Abstract
Considerable salt-soluble protein degradation was observed in pork muscle inoculated with Pseudomonas fragi. During a 20-day incubation period at 10 C, the samples proceeded to rank spoilage or putrefaction. There was a large decrease in the salt-soluble protein fraction and a corresponding increase in nonprotein nitrogen. Disc gel electrophoretic patterns showed that breakdown of the salt-soluble proteins had occurred after incubation for 20 days. During incubation for 10 days at 10 C, P. fragi produced large amounts of extracellular proteolytic activity in ground pork. Most of the proteolytic activity appeared immediately after spoilage occurred. However, a significant increase in the ability to hydrolyze casein and a slight increase in the ability to hydrolyze denatured hemoglobin occurred prior to spoilage.
Full text
PDF
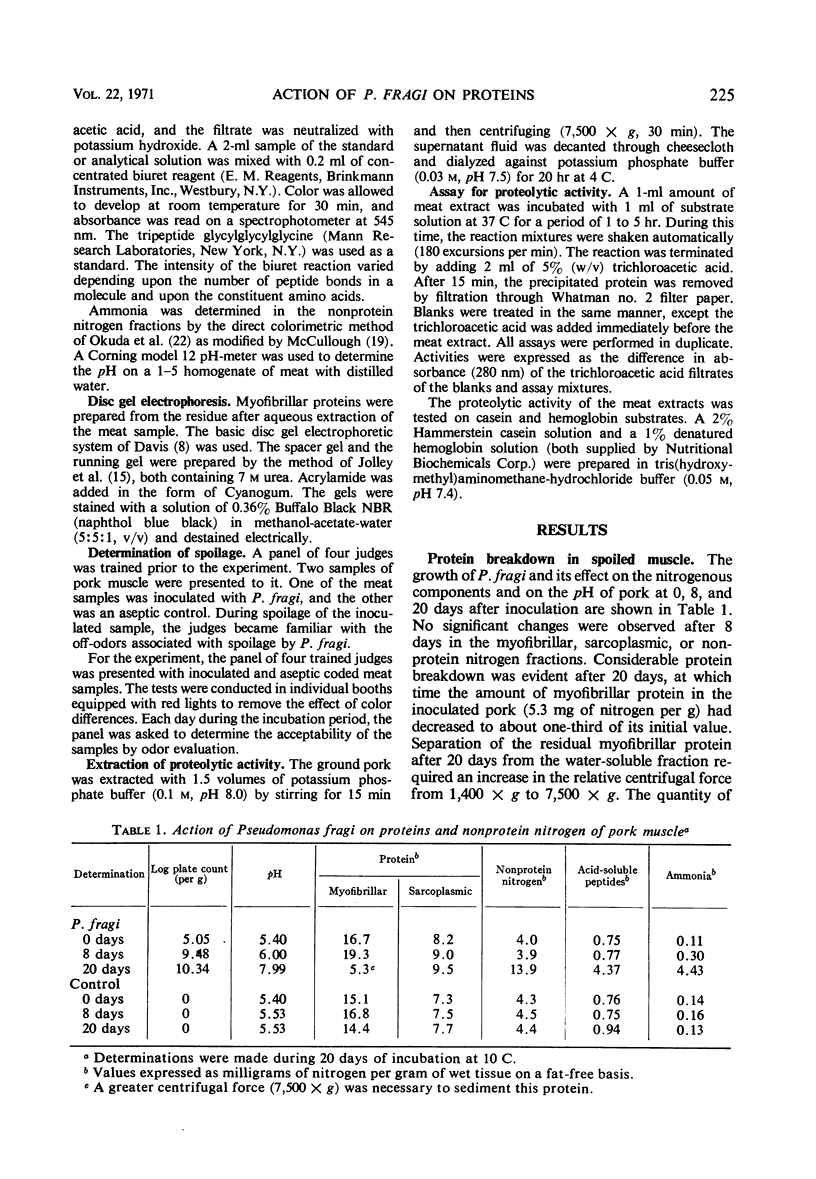
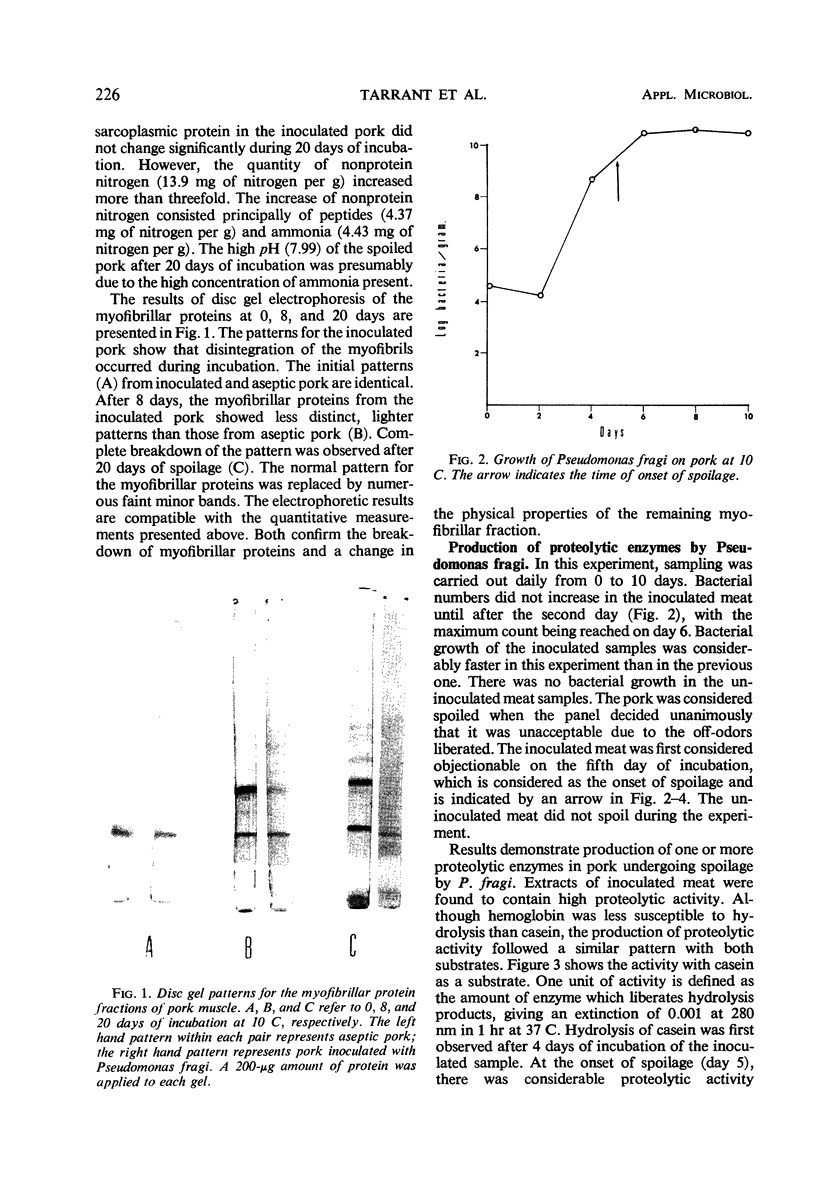
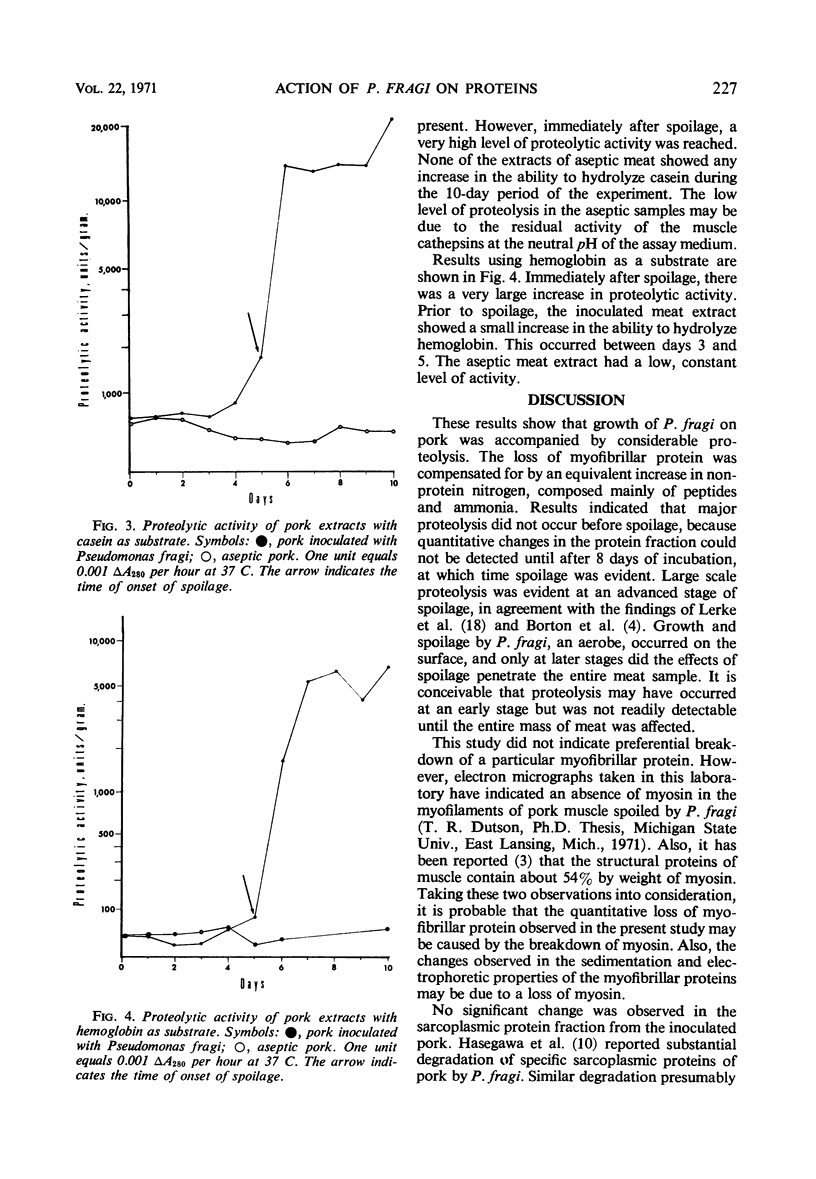

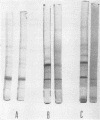
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Pearson A. M., Price J. F., Lechowich R. V. Action of bacterial growth on the sarcoplasmic and urea-soluble proteins from muscle. I. Effects of Clostridium perfringens, Salmonella enteritidis, Achromobacter liquefaciens, Streptococcus faecalis, and Kurthia zopfi. Appl Microbiol. 1970 Jul;20(1):117–122. doi: 10.1128/am.20.1.117-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. M., Kontou K. S. Fate of free amino acids and nucleotides in spoiling beef. Appl Microbiol. 1967 Jul;15(4):759–764. doi: 10.1128/am.15.4.759-764.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. M. Nature, characteristics, and proteolytic properties of beef spoilage bacteria at low and high temperatures. Appl Microbiol. 1967 Jul;15(4):943–944. doi: 10.1128/am.15.4.943-944.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley W. B., Allen H. W., Griffith O. M. Ultracentrifugation using acrylamide gel. Anal Biochem. 1967 Dec;21(3):454–461. doi: 10.1016/0003-2697(67)90320-x. [DOI] [PubMed] [Google Scholar]
- Lerke P., Farber L., Adams R. Bacteriology of spoilage of fish muscle. IV. Role of protein. Appl Microbiol. 1967 Jul;15(4):770–776. doi: 10.1128/am.15.4.770-776.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967 Aug;17(2):297–304. doi: 10.1016/0009-8981(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Okuda H., Fujii S., Kawashima Y. A direct colorimetric determination of blood ammonia. Tokushima J Exp Med. 1965 May;12(1):11–23. [PubMed] [Google Scholar]