Significance
Learning, the foundation of adaptive and intelligent behavior, is based on changes in neural assemblies and reflected by the modulation of electric brain responses. In infancy, long-term memory traces are formed by auditory learning, improving discrimination skills, in particular those relevant for speech perception and understanding. Here we show direct neural evidence that neural memory traces are formed by auditory learning prior to birth. Our findings indicate that prenatal experiences have a remarkable influence on the brain’s auditory discrimination accuracy, which may support, for example, language acquisition during infancy. Consequently, our results also imply that it might be possible to support early auditory development and potentially compensate for difficulties of genetic nature, such as language impairment or dyslexia.
Keywords: mismatch negativity, event-related potentials
Abstract
Learning, the foundation of adaptive and intelligent behavior, is based on plastic changes in neural assemblies, reflected by the modulation of electric brain responses. In infancy, auditory learning implicates the formation and strengthening of neural long-term memory traces, improving discrimination skills, in particular those forming the prerequisites for speech perception and understanding. Although previous behavioral observations show that newborns react differentially to unfamiliar sounds vs. familiar sound material that they were exposed to as fetuses, the neural basis of fetal learning has not thus far been investigated. Here we demonstrate direct neural correlates of human fetal learning of speech-like auditory stimuli. We presented variants of words to fetuses; unlike infants with no exposure to these stimuli, the exposed fetuses showed enhanced brain activity (mismatch responses) in response to pitch changes for the trained variants after birth. Furthermore, a significant correlation existed between the amount of prenatal exposure and brain activity, with greater activity being associated with a higher amount of prenatal speech exposure. Moreover, the learning effect was generalized to other types of similar speech sounds not included in the training material. Consequently, our results indicate neural commitment specifically tuned to the speech features heard before birth and their memory representations.
During the fetal period the brain undergoes extensive developmental changes as new synapses are formed (1) and axonal connections between neurons are myelinated (2), facilitating efficient recognition and analysis of complex information. In audition, the functional maturation of the developing nervous system is driven by external input, which is evidenced by, for instance, the rapid reorganization of the auditory cortex by external stimuli soon after the onset of hearing in rats (3). This was suggested to occur in humans usually by the gestational age of 27 wk (4). Such plastic changes in neural assemblies during early development indicate that humans have some learning capability even before birth (5, 6). However, this learning capability may be based predominantly on the discrimination of low-pitched sounds that can penetrate the intrauterine walls (7–9). This low-pitch information may play an important role in early speech discrimination of newborns (10) by facilitating learning to segment incoming speech into meaningful units.
Consistent with this, previous behavioral studies have shown that fetuses become attuned to a variety of features of the surrounding auditory environment. For example, fetuses habituate to the native language of the environment or of the mother (11, 12), familiar melodies (13) or fragments of stories heard during pregnancy (14), and even the mother’s voice (15). In addition to learning-based habituation involving the laterobasal amygdala only (16), fetuses, for example, react differently to native and nonnative vowels (17) or familiar and unfamiliar melodic contours (18) and discriminate between different vowels of their native language (19). This capability for fine-tuned auditory processing and discrimination suggests that memory traces lasting for several days in the auditory cortex (20) are formed as a result of fetal learning. These neural memory traces are a prerequisite for effective recognition, categorization, and understanding of speech (21), enabling newborns to generate specific learned behaviors. For example, at birth, neonates cry with their native language prosody (22).
If neural memory traces for individual sounds are formed in utero, then this should be reflected after birth by changes in the brain’s electric activity—namely, by the emergence and enhancement of the mismatch response (MMR) to sound changes (23). The MMR, the infant analogy to the adult mismatch negativity (MMN), represents the brain’s automatic change detection system (24) and is elicited by any discriminable change in the learned material, therefore indirectly reflecting the underlying neural representations of learned repetitive (“standard”) stimuli, such as those for native language speech sounds. Consequently, the MMR indices of cortical discrimination accuracy and plasticity (23, 25, 26) are elicited irrespective of whether or not the individual is attending to sound stimuli (27) and can be recorded from sleeping infants (23, 28) and, with magnetoencephalography, even from fetuses (29).
We investigated the prenatal formation of neural memory traces for speech sounds by comparing the neural dynamics and the MMRs of newborns who had or had not been exposed to novel speech material as fetuses with each other. Starting from pregnancy week 29 until birth, the infants in the learning group were exposed to a trisyllabic pseudoword, [tatata], and two infrequently presented changes: a vowel change (in the middle syllable, [tatota]) or a pitch change ([tatata] with pitch modifications of the middle syllable). These speech sequences were presented in three separate parts, which were interspersed with nonvocal music. The learning effects were investigated in these infants after their birth by recording neural responses to the infrequent vowel and pitch changes used in the training material. In addition, generalization of the learning effects was determined by recording neural responses to unfamiliar changes of vowel intensity ([tatata]) and vowel duration ([tata:ta]) in the middle syllable. For comparison, neural responses to all of these stimuli were also recorded from a naive control group. To ensure that the basic auditory abilities of both groups were comparable, neural responses were also recorded for pitch changes of tones equally unfamiliar for both groups.
We expected selective learning effects for the pseudoword with the pitch changes because pitch changes seldom occur in the middle of words in Finnish, the language of the infants’ environment. In contrast, both groups should show similar MMRs for a vowel identity change, previously observed in newborns (30, 31), because both groups had heard vowels in utero, being surrounded by the Finnish language environment, which is rich in vowels (32).
Results
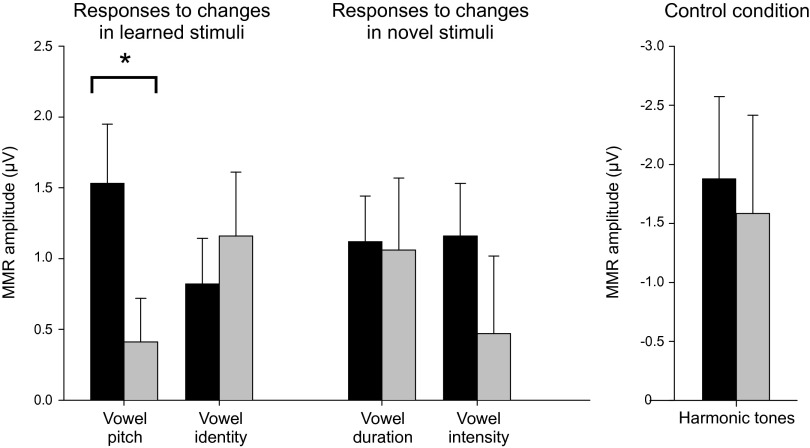
Our results show that exposure to pseudowords modulated the neural responsiveness as predicted. First, infants in the learning group showed statistically significant MMRs for both the vowel identity and pitch changes of the syllable [vowel identity: t(16) = 2.536, P < 0.022; pitch: t(16) = 3.640, P < 0.002]. In contrast, infants not exposed to these stimuli at the fetal stage had a statistically significant MMR for the vowel change [t(15) = 2.577, P < 0.021] only. Furthermore, the response to pitch changes was stronger in infants who had heard these changes as fetuses than in infants in the control group [t(31) = 2.122, P < 0.042, d = 0.763; Fig. 1].
Fig. 1.
(Left) The effects of fetal exposure to pseudowords on the amplitude of neural MMRs in the learning (dark bars; n = 17) and control (light bars; n = 16) groups. Bars denote average MMR response amplitudes (with SEMs) to different changes in the middle syllable of the pseudoword [tɑtɑtɑ]. Neural activity as reflected by the MMR was significantly stronger in the learning group than in the control group for pitch changes to which only the learning group had been prenatally exposed. (Right) No group differences were found in the MMRs for pure tones, equally unfamiliar for both learning (dark bar; n = 17) and control (light bar; n = 16) groups, suggesting that the groups did not differ in basic auditory discrimination abilities (*P < 0.05).
The learning effects were also generalized to speech stimuli not included in the learning material. We found statistically significant MMRs to vowel duration [t(16) = 3.493, P < 0.003] and vowel intensity [t(16) = 3.108, P < 0.028] changes in the learning group but not in the control group. The differences found between the groups in MMRs for infrequent changes in speech sounds cannot be explained by differences in basic auditory abilities because the MMR amplitudes for pitch changes of harmonic tones (1,000 Hz vs. 1,100 Hz), recorded in another condition, equally unfamiliar for all infants, did not differ between the two groups [t(30) = −0.786, P > 0.438; Fig. 1]. Neural response waveforms to pseudowords are shown in Fig. S1.
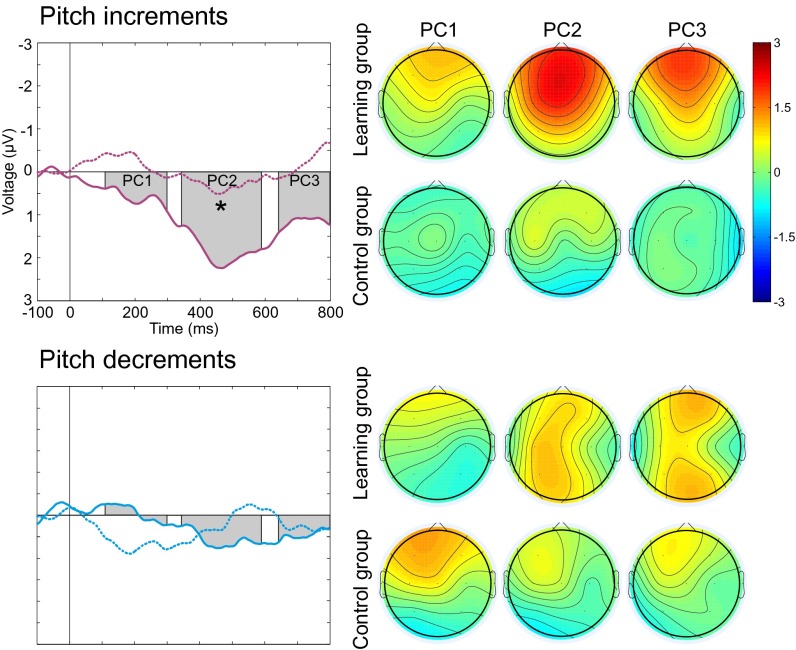
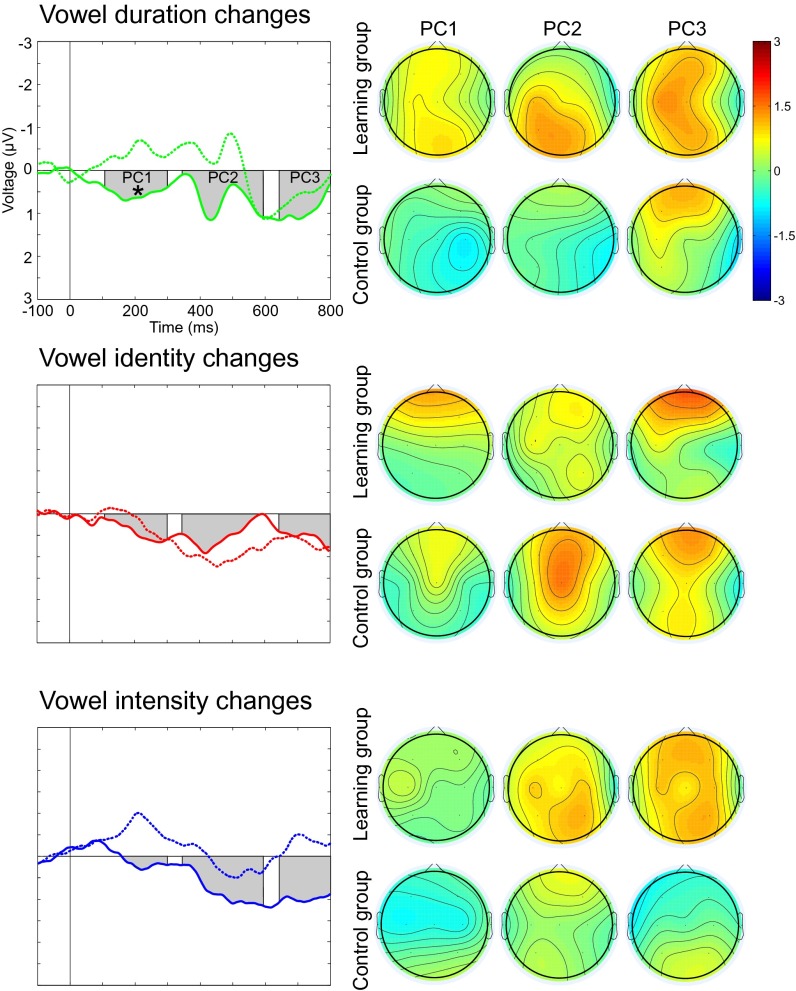
A more detailed analysis assessing the neural dynamics of the responses validated the effects seen in the MMR amplitude analyses (stimuli × component × group interaction, F6,26 = 2.97, P < 0.024, η2 = 0.41). The responses to pitch changes were stronger in the infants who had heard these changes as fetuses than in infants in the control group (340–590 ms time range; F1,31 = 4.357, P < 0.045, η2 = 0.12). Further analysis revealed that the learning group infants had larger responses to pitch increments but not to pitch decrements than their control group peers (340–590 ms time range; F1,31 = 6.497, P < 0.016, η2 = 0.17) (Fig. 2). Furthermore, the amount of prenatal exposure was positively correlated with the neural response amplitude to pitch increments in the learning group infants (340–590 ms time range, C4 electrode; r = 0.61, P < 0.009, R2 = 0.37). Generalization of learning effects was also seen in the detailed analysis of neural dynamics; the responses to vowel duration changes were larger in the learning group than in the control group (110–300 ms time range; F1,31 = 4.988, P < 0.033, η2 = 0.14; Fig. 3).
Fig. 2.
Effects of fetal exposure to pitch increments and decrements in the middle syllable of the pseudoword [tɑtɑtɑ] on the neural responses. The responses of the learning group are shown with solid lines (n = 17), and those of the control group are shown with dotted lines (n = 16). Gray bars denote the latencies of interest indicated by the PCs of the tPCA. The right column shows the distribution of the neural activity across the scalp for each of the PCs. The neural activity was significantly stronger in the learning group than in the control group for pitch increments to which only the learning group had been exposed prenatally (*P < 0.05).
Fig. 3.
Effects of fetal exposure to pseudowords on the neural responses to vowel duration, vowel identity, and vowel intensity changes in the middle syllable of the pseudoword [tɑtɑtɑ]. The responses of the learning group are shown with solid lines (n = 17), and those of the control group are shown with dotted lines (n = 16). Gray bars denote the latencies of interest indicated by the PCs of the tPCA. The right column shows the distribution of the neural activity across the scalp for each of the PCs. Neural activity for the vowel duration change was stronger in the learning group than in the control group (*P < 0.05).
Discussion
Our results indicate the development of neural commitment in fetuses that were systematically exposed to selected speech stimuli during the fetal period. This was evident in the stronger neural activation in the MMR time range elicited in the learning group than in the control group for the middle-syllable increases of pitch, not belonging to the native language of the participants. In addition, the neural activation was significantly greater in infants with more prenatal exposure to the speech material. Furthermore, unlike the control group, the learning group had statistically significant MMRs for changes in vowel intensity and duration not included in the learning material, suggesting generalization of the learning effects. Further supporting the notion of generalization, the learning group showed stronger neural activation for vowel duration changes than the control group. These results reflect genuine learning effects because the basic neural sound processing did not differ between the two groups, as suggested by similar MMRs to pitch changes of tones equally unfamiliar for both groups. Furthermore, because the learning group was not exposed to the learning material for an average of 5 (mean; range 1–27) full days before the recording of the MMRs, the changes in neural responsiveness appear to reflect neural memory traces developed in the fetal brain.
These results generally suggest an improved neural basis of speech perception because the brain processes generating the MMR, reflecting neural discrimination ability, constitute a prerequisite for the accurate auditory perception mandatory for fluent speech functions (23, 26). Previous studies (33–36) have shown that the adult analogy of the infant MMR (MMN) closely correlates with the ability to discriminate changes in speech and nonspeech sounds, reflecting learning-induced brain plasticity (21, 36, 37). For example, this response becomes stronger for changes in foreign language speech sounds in the course of acquiring a good command of that language (21, 38). It also reflects neural tuning to native language speech sounds during early childhood development (23, 39).
However, there may be several different neural mechanisms facilitating the formation of long-term memory traces in the fetal brain. Prenatal exposure to sounds and their changes may lead to the development of a more effective neural network for processing such changes after birth, which is reflected as enhanced neural activation in the MMR time range after birth. Alternatively, the learning group may habituate to the prenatal stimulation more efficiently than the untrained control group, thus facilitating change detection after birth. Because the fetuses heard nonvocal music in addition to the speech material in utero, it is possible that they and their mothers were less stressed than those in the control group, which could have further facilitated the neural plastic changes. Future learning studies should determine such effects, for example, by measuring heart rate variations and cortisol levels during exposure sessions. This would also enable online determination of when the fetuses detect novel learning material (10).
Regardless of the facilitating mechanism, these results show that the neural speech apparatus of fetuses is modulated by the features of speech heard in their environment. As pitch changes in adults can be perceived as changes in intensity and loudness (40), the enhanced responses to increases of pitch due to prenatal exposure may be beneficial for word stress recognition, helping the infant to segment incoming speech into meaningful units. Alternatively, the fetus may be innately more susceptible to learning to discriminate pitch changes because newborns use pitch cues in discriminating between infant-directed and adult-directed speech (41). Furthermore, increased exposure to structured speech material, such as our word [tatata] and its variants, may generally enhance speech discrimination, as suggested by the enhanced neural responsiveness for duration changes not included in these stimuli.
These results indicate that auditory experiences during the fetal period can induce changes in neural processing and therefore have several important practical implications. First, these results indicate that the shaping of the central auditory system begins before birth. Repeated exposure to certain types of sounds leads to the development of neural memory traces for these sounds, as suggested by the strengthening of the activation in the MMR time range to changes in the learned material in the learning group. Thus, it appears likely that hearing a great deal of speech before birth may have positive effects, preparing the neural apparatus for the accurate analysis and discrimination of the fine acoustic features of speech. These early experiences may, then, affect the individual’s later abilities of speech perception and language acquisition.
However, our results also imply that because the fetal brain is malleable to the surrounding sounds, it is also vulnerable to harmful environmental acoustic effects. Although speech directed to the fetus by parents or family members seems to have positive effects on fetal development (6), abnormal, unstructured, and novel sound stimulation, which the fetus could perceive as noise, cannot be recommended until follow-up studies on such stimulation have been thoroughly conducted (42). Harmful effects of abnormal auditory stimulation have been shown in adults, in whom noisy environments may disrupt the neural processes underlying speech perception by decreasing neural responsiveness to speech, especially in the left hemisphere, which is specialized for language (43). Moreover, noise may be even more detrimental to the developing central auditory system, which rapidly matures during the fetal period and infancy. If a fetus is exposed to noisy or unstructured auditory environments at, for example, the workplace of the pregnant mother, this experience may cause an aberrant organization of the infant’s central auditory system structures, which may later affect speech perception and learning. In support of this, experiments with rat pups have shown that even moderate background noise prevents the normal development of their central auditory system (3). Noise-rearing delayed the emergence of refined response selectivity of neurons and topographic sound representation in the auditory cortex. Consistent with this, subsequent rat studies have shown the benefit of the exposure to structured sound environments, such as music, in terms of both cortical organization (44) and long-term cognitive capabilities (45). Our results indicate that the fetal brain possesses similar learning and memory capacities to those of an infant, and improving and optimizing the auditory environment even before birth is warranted.
Materials and Methods
Participants.
Forty-four families took part in the experiment. Twenty-eight mothers recruited from Internet discussion boards participated in the learning group; 17 of the mothers continued the experiment until the EEG recording of the infants. The 16 mothers of the control group were recruited from Internet discussion boards and the delivery ward of Women’s Hospital of the Hospital District of Helsinki and Uusimaa. All mothers gave their informed consent to participate and for their newborns to undergo EEG recording.
Twelve mothers of the 17 participating infants in the learning group had an academic education or were university students (13 of 16 in the control group), 4 mothers had upper secondary school education or were students in upper secondary schools (3 of 16 in the control group), and 1 mother had vocational education. The ages of the mothers in the learning group were between 23 and 39 y, with a mean age of 32 y (ages were between 25 and 38 y, with a mean age of 33 y, in the control group). The EEG of the learning group infants was recorded at the age of 1–27 d, with a mean age of 5.5 d (age was 1–7 d, with a mean age of 4.0 d, for the control group). Thirteen of the infants in the learning group were boys (10 in the control group). Learning group infants were born on pregnancy weeks 38 + 0 to 42 + 1 (weeks + days; mean 39 + 6), and control group infants were born on pregnancy weeks 38 + 0 to 42 + 3 (mean 40 + 2). The birth weights of the infants in the learning group were 2,880–4,740 g (mean 3,652 g), and their Apgar scores at 1 min were 7–10 (mean 8.8). The birth weights for the control group were 2,485–4,840 g (mean 3,589 g), and their Apgar scores at 1 min were 7–9 (mean 8.4). No statistically significant differences were found in the background factors between the groups.
All infants passed the hearing screening and an examination by a neonatologist at the delivery ward. The mothers had no history of substance abuse and no neurological disorders during the pregnancy. None of the mothers had diabetes. All pregnancies and deliveries were normal. Approval of the study protocol was obtained from the Ethics Committees of the Hospital District of Helsinki and Uusimaa and the Department of Psychology, University of Helsinki.
Prenatal Stimulation.
The families of the learning group were given a CD in which two 4-min sequences consisting of three variants of [tɑɑtɑɑtɑɑ] pseudowords were played (Table 1). The sequences contained a frequently presented [tɑtɑtɑ] (P = 0.7) and two types of infrequently presented changes in the middle syllable: a vowel change ([tatota], P = 0.1) and a frequency change ([tɑtɑtɑ]; the pitch of the middle syllable was altered relative to the frequent stimulus as follows: either +8% or −8%, P = 0.05 for both, or +15% or −15%, P = 0.05 for both). To make listening more pleasant, sequences were interspersed with nonvocal music. The mother could choose between a classical piece, a short Latin American melody, or a children’s melody. Although some musical genres may be more facilitative than others (46), unfortunately, the music choices were not recorded.
Table 1.
Stimuli used in the prenatal stimulation and the EEG recording after birth
| Stimulus | Probability of occurrence |
| Stimuli of the prenatal stimulation | |
| Standard ([tɑtɑtɑ]) | P = 0.7 |
| Vowel change ([tɑtotɑ]) | P = 0.1 |
| Pitch change ([tɑtɑtɑ] with a ±8% or ±15% pitch change in the middle syllable) | P = 0.05 each |
| Stimuli of the EEG recording | |
| Standard ([tɑtɑtɑ]) | P = 0.5 |
| Vowel change ([tɑtotɑ]) | P = 0.1 |
| Pitch change ([tɑtɑtɑ] with a ±8% or ±15% pitch change in the middle syllable) | P = 0.05 each |
| Vowel duration change in the middle syllable ([tɑtɑ:tɑ]) | P = 0.1 |
| Intensity change ([tɑtɑtɑ] with a ±6 dB intensity change in the middle syllable) | P = 0.1 |
In each of the two 4-min sequences, the standard [tɑtɑtɑ] was presented 429 times, the vowel change ([tɑtotɑ]) was presented 146 times, and each of the four different pitch changes ([tɑtɑtɑ] with pitch changes in the middle syllable) was presented 74 times. The mothers were instructed to play the CD 5–7 times per week, preferably at approximately the same time of day, starting from pregnancy week 29 + 0 until birth and never during or after birth. The mothers were informed that the study was aimed at assessing whether fetuses perceive music and speech differently. The mothers were explicitly forbidden to sing, hum, or speak during the prenatal stimulation. During the stimulation, the mothers were encouraged but not required to be auditorily masked or, for example, to watch television, read, or listen to music as long as headphones were used. The mothers kept diaries on how often and where they played the CD and reported playing the CD 50–71 times altogether (mean 60). In total, the fetuses heard the standard stimulus ([tɑtɑtɑ]) 21,450–30,459 times (mean 25,740), the stimulus with the vowel change ([tɑtotɑ]) 7,300–10,366 times (mean 8,760), and each of the four pitch changes ([tɑtɑtɑ]) 3,700–5,254 times (mean 4,440).
The mothers in the learning group were also given a questionnaire regarding singing, reading out loud, playing instruments, having the fetus exposed to music played by a family member, and listening to music during the last trimester of pregnancy. All mothers listened to music from, for example, the radio during pregnancy. Eleven mothers sang or hummed to the background music, and one mother sang occasionally in a semiprofessional fashion. Two mothers played instruments occasionally, and two other mothers had family members who played instruments on occasion.
Stimuli of EEG Recording.
The stimuli consisted of pseudowords of 480 ms in duration: the standard [tɑtɑtɑ] (probability of occurrence, P = 0.5) and four types of changes in the middle syllable. These were a vowel change ([tɑtotɑ]) (P = 0.1); four different pitch changes (F0 of the middle syllable being equiprobably varied either 8% or 15% up or down, P = 0.05 for each); a duration change ([tɑtɑ:tɑ]) (P = 0.1), the vowel duration being lengthened by 100% (80 ms); and a vowel intensity change ([tɑtɑtɑ]) (P = 0.1), the intensity of the middle syllable being randomly either increased or decreased by 6 dB (Table 1). The standard and vowel-modified pseudowords were produced by a native female speaker of Finnish. They contained the normal variation present in the Finnish language, and the vowels were matched with normal Finnish vowels in duration. The standard and vowel-modified pseudowords were matched in loudness, pitch, and duration. The word stress was on the initial syllable. The intensities of the middle and final syllables were lower than that of the initial syllable by 2 dB and 3 dB, respectively. The frequency of the sound was 177 Hz in the initial syllable and 167 Hz in the final syllable (see also ref. 47). The standard, frequency-modified, and vowel-modified pseudowords were the same ones used in the learning CD. In the control experiment, the infants were presented with 100-ms tones of 1,000 Hz (P = 0.9) and 1,100 Hz (P = 0.1), which were not included in the learning CD.
EEG Recording, Data Analysis, and Statistical Testing.
The EEG was recorded at a 500-Hz sampling rate from nine channels (F3, F4, C3, Cz, C4, P3, P4, T3, and T4), with the average of the mastoid electrodes used as a common reference. Eye movements were monitored using two electrodes placed below and on the right side of the right eye. The EEG was recorded by a trained nurse while infants were lying on their backs in cribs.
Sounds were presented in a sequence in which the standard [tɑtɑtɑ] and the deviants (changes in middle syllable) were alternated. Stimuli were presented at a 60-dB (spl) volume from two loudspeakers placed at a distance of about 1 m from the infant. The stimulus onset asynchrony was 1 s (600 ms in the control experiment). In the control experiment, one infant from the control group was rejected because the data were not registered due to hardware malfunction.
The infant sleep stages were determined by using the EEG, electrooculogram (EOG), and behavioral measures. Data recorded while the infant was awake were discarded due to extensive movement artifacts. The learning group infants spent 53% of their time in active sleep (59% for the control group); no significant difference emerged between the groups. Data were offline-filtered from 0.5 to 20 Hz, and epochs containing external artifacts exceeding ±200 µV were removed. Data were split into epochs from −100 ms to 800 ms from stimulus onset and baseline-corrected to the prestimulus interval.
To assess the presence of the MMN, responses from F3, F4, C3, Cz, and C4 electrodes were averaged together, and the MMR amplitude was determined in a 60-ms window centered at the peak latency of the largest positive peak in the grand average deviant-minus-standard waveform. A Shapiro–Wilk W test indicated that the data were normally distributed. The significance of the MMRs was determined using two-tailed t tests comparing the MMR amplitude with 0. Group differences in MMR amplitudes were studied with two-tailed t tests with a correction for unequal variances if Levene’s test was smaller than P < 0.05, and effect sizes were calculated using Cohen’s d.
In addition, the neural dynamics of the event-related potentials (ERPs) were assessed in greater detail. First, temporal principal component analysis (tPCA) was used to locate the latencies of interest (e.g., refs. 48–50). The mean amplitudes in successive 10-ms windows between −100 and 800 ms were used as variables, and ERPs recorded from different electrodes, stimuli, and participants were used as cases. The tPCA components were rotated using the Promax rotation (51). The tPCA showed three principal components (PCs) with factor loadings of 0.8 or greater, which were selected for further analysis. PC1 at the latency of 110–300 ms accounted for 17.2%, PC2 accounted for 51.1% (340–590 ms), and PC3 accounted for 9.8% (640–800 ms) of the variance in the data, for a total of 78.1%. Further statistical analyses were conducted using the mean amplitude values in the deviant-minus-standard difference waveforms from the latencies of interest indicated by the tPCA.
The Shapiro–Wilk W test indicated that the data were normally distributed. Group differences were studied using repeated-measures ANOVA with group (learning and control), stimulus (vowel intensity, pitch, duration, and identity), tPCA component (PC1, PC2, PC3), and electrode (F3, F4, C3, Cz, C4) as factors. Greenhouse-Geisser correction was applied if sphericity was violated (original degrees of freedom are reported). Bonferroni correction was applied to correct for multiple comparisons in all post hoc tests. Effect sizes are reported as partial etas (η2). Pearson correlation was used to assess whether the number of times that infants had been exposed to the stimuli prenatally or the time between the EEG recording and the last exposure to the stimuli affected the response amplitudes. Due to a large number of correlations, a P value of 0.01 or smaller was considered significant. For correlations, coefficients of determinations (R2) are reported.
The effects of background variables such as the mother singing, reading out loud, playing instruments, and listening to music and exposure to music played by a family member during the last trimester of pregnancy were tested using the aforementioned repeated-measures ANOVA with the data from the learning group only using the background factors as covariates. Because all mothers did not give an estimate for these background factors, they were given a value of 1 if the mother, for example, sang during the last trimester of pregnancy and 0 if the mother did not. None of the effects were statistically significant (P > 0.373 for all comparisons).
Supplementary Material
Acknowledgments
We thank all families participating in the study. We thank Dr. Martti Vainio for recording the stimuli and Tarja Ilkka for conducting the newborn EEG recordings. The study was supported by the Academy of Finland (Grants 128840, 122745, 1135304, and 1135161), the European Commission's Network of European funding for Neuroscience research (ERANET-NEURON) Project Probing the Auditory Novelty System, the Finnish Cultural Foundation, and a University of Helsinki Graduate School grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302159110/-/DCSupplemental.
References
- 1.Kostović I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99(8):1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 2.Moore JK, Linthicum FH., Jr The human auditory system: A timeline of development. Int J Audiol. 2007;46(9):460–478. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- 3.Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300(5618):498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- 4.Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child. 1994;71(2):F81–F87. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vouloumanos A, Werker JF. Listening to language at birth: Evidence for a bias for speech in neonates. Dev Sci. 2007;10(2):159–164. doi: 10.1111/j.1467-7687.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 6.Moon CM, Fifer WP. Evidence of transnatal auditory learning. J Perinatol. 2000;20(8 Pt 2):S37–S44. doi: 10.1038/sj.jp.7200448. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt KJ, Abrams RM. Fetal exposures to sound and vibroacoustic stimulation. J Perinatol. 2000;20(8 Pt 2):S21–S30. doi: 10.1038/sj.jp.7200446. [DOI] [PubMed] [Google Scholar]
- 8.Lecanuet JP, Schaal B. Fetal sensory competencies. Eur J Obstet Gynecol Reprod Biol. 1996;68(1–2):1–23. doi: 10.1016/0301-2115(96)02509-2. [DOI] [PubMed] [Google Scholar]
- 9.Querleu D, Renard X, Boutteville C, Crepin G. Hearing by the human fetus? Semin Perinatol. 1989;13(5):409–420. [PubMed] [Google Scholar]
- 10.Lecanuet JP, Graniere-Deferre C, Jacquet AY, DeCasper AJ. Fetal discrimination of low-pitched musical notes. Dev Psychobiol. 2000;36(1):29–39. [PubMed] [Google Scholar]
- 11.DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 12.Moon C, Cooper RP, Fifer WP. Two-day-olds prefer their native language. Infant Behav Dev. 1993;16(4):495–500. [Google Scholar]
- 13.Hepper PG. Fetal “soap” addiction. Lancet. 1988;1(8598):1347–1348. doi: 10.1016/s0140-6736(88)92170-8. [DOI] [PubMed] [Google Scholar]
- 14.DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behav Dev. 1986;9(2):133–150. [Google Scholar]
- 15.Kisilevsky BS, et al. Effects of experience on fetal voice recognition. Psychol Sci. 2003;14(3):220–224. doi: 10.1111/1467-9280.02435. [DOI] [PubMed] [Google Scholar]
- 16.Mutschler I, et al. Time scales of auditory habituation in the amygdala and cerebral cortex. Cereb Cortex. 2010;20(11):2531–2539. doi: 10.1093/cercor/bhq001. [DOI] [PubMed] [Google Scholar]
- 17.Moon C, Lagercrantz H, Kuhl PK. Language experienced in utero affects vowel perception after birth: A two-country study. Acta Paediatr. 2013;102(2):156–160. doi: 10.1111/apa.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granier-Deferre C, Bassereau S, Ribeiro A, Jacquet AY, Decasper AJ. A melodic contour repeatedly experienced by human near-term fetuses elicits a profound cardiac reaction one month after birth. PLoS ONE. 2011;6(2):e17304. doi: 10.1371/journal.pone.0017304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecanuet JP, et al. Perception et discrimination foetales de stimuli langagiers; mise en évidence à partir de la réactivité cardiaque; résultats préliminaires. C R Acad Sci III. 1987;305(5):161–164. [PubMed] [Google Scholar]
- 20.Shestakova A, Brattico E, Soloviev A, Klucharev V, Huotilainen M. Orderly cortical representation of vowel categories presented by multiple exemplars. Brain Res Cogn Brain Res. 2004;21(3):342–350. doi: 10.1016/j.cogbrainres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Winkler I, et al. Brain responses reveal the learning of foreign language phonemes. Psychophysiology. 1999;36(5):638–642. [PubMed] [Google Scholar]
- 22.Mampe B, Friederici AD, Christophe A, Wermke K. Newborns’ cry melody is shaped by their native language. Curr Biol. 2009;19(23):1994–1997. doi: 10.1016/j.cub.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 23.Cheour M, et al. Development of language-specific phoneme representations in the infant brain. Nat Neurosci. 1998;1(5):351–353. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- 24.Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin Neurophysiol. 2007;118(12):2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Näätänen R, et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385(6615):432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- 26.Näätänen R, et al. The mismatch negativity: An index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134(Pt 12):3435–3453. doi: 10.1093/brain/awr064. [DOI] [PubMed] [Google Scholar]
- 27.Tiitinen H, May P, Reinikainen K, Näätänen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994;372(6501):90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- 28.Cheour M, et al. Speech sounds learned by sleeping newborns. Nature. 2002;415(6872):599–600. doi: 10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- 29.Huotilainen M, et al. Short-term memory functions of the human fetus recorded with magnetoencephalography. Neuroreport. 2005;16(1):81–84. doi: 10.1097/00001756-200501190-00019. [DOI] [PubMed] [Google Scholar]
- 30.Cheour-Luhtanen M, et al. Mismatch negativity indicates vowel discrimination in newborns. Hear Res. 1995;82(1):53–58. doi: 10.1016/0378-5955(94)00164-l. [DOI] [PubMed] [Google Scholar]
- 31.Kujala A, et al. Speech-sound discrimination in neonates as measured with MEG. Neuroreport. 2004;15(13):2089–2092. doi: 10.1097/00001756-200409150-00018. [DOI] [PubMed] [Google Scholar]
- 32. Maddieson I (2011) Consonant inventories. The World Atlas of Language Structures, eds Dryer MS, Haspelmath M (Max Planck Digital Library, Munich), Chap 1. Available at http://wals.info/chapter/2. Accessed December 1, 2011.
- 33.Novitski N, Tervaniemi M, Huotilainen M, Näätänen R. Frequency discrimination at different frequency levels as indexed by electrophysiological and behavioral measures. Brain Res Cogn Brain Res. 2004;20(1):26–36. doi: 10.1016/j.cogbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Kujala T, Kallio J, Tervaniemi M, Näätänen R. The mismatch negativity as an index of temporal processing in audition. Clin Neurophysiol. 2001;112(9):1712–1719. doi: 10.1016/s1388-2457(01)00625-3. [DOI] [PubMed] [Google Scholar]
- 35.Kujala T, Näätänen R. The adaptive brain: A neurophysiological perspective. Prog Neurobiol. 2010;91(1):55–67. doi: 10.1016/j.pneurobio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Atienza M, Cantero JL, Stickgold R. Posttraining sleep enhances automaticity in perceptual discrimination. J Cogn Neurosci. 2004;16(1):53–64. doi: 10.1162/089892904322755557. [DOI] [PubMed] [Google Scholar]
- 37.Kujala T, et al. Plastic neural changes and reading improvement caused by audiovisual training in reading-impaired children. Proc Natl Acad Sci USA. 2001;98(18):10509–10514. doi: 10.1073/pnas.181589198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheour M, Korpilahti P, Martynova O, Lang AH. Mismatch negativity and late discriminative negativity in investigating speech perception and learning in children and infants. Audiol Neurootol. 2001;6(1):2–11. doi: 10.1159/000046804. [DOI] [PubMed] [Google Scholar]
- 39.Jansson-Verkasalo E, et al. Atypical perceptual narrowing in prematurely born infants is associated with compromised language acquisition at 2 years of age. BMC Neurosci. 2010;11:88. doi: 10.1186/1471-2202-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gelfand SA (2009) Hearing: An Introduction to Psychological and Physiological Acoustics (Informa Healthcare, London), 5th Ed.
- 41.Fernald A, Kuhl PK. Acoustic determinants of infant preference for motherese speech. Infant Behav Dev. 1987;10(3):279–293. [Google Scholar]
- 42.Krueger C, Horesh E, Crossland BA. Safe sound exposure in the fetus and preterm infant. J Obstet Gynecol Neonatal Nurs. 2012;41(2):166–170. doi: 10.1111/j.1552-6909.2012.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kujala T, et al. Long-term exposure to noise impairs cortical sound processing and attention control. Psychophysiology. 2004;41(6):875–881. doi: 10.1111/j.1469-8986.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, et al. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 2006;28(2):109–114. doi: 10.1016/j.braindev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Aoun P, Jones T, Shaw GL, Bodner M. Long-term enhancement of maze learning in mice via a generalized Mozart effect. Neurol Res. 2005;27(8):791–796. doi: 10.1179/016164105X63647. [DOI] [PubMed] [Google Scholar]
- 46.Trainor LJ, Lee K, Bosnyak DJ. Cortical plasticity in 4-month-old infants: Specific effects of experience with musical timbres. Brain Topogr. 2011;24(3–4):192–203. doi: 10.1007/s10548-011-0177-y. [DOI] [PubMed] [Google Scholar]
- 47.Partanen E, Vainio M, Kujala T, Huotilainen M. Linguistic multifeature MMN paradigm for extensive recording of auditory discrimination profiles. Psychophysiology. 2011;48(10):1372–1380. doi: 10.1111/j.1469-8986.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- 48.Dien J. Addressing misallocation of variance in principal components analysis of event-related potentials. Brain Topogr. 1998;11(1):43–55. doi: 10.1023/a:1022218503558. [DOI] [PubMed] [Google Scholar]
- 49.Leppänen PH, et al. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46(10):1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Partanen E, Pakarinen S, Kujala T, Huotilainen M. Infants’ brain responses for speech sound changes in fast multifeature MMN paradigm. Clin Neurophysiol. 2013;124(8):1578–1585. doi: 10.1016/j.clinph.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clin Neurophysiol. 2005;116(8):1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.