Abstract
Scientific advances have improved our ability to target cancer interventions to individuals who will benefit most, and spare the risks and costs to those who will derive little benefit or even be harmed. Several approaches are currently used for targeting interventions for cancer risk reduction, screening and treatment, including risk prediction algorithms for identifying high-risk subgroups and diagnostic tests for tumor markers and germline genetic mutations. Economic evaluation can inform decisions about the use of targeted interventions, which may be more costly than traditional strategies. However, assessing the impact of a targeted intervention on costs and health outcomes requires explicit consideration of the method of targeting. Here we describe the importance of this principle by reviewing published cost-effectiveness analyses (CEAs) of targeted interventions in breast cancer. Few studies we identified explicitly evaluated the relationship between the method of targeting, the accuracy of the targeting test and outcomes of the targeted intervention. Those that did found that characteristics of targeting tests had a substantial impact on outcomes. We posit that the method of targeting and the outcomes of a targeted intervention are inextricably linked and recommend that CEAs of targeted interventions explicitly consider costs and outcomes of the method of targeting.
Keywords: breast cancer, economic analysis, cost-effectiveness analysis, targeted therapy, personalized medicine, BRCA, trastuzumab, gene expression profiling
Introduction
Recent decades have seen a rapid expansion in the use of genetic information to estimate disease risk, assess prognosis and manage patient care. By identifying risk factors, prognostic factors and predictive factors, the hope is that interventions can be tailored to maximize benefit and minimize toxicity. The role of targeted strategies has been particularly pivotal in oncology, where numerous biomarkers are used to predict cancer risk, response to the therapy, adverse events and other clinical outcomes.
While targeted interventions have the potential to improve health outcomes, they often come at a high cost. These interventions often involve tests or treatments that are considerably more expensive than the prior standard of care. From a societal perspective, however, targeted therapies offer the promise of optimizing resources so that interventions are directed to individuals who will benefit most from them, and not administered to those who are likely to derive little or no benefit or even be harmed by them.
Economic analysis in general and cost-effectiveness analysis (CEA) in particular have been applied to health and medical interventions for more than a quarter-century, with dramatic growth in recent years.1 While standardized methods for conducting and reporting CEAs have been promoted in the US and elsewhere,2–4 a unique feature of targeted interventions requires explicit consideration by analysts and decision makers. Specifically, in the economic assessment of a targeted intervention, the test used to identify candidates for the intervention is inseparable from the intervention itself. An economic analysis that considers the intervention in isolation from the targeting test obfuscates assumptions about the ability of the test to accurately identify candidates for the intervention, and fails to fully capture all relevant health and economic outcomes.
In this paper we describe the relationship between targeting tests and targeted interventions and the importance of this relationship in assessing outcomes of a targeted strategy. We identify examples of targeted interventions relevant to breast cancer and review published CEAs, noting whether and how the authors considered the relationship between test and intervention. Finally we offer recommendations for economic analysis of targeted interventions in breast and other cancers. Our goals are to advance the quality of economic evaluations of targeted interventions and to help consumers of these studies – clinicians, payers, policymakers – better understand and use the information they provide.
Using Test Results to Target Interventions
Nearly all diagnostic tests give an inherently continuous result that is categorized as a basis for action, and nearly all tests must be considered imperfect predictors of a true state, rather than certain indicators of the truth. When the outcome of a targeted intervention depends on the presence or absence of the target, test results are generally dichotomized, based on some threshold applied to the underlying continuous result. In these cases, economic evaluation of a targeted strategy requires explicit consideration of test performance relative to a gold standard, and how the performance characteristics of the test in the population of interest – sensitivity, specificity, positive and negative predictive value – influence the use and outcomes of the intervention. An expensive test may be cost-effective if its high accuracy means that people who will not benefit from the intervention are spared its risks and costs, or if treatment yields substantial health gains for those correctly identified by the targeting test.
When a target is binary the accuracy of the targeting test will influence both effectiveness and cost of the targeted strategy. A comprehensive economic analysis must therefore include the outcomes of all possible test results. There are costs and health impacts for individuals with a positive result, all of whom would receive the targeted intervention, but the consequences of a true-positive result will not be the same as those of a false-positive result. An individual with a true-positive result incurs the cost of the intervention, but experiences a net gain in health outcomes if improvement in the targeted condition exceeds the expected harm of adverse effects. An individual with a false-positive result incurs the cost of the intervention, but may experience a net health decline due to side effects, because in the absence of the target no health improvement would be expected. Similarly there are costs and health impacts for individuals who have a negative test result and thus would not receive the intervention, but the consequences of a true-negative result will differ from those of a false-negative result.
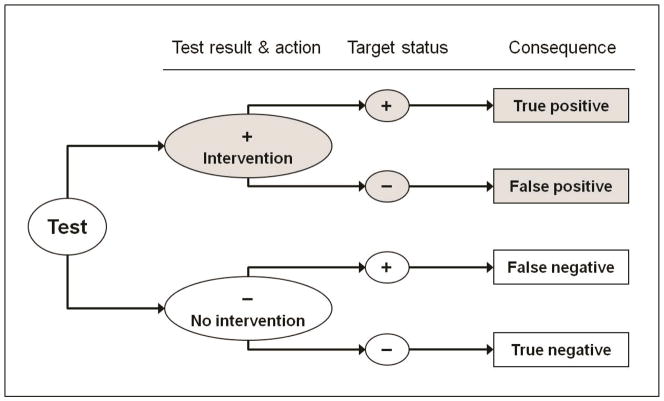
A CEA restricted to a cohort with positive test results only partially captures the full range of health and economic impacts of implementing a targeted intervention, because it ignores negative test results and their consequences (Figure 1). When the targeting test is very expensive, a CEA that ignores individuals with negative test results would exclude these costs. Such an analysis would also exclude the negative health consequences of failing to give the intervention to those with a false-negative result –individuals who truly have the target.
Figure 1.
Possible test results in an economic evaluation of a targeted intervention
The figure depicts test results (positive or negative), actual target status (positive or negative) and the four consequential permutations of these in an economic evaluation which explicitly considers both the targeted intervention and the method of targeting. The shaded segments represent an analysis restricted to a cohort with a positive test result.
Some targeted interventions are directed toward individuals at high risk of a poor outcome or those who have a high likelihood of benefiting from an intervention, where the relevant “test” is a risk prediction algorithm based on multiple pieces of information, rather than a single assay, image or other marker. As with tests for a single target, risk prediction algorithms give a result that is inherently continuous and must be categorized to inform decisions, for example, by defining subgroups of “low”, “moderate” and “high” risk based on a predicted probability of the outcome of interest. However, the accuracy of such an algorithm cannot be assessed by comparison with a gold standard, because the only indicator of test performance is whether or not an individual experiences the predicted outcome, an event that cannot be observed at the time of testing. In these cases, the only available information regarding the relationship between predicted probability and actual outcome is from a dataset in which the algorithm has been validated.
Economic evaluation of risk-targeted interventions requires explicit consideration of the threshold risk criterion. Figure 2 shows hypothetical distributions of the predicted risk of disease recurrence in two groups of women treated for early-stage breast cancer: those who will, in fact, experience a recurrence and those who will not. In Panel A the threshold is relatively strict; the intervention is given to a small proportion of patients, all of whom would have had a disease recurrence in the absence of the intervention. When the criterion is more lenient and individuals with a lower predicted risk are eligible for the intervention (Panel B), more patients who would have recurred receive the intervention, but so too will some women who never would have had a disease recurrence. The tradeoffs associated with the threshold risk criterion – and the resulting costs, risks and benefits of the test and intervention – will influence the cost-effectiveness of a risk-targeted strategy.
Figure 2.
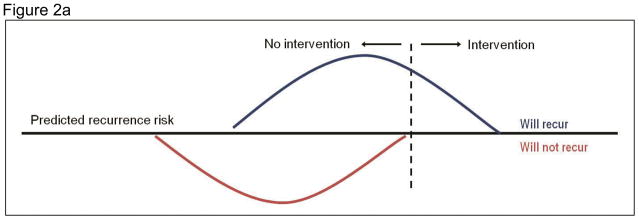
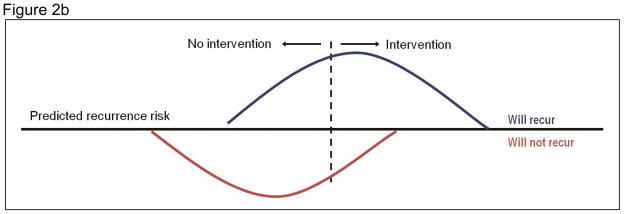
Thresholds for a targeted intervention by predicted risk and actual outcome
Each panel shows hypothetical distributions of the predicted risk of disease recurrence in two groups of women treated for early-stage breast cancer: those who will experience a recurrence (top curve) and those who are not (bottom curve). The patients in each distribution to the right of the threshold risk criterion (dashed line) receive the intervention. Panel A depicts a stricter threshold for a targeted intervention; Panel B depicts a more lenient threshold.
Targeted Interventions in Breast Cancer
Several targeted interventions have been widely implemented or recommended for breast cancer risk reduction and treatment (Figure 3). Tests to identify target populations for these interventions include germline genotyping, risk prediction algorithms, tumor protein assays, tumor single-gene assays and tumor multi-gene signatures. The maturity of targeted breast cancer interventions and the availability of CEAs of these interventions provide useful examples for illustrating the relationship between targeting tests and interventions, and the importance of this relationship in economic analysis.
Figure 3.
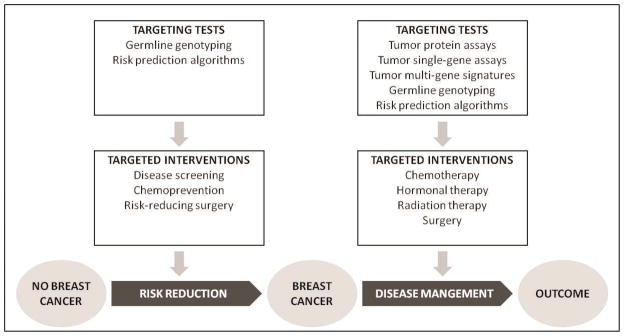
Targeting test modalities and targeted interventions in the breast cancer disease continuum
Economic Evaluations of Targeted Risk Reduction and Screening Strategies
Germline genetic testing and multifactorial risk prediction algorithms can identify women at high risk of developing breast cancer and target preventive or screening interventions to them. Specific mutations in the BRCA1 and BRCA2 genes are associated with a substantially increased risk of breast cancer among carriers.5 Breast cancer risk prediction models use relevant health information to predict a woman’s risk of developing breast cancer. For example, the NCI Breast Cancer Risk Assessment Tool, based on the Gail model, predicts the risk of breast cancer based on a woman’s age, the number of first-degree relatives with a history of breast cancer, number of prior breast biopsies, atypical hyperplasia in a biopsy specimen, age at menarche, and age at first live birth.6 Other models use a more limited set of risk factors and may or may not include information about BRCA1 and BRCA2 mutations.7
Among women at high risk of developing breast cancer, chemoprevention, risk-reducing surgery and intensive screening may prevent breast cancer or facilitate its detection at an early stage. In randomized clinical trials, two selective estrogen receptor modulators – tamoxifen and raloxifene – reduced the incidence of breast cancer in high-risk women.8, 9 Risk-reducing bilateral mastectomy, with or without bilateral oophorectomy, has been evaluated predominantly in women with BRCA mutations, and has been shown to substantially reduce breast cancer incidence in these women.10, 11 Screening with advanced imaging modalities, including magnetic resonance imaging (MRI), ultrasound and digital mammography, detects more cancers in high-risk women, compared with conventional mammography alone, although the impact of these strategies on breast cancer outcomes is uncertain.12–14
Several studies have assessed the cost-effectiveness of interventions to prevent breast cancer or detect it early in high-risk women, with estimated risk based on BRCA mutation status or a risk prediction algorithm (Table 1). Hershman et al. and Noe et al. evaluated the cost-effectiveness of tamoxifen chemoprevention in high-risk women using data and assumptions from the Breast Cancer Prevention Trial (BCPT).15, 16 These studies focused on subgroups of women defined by breast cancer risk as predicted by the Gail model or by specific individual risk factors. Other studies have compared the cost-effectiveness of multiple risk reduction strategies including chemoprevention, risk-reducing surgery and intensive surveillance, in women with a BRCA1 or BRCA2 mutation.17, 18 Several investigators have studied the cost-effectiveness of MRI screening in high-risk cohorts defined by BRCA mutation status or an algorithm-based risk prediction.19–21
Table 1.
Cost-effectiveness studies of targeted breast cancer risk reduction interventions
| Study | Intervention | Targeted group | Model inception cohort | Method of targeting evaluated? |
|---|---|---|---|---|
| Hershman et al. (2002) | Chemoprevention | High-risk women | Women with elevated risk by specific characteristics | Separate analysis by risk group |
| Noe et al. (1999) | Chemoprevention | High-risk women | Women with elevated risk by specific characteristics | No |
| Anderson et al. (2006) | Risk-reducing surgery; screening; chemoprevention | BRCA1/2 mutation carriers | BRCA1/2 mutation carriers | No |
| Moore et al. (2009) | MRI screening | High-risk women | Women with ≥15% lifetime risk by Claus algorithm | No |
| Plevritis et al. (2006) | MRI screening | BRCA1/2 mutation carriers | BRCA1/2 mutation carriers | Separate analysis by BRCA mutation; sensitivity analysis of breast cancer risk |
| Taneja et al. (2009) | MRI screening | High-risk women | BRCA1/2 mutation carriers or high risk by specific characteristics | Separate analysis for gene mutation carriers and others |
| Grann et al. (1999) | Risk-reducing surgery; screening | BRCA1/2 mutation carriers | Cancer-free women of Ashkenazi Jewish descent | Analysis explicitly focused on testing |
| Holland et al. (2009) | Risk-reducing surgery | BRCA1/2 mutation carriers | Cancer-free women with concern or family history | Analysis explicitly focused on testing |
| Kwon et al. (2010) | Risk-reducing surgery | BRCA1/2 mutation carriers | Breast cancer patients under age 50 | Analysis explicitly focused on testing |
The aforementioned studies all used decision-analytic modeling to simulate long-term health outcomes and costs, using information about breast cancer risk from clinical trials. While most performed sensitivity analysis of breast cancer risk estimates, not all explicitly assessed the relationship between the method of targeting and the outcomes of the targeted intervention. Hershman et al. performed separate analysis of four subgroups characterized by observable risk factors, finding substantial variation in the incremental cost-effectiveness of tamoxifen chemoprevention as a function of breast cancer risk.15 Anderson et al. varied breast cancer risk estimates in BRCA mutation carriers and found that while the magnitude of incremental cost-effectiveness ratios (ICERs) changed, risk-reducing surgery remained preferable to chemoprevention or surveillance.18 Similarly, Grann et al. separately analyzed women with a BRCA1 mutation, BRCA2 mutation or mutation in both genes, and found that the incremental cost-effectiveness of the strategies evaluated varied among these groups.17 Taneja et al. separately analyzed BRCA mutation carriers and women at increased risk due to other factors, finding that the incremental cost-effectiveness of targeted MRI screening varied considerably with estimated breast cancer risk.21 Plevritis et al. found a similar result, reporting that the incremental cost-effectiveness of targeted MRI screening was more sensitive to predicted breast cancer risk than to any other model parameter.20 In most of these studies, breast cancer risk was treated as a source of variability, not uncertainty. Thus, they did not directly address the impact of test performance on costs and health outcomes.
Most studies of targeted risk-reduction strategies have compared interventions for cohorts of women whose breast cancer risk is assumed to be known. Few studies have assessed the economic impact of the decision to perform a test that yields information about breast cancer risk. Grann et al. examined the cost-effectiveness of BRCA testing in cancer-free women of Ashkenazi Jewish descent.22 This analysis explicitly considered the costs and outcomes of the test itself, and found that the cost-effectiveness of testing varied based on the risk-reduction strategy (mastectomy, oophorectomy or surveillance) that was assumed to be administered to women who test positive for a BRCA mutation. Using a similar approach, Holland et al. estimated the cost-effectiveness of BRCA testing in cancer-free women concerned about a mutation or with a family history of breast or ovarian cancer.23 They found that the incremental cost-effectiveness of BRCA testing varied with the probability of a mutation and with the quality-of-life benefit associated with a negative test result.
Kwon et al. estimated the economic impact of BRCA testing in women newly diagnosed with breast cancer, finding that testing was most cost-effective for women with high-risk tumor features.24 Although this study focused on a different point in the breast cancer continuum, addressing a targeted disease-management intervention rather than a disease prevention intervention (Figure 3), it followed a design quite similar to the analyses of BRCA testing in cancer-free women, and, notably, explicitly considered costs and outcomes of the targeting test itself.
Economic Evaluations of Targeted Treatment Strategies
Efforts to target breast cancer treatment have focused primarily on systemic therapy. Beyond standard tumor characteristics such as size, regional lymph node involvement and hormone receptor status, newer methods of guiding systemic therapy decisions include assays to detect individual tumor genes and proteins and gene expression profiles based on the activity of multiple tumor genes. Examples of these strategies include HER2 testing to identify candidates for the monoclonal antibody trastuzumab and tumor gene expression profiling to guide the use of adjuvant chemotherapy.
Approximately 20–30% of breast cancers overexpress the human epidermal growth factor receptor-2 (HER2) protein, a product of the HER2/neu oncogene.25, 26 Trastuzumab, a monoclonal antibody, has demonstrated anti-tumor effects in HER2-overexpressing breast cancer, increasing progression-free survival in women with HER2-positive metastatic breast cancer and improving both disease-free and overall survival in women with HER2-positive early-stage breast cancer.27–29 The American Society of Clinical Oncology (ASCO), the College of American Pathologists (CAP) and the National Comprehensive Cancer Network (NCCN) recommend routine testing of all newly diagnosed breast cancers with immunohistochemistry (IHC), fluorescence in situ hybridization (FISH) or a combination of the two, and trastuzumab is only indicated for women with a positive HER2 test result.30, 31 However, IHC and FISH vary in their accuracy and performance characteristics, and the laboratories that perform these tests may vary in their procedures, accreditation and proficiency.
Many investigators have evaluated the cost-effectiveness of trastuzumab, but few explicitly examined methods of identifying trastuzumab candidates (Table 2). Elkin et al. compared seven strategies for targeting trastuzumab in women with metastatic breast cancer.32 Lidgren et al. performed a similar analysis in the adjuvant setting, comparing five strategies for targeting trastuzumab in women with early-stage breast cancer.33 Both studies found that strategies involving FISH, used alone or as confirmation of positive IHC results, were optimal for targeting trastuzumab therapy. They also demonstrated the sensitivity of results to assumptions about the characteristics of the targeting test. Both found that IHC alone was dominated by a strategy involving FISH if the specificity of IHC was less than 100%, and Elkin et al. found a three-fold difference in the incremental cost-effectiveness ratio (ICER) for FISH alone as the sensitivity of IHC varied between 50% and 99%. A third study compared seven different HER2 testing strategies, but measured effectiveness as the number of cases with accurately determined HER2 status, thus ignoring costs and outcomes of targeted treatment with trastuzumab.34
Table 2.
Cost-effectiveness studies of targeted breast cancer disease management interventions
| Study | Intervention | Targeted group | Model inception cohort | Method of targeting evaluated? |
|---|---|---|---|---|
| Elkin et al. (2004) | Trastuzumab in metastatic setting | Patients with HER2- positive disease | Patients with metastatic disease | HER2 testing strategies modeled explicitly |
| Norum et al. (2005) | Trastuzumab in metastatic setting | Patients with HER2- positive disease | Patients with HER2- positive disease | Included test costs, but did not compare testing strategies or evaluate test performance |
| Lidgren et al. (2008) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients post surgery and adjuvant chemotherapy | HER2 testing strategies modeled explicitly |
| Kurian et al. (2007) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Dendukuri et al. (2007) | Trastuzumab in adjuvant or metastatic setting | Patients with HER2- positive disease | All newly diagnosed patients | Analysis explicitly focused on testing; treatment costs and outcomes excluded |
| Garrison et al. (2007) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | Included test costs, but did not compare testing strategies or evaluate test performance |
| Skedgel et al. (2009) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Liberato et al. (2007) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Norum et al. (2007) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| NICE (2006) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Millar et al. (2007) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Dedes et al. (2007) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Neyt et al. (2008) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Shiroiwa et al. (2008) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Chen et al. (2009) | Adjuvant trastuzumab | Patients with HER2- positive disease | Patients with HER2- positive disease | No |
| Hornberger et al. (2005) | Adjuvant chemotherapy | Patients at high risk of recurrence by GEP | Patients with node- negative, HR-positive disease | Yes, but did not evaluate test result thresholds |
| Lyman et al. (2007) | Adjuvant chemotherapy | Patients at high risk of recurrence by GEP | Patients with node- negative, HR-positive disease | Yes, but did not evaluate test result thresholds |
| Cosler et al. (2009) | Adjuvant chemotherapy | Patients at high risk of recurrence by GEP | Patients with node- negative, HR-positive disease | Yes, but did not evaluate test result thresholds |
| Klang et al. (2010) | Adjuvant chemotherapy | Patients at high risk of recurrence by GEP | Patients with node- negative, HR-positive disease | Yes, but did not evaluate test result thresholds |
| Tsoi et al. (2010) | Adjuvant chemotherapy | Patients at high risk of recurrence by GEP | Patients with node- negative, HR-positive disease | Yes, but did not evaluate test result thresholds |
| Oestreicher et al. (2005) | Adjuvant chemotherapy | Patients at high risk of recurrence by GEP | Patients with node- negative, HR-positive disease | Comparison of test-treat strategies and sensitivity analysis of test performance |
BC: breast cancer
GEP: gene expression profile
HR: hormone receptor
Numerous other studies have examined the cost-effectiveness of trastuzumab but did not explicitly evaluate the impact of HER2 testing strategies. Rather, they assessed the cost-effectiveness of trastuzumab in a group already identified as having HER2-positive disease, generally by the same methods employed in one of the clinical trials from which they derived estimates of trastuzumab’s efficacy.35–45 Most ignored the costs of testing. Some assigned a total cost of testing per HER2-positive patient, reflecting the costs of testing all breast cancer patients in the relevant clinical setting, but they explicitly addressed neither test performance nor outcomes in women with a negative test result.37, 46 As illustrated in Figure 1, analyses that focused on a group already identified by a positive HER2 test result necessarily excluded the economic and health consequences of omitting trastuzumab in women with a negative – true or false –test result. Authors of one of these studies, commenting on the omission of testing strategies from their model, noted that if testing had been included, “the ICER for trastuzumab-based therapies would be less favorable.”38
In women with early-stage breast cancer, systemic adjuvant chemotherapy is often given to reduce the risk of disease recurrence following primary surgical treatment. The use of adjuvant chemotherapy has traditionally been guided by basic tumor characteristics, and it is generally the standard of care for women with large tumors, axillary lymph node involvement or tumors that are not hormone-responsive.30 In women with more favorable tumor characteristics, the absolute decrease in recurrence risk associated with adjuvant chemotherapy may be quite small, and therefore the benefits and risks of adjuvant chemotherapy must be weighed carefully. Gene expression profiling (GEP) involves analysis of tumor genes using DNA microarray or real-time polymerase chain reaction (RT-PCR) technology.47 The GEP tests commercially available in the US use tumor gene signatures from selected candidate genes to estimate a patient’s risk of disease recurrence, based on proprietary algorithms. GEP has been recommended as a tool for risk-stratifying patients who would not be candidates for adjuvant chemotherapy based solely on tumor features such as size, hormone receptor status and lymph node involvement.30, 31 In these women GEP may distinguish those who will benefit most and least from adjuvant chemotherapy.
Six studies have assessed the cost-effectiveness of GEP for targeting adjuvant chemotherapy (Table 2). Five evaluated adjuvant chemotherapy assignment based on the OncotypeDX 21-gene RT-PCR assay in women with node-negative, estrogen receptor-positive disease, compared with NCCN treatment guidelines,48 with universal chemotherapy or no chemotherapy,49, 50 with treatment guided by the Adjuvant! Online risk prediction algorithm51 and with pre-test physician recommendations.52 Targeting adjuvant chemotherapy with GEP was either cost-saving or was associated with a modest ICER, compared with NCCN guidelines and with strategies of universal chemotherapy or no chemotherapy.48–50 Compared with Adjuvant! Online, GEP-based chemotherapy assignment had cost about $63,000 per QALY gained.51 In all of these studies treatment assignment based on GEP relied on the manufacturer’s classification of the test result, or recurrence score, into groups of “low,” “intermediate” and “high” risk, and all assumed that in a GEP-based strategy, chemotherapy would be given to those in the intermediate- and high-risk groups. Although all performed sensitivity analysis on the recurrence risk estimates associated with each group, none evaluated strategies that involved alternative risk-stratified treatment assignment, and none explored the relationship between the thresholds for classifying recurrence scores into risk groups and the cost-effectiveness of using GEP to target chemotherapy. One study reported that results were most sensitive to assumptions about probabilities “relating to risk groups and recurrence rates.”51
Oestreicher et al. evaluated the Mammaprint 70-gene microarray-based assay in a hypothetical cohort of women younger than 55 years with Stage I or node-negative Stage II disease.53 Compared with NIH consensus treatment guidelines, adjuvant chemotherapy assignment based on GEP was less costly, but also less effective. In sensitivity analysis the authors evaluated the impact of each strategy’s performance characteristics, which were estimated from the dataset in which the GEP assay was originally validated. They also explored the relationship between cost-effectiveness and the test result cutoff for defining high-risk women, and they found that at any threshold, the GEP-based strategy did not attain the sensitivity of at least 95% which would make it more cost-effective than the guideline-based strategy.
Recommendations for Economic Assessment of Targeted Interventions
While targeted breast cancer interventions have been the subject of numerous CEAs, deficiencies in these analyses prompt concern. Most importantly, few of the studies we identified explicitly evaluated the relationship between the method of targeting, the accuracy of the targeting test and the outcomes of the targeted intervention. Of those that did, some directly compared alternative targeting strategies in their base-case models, while others explored assumptions about alternative targeting strategies or test accuracy in sensitivity analysis. Regardless of the analytic method by which they evaluated alternative targeting strategies or test accuracy, all of the studies that did so reached a similar conclusion: assumptions about the targeting test had a substantial impact on the estimated cost-effectiveness of the targeted intervention. Based on these findings, we offer the following recommendations for economic analyses of targeted cancer interventions:
CEAs of targeted interventions should explicitly consider the costs and outcomes of the method of targeting.
When the method of targeting is a test that can be compared with a gold standard, the analyst should explicitly consider the impact of the test’s performance characteristics on costs and health outcomes.
When the method of targeting is a risk prediction algorithm, the analyst should explicitly consider the impact of thresholds for risk group definitions and subsequent actions on costs and health outcomes.
The manner in which testing and risk prediction are explicitly considered may vary, depending on the clinical context. In cases where a single test and its positivity criterion are already established and widely used in routine practice, then an economic analysis of newer or experimental interventions for the targeted group might reasonably exclude test costs and accuracy from the quantitative analysis. In these cases, analysts should, at a minimum, acknowledge the role of the targeting test and comment on its cost and accuracy. However, the impacts of test cost and performance merit formal analysis when there is more than one testing option, when test timing is variable, when the positivity criterion or risk threshold is not well established or when a new test becomes available. In addition, economic evaluations may be used to challenge established testing practices if new scientific discoveries suggest that alternative tests or non-targeted strategies may be more effective or cost-effective.
Our recommendations should not be excessively burdensome to analysts using decision-analytic simulation. Decision analysis is a formal, systematic and quantitative method for comparing the outcomes of alternative clinical strategies under circumstances of uncertainty.54, 55 Although cost-effectiveness may also be estimated in the context of a randomized clinical trial, decision analysis offers a number of unique advantages. In a decision analysis, the analyst can compare more strategies over a longer time horizon than are typically feasible in the context of a clinical trial. Decision analysis is well suited to answering the important questions of how and when to use a targeting test, which of several available tests to use, and which action to take in response to a test result. A decision analysis can also simulate factors such as patient non-adherence which influence outcomes in routine practice settings but are often minimized in a clinical trial.
While the usefulness of a decision-analytic simulation depends on the quality of available data, decision analysis lends itself quite readily to the evaluation of alternative strategies and assumptions about important parameters such as test performance. Even with limited data, the analyst can model hypothetical scenarios to assess the robustness of results to assumptions about the method of targeting. And as technologies evolve over time, decision-analytic simulations can evaluate new scenarios. Such information is essential to clinicians, payers and others who must often make decisions about the use of new technologies before clinical trial results are available.
Conclusions and Future Applications
Targeted cancer prevention, screening and treatment offer the promise of maximizing health benefits while minimizing health risks to yield the optimal gain from limited resources. The costs of cancer care have increased dramatically over time, and out-of-pocket spending can pose a serious financial burden to patients and their families.56 The high cost of many new targeted interventions, especially targeted therapeutic agents, warrants their economic evaluation. As studies of targeted breast cancer interventions demonstrate, the strategy used to target an intervention is likely to have a substantial impact on the cost-effectiveness of the intervention. Consequently, an economic analysis that fails to consider the accuracy of the targeting test or alternative methods of targeting does not sufficiently inform decisions about adoption of the targeted intervention.
The examples reviewed here represent the most mature applications of targeted interventions in breast cancer. Numerous other targeted interventions are currently under investigation. These include selection of endocrine therapy based on CYP2D6 genotype57, 58 and omission of radiation therapy in women with specific single nucleotide polymorphisms associated with an increased risk of radiation toxicity.59 The economic assessment of targeted interventions is relevant to other cancers as well. In colorectal cancer, for example, emerging targeted interventions include intensive screening and prophylactic surgery in individuals with Lynch syndrome or familial adenomatous polyposis who are at high risk of disease due to a germline genetic mutation,60–63 and chemotherapy assignment based on mutations in the KRAS gene and UGT1a1 gene,64, 65 genes associated with response to or adverse effects of specific chemotherapeutic agents.
Targeted risk reduction, prevention, screening and treatment are motivating substantial research activity in breast, colorectal and other cancers. As discoveries move from bench to bedside, their high costs prompt concern about the allocation of limited health care resources. Comparative effectiveness research – including cost-effectiveness analysis – has been enthusiastically promoted to support informed medical decision making.66, 67 Analyses that follow the recommendations offered here will best inform the optimal deployment and utilization of targeted cancer interventions.
Contributor Information
Elena B. Elkin, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY.
Deborah A. Marshall, Department of Community Health Sciences, University of Calgary, Calgary, Alberta. Centre for Evaluation of Medicines, McMaster University, Hamilton, Ontario.
Nathalie A. Kulin, Department of Community Health Sciences, University of Calgary, Calgary, Alberta.
Ilia L. Ferrusi, Centre for Evaluation of Medicines, McMaster University, Hamilton, Ontario.
Michael J. Hassett, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA.
Uri Ladabaum, Department of Medicine, Stanford University, Stanford, CA.
Kathryn A. Phillips, UCSF Center for Translational and Policy Research on Personalized Medicine (TRANSPERS) and Department of Clinical Pharmacy, University of California-San Francisco, San Francisco, CA.
References
- 1.Neumann PJ. Costing and perspective in published cost-effectiveness analysis. Med Care. 2009;47:S28–32. doi: 10.1097/MLR.0b013e31819bc09d. [DOI] [PubMed] [Google Scholar]
- 2.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. New York: Oxford University Press; 2005. [Google Scholar]
- 3.Gold MR, Siegel JE, Russell LB, et al. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 4.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford University Press; 2006. [Google Scholar]
- 5.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi CE, de Bock GH, Siegerink B, et al. Differences and similarities in breast cancer risk assessment models in clinical practice: which model to choose? Breast Cancer Res Treat. 2009;115:381–390. doi: 10.1007/s10549-008-0070-x. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. J Amer Med Assoc. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 10.Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olopade OI, Artioli G. Efficacy of risk-reducing salpingo-oophorectomy in women with BRCA-1 and BRCA-2 mutations. Breast J. 2004;10(Suppl 1):S5–9. doi: 10.1111/j.1524-4741.2004.101s3.x. [DOI] [PubMed] [Google Scholar]
- 12.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. J Amer Med Assoc. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 14.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 15.Hershman D, Sundararajan V, Jacobson JS, et al. Outcomes of tamoxifen chemoprevention for breast cancer in very high-risk women: a cost-effectiveness analysis. J Clin Oncol. 2002;20:9–16. doi: 10.1200/JCO.2002.20.1.9. [DOI] [PubMed] [Google Scholar]
- 16.Noe LL, Becker RV, 3rd, Gradishar WJ, et al. The cost effectiveness of tamoxifen in the prevention of breast cancer. Am J Manag Care. 1999;5:S389–406. [PubMed] [Google Scholar]
- 17.Grann V, Patel P, Jacobson J, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2010:1–11. doi: 10.1007/s10549-010-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson K, Jacobson JS, Heitjan DF, et al. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann Intern Med. 2006;144:397–406. doi: 10.7326/0003-4819-144-6-200603210-00006. [DOI] [PubMed] [Google Scholar]
- 19.Moore SG, Shenoy PJ, Fanucchi L, et al. Cost-effectiveness of MRI compared to mammography for breast cancer screening in a high risk population. BMC Health Serv Res. 2009;9:9. doi: 10.1186/1472-6963-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. J Amer Med Assoc. 2006;295:2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- 21.Taneja C, Edelsberg J, Weycker D, et al. Cost effectiveness of breast cancer screening with contrast-enhanced MRI in high-risk women. J Am Coll Radiol. 2009;6:171–179. doi: 10.1016/j.jacr.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Grann VR, Whang W, Jacobson JS, et al. Benefits and costs of screening ashkenazi Jewish women for BRCA1 and BRCA2. J Clin Oncol. 1999;17:494. doi: 10.1200/JCO.1999.17.2.494. [DOI] [PubMed] [Google Scholar]
- 23.Holland ML, Huston A, Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health. 2009;12:207–216. doi: 10.1111/j.1524-4733.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Kwon JS, Gutierrez-Barrera AM, Young D, et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol. 2010;28:4214–4220. doi: 10.1200/JCO.2010.28.0719. [DOI] [PubMed] [Google Scholar]
- 25.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 26.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the Her-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 27.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 28.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 29.Slamon D, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 30.NCCN Clinical Practice Guidelines in Oncology™ Breast Cancer (Version 1.2009) National Comprehensive Cancer Network, Inc; 2009. [Google Scholar]
- 31.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 32.Elkin EB, Weinstein MC, Winer EP, et al. HER-2 testing and trastuzumab therapy for metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol. 2004;22:854–863. doi: 10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 33.Lidgren M, Jonsson B, Rehnberg C, et al. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol. 2008;19:487–495. doi: 10.1093/annonc/mdm488. [DOI] [PubMed] [Google Scholar]
- 34.Dendukuri N, Khetani K, McIsaac M, et al. Testing for HER2-positive breast cancer: a systematic review and cost-effectiveness analysis. Cmaj. 2007;176:1429–1434. doi: 10.1503/cmaj.061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Jiang Z, Shao Z, et al. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health. 2009;12(Suppl 3):S82–84. doi: 10.1111/j.1524-4733.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- 36.Dedes KJ, Szucs TD, Imesch P, et al. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a model-based analysis of the HERA and FinHer trial. Ann Oncol. 2007;18:1493–1499. doi: 10.1093/annonc/mdm185. [DOI] [PubMed] [Google Scholar]
- 37.Garrison LP, Lubeck D, Lalla D, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007;110:489–498. doi: 10.1002/cncr.22806. [DOI] [PubMed] [Google Scholar]
- 38.Kurian AW, Thompson RN, Gaw AF, et al. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25:634–641. doi: 10.1200/JCO.2006.06.3081. [DOI] [PubMed] [Google Scholar]
- 39.Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2007;25:625–633. doi: 10.1200/JCO.2006.06.4220. [DOI] [PubMed] [Google Scholar]
- 40.Millar JA, Millward MJ. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoecon. 2007;25:429–442. doi: 10.2165/00019053-200725050-00006. [DOI] [PubMed] [Google Scholar]
- 41.National Institutes for Health and Clinical Excellence. NICE technology appraisal guidance. 2006. Trastuzumab for the adjuvant treatment of earlystage HER2-positive breast cancer. [Google Scholar]
- 42.Neyt M, Huybrechts M, Hulstaert F, et al. Trastuzumab in early stage breast cancer: a cost-effectiveness analysis for Belgium. Health Policy. 2008;87:146–159. doi: 10.1016/j.healthpol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Norum J, Olsen JA, Wist EA, et al. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol. 2007;46:153–164. doi: 10.1080/02841860601096841. [DOI] [PubMed] [Google Scholar]
- 44.Shiroiwa T, Fukuda T, Shimozuma K, et al. The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat. 2008;109:559–566. doi: 10.1007/s10549-007-9679-4. [DOI] [PubMed] [Google Scholar]
- 45.Skedgel C, Rayson D, Younis T. The Cost-Utility of Sequential Adjuvant Trastuzumab in Women with Her2/Neu-Positive Breast Cancer: An Analysis Based On Updated Results from the HERA Trial. Value in Health. 2009;12:641–648. doi: 10.1111/j.1524-4733.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 46.Norum J, Risberg T, Olsen JA. A monoclonal antibody against HER-2 (trastuzumab) for metastatic breast cancer: a model-based cost-effectiveness analysis. Ann Oncol. 2005;16:909–914. doi: 10.1093/annonc/mdi188. [DOI] [PubMed] [Google Scholar]
- 47.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 48.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 49.Cosler LE, Lyman GH. Economic analysis of gene expression profile data to guide adjuvant treatment in women with early-stage breast cancer. Cancer Invest. 2009;27:953–959. doi: 10.3109/07357900903275217. [DOI] [PubMed] [Google Scholar]
- 50.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 51.Tsoi DT, Inoue M, Kelly CM, et al. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist. 2010;15:457–465. doi: 10.1634/theoncologist.2009-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klang SH, Hammerman A, Liebermann N, et al. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health. 2010;13:381–387. doi: 10.1111/j.1524-4733.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 53.Oestreicher N, Ramsey SD, Linden HM, et al. Gene expression profiling and breast cancer care: what are the potential benefits and policy implications? Genet Med. 2005;7:380–389. doi: 10.1097/01.gim.0000170776.31248.75. [DOI] [PubMed] [Google Scholar]
- 54.Hunink M, Glasziou P, Siegel J, et al. Decision making in health and medicine: integrating evidence and values. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 55.Marshall DA, O’Brien BJ. Economic evaluation of diagnostic tests. In: Price C, Christenson R, editors. Evidence-Based Laboratory Medicine: From Principles to Outcomes. Washington, DC: American Association for Clinical Chemistry; 2003. pp. 159–18. [Google Scholar]
- 56.Elkin EB, Bach PB. Cancer’s next frontier: addressing high and increasing costs. J Amer Med Assoc. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. J Amer Med Assoc. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Punglia RS, Burstein HJ, Winer EP, et al. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst. 2008;100:642–648. doi: 10.1093/jnci/djn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang-Claude J, Ambrosone CB, Lilla C, et al. Genetic polymorphisms in DNA repair and damage response genes and late normal tissue complications of radiotherapy for breast cancer. Br J Cancer. 2009;100:1680–1686. doi: 10.1038/sj.bjc.6605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 61.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 62.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. J Amer Med Assoc. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 63.Giardiello FM, Brensinger JD, Petersen GM, et al. The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. N Engl J Med. 1997;336:823–827. doi: 10.1056/NEJM199703203361202. [DOI] [PubMed] [Google Scholar]
- 64.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 65.Massacesi C, Terrazzino S, Marcucci F, et al. Uridine diphosphate glucuronosyl transferase 1A1 promoter polymorphism predicts the risk of gastrointestinal toxicity and fatigue induced by irinotecan-based chemotherapy. Cancer. 2006;106:1007–1016. doi: 10.1002/cncr.21722. [DOI] [PubMed] [Google Scholar]
- 66.Information on cost-effectiveness: an essential product of a national comparative effectiveness program. Ann Intern Med. 2008;148:956–961. doi: 10.7326/0003-4819-148-12-200806170-00222. [DOI] [PubMed] [Google Scholar]
- 67.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]