Abstract
Bcl-XL is a major anti-apoptotic protein in the Bcl-2 family whose overexpression is more widely observed in human lung cancer cells than that of Bcl-2, suggesting that Bcl-XL is more biologically relevant and therefore a better therapeutic target for lung cancer. Here, we screened small molecules that selectively target the BH3 domain (aa 90–98) binding pocket of Bcl-XL using the UCSF DOCK 6.1 program suite and the NCI chemical library database. We identified two new Bcl-XL inhibitors (BXI-61 and BXI-72) that exhibit selective toxicity against lung cancer cells compared with normal human bronchial epithelial cells. Fluorescence polarization assay reveals that BXI-61 and BXI-72 preferentially bind to Bcl-XL protein but not Bcl2, Bcl-w, Bfl-1/A1 or Mcl-1 in vitro with high binding affinities. Treatment of cells with BXI-72 results in disruption of Bcl-XL/Bak or Bcl-XL/Bax interaction, oligomerization of Bak and cytochrome c release from mitochondria. Importantly, BXI-61 and BXI-72 exhibit more potent efficacy against human lung cancer than ABT-737 but less degree in platelet reduction in vivo. BXI-72 overcomes acquired radioresistance of lung cancer. Based on our findings, the development of BXI(s) as a new class of anticancer agents is warranted and represents a novel strategy for improving lung cancer outcome.
Introduction
Lung cancer is the leading cause of cancer-related death in the United states, accounting for 28% of the total estimated cancer deaths (1). The estimated cancer-related mortality from lung cancer alone has surpassed the combined mortality from prostate (11%), colon (9%), and pancreatic cancer (6%) in male cancer patients (1). The two major categories of lung cancer, non-small cell lung cancer (NSCLC; approximately 85%) and small cell lung cancer (SCLC; approximately 15%), both have very dismal overall survival rates of 16% and 6%, respectively. Despite recent therapeutic advances, virtually all patients with advanced NSCLC eventually develop resistance to currently available cytotoxic and targeted therapy (2–4).
One of the well-known hallmarks of cancer is the dysregulation of programmed cell death (i.e. apoptosis), resulting in evasion of apoptosis (5). Impaired apoptosis is a critical step in tumor development and renders the tumor cells more resistant to conventional cytotoxic therapy. Despite the frequent dysregulation of apoptosis in tumors, nearly all tumors maintain the core apoptotic regulatory machinery: Bcl2 family proteins, cytochrome c (Cyt c), caspases, etc. Among the components of the apoptotic machinery, overexpression of the anti-apoptotic Bcl-2 family proteins such as Bcl2, Bcl-XL, and Mcl-1 plays critical roles in mediating resistance to apoptosis induced by chemotherapy or radiotherapy (6). Elevated expression levels of anti-apoptotic Bcl2 family proteins have been observed in lung cancers and are associated with chemo- and radio-resistance and poor prognosis (3, 4). Bcl-XL, an anti-apoptotic member of the Bcl2 family, is widely expressed in both SCLC and NSCLC cells (4, 7, 8). Bcl-XL also plays important roles in the crosstalk between autophagy and apoptosis (9). Expression of Bcl-XL is associated with resistance against chemotherapeutic agents (4). Therefore, an attempt to overcome this inherent resistance against apoptosis by inactivating Bcl-XL is a very attractive approach for anti-cancer therapeutics.
Several small molecule BH3 mimetic compounds such as ABT-737, ABT-263 (the oral form of ABT-737), AT-101, GX15-070 and TW-37 have been developed and evaluated in clinical trials with limited success (8, 10–21). Although ABT-737 is considered a potent anti-cancer drug, cancer cells expressing high levels of endogenous Bcl-XL exhibit dramatically less sensitivity to ABT-737 (17, 18, 22). To overcome the limitations of currently available Bcl-2 inhibitors for lung cancer treatment, we have developed two additional effective anti-cancer agents (BXI-61 and BXI-72) that specifically target Bcl-XL for the treatment of lung cancers and, potentially, other cancers with high expression levels of endogenous Bcl-XL. Our findings demonstrate the efficacy of BXIs in potently repressing lung cancer and also overcoming acquired radioresistance of lung cancer cells both in vitro and in vivo.
Materials and Methods
Materials
Small molecules, including NSC354961 (BXI-61) and NSC334072 (BXI-72), were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutic Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (NCI, Bethesda, MD) (http://dtp.nci.nih.gov/RequestCompounds). ABT-737 and antibodies, including PARP, Bax, Mcl-1, cytochrome c, and Actin, were purchased from Santa Cruz (Santa Cruz, CA). Bcl2 antibody was obtained from Calbiochem (Darmstadt, Germany). Bcl-XL and Bak antibodies were purchased from Epitomics (Burlingame, CA). NanoJuice™ Transfection Kit was obtained from Novagene (Madison, WI). Active caspase 3-specific antibody was purchased from Cell Signaling Technology. Fluorescent Bak BH3 domain peptide (FAM-GQVGRQLAIIGDDINR) and purified Bcl-XL protein were purchased from NeoBioSci™ (Cambridge, MA). Purified Bcl2 protein was obtained from ProteinX Lab (San Diego, CA). Purified recombinant Mcl-1 protein was purchased from GenWay Biotech, Inc. (San Diego, CA). Purified recombinant Bcl-w and Bfl-1/A1 proteins were obtained from R&D systems (Minneapolis, MN). Bis (maleimido) hexane (BMH) was purchased from Thermo Scientific (Rockford, IL). All other reagents used were obtained from commercial sources unless otherwise stated.
Cell lines and cell culture
Normal lung epithelial and lung cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). SCLC cell lines DMS53, DMS114 and DMS153 were cultured in Weymouth’s medium (Gibco, Grand Island, NY) supplemented with 5% fetal bovine serum (FBS) and 5% bovine serum (BS) as described (23). Normal human bronchial epithelial cell line (BEAS-2B) and A549 were cultured in DMEM/F-12 medium supplemented with 10% FBS. H69, H292, H358, H460, H1299, H1792, and H1944 were cultured in RPMI 1640 medium supplemented with 5% FBS and 5% BS. These cell lines were employed for the described experiments without further authentication.
Sulforhodamine B (SRB) colorimetric assay
Cells were seeded at a density of 6 × 103 – 8 × 103 per well in 96-well plates and allowed to grow overnight. Cells were treated with BXI or other agent(s) for 72h. The surviving cell fraction was determined using the sulforhodamine B (SRB) assay as described (24).
Fluorescence polarization assay
Fluorescent Bak BH3 domain peptide (FAM-GQVGRQLAIIGDDINR) and Bcl-XL protein were purchased from NeoBioSci™ (Cambridge, MA). To measure the binding affinity of BXI to Bcl-XL protein, a competition fluorescence polarization assay was employed as previously described (25–27). Fluorescent Bak BH3 domain peptide (3nM) was incubated with purified, human Bcl-XL protein (6nM) in the absence or presence of increasing concentrations (i.e. 0.1~500nM) of BXI (s) in the binding affinity assay buffer (50mM Tris (pH8.0), 150mM NaCl, 0.1% BSA, and 5mM DTT) in a 96-well assay plate. The plate was mixed on a shaker for 1 min and incubated at room temperature for an additional 15 min. Polarization, defined as millipolarization units (mP), was measured at room temperature with a fluorescence microplate reader at 485/530nm (Gemini XPS™, Molecular Devices, CA). A negative control (DMSO, 3nM peptide and assay buffer) and a positive control (DMSO, 3nM peptide, 6nM Bcl-XL and assay buffer) were used to determine the range of the assay. The percentage of inhibition was determined by the equation: 1−[(mP value of well − negative control)/range)] × 100%. Inhibitory constant (Ki) value was determined by the formula: Ki = [I]50/([L]50/Kd + [P]0/Kd + 1) as described (27). Reported values are the mean ± S.D. for three separate experiments run in duplicate.
Cytochrome c (Cyt c) release and Bak oligomerization
Subcellular fractionation for isolation of mitochondria and cytosol was performed as previously described (28). Cyt c was analyzed by Western blot. Bak oligomerization was analyzed as described (29). Briefly, 10mM Bis (maleimido) hexane (BMH) was added to the mitochondrial fraction dissolved in conjugation buffer (PBS, pH7.2) and 5mM EDTA was added for crosslinking between sulfhydryl groups of Bak proteins. The reaction mixture was incubated for 1h at room temperature. The reaction was stopped by adding quench solution (1M DTT) for 15min at room temperature. The reaction product was subjected to SDS-PAGE gels and analyzed by Western blotting using a Bak antibody.
Establishment of irradiation resistant (IRR) cell lines
We chose A549, H157 and H358 cell lines to establish ionizing radiation resistant lung cancer cell lines (A549-IRR, H157-IRR and H358-IRR) as described (30). Briefly, A549, H157, and H358 cells (1×106) were serially irradiated with 2Gy of X-rays to a final dose of 80Gy using X-RAD 320 (Precision X-ray, Inc., North Branford, CT). Culture medium was renewed immediately after each dose of radiation. After growing to approximately 90% confluence, cells were trypsinized and passaged into new culture dishes. Re-irradiation of the newly passaged cells with 2Gy of X-rays occurred at about 60% confluence and this was repeated 40 times over a period of 5 months, for a total dose of 80Gy. The parental cells (A549-P, H157-P and H358-P) were trypsinized, counted, and passaged under the same conditions without ionizing irradiation as described (30).
Lung cancer xenografts and treatments
Six-week-old female Nu/Nu nude mice were purchased from Harlan and housed under pathogen-free conditions in microisolator cages. All animal treatments were undertaken in accordance with protocols approved by the Institutional Animal Care and Use Committee at Emory University. 3×106 H1299 cells in Hanks' Balanced Salt Solution (HBSS, Gibco) were injected into subcutaneous tissue at the flank region of nude mice. The tumors were allowed to grow to an average volume of ~250 mm3 prior to initiation of therapy as described (18). Mice were treated with BXI-72 or BXI-61 intraperitoneally (i.p.) as indicated. For radiotherapy, mice with A549 parental (A549-P) or A549-irradiation resistant (A549-IRR) xenografts were irradiated with 2 Gy every other day for 5 treatments using an X-RAD 320 irradiator (Precision X-ray) to deliver whole body irradiation to the mice at a rate of 0.8 Gy per minute as described (31). During treatment, tumor volume (V) was measured by caliper measurements once every two days and calculated with the formula: V=(L×W2)/2 (L: length; W: width). Mice were sacrificed by inhaled CO2 at the end of treatment. Harvested tumors were weighed and immediately fixed in formalin for immunohistochemistry.
Mouse blood analysis
Whole blood (250µL) was collected in EDTA-coated tubes via cardiac puncture of anesthetized mice for hematology studies. Specimens were analyzed for white blood cells (WBC), red blood cells (RBC), platelets (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and blood urea nitrogen (BUN) in the Clinical Pathology Laboratory at the University of Georgia (Athens, GA).
Statistical analysis
The statistical significance of differences between groups was analyzed with two-sided unpaired student’s t-test or Fisher’s exact test. Results were considered statistically significant at P<0.05. The IC50 values were calculated using SPSS Statistics software 18 (IBM). All data are presented as mean ± standard deviation (S.D.).
Results
Screening of small molecule Bcl-XL inhibitors (BXIs) that target the BH3 domain of Bcl-XL (aa 90–98) and suppress lung cancer cell growth
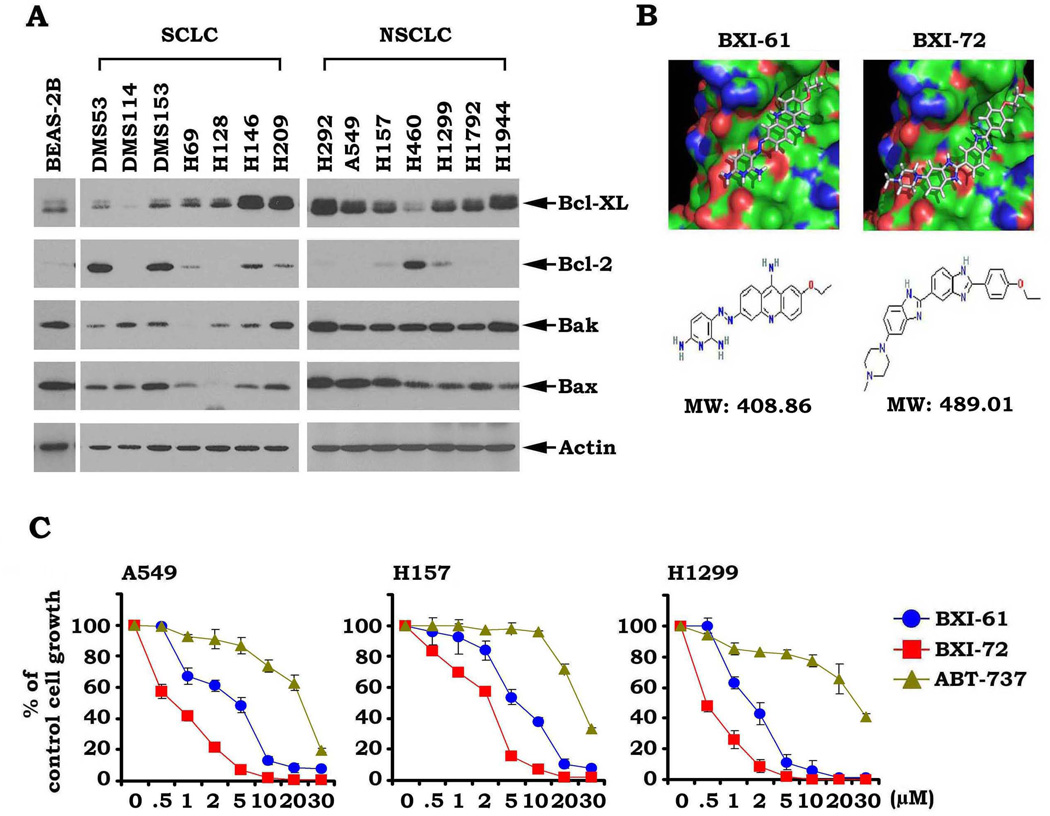
Our findings and those of others have shown that Bcl-XL is more widely expressed than Bcl2 in SCLC and NSCLC cell lines (Fig. 1A) (4, 7, 8), suggesting that Bcl-XL may be a more biologically relevant therapeutic target for lung cancer. To screen for small molecules that specifically target Bcl-XL, a library containing approximately 300,000 small molecules from the NCI was used to dock the structural pocket of the BH3 domain (aa90–98; accession number: 1LXL and 1MAZ) using the UCSF DOCK 6.1 program suite as described (32). The small molecules were ranked according to their energy scores. The top 200 small molecules were selected for screening of cytotoxicity in human lung cancer cells (i.e. H1299 or A549 cells) by SRB assay. Among these small molecules, two compounds (i.e. NSC354961 and NSC334072) had the most potent activities against human lung cancer cells. We named these two lead compounds Bcl-XL inhibitor BXI-61 (C20H19ClN7O, MW: 408.86) and BXI-72 (C27H29ClN6O, MW: 489.01). In this report, we focus on characterizing BXI-61 and BXI-72. The molecular modeling of these two leads in complex with Bcl-XL is shown in Fig. 1B. To compare sensitivities of BXIs with ABT-737 in human lung cancer cells, A549, H159 and H1299 cells were treated with increasing concentrations (0, 0.5, 1, 2, 5, 10, 30µM) of BXI-61, BXI-72 or ABT-737 for 72h. The surviving cell fraction was determined using SRB assay as described (33). SRB assay estimates cell density based on the amount of cellular protein content and is an established and optimized assay for the toxicity screening of compounds on adherent cells in a 96-well format. Results indicate that BXI-61 and BXI-72 are superior to ABT-737 in suppressing lung cancer cell growth (Fig. 1C). BXI-72 showed the greatest potency based on its low IC50 concentrations against the tested cell lines (i.e. H1299: 0.49 ± 0.12 umol/L A549: 0.68 ± 0.08 umol/L; Table1). Because most lung cancer cell lines expressed higher levels of Bcl-XL as compared to normal human bronchial epithelial cells (BEAS-2B) (Fig. 1A), both BXI-61 and BXI-72 displayed relatively selective cytotoxicity against lung cancer cells compared to normal human bronchial epithelial cells (Fig. S1). These findings suggest that BXIs may have potential selectivity for tumor cells in vitro and/or in vivo.
Figure 1.
BXIs potently suppress human lung cancer cell growth. A, Expression levels of Bcl-XL, Bcl2, Bax and Bak in various lung cancer cell lines were analyzed by Western blot. B, Structural modeling of BXI-61 and BXI-72 in the BH3 domain binding pocket of Bcl-XL protein. C, A549, H157, and H1299 cells were treated with increasing concentrations of BXI-61, BXI-72 or ABT-737 for 72 h. Cell growth was analyzed by SRB assay. Error bars represent ± S.D.
Table 1.
IC50 values of BXI-61, BXI-72 and ABT-737
| IC50 (µM) | BXI-61 | BXI-72 | ABT-737 |
|---|---|---|---|
| A549 | 3.17±0.28 | 0.68±0.08 | 19.40±1.23 |
| H157 | 5.71±0.49 | 1.87±0.16 | 27.55±1.49 |
| H1299 | 1.79±0.12 | 0.49±0.12 | 40.48±3.56 |
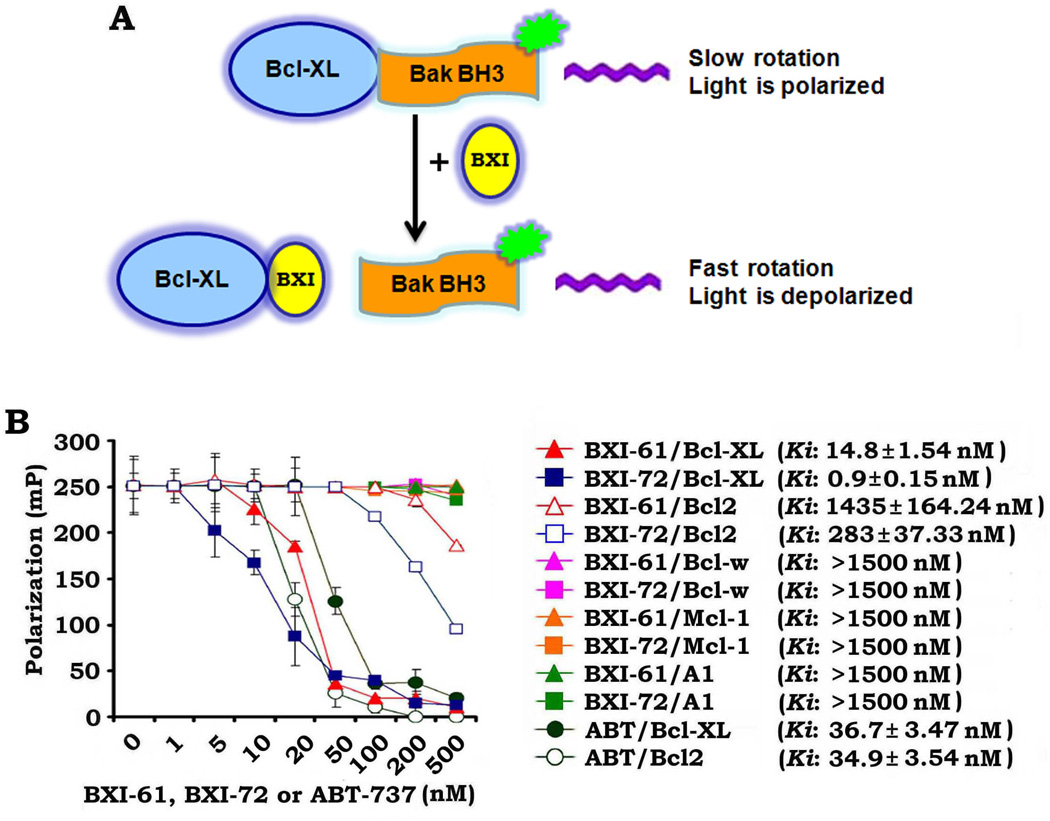
To directly measure BXI/Bcl-XL binding, we used an in vitro fluorescence polarization assay with a fluorescent Bak BH3 domain peptide (5'-FAM-GQVGRQLAIIGDDINR) and purified Bcl-XL protein (25–27, 34). We found that BXIs directly bind to Bcl-XL with high binding affinity (BXI-61: Ki =14.8 ± 1.54 nM; BXI-72: Ki = 0.9 ± 0.15 nM) (Fig. 2). Importantly, both BXI-61 and BXI-72 have very low binding affinities with Bcl2, Mcl-1, Bcl-w and Bfl-1/A1, indicating the specificity of their binding to Bcl-XL (Fig. 2). Similar ABT-737 binding affinities were observed for Bcl2 and Bcl-XL (Fig. 2). In contrast, BXI-61 and BXI-72 bind significantly tighter to Bcl-XL than Bcl2. This suggests that BXI-61 and BXI-72 specifically target Bcl-XL.
Figure 2.
BXI-61 and BXI-72 preferentially bind to Bcl-XL but not Bcl2, Bcl-w, A1 and Mcl-1. A, Schematic representation of fluorescence polarization assay for Bcl-XL/BXI binding. B, Fluorescent Bak BH3 domain peptide (3nM) was incubated with purified human Bcl-XL, Bcl2, Bcl-w, A1 or Mcl-1 protein (6nM) in the absence or presence of increasing concentrations (i.e. 0.1~500nM) of BXI-61 or BXI-72 in the binding affinity assay buffer. Binding affinities were analyzed using a competition fluorescence polarization assay. Reported values are the mean ± S.D. for three separate experiments run in duplicate.
Bcl-XL is a required target for BXI suppression of human lung cancer growth
To further test whether Bcl-XL is an essential target for BXI suppression of human lung cancer, endogenous Bcl-XL was knocked down by RNAi using Bcl-XL shRNA in H1299 cells (Fig. S2A). Approximately 50–60% of cells died following transfection with Bcl-XL shRNA at 48h. The surviving cells that remained were selected by puromycin for stable clones. The selected stable clones were then identified by Western bot, and treated with BXI compounds. As expected, the effect of Bcl-XL shRNA on Bcl-XL expression was highly specific, as shown by the control shRNA having no effect (Fig. S2A). Colony formation assay revealed that depletion of endogenous Bcl-XL significantly reduced the sensitivity of H1299 cells to BXI-61 and BXI-72 (Fig. S2B). These findings indicate that Bcl-XL may be a selective target for BXI’s effect on growth inhibition.
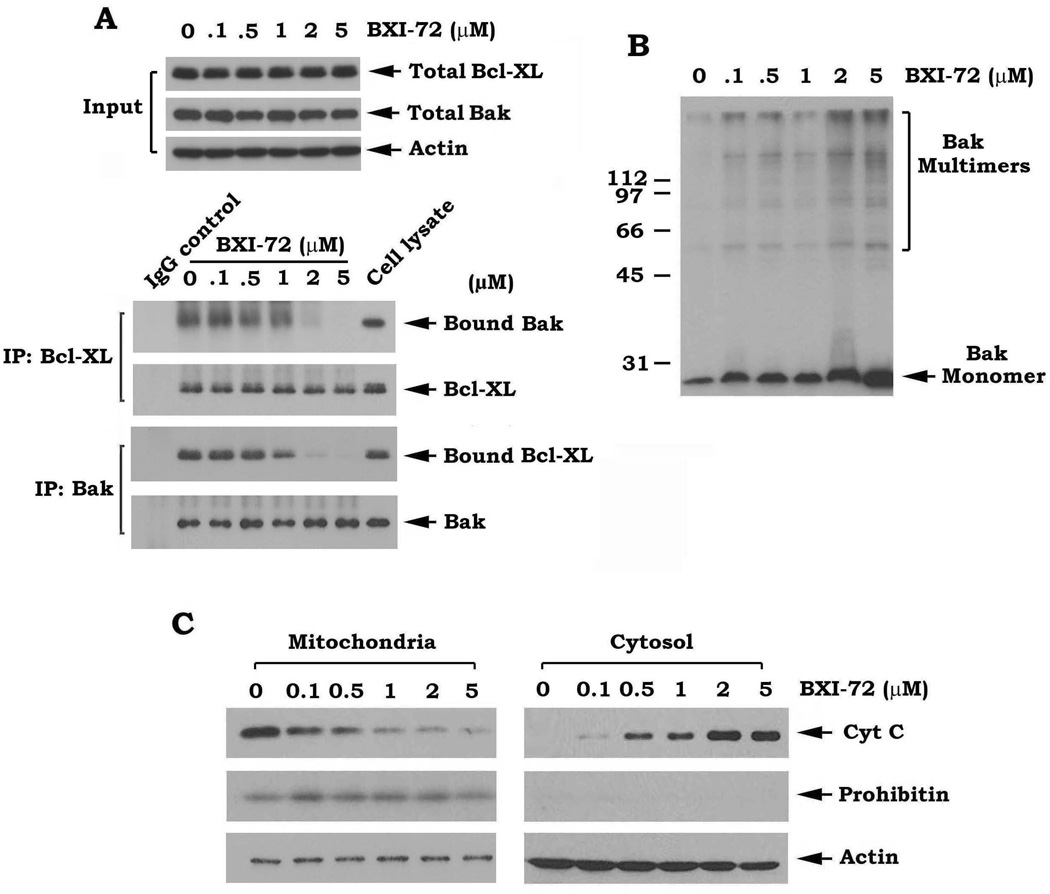
Treatment of human lung cancer cells with BXI results in disruption of Bcl-XL/Bak or Bcl-XL/Bax association, Bak oligomerization and Cyt c release
Bcl-XL forms a heterodimer with Bak through the BH3 domain and suppresses apoptosis (35, 36). Since BXIs are predicted to target the BH3 binding pocket of Bcl-XL, BXIs may disrupt the Bcl-XL/Bak heterodimerization leading to dissociation of Bak from the Bcl-XL/Bak complex and subsequent Bak homo-oligomerization and activation. To test this hypothesis, H1299 cells expressing high levels of endogenous Bcl-XL and Bak were treated with increasing concentrations of BXI-72 (i.e. 0.1–5µM) for 24 h. Co-immunoprecipitation experiments were performed using a Bcl-XL or Bak antibody, respectively. Results indicated that treatment of cells with BXI-72 resulted in a dose-dependent Bcl-XL/Bak dissociation (Fig. 3A). A recent report indicates that Bcl-XL can also interact with Bax (37). Similarly, BXI-72 also disrupts Bcl-XL/Bax complexes (Fig. S3). BXI-72 itself does not affect Bak expression in cells (Fig. 3A). To assess whether BXI-dissociated Bak molecules form homo-oligomers in the mitochondrial membrane, a cross-linking study using BMH was carried out following treatment of cells with BXI-72 as described in “Methods.” Intriguingly, treatment of cells with BXI-72 facilitated the formation of Bak dimers, trimers and multimers (Fig. 3B). The molecular sizes of these oligomers obtained were estimated to be multiples of ~28 kDa, suggesting the formation of Bak homo-oligomers. It is known that formation of Bak oligomers can result in Cyt c release to induce apoptosis (38, 39). Our results show that BXI-72-initiated Bak oligomerization can also facilitate Cyt c release from mitochondria (Fig. 3C).
Figure 3.
Treatment of human lung cancer cells with BXI-72 results in Bcl-XL/Bak dissociation, oligomerization of Bak and Cyt c release. A, H1299 cells were treated with increasing concentrations of BXI-72 for 24h. Levels of Bcl-XL and Bak in total cell lysate were analyzed by Western blot. Meantime, a co-immunoprecipitation (IP) was performed using a Bcl-XL antibody. Bcl-XL-associated Bak and Bcl-XL were analyzed by Western blot. Normal IgG was used as control for IP. B and C, H1299 cells were treated as in (A). Bak oligomerization and Cyt c release were analyzed as described in “Methods”. Prohibitin was used as a mitochondrial marker.
BXI potently represses lung cancer in animal models
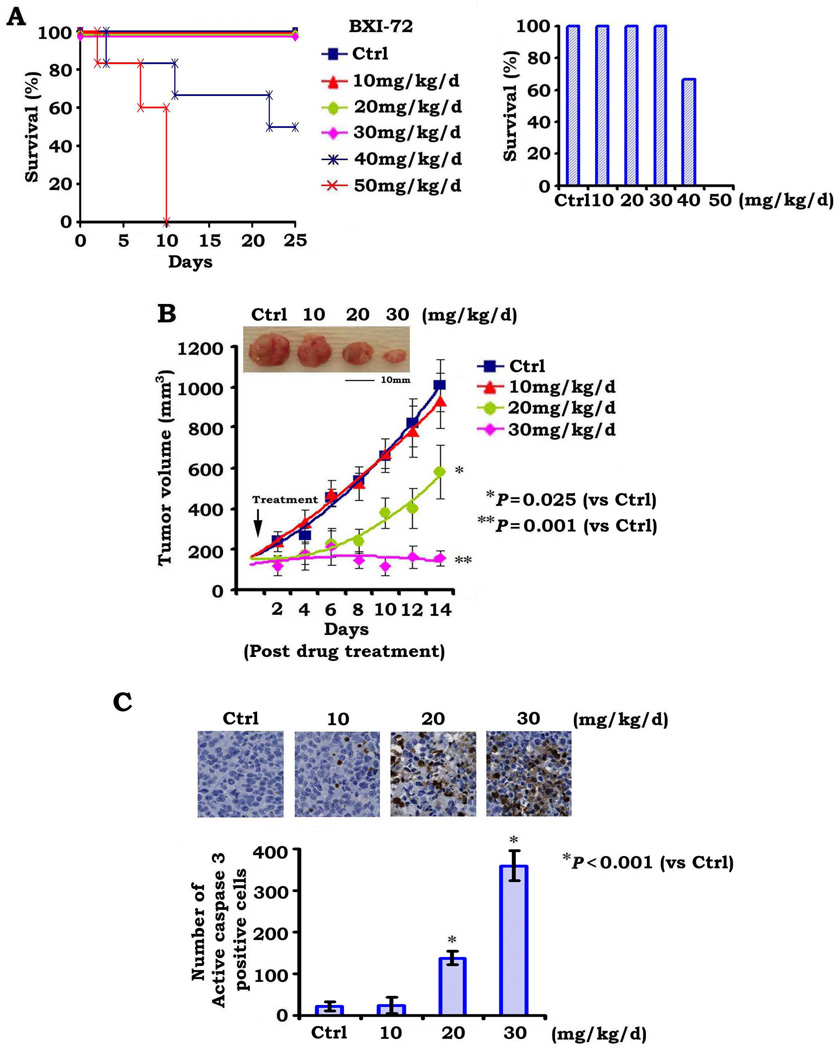
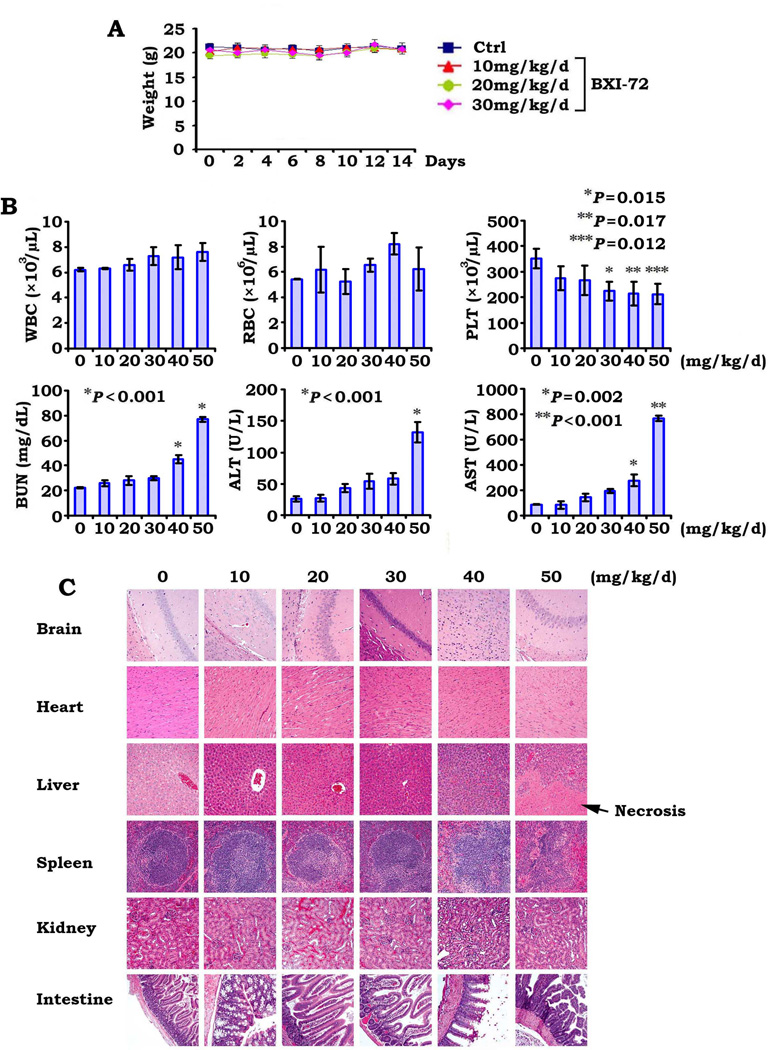
In order to define the appropriate doses for in vivo experimentation, we first determined the maximum tolerated dose (MTD) as previously described (11). Mice were treated in groups of six per dose level with increasing doses of BXI-72 (10–50mg/kg/d) intraperitoneally (i.p.) for up to 25 days. The 50mg/kg/d was uniformly lethal in the six mice within 10 days while 65% of mice treated at the 40mg/kg/d dose died within 25 days. The dose range between 10 and 30mg/kg/d was tolerable with no death recorded after 25 days of daily administration (Fig. 4A). We therefore determined the MTD of BXI-72 (i.p.) with 25-day treatment to be approximately 30~40mg/kg/d.
Figure 4.
BXI-72 potently represses lung cancer in vivo. A, Nu/Nu nude mice were treated with increasing doses of BXI for 25 days (6 mice each dose). Percentage of survival of mice was calculated. B, Nu/Nu mice with H1299 lung cancer xenografts were treated with increasing doses (0, 10, 20 and 30mg/kg/d) by i.p. for 14 days. Each group includes 8 mice. Tumor volume was measured once every 2 days. After 14 days, the mice were sacrificed and the tumors were removed and analyzed. All P values are compared with control group. C, Active caspase 3 was analyzed in tumor tissues at the end of experiments by IHC staining and quantified as described in “Methods.
To test whether BXI is active in vivo, we tested the anti-lung cancer efficacy of BXI-72 in lung tumor xenografts as described (18). Results indicate that treatment with BXI-72 resulted in a dose-dependent regression of established lung cancer xenografts (Fig. 4B). To assess whether BXI-72 induced tumor growth regression via apoptosis in vivo, representative samples from harvested tumor tissues were analyzed by IHC for active caspase 3 as described (18). A dose-dependent apoptosis was observed in tumor tissues after BXI-72 treatment (Fig. 4C). Importantly, doses of 20–30mg/kg not only potently suppressed tumor growth but were also well tolerated without weight loss (Figs. 4 and 5A). However, treatment of mice with increased concentrations of BXI-72 resulted in a dose-dependent reduction of platelets. There were no significant increases in ALT, AST and BUN or decreases in WBC and RBC (Fig. 5B). Histopathology of harvested normal tissues (heart, liver, lung, brain, spleen, kidney, intestine, etc.) revealed no evidence of normal tissue toxicities after treatment with doses of 10–30mg/kg/d (Fig. 5C). However, treatment of mice with doses of 40–50mg/kg/d for two weeks resulted in increases in ALT, AST and BUN, indicating renal and hepatic toxicities at these higher doses (Fig. 5B). Elevated liver function test was associated with hepatocellular necrosis in mice treated with 50mg of BXI-72 (Fig. 5C). These findings suggest that doses between 20mg/kg and 30mg/kg provide the optimal therapeutic index for BXI-72 for in vivo experimentation involving lung cancer xenografts. Additionally, BXI-61 at dose of 40mg/kg/d also exhibited high potency against lung cancer without significant normal tissue toxicities except for a slight decrease in platelets (Fig. S4).
Figure 5.
Analysis of BXI-72 toxicity in vivo. A, Body weight of mice was measured once every other day during treatment with various doses of BXI-72. B, Blood analysis of mice after treatment with various doses of BXI-72. C, H&E histology of various organs from mice after treatment with various doses of BXI-72.
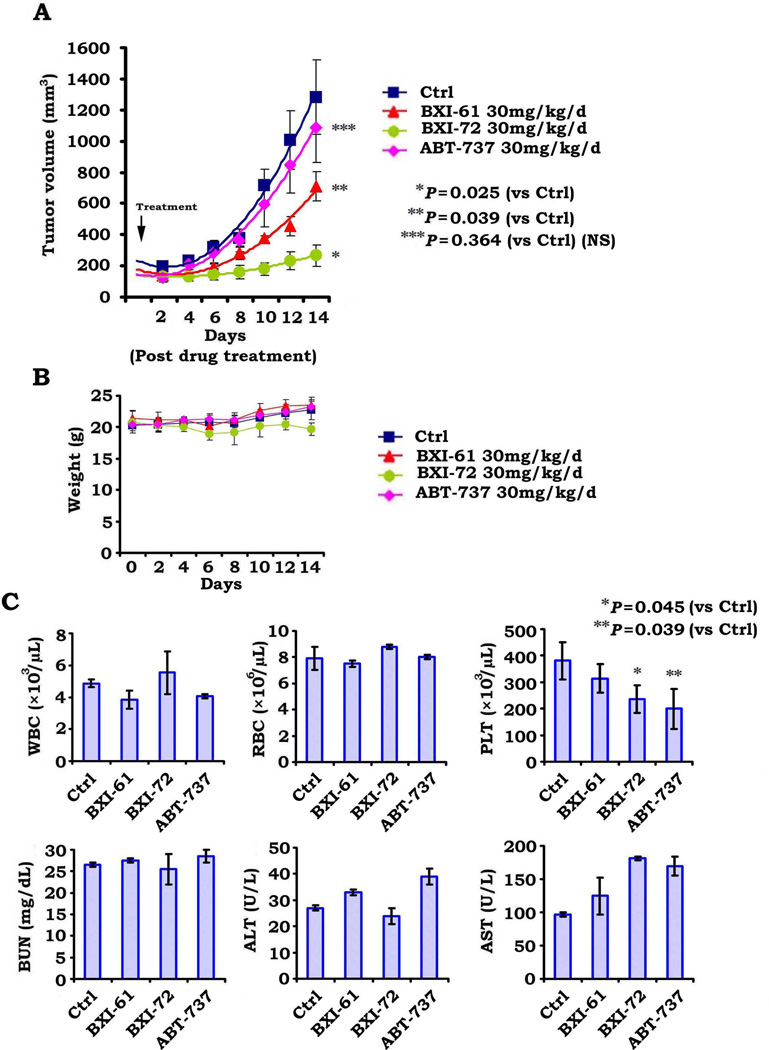
To compare the anti-tumor efficacy between BXI-72, BXI-61 and ABT-737 in vivo, mice with H1299 lung cancer xenografts were treated with same dose (i.e.30mg/kg/d) of BXI-61, BXI-72 or ABT-737 by i.p. for 14 days. Results show that BXI-72 and BXI-61 have more potent efficacy against human lung cancer than ABT-737 without weight loss (Fig. 6A, B). Although three compounds caused various degrees of platelet reduction, BXI-61 and BXI-72 had less effect on platelet than ABT-737 (Fig. 6C).
Figure 6.
BXI has more potent efficacy against lung cancer than ABT-737 in vivo. A, B, and C, Nu/Nu mice with H1299 lung cancer xenografts were treated with 30mg/kg/d of BXI-61, BXI-72 or ABT-737 by i.p. for 14 days. Tumor volume (A), body weight (B) and blood (C) were analyzed described in “Methods”.
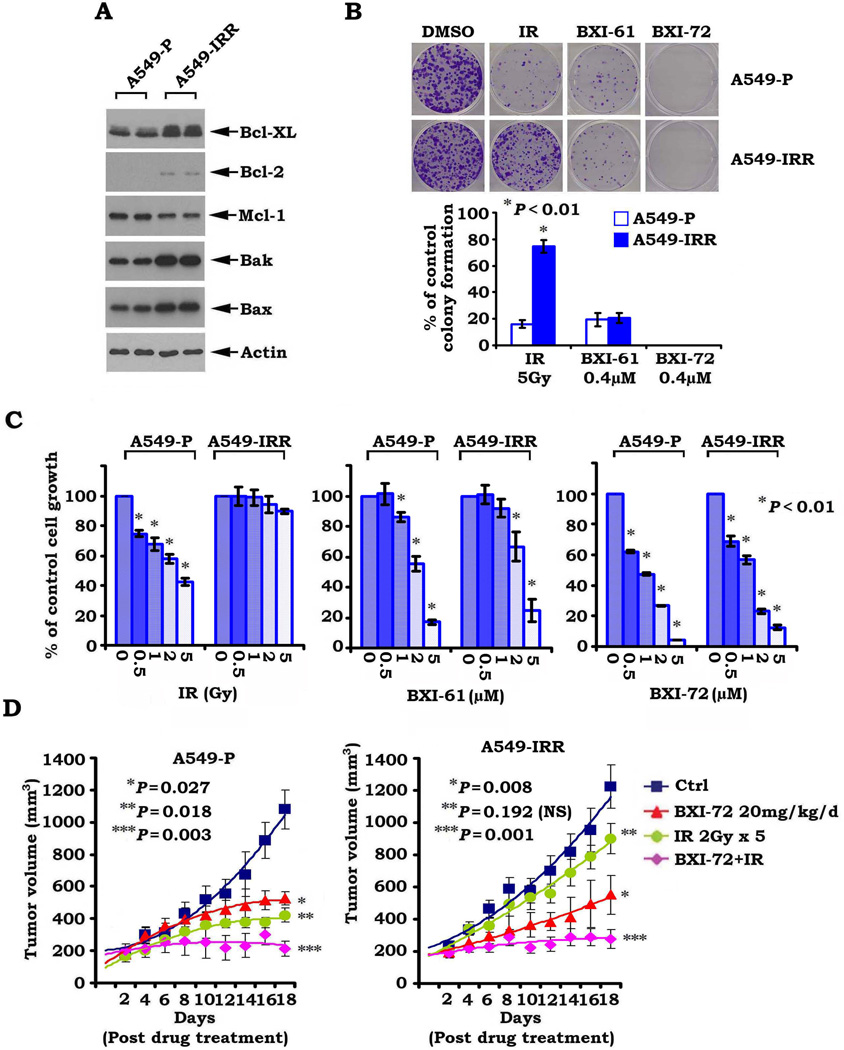
BXI overcomes acquired IR-resistance both in vitro and in vivo
To determine whether Bcl2 or Bcl-XL contributes to acquired resistance to radiation, we established an A549 cell line with acquired resistance to ionizing radiation (i.e. A549-IRR) as described (30). Increased levels of Bcl-XL and Bcl2 were observed in A549-IRR cells as compared to A549-P cells (Fig. 7A). Similar findings were also observed in other lung cancer cells (i.e. H157-P/H157-IRR and H358-P/H358-IRR, Fig. S5). Importantly, parental A549 (A549-P) cells remained sensitive but A549-IRR became insensitive to IR (Fig. 7B, C). These results provide strong evidence that IR-enhanced Bcl-XL contributes to acquired radioresistance. Intriguingly, both A549-P and A549-IRR cells remained sensitive to BXI-61 and BXI-72 (Fig. 7B, C), suggesting that BXIs are able to overcome acquired radioresistance through their suppression of Bcl-XL. To further test whether BXI overcomes radioresistance in vivo, NSCLC xenografts derived from A549-P and A549-IRR cell lines were treated with IR (2Gy ×5) or BXI-72 (20mg/kg/d ×18) alone or in combination as indicated. We observed that lung cancer xenografts derived from A549-IRR cells were resistant to IR treatment whereas xenografts derived from A549-P were sensitive to IR treatment (Fig. 7D). Consistent with the in vitro observation, BXI-72 repressed tumors derived from both A549-P and A549-IRR cells, indicating that BXI-72 can overcome acquired radioresistance in vivo. Other than a slight decrease in WBC or PLT count in IR- or BXI-72-treated mice, there were no significant normal tissue toxicities (Fig. S6).
Figure 7.
BXI-72 overcomes radioresistance of lung cancer in vivo. A, Expression levels of Bcl-XL, Bcl-2, Mcl-1, Bak and Bax in A549-P and A549-IRR cells were analyzed by Western blot. B and C, A549-P and A549-IRR cells were treated with IR, BXI-61 or BXI-72 as indicated. Cell growth was analyzed by colony formation assay after 10 days (B) or SRB assay after 72h (C). D, Mice bearing A549-P or A549-IRR lung cancer xenografts were treated with IR, BXI-72 or their combination as indicated for 14 days. Each group includes 8 mice. Tumor volume was measured once every 2 days. After 14 days, the mice were sacrificed and the tumors were removed and analyzed. All P values are compared with control group. NS: non-significant.
Discussion
Mimicking the BH3 domain to kill cancers is a strategy that is presently being explored in the development of Bcl2 inhibitors as anticancer drugs (40, 41). By binding to the hydrophobic cleft of Bcl2/Bcl-XL, the BH3 mimetics function as competitive inhibitors (18, 40). Three small molecule Bcl2 inhibitors, including gossypol (AT-101), obatoclax (GX15-070) and ABT-263, are in clinical trials (Phase I~II) (40, 41). Gossypol and obatoclax are BH3 mimetics that act as pan-Bcl2 inhibitors (12, 41). However, gossypol and obatoclax are not entirely dependent on Bax and Bak for apoptosis induction and toxicity to normal cells (40, 42). In contrast, the Bad BH3 mimetic ABT-737 was ineffective in inducing apoptosis in cells doubly deficient in Bax and Bak, indicating that its mechanism of action may be predominantly through the Bcl2 family (40, 43). ABT-737 selectively binds to Bcl2, Bcl-XL and Bcl-W but not Mcl-1 and Bfl-1/A1 (18). However, ABT-737 resistance can be caused by expression of Mcl-1 and Bcl-XL (17, 43–45). Here we chose the BH3 domain of Bcl-XL (aa90–98) as a docking site for screening of small molecules that may inactivate Bcl-XL using the UCSF DOCK program 6 and a database of small molecules from the NCI. We found two new Bcl-XL inhibitors (BXI-61 and BXI-72) that preferentially bind to Bcl-XL with inhibitory constant (Ki) values at nanomolar levels (Fig. 2). These two compounds exhibited significantly lower binding affinity for Bcl-2, Mcl-1, Bcl-w and Bfl-1/A1 (Fig. 2), indicating a more selective binding to Bcl-XL. This is especially important because Bcl-XL is more widely expressed in NSCLC and SCLC cells than Bcl-2 (Fig. 1A) (8, 22). A relatively high dose of ABT-737 (i.e. Bcl-2 inhibitor) is required to effectively inhibit the growth of lung cancer cells expressing low levels of endogenous Bcl-2 and high levels of endogenous Bcl-XL (17, 18, 21, 22). By contrast, our new Bcl-XL inhibitors (i.e. BXI-61 and BXI-72) showed superior efficacy to ABT-737 against lung cancer cells that express high levels of endogenous Bcl-XL (Fig. 1). Consistent with our discovery approach, BXI repression of lung cancer growth occurs in a Bcl-XL-dependent manner since depletion of Bcl-XL significantly reduces sensitivity of lung cancer cells to BXI (Supplementary Fig. S2). Importantly, BXI-72, which showed a stronger Bcl-XL binding affinity (Ki→ 0.9 ± 0.15 nM), also displayed greater cytotoxicity against human lung cancer cells as compared to BXI-61 (Ki→14.8 ± 1.54 nM) (Fig. 1C, D). This thus suggests that the anticancer potency of this new class of agents may be dependent on their Bcl-XL binding affinity.
Although silencing of Bcl-XL could cause some H1299 cells undergoing apoptosis due to disruption of Bcl-XL/Bak heterodimerization, certain populations of cells may still be alive via a compensatory mechanism (i.e. formation of Bak/Mcl-1 complex) during long-term stable selection because H1299 cells also express high levels of endogenous Mcl-1 protein (46). Thus, there are no or less Bcl-XL molecules in such type of survived cells for BXI targeting. This not only helps explain why the stable Bcl-XL silenced and survived cells were insensitive to BXI treatment (Fig. S2) but also suggests that Bcl-XL is a selective target for BXI in cells.
A distinctive feature of Bcl-2 family proteins is that they interact with one another to form heterodimers or homodimers through the Bcl-2 homology (BH) domains (35). Anti-apoptotic Bcl-XL preferentially interacts with Bak and forms a heterodimer that inhibits the pro-apoptotic function of Bak (35). Bak is thought to drive apoptosis by forming homo-oligomers that permeabilize mitochondria (29). This homo-oligomerization of Bak is essential for activation of its pro-apoptotic function. Oligomerization involves insertion of the BH3 domain of one Bak molecule into the groove of another and may produce symmetrical Bak dimers. Our results reveal that treatment of lung cancer cells with BXI-72 resulted not only in dissociation of Bak/Bcl-XL or Bax/Bcl-XL complexes but also in Bak oligomerization and Cyt C release (Fig. 3), suggesting that the binding of BXI with Bcl-XL could disrupt Bcl-XL/Bak heterodimers and subsequently facilitate Bak activation via its homo-oligomerization. This may be a key mechanism by which BXI activates apoptosis leading to cell death and tumor regression in vitro and in vivo, respectively.
The anti-tumor activity of BXI in vivo was evaluated in lung cancer xenografts. Both BXI-61 and BXI-72 potently repressed lung cancer in animal models (Figs. 4 and S4). We determined the MTD of BXI-72 with a 25-day treatment to be between 30~40mg/kg/d (Fig. 4A). Dose-response experiments indicated that doses of BXI-72 between 20 and 30mg/kg/d potently repress lung cancer in vivo with a slight decrease in platelet count but no other normal tissue toxicity (Figs. 4 and 5), indicating that these doses should be effective and safe for further characterization of this compound in murine lung cancer models. Since a dose-dependent increase of apoptosis in tumor tissues was observed in the BXI treatment group, this suggests that repression of lung cancer by BXI occurs through induction of apoptosis in tumors (Fig. 4C).
Recent reports indicate that, in addition to antitumor activity, ABT-737 and ABT-263 can induce thrombocytopenia (47–49). Our findings here show that two BXI compounds have more potent efficacy than ABT-737 against lung cancer but less effect on platelet reduction in vivo (Fig. 6). It is known that ABT-737 or ABT-263 not only inhibits Bcl-XL but also inhibit Bcl2 and Bcl-w (18), suggesting that ABT-737 or ABT-263 is not a specific Bcl-XL inhibitor, which is a pan-inhibitor for Bcl2, Bcl-XL and Bcl-w. This could render ABT-737 or ABT-263 more toxicity to normal cells, such as platelets. In contrast, BXI-61 or BXI-72 only specifically binds Bcl-XL but not Bcl2, Bcl-w, Mcl-1 and Bfl-1A1 (Fig. 2), indicating that BXI but not ABT-737 is more specific Bcl-XL inhibitor. Thus, BXI may have better chance to let platelet survival because Bcl2 and Bcl-w could be functional after BXI treatment in platelets. This may help explain why BXIs have less effect on platelet reduction than ABT-737 or ABT-263. Because thrombocytopenia is a major problem in cancer therapy, BXI compounds may have advantage and be more valuable in clinic use as compared to ABT-737 or ABT-263.
Radiotherapy is a major therapeutic intervention for patients with lung cancer and is administered to up to 75% of lung cancer patients during the course of their disease (50). A major challenge affecting outcomes of patients with lung cancer is the development of acquired radioresistance. To test whether BXI could overcome radioresistance of lung cancer, we established acquired radiation-resistant lung cancer cell model systems (Figs. 7 and S5). Elevated levels of the anti-apoptotic proteins Bcl-XL and Bcl2 but not Mcl-1were observed in ionizing radiation resistant cells (i.e. A549-IRR vs. A549-P, H157-IRR vs. H157-P, H358-IRR vs. H358-P) (Figs. 7A and S5), suggesting that this upregulation could, at least in part, contribute to radioresistance. Intriguingly, the BXI lead compound not only reversed radiation resistance in vitro but also overcame radiation resistance in vivo at a relatively low dose (i.e. 20mg/kg/d), leading to the effective suppression of lung cancer xenografts that were resistant to radiation (Fig. 7). Mice tolerated the combination treatment with BXI and IR well without significant normal tissue toxicities except for a reversible decrease in white blood cells that resulted from radiotherapy or a tolerated decrease in platelets that resulted from BXI (Fig. S6).
In summary, we have discovered new Bcl-XL inhibitors (BXI-61 and BXI-72) that specifically bind the BH3 domain pocket of Bcl-XL, disrupt Bcl-XL/Bak or Bcl-XL/Bax heterodimerization, and facilitate Bak homo-oligomerization leading to Bak activation and apoptosis in lung cancer cells. These lead compounds have potent activities against lung cancer in vitro and in vivo, and potentially offer superior efficacy over the BH3 mimetic ABT-737 or ABT-263 in lung cancer therapy. The increased levels of Bcl-XL in lung cancer cells with acquired radioresistance make Bcl-XL an ideal target for overcoming radioresistance. Since our findings demonstrate that BXI can overcome acquired radioresistance of lung cancer in vitro and in vivo, a combination of BXI with IR may represent an effective new strategy for the treatment of lung cancer, including those patients who are resistant to radiotherapy, leading to long-term tumor-free survival.
Supplementary Material
Acknowledgments
This work was supported by NCI, National Institutes of Health grants R01CA112183, R01CA136534, Flight Attendant Medical Research Institute Clinical Innovator Awards, and by NASA grant NNX12AC30G.
We thank Anthea Hammond for editing of the manuscript.
Footnotes
The authors disclose no potential conflicts of interest
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 3.Haura EB, Cress WD, Chellappan S, Zheng Z, Bepler G. Antiapoptotic signaling pathways in non-small-cell lung cancer: biology and therapeutic strategies. Clin Lung Cancer. 2004;6:113–122. doi: 10.3816/CLC.2004.n.025. [DOI] [PubMed] [Google Scholar]
- 4.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Datta R, Manome Y, Taneja N, Boise LH, Weichselbaum R, Thompson CB, et al. Overexpression of Bcl-XL by cytotoxic drug exposure confers resistance to ionizing radiation-induced internucleosomal DNA fragmentation. Cell Growth Differ. 1995;6:363–370. [PubMed] [Google Scholar]
- 7.Karczmarek-Borowska B, Filip A, Wojcierowski J, Smolen A, Korobowicz E, Korszen-Pilecka I, et al. Estimation of prognostic value of Bcl-xL gene expression in non-small cell lung cancer. Lung Cancer. 2006;51:61–69. doi: 10.1016/j.lungcan.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan SL, Lee MC, Tan KO, Yang LK, Lee AS, Flotow H, et al. Identification of chelerythrine as an inhibitor of BclXL function. J Biol Chem. 2003;278:20453–20456. doi: 10.1074/jbc.C300138200. [DOI] [PubMed] [Google Scholar]
- 11.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048) Blood. 2008;111:3211–3219. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoluzzi L, Gonen M, Gardner JR, Mastrella J, Yang D, Holmlund J, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood. 2008;111:5350–5358. doi: 10.1182/blood-2007-12-129833. [DOI] [PubMed] [Google Scholar]
- 14.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 15.Azmi AS, Mohammad RM. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol. 2009;218:13–21. doi: 10.1002/jcp.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 17.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Breast Cancer Special Feature: Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogler M, Dinsdale D, Sun XM, Young KW, Butterworth M, Nicotera P, et al. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008;15:820–830. doi: 10.1038/cdd.2008.25. [DOI] [PubMed] [Google Scholar]
- 21.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 22.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball ED, Sorenson GD, Pettengill OS. Expression of myeloid and major histocompatibility antigens on small cell carcinoma of the lung cell lines analyzed by cytofluorography: modulation by gamma-interferon. Cancer Res. 1986;46:2335–2339. [PubMed] [Google Scholar]
- 24.Liu Y, Sun SY, Owonikoko TK, Sica GL, Curran WJ, Khuri FR, et al. Rapamycin induces bad phosphorylation in association with its resistance to human lung cancer cells. Mol Cancer Ther. 2012;11:45–56. doi: 10.1158/1535-7163.MCT-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M. Development of a high-throughput fluorescence polarization assay for Bcl-x(L) Anal Biochem. 2002;307:70–75. doi: 10.1016/s0003-2697(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 27.Bruncko M, Oost TK, Belli BA, Ding H, Joseph MK, Kunzer A, et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J Med Chem. 2007;50:641–662. doi: 10.1021/jm061152t. [DOI] [PubMed] [Google Scholar]
- 28.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–40219. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 29.Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Lee YS, Oh JH, Yoon S, Kwon MS, Song CW, Kim KH, et al. Differential gene expression profiles of radioresistant non-small-cell lung cancer cell lines established by fractionated irradiation: tumor protein p53-inducible protein 3 confers sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;77:858–866. doi: 10.1016/j.ijrobp.2009.12.076. [DOI] [PubMed] [Google Scholar]
- 31.Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrov DA, Magis AT, Wronski TJ, Chan EK, Toro EJ, Donatelli RE, et al. Identification of enoxacin as an inhibitor of osteoclast formation and bone resorption by structure-based virtual screening. J Med Chem. 2009;52:5144–5151. doi: 10.1021/jm900277z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 34.Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44:4313–4324. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- 35.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 36.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Xing D, Liu L. PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-X L during UV-induced apoptosis. Mol Biol Cell. 2009;20:3077–3087. doi: 10.1091/mbc.E08-11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 39.Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J Cell Biol. 2011;194:39–48. doi: 10.1083/jcb.201102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(Suppl 1):S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Xin M, Wang T, Zhang Y, Deng X. Nicotine enhances the antiapoptotic function of Mcl-1 through phosphorylation. Mol Cancer Res. 2009;7:1954–1961. doi: 10.1158/1541-7786.MCR-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogler M, Hamali HA, Sun XM, Bampton ET, Dinsdale D, Snowden RT, et al. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood. 2011;117:7145–7154. doi: 10.1182/blood-2011-03-344812. [DOI] [PubMed] [Google Scholar]
- 48.Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 49.Kodama T, Hikita H, Kawaguchi T, Shigekawa M, Shimizu S, Hayashi Y, et al. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ. 2012;19:1856–1869. doi: 10.1038/cdd.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coate LE, Massey C, Hope A, Sacher A, Barrett K, Pierre A, et al. Treatment of the elderly when cure is the goal: the influence of age on treatment selection and efficacy for stage III non-small cell lung cancer. J Thorac Oncol. 2011;6:537–544. doi: 10.1097/JTO.0b013e31820b8b9b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.