 74 | 103 | 161 |
Mathematical modeling and mass balance
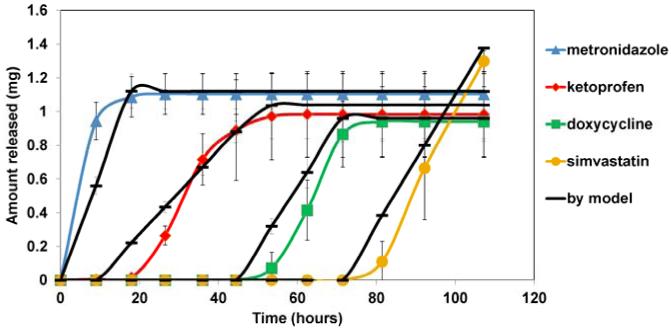
In vitro release of four drugs from the multilayered devices was compared to the profiles predicted using Hopfenberg’s model. Figure 5 shows representative results for the double blank layer devices. The predicted release profiles were similar to those measured for sequential release of the four drugs from the multilayered CAPP devices. Linear regression showed that there were no statistically significant differences between the slopes of the predicted and experimental drug release profiles. The measured amounts of drugs released were not significantly different from the expected amounts.
Figure 5.
Cumulative drug release from double blank layer devices along with mathematical modeling.
Bioactivity
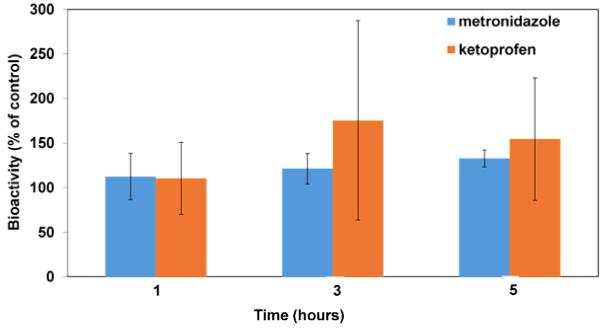
Figure 6 shows the bioactivity of the metronidazole supernatants at 1, 3, and 5 hours of release from single-layered CAPP films as measured by the ability to kill P. gingivalis. The results of the Kirby-Bauer assay showed that the observed area of inhibition caused by the metronidazole supernatants from all the release/erosion supernatants was about 10% higher than that expected from fresh antibiotic solutions of the same concentration. Even though the observed area of inhibition was higher than expected, statistical analysis showed that the difference was statistically significant at only the 5 hour time point (p = 0.0152). Release supernatants from blank CAPP films, which were used as controls, did not produce any clear area (not shown).
Figure 6.
Percentage of bioactivity retained by metronidazole and ketoprofen released from CAPP films. Data are mean ± standard deviation (n=3).
By measuring inhibition of COX-1 enzyme activity, ketoprofen in release supernatants was also found to have retained its bioactivity (Figure 6). Even though the assay results showed that the ketoprofen from the release supernatant had more than 100% bioactivity retention, about 120% on an average, statistical analysis showed that there were no significant differences.
DISCUSSION
The complexity of periodontal pathogenesis necessitates use of more than one type of drug for complete treatment of the condition. For this purpose, a localized delivery system capable of releasing multiple drugs in a sequential order would be ideal. Chen et al. [22] and Santo et al. [23] have given a detailed account of current research on multiple drug delivery systems for tissue engineering. The majority of these multiple drug delivery systems were designed for release of more than one type of drug simultaneously [24-30] or sequential release of more than one type of drug by using composite devices comprising more than one polymer [31-35]. The multilayered delivery devices designed for the present research using a single association polymer system successfully released four different drugs in the desired temporal sequence for potential treatment of periodontitis. The sequence in which these four drugs are delivered is of critical importance for the treatment of the disease. For example, administration of an osteogenic agent before elimination of the bacterial infection and inflammation would not be as effective for tissue regeneration.
Delivery devices based on the CAPP association polymer system have been used for drug release both in the form of films and microspheres [36, 37]. For the present research, the physical form of films was selected for designing a system for sequential drug release. Previous research demonstrated that CAPP films can deliver drugs in a near-zero-order fashion [38]. In agreement, the mass loss profile followed a linear pattern with the mass loss occurring at a rate of 2.5 mg/hour, suggesting surface erosion and drug release. Use of the surface-eroding polyanhydride poly(sebacic acid), which erodes more slowly than does CAPP, as a barrier layer limited erosion to only one surface and, therefore, unidirectional erosion and drug release.
Selection of the appropriate drugs is also an important factor for successful therapy. The first step in the treatment of periodontitis involves elimination of the bacterial infection. The initial stages of wound management are critical for repair and regeneration of periodontal tissues, because the innate regenerative potential of the periodontium is dependent on wound stability [7]. The localized delivery of various antimicrobial agents for treatment of chronic periodontitis and its advantages have been discussed by Etienne [6]. Of the various antimicrobial agents used, including tetracycline, chlorhexidine, doxycycline, and minocycline [6], metronidazole was chosen for this research because it is one of the most commonly used antibiotics against periodontal pathogens. Metronidazole concentrations achieved by systemic administration might not be effective against biofilm-associated P. gingivalis [39], however, which further increases the need for localized antibiotic delivery for the treatment of chronic periodontal conditions. Porphyromonas gingivalis is a pathogenic bacterium commonly associated with periodontal infections [40, 41]. Metronidazole has been shown to significantly reduce P. gingivalis infection compared to other antibiotics in vitro [42], and it is effective against different strains [43]. Localized delivery of metronidazole has been achieved in the form of gels for treatment [44], and studies have shown that metronidazole can readily attain minimum inhibitory concentrations in gingival tissue and crevicular fluid [45, 46]. Besides gels, metronidazole has also been loaded and released using CAPP films by Gates et al. [36] and by the authors [38]. The effectiveness of metronidazole as an antimicrobial agent against periodontal pathogens and its successful localized delivery using CAPP films makes it an appropriate option for the first drug to be delivered for the treatment of periodontitis.
The bacterial infection associated with periodontitis results in an exuberant host response, with chronic inflammation leading to the loss of soft tissue and resorption of the alveolar bone [47]. The persistence of inflammation will affect the regenerative process even if growth factors or other osteogenic agents are provided [8]. Consequently, the inflammatory response must be controlled before attempting to prevent bone resorption and aid regeneration. The present CAPP system was capable of releasing the anti-inflammatory drug ketoprofen before delivering antiresorptive and osteogenic agents. Ketoprofen has been successfully used in the treatment of periodontitis [48, 49]. Both systemic and topical administration of ketoprofen in the form of gels was effective in reducing prostaglandin levels in gingival crevicular fluid in adult periodontal patients, which thus aided inhibition of disease progression [50, 51]. Both metronidazole and ketoprofen retained essentially 100% of their bioactivity, which shows that the loading of drugs in the CAPP films and their subsequent release did not adversely affect the drugs.
After ketoprofen, doxycycline was released from the CAPP delivery system. Doxycycline, a tetracycline derivative, has significant anti-matrix metalloproteinase activity and also inhibits osteoclast development, structure, and function [52]. Furthermore, doxycycline also has antimicrobial properties and has been shown to be effective against P. gingivalis [43]. Llindhe et al. showed the effectiveness of locally delivered tetracycline using hollow fibers in periodontal pockets for the elimination or reduction of clinical symptoms of periodontitis [53]. These factors make doxycycline an appropriate third drug released to inhibit tissue loss. The antimicrobial properties of doxycycline along with metronidazole which was delivered first, provide continuous protection against bacteria at the site of repair and regeneration. In the case of severe bacterial infection, a combination of antibiotics might be more effective for periodontal treatment [54], and this type of multiple antibiotic delivery can be readily achieved using this CAPP system.
Simvastatin was the final drug released from the CAPP system. Beside its common use for cholesterol control [55], simvastatin serves as an osteogenic agent [56]. Apart from aiding general bone regeneration by increasing the expression of BMP-2 [57], simvastatin has been specifically used for periodontal regeneration [58]. Simvastatin is a cost-effective option when compared to BMP-2, and topical administration of simvastatin has the potential to effectively recover alveolar bone loss [15]. Simvastatin has been shown to have a positive effect on the periodontal ligament cells [16], which have the capacity to regenerate the periodontal attachment [59]. Other studies have shown that simvastatin inhibits inflammation and encourages angiogenesis, which might have further positive effects on bone formation [60]. Simvastatin has been loaded into and released from CAPP microspheres m compressed in the form of films for in vivo bone regeneration [61]. Along with its effectiveness in overall periodontal regeneration, prior knowledge on delivery of simvastatin using CAPP system makes it a suitable final drug to be released from this multilayered CAPP delivery system.
Two main advantages of this system include: 1) the relative ease with which the CAPP films can be fabricated using the solvent evaporation technique and 2) the capability of the devices to release a variety of bioactive drugs in a sequential manner. The dose of drugs loaded in the CAPP films can be altered based on the severity of the condition and the type of drug used. The time of drug release can also be controlled by altering the thickness of the layers of CAPP films. Use of two blank layers instead of one resulted in more distinct separation of drug release peaks and also increased the total erosion time of the devices, in spite of slight overlapping that was observed between the adjacent drug release peaks. The use of approximately 300 μm thick PSA layers between the CAPP blank layers further increased the total erosion time of the device by approximately a factor of two in comparison with the devices with single blank layers, with only a slight increase in the overall thickness. Thus, the PSA layers can be increased in thickness or they can replace the blank CAPP layers completely to achieve an even longer erosion time, depending on the treatment requirements. Adjustments in the device design make it possible to achieve different erosion times as well as different release times for the drugs. This type of versatility will be critical in designing devices based on the specificity of disease conditions.
CONCLUSION
The multilayered, bioerodible CAPP delivery system was successful in releasing four different drugs in a predetermined temporal sequence based on the pathogenesis of periodontitis. This device serves as an initial step in the development of multiple drug delivery systems for use against periodontitis or other complex disease condition, which ideally will intervene at different stages of the disease to provide a more comprehensive treatment for the condition.
ACKNOWLEDGEMENTS
This research was supported by NIH (DE019645 and AR060964) and Kentucky NASA EPSCoR (NNX08BA13A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:30–43. doi: 10.1902/jop.1999.70.1.30. [DOI] [PubMed] [Google Scholar]
- [2].Brown LJ, Oliver RC, Loe H. Periodontal diseases in the U.S. in 1981: prevalence, severity, extent, and role in tooth mortality. J Periodontol. 1989;60:363–70. doi: 10.1902/jop.1989.60.7.363. [DOI] [PubMed] [Google Scholar]
- [3].Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010;16:219–55. doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- [4].Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol. 2001;6:91–8. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- [5].Rosen PS. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J Periodontol. 2001;72:1790–800. doi: 10.1902/jop.2001.72.12.1790. [DOI] [PubMed] [Google Scholar]
- [6].Etienne D. Locally delivered antimicrobials for the treatment of chronic periodontitis. Oral Dis. 2003;9(Suppl 1):45–50. doi: 10.1034/j.1601-0825.9.s1.8.x. [DOI] [PubMed] [Google Scholar]
- [7].Polimeni G, Xiropaidis AV, Wikesjö UME. Biology and principles of periodontal wound healing/regeneration. Periodontology 2000. 2006;41:30–47. doi: 10.1111/j.1600-0757.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- [8].Thomas MV, Puleo DA. Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res. 2011;90:1052–61. doi: 10.1177/0022034510393967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cochran DL, Wennström JL, Funakoshi E, Heijl L. Biomimetics in Periodontal Regeneration. Quintessence Publishing Co, Inc; Hanover Park, IL: 2003. [Google Scholar]
- [10].Vyas SP, Sihorkar V, Mishra V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J Clin Pharm Ther. 2000;25:21–42. doi: 10.1046/j.1365-2710.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- [11].Schwach-Abdellaoui K, Vivien-Castioni N, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm. 2000;50:83–99. doi: 10.1016/s0939-6411(00)00086-2. [DOI] [PubMed] [Google Scholar]
- [12].Srinivas M, Medaiah S, Girish S, Anil M, Pai J, Walvekar A. The effect of ketoprofen in chronic periodontitis: A clinical double-blind study. J Ind Soc Periodontol. 2011;15:255–9. doi: 10.4103/0972-124X.85670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Queiroz-Junior CM, Pacheco CM, Maltos KL, Caliari MV, Duarte ID, Francischi JN. Role of systemic and local administration of selective inhibitors of cyclo-oxygenase 1 and 2 in an experimental model of periodontal disease in rats. J Periodontal Res. 2009;44:153–60. doi: 10.1111/j.1600-0765.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- [14].Binderman I, Adut M, Yaffe A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. J Periodontol. 2000;71:1236–40. doi: 10.1902/jop.2000.71.8.1236. [DOI] [PubMed] [Google Scholar]
- [15].Seto H, Ohba H, Tokunaga K, Hama H, Horibe M, Nagata T. Topical administration of simvastatin recovers alveolar bone loss in rats. J Periodontal Res. 2008;43:261–7. doi: 10.1111/j.1600-0765.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- [16].Yazawa H, Zimmermann B, Asami Y, Bernimoulin JP. Simvastatin promotes cell metabolism, proliferation, and osteoblastic differentiation in human periodontal ligament cells. J Periodontol. 2005;76:295–302. doi: 10.1902/jop.2005.76.2.295. [DOI] [PubMed] [Google Scholar]
- [17].King GN, King N, Hughes FJ. Effect of two delivery systems for recombinant human bone morphogenetic protein-2 on periodontal regeneration in vivo. J Periodontal Res. 1998;33:226–36. doi: 10.1111/j.1600-0765.1998.tb02194.x. [DOI] [PubMed] [Google Scholar]
- [18].Selvig KA, Sorensen RG, Wozney JM, Wikesjo UM. Bone repair following recombinant human bone morphogenetic protein-2 stimulated periodontal regeneration. J Periodontol. 2002;73:1020–9. doi: 10.1902/jop.2002.73.9.1020. [DOI] [PubMed] [Google Scholar]
- [19].Xu X, Lee PI. Programmable drug delivery from an erodible association polymer system. Pharm Res. 1993;10:1144–52. doi: 10.1023/a:1018960016756. [DOI] [PubMed] [Google Scholar]
- [20].Raiche AT, Puleo DA. Association polymers for modulated release of bioactive proteins. IEEE Eng Med Biol Mag. 2003;22:35–41. doi: 10.1109/memb.2003.1256270. [DOI] [PubMed] [Google Scholar]
- [21].Hopfenberg HB. Controlled Release Polymeric Formulations. Americal Chemical Society; Washington, D.C.: 1976. Controlled release from erodible slabs, cylinders, and spheres; pp. 26–32. [Google Scholar]
- [22].Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- [23].Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering-Part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B Rev. 2013;19:327–52. doi: 10.1089/ten.teb.2012.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–9. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- [25].Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991;62:458–67. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- [26].Chen FM, Chen R, Wang XJ, Sun HH, Wu ZF. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009;30:5215–24. doi: 10.1016/j.biomaterials.2009.06.009. [DOI] [PubMed] [Google Scholar]
- [27].Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peattie RA, Rieke ER, Hewett EM, Fisher RJ, Shu XZ, Prestwich GD. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials. 2006;27:1868–75. doi: 10.1016/j.biomaterials.2005.09.035. [DOI] [PubMed] [Google Scholar]
- [29].Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dogan AK, Gumusderelioglu M, Aksoz E. Controlled release of EGF and bFGF from dextran hydrogels in vitro and in vivo. J Biomed Mater Res Part B Appl Biomater. 2005;74:504–10. doi: 10.1002/jbm.b.30231. [DOI] [PubMed] [Google Scholar]
- [31].Buket Basmanav F, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- [32].Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–64. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- [33].Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Sequential release of bioactive IGF-I and TGF-β from PLGA microsphere-based scaffolds. Biomaterials. 2008;29:1518–25. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [34].Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–25. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- [35].Tengood JE, Kovach KM, Vescovi PE, Russell AJ, Little SR. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials. 2010;31:7805–12. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gates KA, Grad H, Birek P, Lee PI. A new bioerodible polymer insert for the controlled release of metronidazole. Pharm Res. 1994;11:1605–9. doi: 10.1023/a:1018913921956. [DOI] [PubMed] [Google Scholar]
- [37].Jeon JH, Puleo DA. Alternating release of different bioactive molecules from a complexation polymer system. Biomaterials. 2008;29:3591–8. doi: 10.1016/j.biomaterials.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sundararaj SC, Thomas MV, Dziubla TD, Puleo DA. Bioerodible System for Sequential Release of Multiple Drugs. (in review) 2013 doi: 10.1016/j.actbio.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wright TL, Ellen RP, Lacroix JM, Sinnadurai S, Mittelman MW. Effects of metronidazole on Porphyromonas gingivalis biofilms. J Periodontal Res. 1997;32:473–7. doi: 10.1111/j.1600-0765.1997.tb00560.x. [DOI] [PubMed] [Google Scholar]
- [40].Japoni A, Vasin A, Noushadi S, Kiany F, Japoni S, Alborzi A. Antibacterial susceptibility patterns of Porphyromonas gingivalis isolated from chronic periodontitis patients. Med Oral Patol Oral Cir Bucal. 2011;16:e1031–5. doi: 10.4317/medoral.17174. [DOI] [PubMed] [Google Scholar]
- [41].Tribble GD, Lamont GJ, Progulske-Fox A, Lamont RJ. Conjugal transfer of chromosomal DNA contributes to genetic variation in the oral pathogen Porphyromonas gingivalis. J Bacteriol. 2007;189:6382–8. doi: 10.1128/JB.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Eick S, Pfister W. Efficacy of antibiotics against periodontopathogenic bacteria within epithelial cells: an in vitro study. J Periodontol. 2004;75:1327–34. doi: 10.1902/jop.2004.75.10.1327. [DOI] [PubMed] [Google Scholar]
- [43].Larsen T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol Immunol. 2002;17:267–71. doi: 10.1034/j.1399-302x.2002.170501.x. [DOI] [PubMed] [Google Scholar]
- [44].Sato S, Fonseca MJ, Ciampo JO, Jabor JR, Pedrazzi V. Metronidazole-containing gel for the treatment of periodontitis: an in vivo evaluation. Braz Oral Res. 2008;22:145–50. doi: 10.1590/s1806-83242008000200009. [DOI] [PubMed] [Google Scholar]
- [45].Tenenbaum H, Cuisinier FJ, Le Liboux A, Pichard E, Montay G, Frydman A. Secnidazole concentrations in plasma and crevicular fluid after a single oral dose. J Clin Periodontol. 1993;20:505–8. doi: 10.1111/j.1600-051x.1993.tb00398.x. [DOI] [PubMed] [Google Scholar]
- [46].Van Oosten MA, Notten FJ, Mikx FH. Metronidazole concentrations in human plasma, saliva, and gingival crevice fluid after a single dose. J Dent Res. 1986;65:1420–3. doi: 10.1177/00220345860650120801. [DOI] [PubMed] [Google Scholar]
- [47].Williams RC. Periodontal disease. New England J Med. 1990;322:373–82. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- [48].Paquette DW, Fiorellini JP, Martuscelli G, Oringer RJ, Howell TH, McCullough JR, et al. Enantiospecific inhibition of ligature-induced periodontitis in beagles with topical (S)-ketoprofen. J Clin Periodontol. 1997;24:521–8. doi: 10.1111/j.1600-051x.1997.tb00223.x. [DOI] [PubMed] [Google Scholar]
- [49].Salvi GE, Lang NP. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr Pharm Des. 2005;11:1757–69. doi: 10.2174/1381612053764878. [DOI] [PubMed] [Google Scholar]
- [50].Paquette DW, Lawrence HP, McCombs GB, Wilder R, Binder TA, Troullos E, et al. Pharmacodynamic effects of ketoprofen on crevicular fluid prostanoids in adult periodontitis. J Clin Periodontol. 2000;27:558–66. doi: 10.1034/j.1600-051x.2000.027008558.x. [DOI] [PubMed] [Google Scholar]
- [51].Lawrence HP, Paquette DW, Smith PC, Maynor G, Wilder R, Mann GL, et al. Pharmacokinetic and safety evaluations of ketoprofen gels in subjects with adult periodontitis. J Dent Res. 1998;77:1904–12. doi: 10.1177/00220345980770110701. [DOI] [PubMed] [Google Scholar]
- [52].Vernillo AT, Rifkin BR. Effects of tetracyclines on bone metabolism. Adv Dent Res. 1998;12:56–62. doi: 10.1177/08959374980120012101. [DOI] [PubMed] [Google Scholar]
- [53].Llindhe J, Heijl L, Goodson JM, Socransky SS. Local tetracycline delivery using hollow fiber devices in periodontal therapy. J Clin Periodontol. 1979;6:141–9. doi: 10.1111/j.1600-051x.1979.tb02193.x. [DOI] [PubMed] [Google Scholar]
- [54].Griffiths GS, Ayob R, Guerrero A, Nibali L, Suvan J, Moles DR, et al. Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or re-treatment: a randomized controlled clinical trial. J Clin Periodontol. 2011;38:43–9. doi: 10.1111/j.1600-051X.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- [55].Todd PA, Goa KL. Simvastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1990;40:583–607. doi: 10.2165/00003495-199040040-00007. [DOI] [PubMed] [Google Scholar]
- [56].Chen PY, Sun JS, Tsuang YH, Chen MH, Weng PW, Lin FH. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res. 2010;30:191–9. doi: 10.1016/j.nutres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [57].Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- [58].Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol. 2010;81:214–22. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- [59].Karring T, Nyman S, Gottlow JAN, Laurell L. Development of the biological concept of guided tissue regeneration: animal and human studies. Periodontology 2000. 1993;1:26–35. [PubMed] [Google Scholar]
- [60].Edwards C, Spector T. Statins as modulators of bone formation. Arthritis Res. 2002;4:151–3. doi: 10.1186/ar399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jeon JH, Piepgrass WT, Lin YL, Thomas MV, Puleo DA. Localized intermittent delivery of simvastatin hydroxyacid stimulates bone formation in rats. J Periodontol. 2008;79:1457–64. doi: 10.1902/jop.2008.080004. [DOI] [PubMed] [Google Scholar]