Abstract
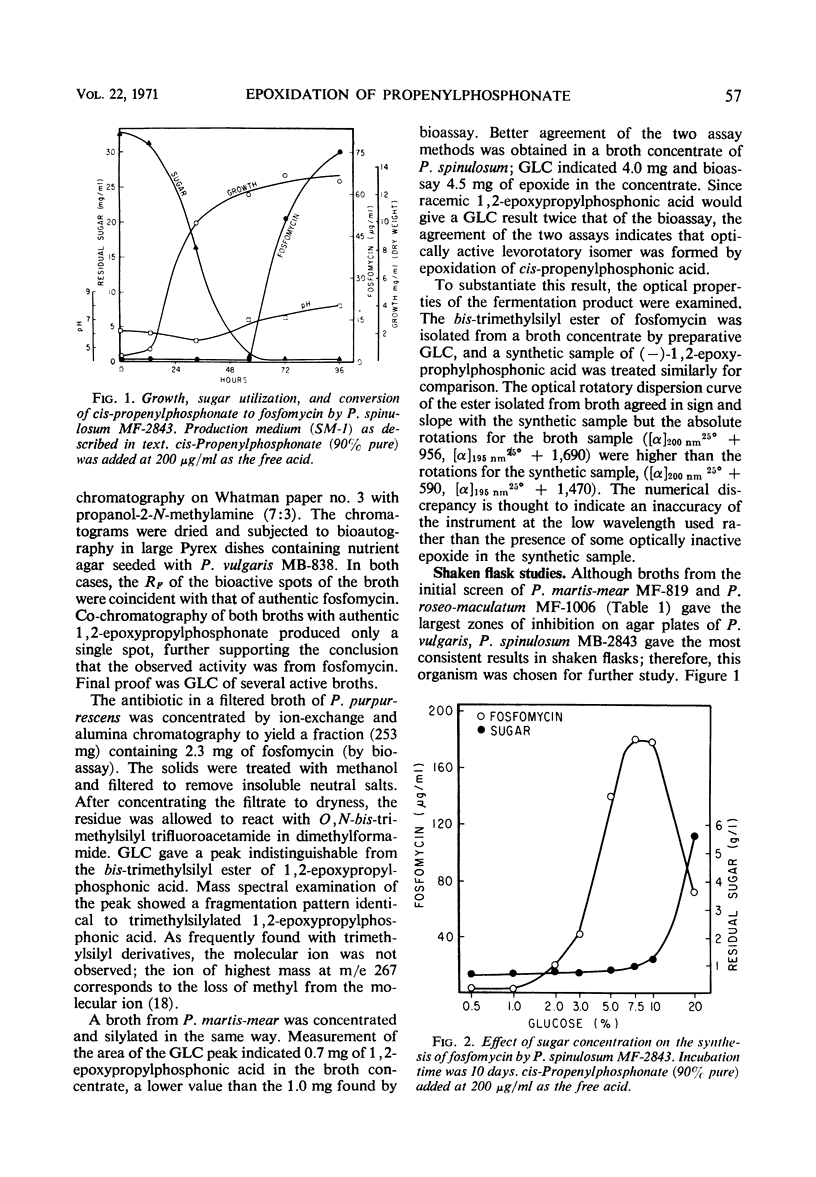
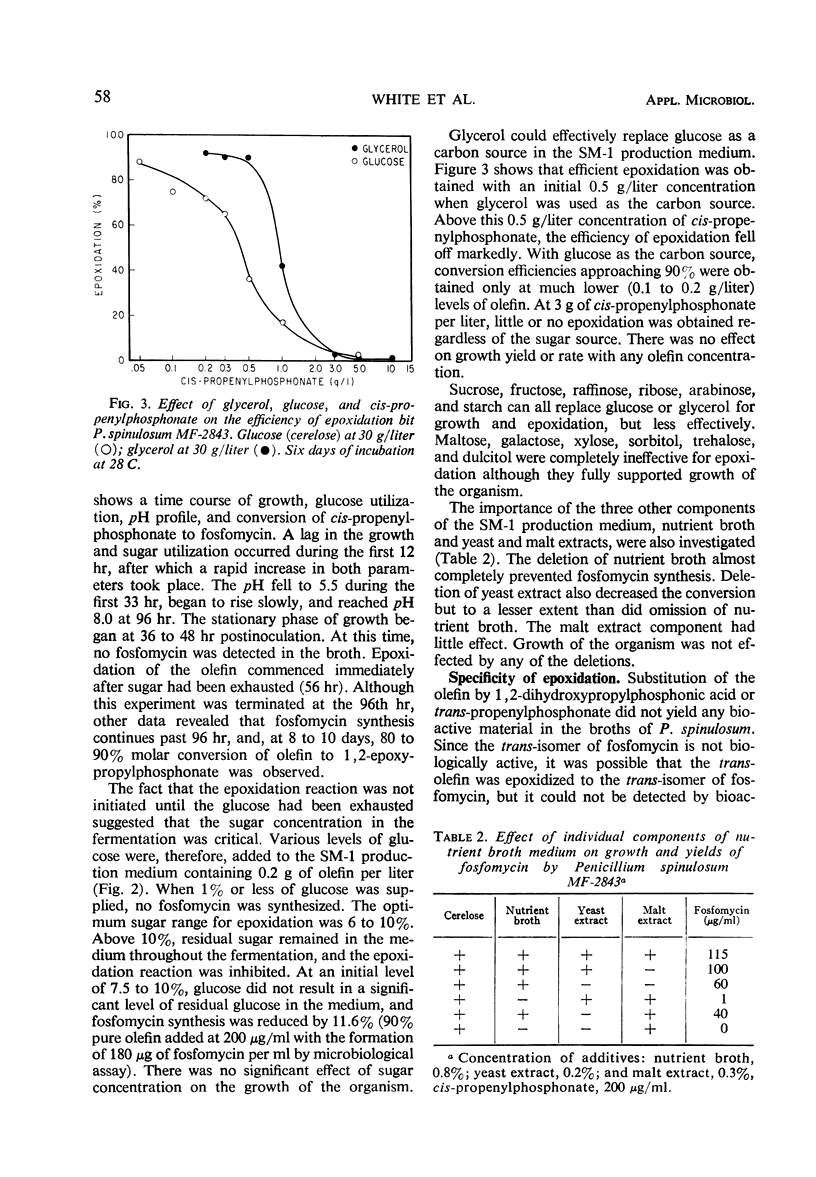
Eighteen species of Penicillium, one of Oidium and one of Paecilomyces were found to effect a stereospecific conversion of cis-propenylphosphonate to fosfomycin which was identified by paper chromatography and gas-liquid chromatography (GLC) of the trimethylsilyl esters. Penicillium spinulosum carried out the epoxidation only after the glucose substrate had been utilized. Glucose controlled the epoxidation since its residual concentrations in the broth severely depresses the reaction. At optimum levels of glucose, an epoxidation efficiency approaching 90% of olefin charged (0.2 g/liter) was obtained after 10 days of incubation. The olefin concentration could be increased to 0.5 g/liter when glucose was replaced by glycerol, whereby a 90% conversion to fosfomycin was attainable in 6 days. The high conversion efficiency, a good agreement between the GLC assay and bioactivity, are indicative of the levorotatory nature of the product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argoudelis A. D., Zieserl J. F. The structure of U-13,933, a new antibiotic. Tetrahedron Lett. 1966 May;18:1969–1973. doi: 10.1016/s0040-4039(00)76280-0. [DOI] [PubMed] [Google Scholar]
- CHANG F. N., SIH C. J. MECHANISMS OF STEROID OXIDATION BY MICROORGANISMS. 7. PROPERTIES OF THE 9-ALPHA-HYDROXYLASE. Biochemistry. 1964 Oct;3:1551–1557. doi: 10.1021/bi00898a028. [DOI] [PubMed] [Google Scholar]
- Christensen B. G., Leanza W. J., Beattie T. R., Patchett A. A., Arison B. H., Ormond R. E., Kuehl F. A., Jr, Albers-Schonberg G., Jardetzky O. Phosphonomycin: structure and synthesis. Science. 1969 Oct 3;166(3901):123–125. doi: 10.1126/science.166.3901.123. [DOI] [PubMed] [Google Scholar]
- Friis P., Daves G. D., Jr, Folkers K. New epoxyubiquinones. Biochemistry. 1967 Nov;6(11):3618–3624. doi: 10.1021/bi00863a037. [DOI] [PubMed] [Google Scholar]
- Hendlin D., Frost B. M., Thiele E., Kropp H., Valiant M. E., Pelak B., Weissberger B., Cornin C., Miller A. K. Phosphonomycin. 3. Evaluation in vitro. Antimicrob Agents Chemother (Bethesda) 1969;9:297–302. [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- MARTIN W. R., FOSTER J. W. Production of trans-L-epoxysuccinic acid by fungi and its microbiological conversion to meso-tartartic acid. J Bacteriol. 1955 Oct;70(4):405–414. doi: 10.1128/jb.70.4.405-414.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. K., Frost B. M., Valiant M. E., Kropp H., Hendlin D. Phosphonomycin. V. Evaluation in mice. Antimicrob Agents Chemother (Bethesda) 1969;9:310–315. [PubMed] [Google Scholar]
- Neuss N., Molloy B. B., Shah R., DeLaHiguera N. The structure of anticapsin, a new biologically active metabolite of Streptomyces griseoplanus. Biochem J. 1970 Jul;118(4):571–575. doi: 10.1042/bj1180571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Katagiri M., Nakagawa A., Sano Y., Nomura S. Studies on cerulenin. V. Structure of cerulenin. J Antibiot (Tokyo) 1967 Nov;20(6):349–354. [PubMed] [Google Scholar]
- Omura S., Nakagawa A., Sekikawa K., Otani M., Hata T. Studies on cerulenin. VI. Some spectroscopic features of cerulenin. Chem Pharm Bull (Tokyo) 1969 Nov;17(11):2361–2363. doi: 10.1248/cpb.17.2361. [DOI] [PubMed] [Google Scholar]
- Read G., Westlake D. W., Vining L. C. Quinone epoxides. V. The biosynthesis of terreic acid. Can J Biochem. 1969 Nov;47(11):1071–1079. doi: 10.1139/o69-171. [DOI] [PubMed] [Google Scholar]
- Shafer H., VandenHeuvel W. J., Ormond R., Kuehl F. A., Wolf F. J. Characterization of phosphonomycin by microchromatographic and related techniques. J Chromatogr. 1970 Oct 7;52(1):111–117. doi: 10.1016/s0021-9673(01)96550-1. [DOI] [PubMed] [Google Scholar]
- Stapley E. O., Hendlin D., Mata J. M., Jackson M., Wallick H., Hernandez S., Mochales S., Currie S. A., Miller R. M. Phosphonomycin. I. Discovery and in vitro biological characterization. Antimicrob Agents Chemother (Bethesda) 1969;9:284–290. [PubMed] [Google Scholar]
- VAN DER LINDEN A. C. EPOXIDATION OF ALPHA-OLEFINS BY HEPTANE-GROWN PSEUDOMONAS CELLS. Biochim Biophys Acta. 1963 Sep 3;77:157–159. doi: 10.1016/0006-3002(63)90484-0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Abraham E. P. The structure of bacilysin and other products of Bacillus subtilis. Biochem J. 1970 Jul;118(4):563–570. doi: 10.1042/bj1180563. [DOI] [PMC free article] [PubMed] [Google Scholar]


