Abstract
Epithelial ion transport is essential to renal homeostatic function, and it is dysregulated in several diseases, such as hypertension. An understanding of the insect renal (Malpighian) tubule yields insights into conserved epithelial ion transport processes in higher organisms and also has implications for the control of insect infectious disease vectors. Here, we examine the role of the Na+-K+-2Cl− (NKCC) cotransporter Ncc69 in Drosophila tubule function. Ncc69 mutant tubules have decreased rates of fluid secretion and K+ flux, and these phenotypes were rescued by expression of wild-type Ncc69 in the principal cells of the tubule. Na+ flux was unaltered in Ncc69 mutants, suggesting Na+ recycling across the basolateral membrane. In unstimulated tubules, the principal role of the Na+-K+-ATPase is to generate a favorable electrochemical gradient for Ncc69 activity: while the Na+-K+-ATPase inhibitor ouabain decreased K+ flux in wild-type tubules, it had no effect in Ncc69 mutant tubules. However, in the presence of cAMP, which stimulates diuresis, additional Na+-K+-ATPase-dependent K+ transport pathways are recruited. In studying the effects of capa-1 on wild-type and Ncc69 mutant tubules, we found a novel antidiuretic role for this hormone that is dependent on intact Ncc69, as it was abolished in Ncc69 mutant tubules. Thus, Ncc69 plays an important role in transepithelial ion and fluid transport in the fly renal tubule and is a target for regulation in antidiuretic states.
Keywords: Malpighian tubule, epithelial ion transport, capa-1, Na+-K+-ATPase, diuretic
epithelial ion transport is central to the kidney's role in maintaining internal homeostasis in the face of external challenges, such as fluid or solute ingestion. The insect renal epithelium, the Malpighian tubule, is a relatively simple system in which to study these processes, with implications for conserved processes in human kidney and for the eradication of insect disease vectors (17). The physiologic study of Drosophila melanogaster tubules, in which powerful genetics can be combined with pharmacologic techniques, was established in 1994 by Dow and colleagues (16).
The Drosophila renal excretory system consists of four Malpighian tubules, an anterior pair and a posterior pair, which empty into the gut at the midgut/hindgut junction (18). Because the tubules are blind-ended, all ion and water transport occurs by transepithelial flux. There are four segments and two cell types in the Drosophila renal tubule (73). In the main segment, which is K+- and water-secreting (53), cation flux occurs through principal cells, in which the apical H+-ATPase drives proton secretion into the lumen (1, 10, 14, 16, 20, 52) (see Fig. 4B). It is thought that protons are then exchanged for Na+ and K+ through apical H+/cation exchangers, allowing cation secretion (13). Chloride flux occurs through stellate cells, probably through chloride channels, although the genes encoding these channels have not been identified (52, 55). Water follows the movement of KCl and NaCl, possibly through aquaporins, several of which are expressed in the fly tubule (39).
Fig. 4.
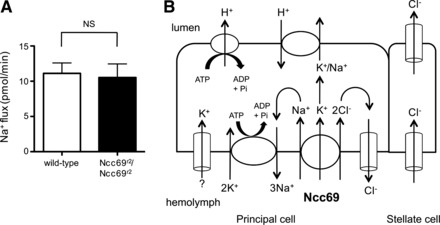
Ncc69 does not serve as a pathway for transepithelial Na+ flux. A: Na+ flux (pmol/min per tubule) was measured in tubules from wild-type (n = 14) and Ncc69r2 homozygous mutants (n = 13). Transepithelial Na+ flux was not impaired in Ncc69 mutants, implying that Na+ transported by Ncc69 is recycled across the basolateral membrane. B: model for transepithelial ion fluxes in unstimulated tubules. K+ is taken up from the hemolymph across the principal cell basolateral membrane, together with Na+ and Cl−, by Ncc69, as well as by other unknown K+ transport pathways (indicated by “?”), and is secreted into the lumen through the H+/cation (Na+ or K+) exchanger. Na+ and Cl− are likely both recycled across the basolateral membrane. The Na+-K+-ATPase generates the electrochemical gradient that allows secondary active transport through Ncc69. The apical H+-ATPase, which secretes H+ into the tubule lumen against its concentration gradient, generates the electrochemical gradient, allowing H+/K+ exchange. Transepithelial Cl− flux occurs through the stellate cell.
Work in several insect species, including Drosophila, has implicated Na+-K+-2Cl− (NKCC) cotransport in the uptake of cations from the hemolymph into the principal cell across the basolateral membrane (27, 30, 31, 33, 35, 37, 44, 54). These studies have relied on the use of the pharmacologic NKCC inhibitor bumetanide, as well as the determination of electrochemical gradients for Na+, K+, and Cl−. To date, the genes encoding insect tubule NKCCs have not been identified. Two genes with sequence homology to the mammalian sodium-chloride cotransporter (NCC)/NKCC family exist in Drosophila: CG31547 (also called Ncc83) and Ncc69 (42, 75). Of these, Ncc69 has been shown to encode a bona fide Na+-K+-2Cl− cotransporter (42, 75). Ncc69 mutants have a glial defect, possibly due to impaired ion transport across the glial cell membrane (42), but tubule function has not previously been studied.
The apical H+-ATPase is thought to be the primary driver of transepithelial ion and water flux in the insect tubule, while the role of the Na+-K+-ATPase has been less clear. Bafilomycin, a H+-ATPase inhibitor, completely abolishes fluid secretion from the fly tubule, while ouabain, a Na+-K+-ATPase inhibitor, does not inhibit fluid secretion (16, 45). This led to the initial proposal that the Na+-K+-ATPase was not important in fly tubule function (16). However, Na+-K+-ATPase expression has been demonstrated in the Drosophila Malpighian tubule, including on the basolateral membrane of principal cells, by transgenic (74), enhancer trap (71), immunofluorescence (41, 72), and electrophysiologic (45) techniques, and more recent studies have suggested that the Na+-K+-ATPase does play a functional role in tubule physiology (35, 45, 78).
Insect tubules, like other renal epithelia, are under the regulatory control of peptide hormones. In Drosophila, three classes of hormones have been described: those that are cAMP-coupled (Drome-DH31 and Drome-DH44) (8, 9); the Ca2+/nitric oxide (NO)/cGMP-coupled capability-1 (capa-1) (6, 7, 11, 12, 15, 40, 70); and Ca2+-coupled leucokinin (66, 77). All three classes of peptides have homologs in other insect species. In Drosophila, the cAMP and cGMP-coupled hormones stimulate the principal cell by increasing apical H+-ATPase activity, while leucokinin stimulates chloride flux through the stellate cells via Ca2+ signaling (9, 52, 55, 66, 77). Thus, these hormones all stimulate diuresis, allowing the fly to avoid retaining excess fluid and solute after food ingestion. However, between meals, insects, which have a high surface area-to-volume ratio, must avoid desiccation, and in other insects, antidiuretic hormones have been described. The best studied example is the Chagas' disease vector Rhodnius prolixus. The transport mechanisms of the Rhodnius tubule are similar to those found in the Drosophila principal cell, with an apical H+-ATPase and cation/H+ exchanger and basolateral Na+-K+-ATPase and NKCC (33, 34, 36, 37, 47, 54). In Rhodnius, two peptides related to Drosophila capa-1, MasCAP2b and RhoprCAPA-α2, have antidiuretic effects (38, 58–61, 64, 65). To date, however, no antidiuretic hormones have been described in Drosophila.
Here, we demonstrate that the NKCC Ncc69 is important for transepithelial fluid and K+ flux in Drosophila by acting in principal cells. A major role for the Na+-K+-ATPase in unstimulated tubules is to support Ncc69 cotransport and prevent excess urinary loss of Na+. In stimulated tubules, additional Na+-K+-ATPase-dependent pathways are also important. While Ncc69 is not required for the diuretic actions of cAMP and leucokinin, the transporter is required for a novel antidiuretic activity of capa-1. Thus, Ncc69 is important for an integrated response to the homeostatic challenges posed by a varying external milieu, in which periods of desiccation threat, characterized by the lack of food and water, are punctuated by bouts of fluid and solute intake.
MATERIALS AND METHODS
Chemicals and reagents.
All chemicals and reagents were from Sigma (St. Louis, MO) unless otherwise specified.
Fly stocks and genetics.
The following Drosophila melanogaster strains were used: w−Berlin (wild-type), obtained from Dr. Adrian Rothenfluh [Univ. of Texas (UT) Southwestern Medical Center, Dallas, TX]; w; Ncc69r2 (crossed to w−Berlin to obtain w; Ncc69r2/+ heterozygotes) and w; UAS-Ncc69-HA Ncc69r2, obtained from Dr. William Leiserson (Yale University, New Haven, CT) (42); w; Df(3L)BSC380/TM6C Sb, a genomic deletion in which the Ncc69 locus is completely deleted, obtained from the Bloomington Stock Center (Bloomington, IN); and w; c42-GAL4, expressing GAL4 in the principal cells of the main and lower segments, as well as bar-shaped cells in the initial and transitional segments (70), obtained from Dr. Julian Dow (University of Glasgow, Glasgow, UK) and outcrossed for five generations to w−Berlin. Flies were reared at room temperature (22–23°C) under ambient humidity and light-dark conditions on cornmeal/yeast/molasses food prepared in a central kitchen at University of Texas Southwestern. Female flies were collected within 48 h of eclosion and kept on standard food for 3–5 days before tubule dissection.
Ramsay assay and ion-specific electrodes.
The Ramsay assay was set up as previously described (16). Malpighian tubules were dissected from adult females under Drosophila saline consisting of the following (in mM): 117.5 NaCl, 20 KCl, 2 CaCl2, 8.5 MgCl2, 10.2 NaHCO3, 4.3 NaH2PO4, 15 HEPES, and 20 glucose, pH 7.0. Anterior tubules are more easily dissected and were used ∼90% of the time. Tubule pairs were transferred to a 10–20 μl bathing droplet consisting of standard bathing medium, a 1:1 mixture of Drosophila saline and Schneider's medium (Invitrogen, Carlsbad, CA), under mineral oil (Fisher, Pittsburgh, PA). The composition of Schneider's medium is as follows (in mM): 3.33 glycine, 2.3 l-arginine, 3.01 l-aspartic acid, 0.496 l-cysteine, 0.417 l-cystine, 5.44 l-glutamic acid, 12.33 l-glutamine, 2.58 l-histidine, 1.15 l-isoleucine, 1.15 l-leucine, 9.02 l-lysine hydrochloride, 5.37 l-methionine, 0.909 l-phenylalanine, 14.78 l-proline, 2.38 l-serine, 2.94 l-threonine, 0.49 l-tryptophan, 2.76 l-tyrosine, 2.56 l-valine, 5.62 β-alanine, 5.41 CaCl2, 15.06 MgSO4, 21.33 KCl, 3.31 KH2PO4, 4.76 NaHCO3, 36.21 NaCl, 4.94 Na2HPO4, 1.37 α-ketoglutaric acid, 11.11 d-glucose, 0.862 fumaric acid, 0.746 malic acid, 0.847 succinic acid, 5.85 trehalose, and 2,000 mg/l yeastolate. One tubule of the pair remained in the droplet, while the other tubule of the pair was wrapped around a Minutien pin (Fine Science Tools, Foster City, CA) as an anchor. Unless otherwise indicated, the secreted fluid droplet was examined at ∼2 h and its diameter was measured using an ocular micrometer in a dissecting stereomicroscope (Nikon, Melville, NY) at ×50 magnification. The volume of the droplet was calculated assuming spherical geometry (4/3 πr3). Secretion rate was calculated for each tubule by dividing volume by time.
Ion-specific and reference electrodes were prepared according to the method of Maddrell et al. (46). Unfilamented borosilicate glass capillaries with an outside diameter of 1.2 mm (Harvard Apparatus, Holliston, MA) were washed for 5 min with nitric acid, rinsed 3–5 times with deionized water, and dried on a hot plate set to 200°C for a minimum of 20 min. Pipettes were pulled to a tip size of 1–2 μm using a vertical pipette puller (Narishige, East Meadow, NY). They were then dried for at least 10 min on the hot plate and lightly silanized by application of a drop of dichlorodimethylsilane inside a 15-cm Pyrex dish, which was inverted over the pipettes on the hot plate for a minimum of 20 min. Silanized pipettes were stored over silica gel (Fisher) until use. For measuring K+ flux, backfill solution of 0.5 M KCl was added to the pipette, and a small amount of potassium ionophore I cocktail B was aspirated into the tip of the pipette by application of negative pressure. The reference electrode was prepared from filamented borosilicate glass capillaries with an outside diameter of 1.2 mm (Harvard Apparatus), pulled in a manner similar to the ion-specific electrode (ISE). The tip and shank were filled with 1 M sodium acetate and the electrode was backfilled with 3 M KCl. For measuring Na+ flux, sodium ionophore X was prepared in a cocktail containing 10% sodium ionophore X, 89.75% nitrophenyl octyl ether, and 0.25% sodium tetraphenyl borate (49, 58). The sodium ISE was backfilled with 150 mM NaCl, and the reference electrode was filled with 150 mM KCl. Selectivity of the potassium ISE for K+ compared with Na+ is >103.9, while selectivity of the sodium ISE for Na+ compared with K+ is >102.6 (49).
For K+ measurement, calibration drops consisting of 15, 75, 150, and 200 mM KCl were measured by immersing the reference and ion-specific electrodes into the fluid drop and recording the potential using a Digidata 1200 amplifier (Axon Instruments, Union City, CA) and an FD223a dual-channel electrometer (World Precision Instruments, Sarasota, FL). The ISE was calibrated before and after each set of experimental measurements. Slope/decile change in K+ concentration was calculated using the Nernst equation for the difference between 15 and 150 mM, 75 and 150 mM, and 150 and 200 mM, and the average slope was then calculated (Table 1). The mean ± SE slope/decile change in K+ concentration across all experiments was 53.67 ± 0.80 (n = 38). K+ activity in the experimental fluid was measured and the concentration was calculated according to the following equation:
where [K+]e is the potassium concentration of the experimental drop, [K+]c is the potassium concentration of the calibration drop, V is the change in potential (mV) between the experimental drop and the calibration drop, and s is the slope (mV) for a tenfold change in K+ concentration, determined by measurements from the calibration drops (Table 1). [K+]c was determined by the mean of the two 200 mM calibration drops (pre- and postexperiment). In the example given in Table 1, [K+]c = 31.25 (mean of 30.6 and 31.9). For Na+ measurement, 15 and 150 mM calibration drops were used, and Na+ concentration was calculated as for K+. The mean ± SE slope/decile change in Na+ concentration across all experiments was 49.34 ± 3.7 (n = 4). The K+ and Na+ flux of each tubule was calculated by multiplying K+ or Na+ concentration by the secretion rate.
Table 1.
Calibration of K+ ISE
| Potential, mV |
||
|---|---|---|
| Pre | Post | |
| KCl concentration, mM | ||
| 15 | −28.4 | −29.4 |
| 75 | 9.1 | 9.4 |
| 150 | 24.4 | 25.0 |
| 200 | 30.6 | 31.9 |
| Slope/Decile* | ||
|---|---|---|
| Interval, mM | ||
| 15–150 (pre) | 52.8 | |
| 15–150 (post) | 54.4 | |
| 75–150 (pre) | 51 | |
| 75–150 (post) | 52 | |
| 150–200 (pre) | 49.6 | |
| 150–200 (post) | 55.2 | |
| Mean | 52.5 | |
For the 15–150 mV interval, slope/decile = mV for the 150 mM calibration drop–mV for the 15 mM calibration drop. For the 75–150 interval, the difference is divided by 0.3 [log (150/75)]. For the 150–200 interval, the difference is divided by 0.125 [log (200/150)]. ISE, ion-specific electrode; Pre, before experiment; post, after experiment.
Pharmacology.
Ouabain was dissolved in hot H2O at a concentration of 20 mM and was added to standard bathing medium to obtain a final concentration of 100 μM. Bumetanide was dissolved in ethanol at a 100 mM concentration and was added to standard bathing medium to obtain a final concentration of 100 μM, or it was dissolved in ethanol at a 10 mM concentration and added to standard bathing medium to obtain a final concentration of 10 μM. Ouabain and bumetanide stocks were prepared fresh each day. Dibutyryl (db)-cAMP was dissolved in H2O at a concentration of 200 mM and was added to standard bathing medium at a final concentration of 1 mM. Tubules were bathed in the drug-containing standard bathing medium for the entirety of the experiment (2 h). As controls, tubules were bathed in standard bathing medium containing vehicle (H2O or ethanol) alone at the same concentration.
The capa-1 (GANMGLYAFPRV-amide) and Drosophila leucokinin (NSVVLGKKQRFHSWG-amide) peptides were synthesized by the UT Southwestern Protein Chemistry Core Facility (http://www.utsouthwestern.edu/research/core-facilities/protein-chemistry-technology-core/peptide-synthesis/index.html) and purified by reverse-phase HPLC to 98.6% (leucokinin) and 100% (capa-1) purity. Peptides were dissolved in H2O at a concentration of 10−4 M and added to standard bathing medium to achieve a final concentration of 10−7 M.
Statistics.
Results comprising two groups were compared using a two-sided unpaired t-test. For results with three or more groups, one-way ANOVA was used. Repeated-measures one-way ANOVA was used when the same tubule was analyzed at multiple time points. Bonferroni's test was used for post hoc testing of one-way ANOVA results. Significance level was set at P < 0.05. Values greater than three standard deviations from the mean were considered outliers and were excluded. All statistical analyses were performed using GraphPad Prism, version 5.0 (GraphPad Software, La Jolla, CA).
RESULTS
A role for the Ncc69 NKCC cotransporter in fluid secretion and K+ flux.
To examine a potential role for the Ncc69 NKCC cotransporter in transepithelial fluid and ion transport in the fly renal tubule, we examined the physiology of Ncc69 mutant tubules using the Ramsay assay. This assay measures fluid secretion by the main segment of the renal tubule (16). By measuring K+ and Na+ concentrations in the secreted fluid using ion-specific electrodes, K+ and Na+ flux can also be calculated (45, 46). Values for the diameter and K+ concentration of secreted fluid droplets from wild-type and Ncc69 mutant tubules are shown in Table 2. In wild-type tubules, rates of fluid secretion and K+ flux were 0.55 ± 0.02 nl/min and 93 ± 3.5 pmol/min, respectively (Fig. 1, A and B), similar to previously published values for fluid secretion and K+ flux in unstimulated tubules (16, 45). Fluid secretion and K+ flux were decreased in Ncc69r2 homozygous mutant tubules compared with controls, to 0.43 ± 0.03 nl/min and 62 ± 3.7 pmol/min, while Ncc69r2/+ heterozygotes had an intermediate phenotype of 0.46 ± 0.02 nl/min and 77 ± 3.5 pmol/min (Fig. 1, A and B). We also tested mutants transheterozygous for the Ncc69r2 mutant allele and a chromosome carrying a deficiency resulting in the deletion of the entire Ncc69 locus. Tubules from Ncc69r2/Df mutant flies had lower rates of fluid secretion (Fig. 1C) and K+ flux (Fig. 1D) compared with tubules from flies heterozygous for the deficiency, but did not have lower rates of fluid secretion (Fig. 1C) or K+ flux (Fig. 1D) compared with Ncc69r2/Ncc69r2 homozygotes. This indicates that Ncc69r2 is an amorphic (null) allele for the fluid secretion and K+ flux phenotypes, as is also the case for the axon-bulging phenotype observed by Leiserson et al. (42).
Table 2.
Droplet size and K+ concentration for wild-type and Ncc69 mutant tubules
| Diameter, μm | [K+], mM | Time, min | |
|---|---|---|---|
| Wild-type | 499 ± 7 | 167 ± 4 | 120 ± 0.2 |
| Ncc69r2/+ | 470 ± 7 | 169 ± 5 | 120 ± 0.1 |
| Ncc69r2/Ncc69r2 | 452 ± 9 | 151 ± 4 | 120 ± 0.1 |
| Df/+ | 526 ± 9 | 151 ± 3 | 120 ± 0.3 |
| Ncc69r2/Df | 476 ± 11 | 155 ± 5 | 120 ± 0.1 |
Values are means ± SE.
Fig. 1.
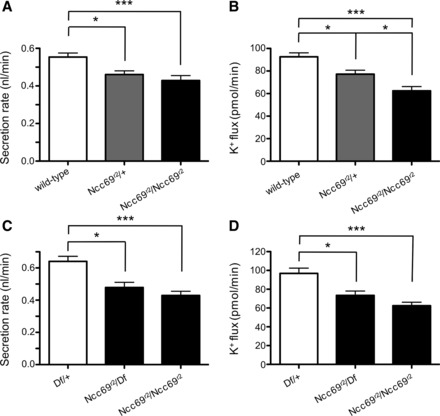
Fluid secretion rate and K+ flux are decreased in Ncc69 mutants. A: fluid secretion rate (nl/min per tubule) was measured in tubules from wild-type (n = 33), Ncc69r2 heterozygous (n = 32), and Ncc69r2 homozygous mutant (n = 34) flies. Secretion was decreased in both Ncc69r2 heterozygotes and homozygotes. In this and subsequent figures, values shown are means ± SE. *P < 0.05; ***P < 0.001. The difference between Ncc69r2/+ and Ncc69r2/Ncc69r2 was not significant (NS). B: K+ flux (pmol/min per tubule) was measured in tubules from wild-type (n = 33), Ncc69r2 heterozygous (n = 32), and Ncc69r2 homozygous mutant (n = 34) flies. K+ flux was decreased in both Ncc69r2 heterozygotes and homozygotes. *P < 0.05; ***P < 0.001. C: fluid secretion rate (nl/min per tubule) was measured in tubules from heterozygotes for a deficiency deleting the entire Ncc69 locus (Df/+, n = 14), in Ncc69r2/Df mutants (n = 14), and in Ncc69r2 homozygous mutants (n = 34, same tubules as in A and B, tested in parallel with Df/+ and Ncc69r2/Df). Ncc69r2/Df transheterozygotes did not have a greater decrease in fluid secretion than Ncc69r2 homozygotes, indicating that Ncc69r2 is an amorphic (null) allele for the fluid secretion phenotype. *P < 0.05; ***P < 0.001. The difference between Ncc69r2/Df and Ncc69r2/Ncc69r2 was NS. D: K+ flux (pmol/min per tubule) was measured in tubules from heterozygotes for a deficiency deleting the entire Ncc69 locus (Df/+, n = 14), in Ncc69r2/Df mutants (n = 14), and in Ncc69r2 homozygous mutants (n = 34, same tubules as in A and B, tested in parallel with Df/+ and Ncc69r2/Df). Ncc69r2/Df transheterozygotes did not have a greater decrease in K+ flux than Ncc69r2 homozygotes, indicating that Ncc69r2 is an amorphic (null) allele for the K+ flux phenotype. *P < 0.05; ***P < 0.001. The difference between Ncc69r2/Df and Ncc69r2/Ncc69r2 was NS.
To confirm that the impairment in secretion and K+ flux seen in Ncc69 mutants is due to the absence of the Ncc69 cotransporter, we expressed a wild-type Ncc69 transgene under the control of the c42-GAL4 driver using the GAL4-UAS system, which allows expression of transgenes in a spatially restricted manner (5). c42-GAL4 is expressed in principal cells of the main segment, but not in the stellate cells (70). Expression of Ncc69 in this pattern restored fluid secretion (Fig. 2A) and K+ flux (Fig. 2B) of Ncc69r2/Ncc69r2 mutant tubules to wild-type levels. This indicates that loss of Ncc69 in the principal cells of the main segment is responsible for the decreased fluid secretion and K+ flux seen in the Ncc69 mutants.
Fig. 2.
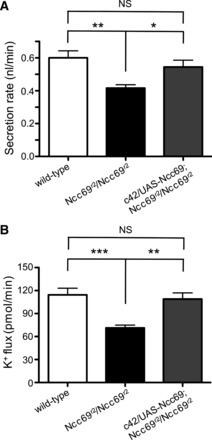
Expression of wild-type Ncc69 in Malpighian tubule principal cells restores fluid secretion rate and K+ flux to wild-type levels. A: fluid secretion rate (nl/min per tubule) was measured in tubules from wild-type, Ncc69r2 homozygous mutants, and Ncc69r2 homozygous mutants in which wild-type Ncc69 was expressed under the control of c42-GAL4 (c42/UAS-Ncc69; Ncc69r2/Ncc69r2; n = 18 for all genotypes). c42-GAL4 drives expression in the principal cells of the main segment. Expression of wild-type Ncc69 in these cells normalizes secretion. *P < 0.05; **P < 0.01. B: K+ flux (pmol/min per tubule) was measured in tubules from wild-type, Ncc69r2 homozygous mutants, and Ncc69r2 homozygous mutants in which wild-type Ncc69 was expressed under the control of c42-GAL4 (n = 18 for all genotypes). Expression of wild-type Ncc69 in the principal cells normalizes K+ flux. **P < 0.01; ***P < 0.001.
Ianowski and O'Donnell (35) have shown that the electrochemical gradients for K+ and Cl− favor movement of these two ions across the basolateral membrane of the principal cell from cell to bath (hemolymph). Therefore, a favorable electrochemical gradient for Na+ movement must exist for Ncc69 to transport K+, along with Na+ and Cl−, from the hemolymph into the principal cell. Typically, this gradient is established by the Na+-K+-ATPase. To test this, we compared the effect of ouabain, an inhibitor of the Na+-K+-ATPase, on wild-type and Ncc69r2 mutant tubules. Ouabain (100 μM) had no effect on fluid secretion in wild-type tubules (Fig. 3, A and D), a finding consistent with previously published data (16, 45), despite a decrease in luminal K+ concentration and decreased K+ flux (Fig. 3, B and C). This is likely due to an increase in luminal Na+ concentration (Ref. 45 and Fig. 3E), which results in increased Na+ flux (Ref. 45 and Fig. 3F). The increased Na+ flux counterbalances the decreased K+ flux (Fig. 3C), resulting in no net change in secretion. Of note, this does not imply that ion and water fluxes that occur in the presence of ouabain are passive, as there is ongoing activity of the apical H+-ATPase. Indeed, inhibition of the H+-ATPase with bafilomycin abolishes fluid secretion in Drosophila tubules (16).
Fig. 3.
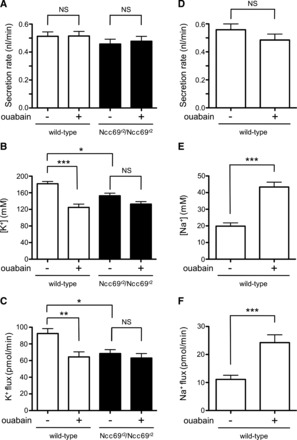
The Na+-K+-ATPase is required for Ncc69 transport. A: secretion rate (nl/min per tubule) was measured in tubules from wild-type and Ncc69r2 homozygous mutants in the absence or presence of 100 μM ouabain (n = 16–17 for each genotype/condition). Ouabain has no effect on fluid secretion. B: K+ concentration (mM) was measured in tubules from wild-type and Ncc69r2 homozygous mutants in the absence or presence of 100 μM ouabain (n = 16–17 for each genotype/condition). Ouabain decreased K+ concentration in wild-type, but not Ncc69r2 mutant tubules. *P < 0.05; ***P < 0.001. For the difference between ouabain-treated wild-type tubules and vehicle-treated Ncc69r2 mutant tubules, P < 0.05. C: K+ flux (pmol/min per tubule) was measured in tubules from wild-type and Ncc69r2 homozygous mutants in the absence or presence of 100 μM ouabain (n = 16–17 for each genotype/condition). Na+-K+-ATPase inhibition decreases K+ flux in wild-type tubules to the level seen in Ncc69r2 mutant tubules, but has no further effect on mutant tubules. *P < 0.05; **P < 0.01. The difference between ouabain-treated wild-type tubules and vehicle-treated Ncc69r2 mutant tubules was NS. D: in a separate experiment, secretion rate was measured in wild-type tubules in the absence (n = 13) or presence (n = 15) of 100 μM ouabain. As in A, Na+-K+-ATPase inhibition has no effect on fluid secretion. E: Na+ concentration (mM) was measured in wild-type tubules in the absence (n = 13) or presence (n = 15) of 100 μM ouabain. Na+ concentration increases in the presence of ouabain. ***P < 0.001. F: Na+ flux (pmol/min per tubule) was measured in wild-type tubules in the absence (n = 13) or presence (n = 15) of 100 μM ouabain. Na+-K+-ATPase inhibition increases Na+ flux. ***P < 0.001.
In wild-type tubules, ouabain decreased K+ flux by 30% (Fig. 3C). This indicates that the Na+-K+-ATPase is required for ∼30% of the normal K+ flux, with the remaining 70% of normal K+ flux mediated by non-Na+-K+-ATPase-dependent K+ uptake pathways. Although there may be residual Na+-K+-ATPase activity, Linton and O'Donnell (45) found no differences in the effects of 10 μM, 100 μM, or 1 mM ouabain on the depolarization of the basolateral membrane potential in Drosophila tubules, suggesting that the 100 μM dose is sufficient to inhibit most of the Na+-K+-ATPase activity. However, ouabain treatment resulted in depolarization of the basolateral membrane potential by only 8 to 10 mV (45), indicating that there are other mechanisms for maintenance of a cell-negative basolateral membrane potential. The ouabain-insensitive Na+-ATPase (69) may be one such mechanism, and it has been proposed to operate in the Rhodnius tubule (27).
Ouabain had no effect on secretion rate, K+ concentration, or K+ flux in Ncc69r2 mutant tubules (Fig. 3, A–C). This indicates that non-Ncc69-mediated K+ transport pathways are not dependent on the Na+-K+-ATPase. Since there was no further inhibition of K+ flux in Ncc69 mutant tubules, Ncc69 is the principal Na+-K+-ATPase-dependent transporter in unstimulated tubules. Consistent with this, there was no difference in K+ flux between ouabain-treated wild-type tubules and untreated Ncc69r2 mutant tubules (Fig. 3C).
In principle, an NKCC cotransporter could mediate transepithelial flux of Na+, K+, or both. It has previously been proposed (35) that Na+ transported across the basolateral membrane of the main segment principal cell is recycled through the Na+-K+-ATPase. To test this, we measured Na+ flux in Ncc69r2 homozygous mutants compared with wild-type and found no difference (Fig. 4A). This suggests that Na+ crossing the basolateral membrane through Ncc69 is recycled back into the hemolymph, while K+ is excreted across the apical membrane into the tubule lumen, resulting in lower levels of Na+ flux compared with K+ flux (compare Fig. 3, C and F). Na+ recycling through the Na+-K+-ATPase likely explains the increase in luminal Na+ concentration and Na+ flux seen in the presence of ouabain (Ref. 45 and Fig. 3, E and F).
Together with previous work, these data are consistent with a model (Fig. 4B) in which the Na+-K+-ATPase, acting in the principal cell, generates a favorable electrochemical gradient for NKCC cotransport through Ncc69 by lowering the intracellular Na+ concentration and generating a cell-negative electrical potential. K+ and Cl− are cotransported along with Na+ against their electrochemical gradients (secondary active transport). Na+, which is scarce in the fly diet, is recycled across the basolateral membrane through the Na+-K+-ATPase and thereby conserved, while K+ is secreted into the lumen through an H+/K+ exchanger, driven by the active transport of H+ into the lumen through the H+-ATPase. Cl− may also be recycled across the basolateral membrane, as Ianowski and O'Donnell (35) have demonstrated a basolateral Cl− conductance and loop current.
Effect of bumetanide.
NKCC cotransporters are potently inhibited by “loop” diuretics, including furosemide and bumetanide. In heterologous expression systems, Ncc69 was inhibited by bumetanide with IC50 values of 49 nM (42) and 1.17 μM (75). We therefore tested the effects of 10 μM bumetanide on wild-type tubules, but found there was no effect on fluid secretion or K+ flux (Fig. 5, A and B). We then tested the effect of 100 μM bumetanide, which has previously been demonstrated to decrease fluid secretion and K+ flux in wild-type Drosophila tubules (45). As previously observed, bumetanide decreased fluid secretion and K+ flux in wild-type tubules, but fluid secretion and K+ flux were also decreased in Ncc69r2 homozygous mutant tubules (Fig. 5, C and D). This indicates that high-dose bumetanide is not a specific inhibitor of Ncc69.
Fig. 5.
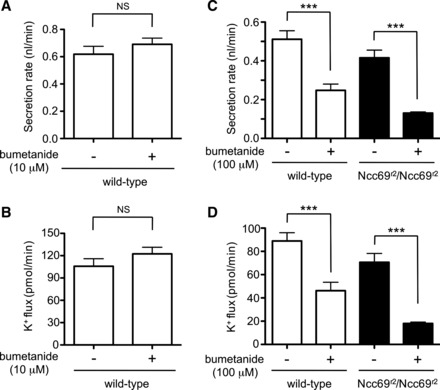
Effect of bumetanide on wild-type and Ncc69 mutant tubules. A: secretion rate (nl/min per tubule) was measured in wild-type tubules in the absence (n = 7) or presence (n = 8) of 10 μM bumetanide. Bumetanide (10 μM) had no effect on fluid secretion. B: K+ flux (pmol/min per tubule) was measured in wild-type tubules in the absence (n = 7) or presence (n = 8) of 10 μM bumetanide. Bumetanide (10 μM) had no effect on K+ flux. C: secretion rate (nl/min per tubule) was measured in wild-type and Ncc69r2 mutant tubules in the absence or presence of 100 μM bumetanide (n = 14–15 tubules per genotype/condition). Bumetanide (100 μM) inhibited fluid secretion in both wild-type and Ncc69r2 mutant tubules, indicating that Ncc69 is not the only target of high-dose bumetanide. ***P < 0.001. D: K+ flux (pmol/min per tubule) was measured in wild-type and Ncc69r2 mutant tubules in the absence or presence of 100 μM bumetanide (n = 14–15 tubules per genotype/condition). Bumetanide (100 μM) inhibited K+ flux in both wild-type and Ncc69r2 mutant tubules, indicating that Ncc69 is not the only target of high-dose bumetanide. ***P < 0.001.
The response of Ncc69 mutant tubules to cAMP, leucokinin, and capa-1.
We next turned our attention to the physiology of stimulated tubules. As is the case for mammalian renal tubular function, insect renal tubule function is modulated by a number of hormones acting through a variety of signaling cascades. Drosophila tubules increase fluid secretion rates in response to cAMP signaling (8, 9, 16, 52), and the peptide hormones leucokinin (66, 77) and capa-1 (40). To determine whether Ncc69 plays a role in the response to any of these modulators, we examined fluid secretion and K+ flux in response to stimulation in wild-type and Ncc69 mutant tubules. First, we examined the effect of cAMP signaling. Both Ncc69r2/+ heterozygous and Ncc69r2 homozygous mutant tubules exhibited a robust response to stimulation with 1 mM db-cAMP, with an increase in fluid secretion (Fig. 6A) and K+ flux (Fig. 6B). This indicates that there are non-Ncc69-mediated K+ transport pathways that are cAMP sensitive. Indeed, there may be compensatory upregulation of these pathways in Ncc69 mutants. This non-Ncc69-mediated, cAMP-sensitive fluid secretion and K+ transport was abolished by 100 μM ouabain (Fig. 6, C and D). Thus, while Ncc69 appears to be the principal Na+-K+-ATPase-dependent K+ flux pathway in unstimulated tubules (Fig. 3C), under cAMP-stimulated conditions, additional Na+-K+-ATPase-dependent pathways are recruited. This suggests either that the Na+-K+-ATPase itself is stimulated by cAMP, allowing increased K+ entry into the principal cell, or that additional secondary active transporters that are dependent on the electrochemical gradient established by the Na+-K+-ATPase are stimulated. Ouabain has also been shown to decrease fluid secretion in wild-type tubules stimulated with cAMP (45).
Fig. 6.
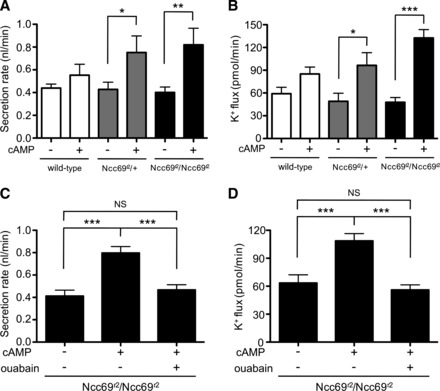
cAMP stimulates fluid secretion and K+ flux in Ncc69 mutant tubules in a Na+-K+-ATPase-dependent manner. A: secretion rate (nl/min per tubule) was measured in tubules from wild-type, Ncc69r2 heterozygous, and Ncc69r2 homozygous mutants in the presence (n = 4–5 tubules/genotype) or absence (n = 11–12 tubules/genotype) of 1 mM dibutyryl (db)-cAMP. cAMP stimulates fluid secretion through a non-Ncc69-dependent pathway. *P < 0.05; **P < 0.01. B: K+ flux (pmol/min per tubule) was measured in tubules from wild-type, Ncc69r2 heterozygous, and Ncc69r2 homozygous mutants in the presence (n = 4–5 tubules/gentoype) or absence (n = 11–12 tubules/genotype) of 1 mM db-cAMP. cAMP stimulates K+ flux through a non-Ncc69-dependent pathway. *P < 0.05; ***P < 0.001. C: secretion rate (nl/min per tubule) was measured in tubules from Ncc69r2 homozygous mutants in the absence of drugs (n = 9), in the presence of 1 mM db-cAMP alone (n = 9), or in the presence of both 1 mM db-cAMP and 100 μM ouabain (n = 11). Non-Ncc69-mediated, cAMP-stimulated secretion is dependent on the Na+-K+-ATPase. ***P < 0.001. D: K+ flux (pmol/min per tubule) was measured in tubules from Ncc69r2 homozygous mutants in the absence of drugs (n = 9), in the presence of 1 mM db-cAMP alone (n = 9), or in the presence of both 1 mM db-cAMP and 100 μM ouabain (n = 11). The non-Ncc69-mediated, cAMP-stimulated K+ flux transport pathway is dependent on the Na+-K+-ATPase. ***P < 0.001.
We next examined tubule response to the leucokinin and capa-1 peptides. In the absence of capa-1, leucokinin-stimulated fluid secretion and K+ flux were similar in wild-type and Ncc69r2 homozygous mutant tubules (Fig. 7, A and B). This suggests that the stellate cells, which are the cells stimulated by leucokinin (52, 55, 66, 77), are not impaired by the loss of Ncc69 function, consistent with our results indicating that Ncc69 is functioning in the principal cell. These results also indicate that Ncc69 mutant tubules do not have general defects in epithelial function, as they are able to support the same rates of fluid secretion and K+ flux as wild-type tubules under these conditions.
Fig. 7.
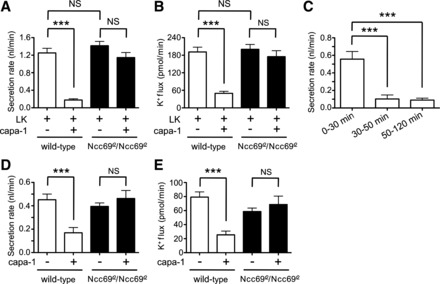
The antidiuretic and antikaliuretic effects of capability-1 (capa-1) are Ncc69 dependent. A: secretion rates (nl/min per tubule) were measured in tubules from wild-type and Ncc69r2 homozygous mutants in the presence of 10−7 M leucokinin (LK), in the absence (n = 15–17) or presence (n = 30–32) of 10−7 M capa-1. There is no difference in leucokinin-stimulated secretion between wild-type and Ncc69 mutant tubules, but the antidiuretic effects of capa-1 are abolished in Ncc69 mutant tubules. ***P < 0.001. For the difference between leucokinin- and capa-1-treated wild-type and Ncc69r2/Ncc69r2, P < 0.001. B: K+ flux (pmol/min per tubule) was measured in tubules from wild-type and Ncc69r2 homozygous mutants in the presence of 10−7 M leucokinin, in the absence (n = 15–17) or presence (n = 30–32) of 10−7 M capa-1. There is no difference in leucokinin-stimulated K+ flux between wild-type and Ncc69 mutant tubules, but the antikaliuretic effects of capa-1 are abolished in Ncc69 mutant tubules. ***P < 0.001. For the difference between leucokinin- and capa-1-treated wild-type and Ncc69r2/Ncc69r2 tubules, P < 0.001. C: secretion rate (nl/min per tubule) was serially measured in wild-type tubules (n = 22) treated with 10−7 M leucokinin and 10−7 M capa-1 over 0–30 min, 30–50 min, and 50–120 min. The antidiuretic effect is apparent after 30 min. ***P < 0.001. The difference between 30–50 min and 50–120 min was NS. D: secretion rates (nl/min per tubule) were measured in wild-type and Ncc69r2 homozygous mutants in the absence or presence of 10−7 M capa-1 (n = 10–11 tubules per genotype/condition). Capa-1 inhibited secretion in wild-type, but not Ncc69 mutant tubules. ***P < 0.001. E: K+ flux (pmol/min per tubule) was measured in wild-type and Ncc69r2 homozygous mutants in the absence or presence of 10−7 M capa-1 (n = 10–11 tubules per genotype/condition). Capa-1 inhibited K+ flux in wild-type, but not Ncc69 mutant tubules. ***P < 0.001.
Leucokinin and capa-1 have previously been shown to additively increase fluid secretion rates (40). We were therefore surprised to see that this combination of peptides resulted in the inhibition of both fluid secretion and K+ flux in wild-type tubules (Fig. 7, A and B). However, we assayed these processes over a period of 2 h, whereas the stimulatory effect of these peptides was previously observed over shorter time periods (30 min). Therefore, we tested fluid secretion rates in stimulated wild-type tubules over three time periods: 0–30 min, 30–50 min, and 50–120 min. As seen in Fig. 7C, the antidiuretic effect was apparent only after 30 min, when secretion rates dropped to very low levels: 0.1 ± 0.05 nl/min for the 30–50 min time period, and 0.09 ± 0.02 nl/min for the 50–120 min time period. This is in contrast to unstimulated tubules, in which fluid secretion occurs at a constant rate over at least 4 h (16). Because rates of secretion are lower than those seen in unstimulated tubules (∼0.5 nl/min), this appears to be an active antidiuretic effect, rather than a loss of hormone action over time, as might occur with receptor desensitization (76). Capa-1 alone also had antidiuretic and antikaliuretic effects on wild-type tubules (Fig. 7, D and E).
Ncc69r2 mutant tubules were resistant to the antidiuretic and antikaliuretic effects of capa-1 both in combination with leucokinin (Fig. 7, A and B) or on its own (Fig. 7, D and E). This indicates that Ncc69 is required for the antidiuretic and antikaliuretic effects of capa-1, and that Ncc69 may be an important target for regulation by capa-1 in antidiuretic states.
DISCUSSION
Cotransport of Na+, K+ and Cl− across the basolateral membrane of insect renal tubule cells through NKCC cotransporters has been proposed in several species, including Drosophila melanogaster (27, 30, 31, 33, 35, 37, 44, 54). However, the genes encoding the involved cotransporters have not been identified. Here, we demonstrate a role for the Drosophila NKCC, encoded by the gene Ncc69, in fluid secretion and transepithelial K+ flux in the fly renal tubule. Ncc69 mutant flies have decreased rates of fluid secretion and K+ flux. Available evidence has indicated that cation flux occurs through principal cells of the fly tubule, while the stellate cells provide a pathway for chloride flux (18, 52, 55). Consistent with this model, expression of wild-type Ncc69 in principal cells only is sufficient to rescue impaired fluid secretion and K+ flux.
Although NKCCs generally transport equimolar Na+ and K+, Ncc69 mutant flies do not have impaired Na+ flux. The data presented here support the proposal by Ianowski and O'Donnell (35) that Na+ is likely recycled across the basolateral membrane through the Na+-K+-ATPase back into the hemolymph, while K+ is secreted across the apical membrane into the tubule lumen, probably through an apical cation/H+ antiporter energized by the apical H+-ATPase (1, 10, 13, 14, 16, 20). Since the fly diet is rich in K+ but poor in Na+, this allows the fly to excrete a high-K+, low-Na+ urine. In contrast, in blood-sucking insects, such as mosquitoes and Rhodnius prolixus, in which a large Na+ load is ingested with the blood meal, NKCC inhibition in stimulated tubules results in decreased Na+ flux, suggesting that the Na+ transported across the basolateral membrane by NKCC is secreted across the apical membrane by the apical cation/H+ transporter (31, 33). Thus, NKCC function in the insect tubule is adapted to the dietary requirements of the organism. In fact, NKCC function is dispensable for Na+ flux in the Drosophila tubule, as robust fluid secretion and Na+ flux can occur in K+-free bathing saline (45).
Two groups have shown that Ncc69 is homologous to the mammalian NCC/NKCC family of SLC12 cotransporters, and both groups demonstrated that Ncc69 transports rubidium in a Na+- and Cl−-dependent manner, consistent with its function as an NKCC (42, 75). They also showed that when Ncc69 is expressed in cultured cells, bumetanide inhibits its activity with an IC50 of ∼1 μM or less (42, 75). In this study, 10 μM bumetanide did not inhibit fluid secretion or K+ flux, but as seen by O'Donnell and coworkers (45), 100 μM bumetanide was inhibitory. It is unclear why the lower dose was ineffective. In perfused Rhodnius tubules, luminally applied bumetanide inhibited fluid secretion, presumably by inhibiting basolateral NKCC (30), suggesting a capacity for transepithelial bumetanide transport in insect tubules. Similarly, higher doses of ouabain than would be predicted by cellular studies are required to see effects on tubule function due to uptake of ouabain by organic anion transporters (78). Finally, there may be differences in Ncc69 in its normally expressed environment compared with heterologous cells that affect sensitivity to bumetanide.
The high-dose bumetanide used here and previously (45) inhibits fluid secretion and K+ flux in both wild-type and Ncc69 mutant tubules, indicating additional targets besides Ncc69. One possibility is that bumetanide inhibits Ncc83. The Ncc83 gene sequence is homologous to the NCC/NKCC branch of SLC12 transporters (42, 75), but the putative cotransporter encoded by this gene has not been studied so far. Additional targets of high-dose bumetanide that have been demonstrated in other systems include potassium-chloride cotransporters (28, 29, 48), parallel Na+/H+ and Cl−/HCO3− exchange in cortical thick ascending limb (22–24), a K+-independent NaCl uptake pathway in rabbit medullary thick ascending limb (2, 21), and the ouabain-insensitive Na+-ATPase (69). Whether any of these mechanisms are operative in the fly renal tubule remains to be determined.
Like the mammalian renal tubule, insect Malpighian tubules are under hormonal control. In Drosophila, a calcitonin-like peptide, Drome-DH31, stimulates fluid secretion and K+ flux through the cAMP signaling pathway (9) by activating the apical vacuolar H+-ATPase. A corticotropin-releasing factor-like peptide, Drome-DH44, also increases tubule cAMP levels and stimulates fluid secretion (8). Whether these peptides have additional effects on basolateral membrane targets is unknown. Here, we demonstrate that cAMP robustly stimulates fluid secretion and K+ flux in Ncc69 mutant tubules, indicating that the Ncc69 transporter is not required for the response to cAMP. However, the response to cAMP is abolished by ouabain, suggesting that either the Na+-K+-ATPase itself, or a secondary transporter dependent on its activity (such as Ncc83), allows K+ uptake across the basolateral membrane, and underscoring the importance of coordinate regulation of apical and basolateral membrane transport.
The peptide capa-1 (or the related peptide MasCAP2b, which has similar effects on the Drosophila tubule) has also been shown to stimulate fluid secretion in Drosophila tubules, through a Ca2+/NO/cGMP signaling pathway (6, 7, 11, 12, 15, 40, 70). Surprisingly, we observed a novel antidiuretic and antikaliuretic effect of capa-1 that was apparent after 30 min of exposure of tubules to capa-1. This is the first observation of antidiuretic peptide activity in a wild-type Drosophila renal tubule, but it is consistent with the antidiuretic activity of capa-1-related peptides in other insects, such as Rhodnius and Tenebrio (38, 58–61, 64, 65, 81). This may serve as a mechanism to limit excess diuresis, preventing desiccation. Indeed, knockdown of the capa-1 receptor in the tubule results in resistance to starvation/desiccation stress, suggesting that excess capa-1 activity is deleterious under these conditions (76).
What is the mechanism by which capa-1 exerts its antidiuretic effect? MasCAP2b and cGMP modulate the transepithelial potential, indicating regulation of the apical H+-ATPase (11, 52). Our data suggest that regulation of basolateral transport pathways is important as well, since Ncc69 mutant tubules escape the antidiuretic effect of capa-1. This is also reminiscent of the Rhodnius tubule, in which the Rhodnius capa homolog, RhoprCAPA-α2, can either stimulate the apical H+-ATPase or inhibit the basolateral NKCC, depending on the hormonal milieu (58). The inhibition of basolateral NKCC by RhoprCAPA-α2 results in antidiuresis, indicating similar regulatory mechanisms of capa signaling in Drosophila and Rhodnius.
The signaling pathways by which capa-1 inhibits diuresis in fly are unknown. The cGMP signaling pathway mediates both the early stimulatory effects of capa-1/MasCAP2b in flies as well as the antidiuretic effects of capa-1/MasCAP2b on Rhodnius tubules and Drosophila tubules overexpressing the cation/proton exchanger CG10806B (6, 11–13, 15, 40, 64, 65). Future studies are needed to determine whether the antidiuretic effect of capa-1 on Drosophila tubules is also mediated by cGMP signaling.
In fly, capa-1/MasCAP2b stimulates cGMP downstream of NO (7, 12, 15, 40, 62). Interestingly, a NO/cGMP signaling pathway inhibits NaCl reabsorption in the thick ascending limb (51, 56) through inhibition of NKCC2 (4, 57). Thus, although NKCC2 is on the apical membrane of the thick ascending limb and functions to absorb NaCl, while Ncc69 is presumably on the basolateral membrane of the fly tubule and is important for K+ secretion, an attractive speculation is that signaling through NO and cGMP may be an evolutionarily conserved mechanism to limit NKCC activity. Dysregulation of this pathway may also be clinically important: in an animal model of hypertension using the NO inhibitor N-nitro-l-arginine-methyl ester (l-NAME), NKCC2 is upregulated, resulting in excess NaCl reabsorption (80). Further understanding of fly Ncc69 regulation may thus lead to insights into human hypertension. Similarly, the three mammalian homologs of Ncc69—NKCC1, NKCC2, and the sodium-chloride cotransporter NCC—are all regulated by two kinase families, the with-no-lysine (WNK) and STE20/SPS1-related proline/alanine-rich kinase (SPAK)/oxidative stress-responsive kinase-1 (OSR1) kinases (3, 19, 25, 26, 50, 63, 67, 68, 79). Study of the regulation of Ncc69 in the Malpighian tubule by the conserved Drosophila WNK and SPAK/OSR1 kinases (32, 43) may lead to further insights into the role of these kinases in epithelial ion transport.
In summary, we have shown that the Drosophila NKCC encoded by Ncc69 is required for normal renal tubule function, including fluid secretion and transepithelial K+ flux. On the other hand, Na+ flux, which is quantitatively less important given the low Na+ content of the fly diet, is not dependent on Ncc69. The inhibition of Ncc69 cotransport activity may also be important in conditions where K+ and water need to be conserved, as Ncc69 is required for the antidiuretic and antikaliuretic effects of the regulatory hormone capa-1.
GRANTS
This research was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-091316 (to A. R. Rodan), DK-007257 (institutional T32), DK-59530 (to C.-L. Huang), DK-41612 and DK-078596 (to M. Baum), and DK-079328 (UTSW O'Brien Center P30).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.R.R. conception and design of the research; A.R.R. performed the experiments; A.R.R. analyzed the data; A.R.R. interpreted the results of the experiments; A.R.R. prepared the figures; A.R.R. drafted the manuscript; A.R.R., M.B., and C.-L.H. edited and revised the manuscript; A.R.R., M.B., and C.-L.H. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Julian Dow, Billy Leiserson, and Adrian Rothenfluh for generous gifts of fly strains. Fly strains were also obtained from the Bloomington Stock Center. Mike O'Donnell provided invaluable advice in setting up the Ramsay assay and the use of ion-specific electrodes. Sungwan An and Chih-Jen Cheng were also extremely helpful in setting up the ISE system. Adrian Rothenfluh participated in many thoughtful discussions and provided comments on the manuscript.
REFERENCES
- 1.Allan AK, Du J, Davies SA, Dow JA. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol Genomics 22: 128–138, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Alvo M, Calamia J, Eveloff J. Lack of potassium effect on Na-Cl cotransport in the medullary thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 249: F34–F39, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci USA 103: 10883–10888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ares GR, Caceres P, Alvarez-Leefmans FJ, Ortiz PA. cGMP decreases surface NKCC2 levels in the thick ascending limb: role of phosphodiesterase 2 (PDE2). Am J Physiol Renal Physiol 295: F877–F887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Broderick KE, Kean L, Dow JA, Pyne NJ, Davies SA. Ectopic expression of bovine type 5 phosphodiesterase confers a renal phenotype in Drosophila. J Biol Chem 279: 8159–8168, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Broderick KE, MacPherson MR, Regulski M, Tully T, Dow JA, Davies SA. Interactions between epithelial nitric oxide signaling and phosphodiesterase activity in Drosophila. Am J Physiol Cell Physiol 285: C1207–C1218, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA, Spana EP, Davies SA, Dow JA. The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol 205: 3799–3807, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol 204: 1795–1804, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Davies SA, Goodwin SF, Kelly DC, Wang Z, Sozen MA, Kaiser K, Dow JA. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem 271: 30677–30684, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Davies SA, Huesmann GR, Maddrell SH, O'Donnell MJ, Skaer NJ, Dow JA, Tublitz NJ. CAP2b, a cardioacceleratory peptide, is present in Drosophila and stimulates tubule fluid secretion via cGMP. Am J Physiol Regul Integr Comp Physiol 269: R1321–R1326, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Davies SA, Stewart EJ, Huesmann GR, Skaer NJ, Maddrell SH, Tublitz NJ, Dow JA. Neuropeptide stimulation of the nitric oxide signaling pathway in Drosophila melanogaster Malpighian tubules. Am J Physiol Regul Integr Comp Physiol 273: R823–R827, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, Dow JA. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci 121: 2612–2619, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Dow JA, Davies SA, Guo Y, Graham S, Finbow ME, Kaiser K. Molecular genetic analysis of V-ATPase function in Drosophila melanogaster. J Exp Biol 200: 237–245, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Dow JA, Maddrell SH, Davies SA, Skaer NJ, Kaiser K. A novel role for the nitric oxide-cGMP signaling pathway: the control of epithelial function in Drosophila. Am J Physiol Regul Integr Comp Physiol 266: R1716–R1719, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Dow JA, Maddrell SH, Gortz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Dow JA, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol 299: F1237–F1244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dow JT, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev 83: 687–729, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 278: 27347–27353, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Du J, Kean L, Allan AK, Southall TD, Davies SA, McInerny CJ, Dow JA. The SzA mutations of the B subunit of the Drosophila vacuolar H+ ATPase identify conserved residues essential for function in fly and yeast. J Cell Sci 119: 2542–2551, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Eveloff JL, Calamia J. Effect of osmolarity on cation fluxes in medullary thick ascending limb cells. Am J Physiol Renal Fluid Electrolyte Physiol 250: F176–F180, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Friedman PA. Bumetanide inhibition of [CO2 + HCO3]-dependent and -independent equivalent electrical flux in renal cortical thick ascending limbs. J Pharmacol Exp Ther 238: 407–414, 1986 [PubMed] [Google Scholar]
- 23.Friedman PA, Andreoli TE. CO2-stimulated NaCl absorption in the mouse renal cortical thick ascending limb of Henle. Evidence for synchronous Na+/H+ and Cl−/HCO3− exchange in apical plasma membranes. J Gen Physiol 80: 683–711, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman PA, Andreoli TE. Effects of (CO2 + HCO3−) on electrical conductance in cortical thick ascending limbs. Kidney Int 30: 325–331, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol 26: 689–698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gamez AD, Gutierrez AM, Garcia R, Whittembury G. Recent experiments towards a model for fluid secretion in Rhodnius Upper Malpighian Tubules (UMT). J Insect Physiol 58: 543–550, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Garay RP, Nazaret C, Hannaert PA, Cragoe EJ., Jr Demonstration of a [K+,Cl−]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl−]-cotransport system. Mol Pharmacol 33: 696–701, 1988 [PubMed] [Google Scholar]
- 29.Gillen CM, Forbush B., 3rd Functional interaction of the K-Cl cotransporter (KCC1) with the Na-K-Cl cotransporter in HEK-293 cells. Am J Physiol Cell Physiol 276: C328–C336, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez AM, Hernandez CS, Whittembury G. A model for fluid secretion in Rhodnius upper Malpighian tubules (UMT). J Membr Biol 202: 105–114, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hegarty JL, Zhang B, Pannabecker TL, Petzel DH, Baustian MD, Beyenbach KW. Dibutyryl cAMP activates bumetanide-sensitive electrolyte transport in Malpighian tubules. Am J Physiol Cell Physiol 261: C521–C529, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Hirata T, Czapar A, Brin L, Haritonova A, Bondeson DP, Linser P, Cabrero P, Thompson J, Dow JA, Romero MF. Ion and solute transport by Prestin in Drosophila and Anopheles. J Insect Physiol 58: 563–569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ianowski JP, Christensen RJ, O'Donnell MJ. Intracellular ion activities in Malpighian tubule cells of Rhodnius prolixus: evaluation of Na+-K+-2Cl− cotransport across the basolateral membrane. J Exp Biol 205: 1645–1655, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ianowski JP, Christensen RJ, O'Donnell MJ. Na+ competes with K+ in bumetanide-sensitive transport by Malpighian tubules of Rhodnius prolixus. J Exp Biol 207: 3707–3716, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Ianowski JP, O'Donnell MJ. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl− cotransport and Cl− conductance. J Exp Biol 207: 2599–2609, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Ianowski JP, O'Donnell MJ. Electrochemical gradients for Na+, K+, Cl− and H+ across the apical membrane in Malpighian (renal) tubule cells of Rhodnius prolixus. J Exp Biol 209: 1964–1975, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Ianowski JP, O'Donnell MJ. Transepithelial potential in Malpighian tubules of Rhodnius prolixus: lumen-negative voltages and the triphasic response to serotonin. J Insect Physiol 47: 411–421, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Ianowski JP, Paluzzi JP, Te Brugge VA, Orchard I. The antidiuretic neurohormone RhoprCAPA-2 downregulates fluid transport across the anterior midgut in the blood-feeding insect Rhodnius prolixus. Am J Physiol Regul Integr Comp Physiol 298: R548–R557, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann N, Mathai JC, Hill WG, Dow JA, Zeidel ML, Brodsky JL. Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. Am J Physiol Cell Physiol 289: C397–C407, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JA. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 282: R1297–R1307, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na+ + K+)-ATPase alpha-subunit in Drosophila melanogaster. EMBO J 8: 193–202, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiserson WM, Forbush B, Keshishian H. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia 59: 320–332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron 28: 793–806, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Leyssens A, Dijkstra S, Van Kerkhove E, Steels P. Mechanisms of K+ uptake across the basal membrane of malpighian tubules of Formica polyctena: the effect of ions and inhibitors. J Exp Biol 195: 123–145, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Linton SM, O'Donnell MJ. Contributions of K+:Cl− cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 202: 1561–1570, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Maddrell SH, O'Donnell MJ, Caffrey R. The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. J Exp Biol 177: 273–285, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Maddrell SH, Overton JA. Stimulation of sodium transport and fluid secretion by ouabain in an insect malpighian tubule. J Exp Biol 137: 265–276, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Mercado A, Vazquez N, Song L, Cortes R, Enck AH, Welch R, Delpire E, Gamba G, Mount DB. NH2-terminal heterogeneity in the KCC3 K+-Cl− cotransporter. Am J Physiol Renal Physiol 289: F1246–F1261, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Messerli MA, Kurtz I, Smith PJ. Characterization of optimized Na+ and Cl− liquid membranes for use with extracellular, self-referencing microelectrodes. Anal Bioanal Chem 390: 1355–1359, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Neant F, Bailly C. Luminal and intracellular cGMP inhibit the mTAL reabsorptive capacity through different pathways. Kidney Int 44: 741–746, 1993 [DOI] [PubMed] [Google Scholar]
- 52.O'Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 199: 1163–1175, 1996 [DOI] [PubMed] [Google Scholar]
- 53.O'Donnell MJ, Maddrell SH. Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. J Exp Biol 198: 1647–1653, 1995 [DOI] [PubMed] [Google Scholar]
- 54.O'Donnell MJ, Maddrell SH. Secretion by the Malpighian tubules of Rhodnius prolixus stal: electrical events. J Exp Biol 110: 275–290, 1984 [DOI] [PubMed] [Google Scholar]
- 55.O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, Kaiser K, Dow JA. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol Regul Integr Comp Physiol 274: R1039–R1049, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Ortiz PA, Garvin JL. NO inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Paluzzi JP, Naikkhwah W, O'Donnell MJ. Natriuresis and diuretic hormone synergism in R. prolixus upper Malpighian tubules is inhibited by the anti-diuretic hormone, RhoprCAPA-alpha2. J Insect Physiol 58: 534–542, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Paluzzi JP, Orchard I. Distribution, activity and evidence for the release of an anti-diuretic peptide in the kissing bug Rhodnius prolixus. J Exp Biol 209: 907–915, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Paluzzi JP, Park Y, Nachman RJ, Orchard I. Isolation, expression analysis, and functional characterization of the first antidiuretic hormone receptor in insects. Proc Natl Acad Sci USA 107: 10290–10295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paluzzi JP, Russell WK, Nachman RJ, Orchard I. Isolation, cloning, and expression mapping of a gene encoding an antidiuretic hormone and other CAPA-related peptides in the disease vector, Rhodnius prolixus. Endocrinology 149: 4638–4646, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Pollock VP, McGettigan J, Cabrero P, Maudlin IM, Dow JA, Davies SA. Conservation of capa peptide-induced nitric oxide signalling in Diptera. J Exp Biol 207: 4135–4145, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quinlan MC, O'Donnell MJ. Anti-diuresis in the blood-feeding insect Rhodnius prolixus Stal: antagonistic actions of cAMP and cGMP and the role of organic acid transport. J Insect Physiol 44: 561–568, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Quinlan MC, Tublitz NJ, O'Donnell MJ. Anti-diuresis in the blood-feeding insect Rhodnius prolixus Stal: the peptide CAP2b and cyclic GMP inhibit Malpighian tubule fluid secretion. J Exp Biol 200: 2363–2367, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Radford JC, Davies SA, Dow JA. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 277: 38810–38817, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocafull MA, Romero FJ, Thomas LE, del Castillo JR. Isolation and cloning of the K+-independent, ouabain-insensitive Na+-ATPase. Biochim Biophys Acta 1808: 1684–1700, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Rosay P, Davies SA, Yu Y, Sozen MA, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Schubiger M, Feng Y, Fambrough DM, Palka J. A mutation of the Drosophila sodium pump alpha subunit gene results in bang-sensitive paralysis. Neuron 12: 373–381, 1994 [DOI] [PubMed] [Google Scholar]
- 72.Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na+-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol 281: C449–C463, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Sozen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun B, Xu P, Wang W, Salvaterra PM. In vivo modification of Na+,K+-ATPase activity in Drosophila. Comp Biochem Physiol B Biochem Mol Biol 130: 521–536, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Sun Q, Tian E, Turner RJ, Ten Hagen KG. Developmental and functional studies of the SLC12 gene family members from Drosophila melanogaster. Am J Physiol Cell Physiol 298: C26–C37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terhzaz S, Cabrero P, Robben JH, Radford JC, Hudson BD, Milligan G, Dow JA, Davies SA. Mechanism and function of Drosophila capa GPCR: a desiccation stress-responsive receptor with functional homology to human neuromedinU receptor. PLos One 7: e29897, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JA. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol 202: 3667–3676, 1999 [DOI] [PubMed] [Google Scholar]
- 78.Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, Dow JA. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA 101: 13689–13693, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397: 223–231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wangensteen R, Rodriguez-Gomez I, Moreno JM, Vargas F, Alvarez-Guerra M. Chronic nitric oxide blockade modulates renal Na-K-2Cl cotransporters. J Hypertens 24: 2451–2458, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Wiehart UI, Nicolson SW, Eigenheer RA, Schooley DA. Antagonistic control of fluid secretion by the Malpighian tubules of Tenebrio molitor: effects of diuretic and antidiuretic peptides and their second messengers. J Exp Biol 205: 493–501, 2002 [DOI] [PubMed] [Google Scholar]


