Abstract
Introduction
Substance abuse treatment programs are often characterized by high rates of premature treatment dropout, which increases the likelihood of relapse to drug use. Negative reinforcement models of addiction emphasize an individual’s inability to tolerate stress as a key factor for understanding poor substance use treatment outcomes, and evidence indicates that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis contributes to an individual’s inability to respond adaptively to stress. The aim of the current study was to examine whether HPA axis response to stress is predictive of treatment retention among a sample of drug users in residential substance abuse treatment.
Method
Prospective study assessing treatment retention among 102 individuals enrolled in residential substance abuse treatment. Participants completed two computerized stress tasks, and HPA axis response to stress was measured via salivary cortisol at five time points from baseline (pre-stress) to 30 min post-stress exposure.
Results
The main outcome measures were treatment dropout (categorical) and total number of days in treatment (continuous). A significantly higher salivary cortisol response to stress was observed in treatment dropouts compared to treatment completers. Further, Cox proportional hazards survival analyses indicated that a higher peak cortisol response to stress was associated with a shorter number of days to treatment dropout.
Conclusions
Results indicate that a higher salivary cortisol level in response to stress is associated with an inability to remain in substance abuse treatment. These findings are the first to document a biological marker of stress as a predictor of substance abuse treatment dropout, and support the development and implementation of treatments targeting this vulnerability.
Keywords: Endocrinology, Stress, Cortisol, HPA axis, Residential, Treatment
1. Introduction
An estimated 10–30% of individuals entering substance abuse treatment programs drop out prior to treatment completion, and the highest rates of treatment dropout occur in long-term residential treatment programs (Substance Abuse and Mental Health Services Administration, 2005a). This is of great public health significance given that more than 40% of individuals seeking substance abuse treatment nationwide receive care at residential facilities (Substance Abuse and Mental Health Services Administration, 2005b), and longer treatment duration is one of the strongest predictors of successful substance use outcomes (Simpson et al., 1997). Understanding the factors underlying an individuals’ inability to remain in treatment is of particular importance in informing future treatment development efforts.
Of particular interest is the extent to which negative affective states play a role in treatment duration and subsequent substance use outcomes. Theoretical accounts of the underlying mechanisms responsible for relapse to drug use implicate negative reinforcement processes (Baker et al., 2004), which collectively emphasize that the motivational basis of addictive drug use is the reduction or avoidance of aversive internal states. In support of this theoretical approach, prospective studies indicate that individuals with an inability to tolerate affective distress are significantly more likely to dropout of residential substance abuse treatment (Daughters et al., 2005a), and have shorter abstinence durations across addictions (Brandon et al., 2003; Brown et al., 2002; Daughters et al., 2005b).
Previous studies examining an individuals’ ability to tolerate affective distress have relied primarily on self-report and behavioral assessment approaches, yet evidence indicates that one’s biological response to stress may also play a key role in substance use outcomes. The most commonly studied biological component associated with stress is the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis, which controls the secretion of hormones from the pituitary gland and adrenal cortex, plays a central role in mediating the body’s response to stress and is extremely sensitive to inputs from the limbic system and prefrontal cortex (Carmichael and Price, 1995; Sinha, 2001), two brain areas that are important in modulating reinforcement and motivational processes.
In animal models of substance use, a series of studies by Piazza and colleagues provide evidence to suggest that a dysfunctional HPA axis response to stress is associated with increases in self-administration of addictive substances (Piazza et al., 1991, 1996, 1998). Specifically, rats who respond to stressful stimuli with prolonged secretion of the HPA stress hormone (i.e., corticosterone), were more likely to self-administer amphetamines than those with lower level HPA reactivity (Piazza et al., 1991). Further, administration of corticosteroids to rats that were low level responders increased the risk that these rats would begin to self-administer amphetamines. Additional studies have demonstrated that drug experienced laboratory animals robustly reinstate drug seeking behavior after exposure to footshock stress and that these effects are mediated by the extrahypothalamic corticotrophin-releasing factor (CRF) and brain noradrenergic pathways (Shaham and Miczek, 2003). Studies with human cocaine users have corroborated animal studies in that increased stress-related corticotrophin and cortisol responses to an emotional imagery stressor have been associated with greater amounts of cocaine consumption per occasion at 90 days post-treatment (Sinha et al., 2006).
Taken together, biobehavioral studies examining an individual’s response to stress provide support for the role of negative reinforcement processes in substance using behavior. These processes are also applicable to treatment retention, as behavioral assessments indicate that an inability to tolerate affective distress predicts premature substance abuse treatment dropout. However, important biological markers have yet to be examined as a concurrent risk factor for treatment retention. Thus, the aim of the current study was to examine whether HPA axis response to stress at treatment entry is predictive of treatment retention among a sample of drug users entering residential substance abuse treatment. In line with previous findings, it was hypothesized that higher salivary cortisol levels in response to stress would be significantly related to an inability to complete treatment.
2. Method
2.1. Sample
Study participants included individuals entering a residential drug treatment center in Northeast Washington, DC. Treatment at this center involves a mix of strategies adopted from Alcoholics and Narcotics Anonymous as well as group sessions focused on relapse prevention. Complete abstinence from drugs and alcohol (verified by a clean urine drug test) is required upon entry into the center and through the duration of the program, with the exception of nicotine; regular drug testing is provided and any drug or alcohol use results in immediate dismissal from the center. When needed, detoxification from an outside source is required prior to entry into the center; therefore, acute drug effects likely did not influence the current findings.
A total of 123 participants provided informed consent and were enrolled in the study. Of these, 18 were excluded for meeting criteria for DSM-IV psychosis (n = 4), indicating use of corticosteroids (n = 4), or providing undetectable salivary cortisol data (n = 10). A total of three participants began study procedures and chose to stop participation because they preferred to attend treatment center activities (e.g., chorus practice). Thus, the data analysis for the current study is based on a sample of 102 participants. Participants were primarily male (81.4%, n = 83) and ranged in age from 19 to 66 (M = 41.9, SD = 10.8). With regard to racial/ethnic background, 89.2% (n = 91) of the participants were African American, 7.8% (n = 8) were White, and 2.9% (n = 3) were Hispanic/Latino. In terms of highest education level, 23.5% (n = 24) reported less than a high school education, 34.3% (n = 35) reported completing high school or obtaining a GED, and 42.2% (n = 43) reported some college or technical school. The majority of the sample reported current unemployment (79.6%, n = 81) and a household income of less than $20,000 a year (68.6%, n = 70). Participants entered treatment under 30-day (47.1%, n = 48), 60-day (33.3%, n = 34), 90-day (2.9%, n = 3), or 180-day (16.7%, n = 17) contract lengths, with 55.9% (n = 57) of the participants being legally mandated by the court to attend treatment by a pre-trial release to treatment program, in which drug offenders who are awaiting trial are granted the option to receive substance abuse treatment prior to their court appearance.
2.2. Procedure
All participants were approached on the Monday of their first week of treatment. After providing a complete description of the study to the subjects, written informed consent was obtained. All aspects of the study and the consent forms were approved by the University Institutional Review Board. Following informed consent, participants were administered the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1997, 2002) to assess Axis I psychiatric and substance use history and diagnoses, as well as Axis II borderline personality disorder and antisocial personality disorder. Residents were then scheduled for participation in an experimental stress session on the Tuesday evening following the Monday evening SCID-IV interview. All testing sessions were conducted between 6 and 8 pm to control for the effects of circadian variation in cortisol levels. Each testing session began with participants completing a 5-min deep breathing exercise for purposes of relaxation, followed by collection of a baseline salivary cortisol sample via the “passive drool” technique (Granger et al., 2006). Specifically, at each collection time point participants were instructed to spit into a straw to fill a cryovial (i.e., resealable plastic tube). Participants then completed the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) to assess baseline state negative mood. Two psychological stressor tasks were then administered (counterbalanced), after which salivary cortisol was immediately collected at 0, 10, 20, and 30 min post-stressor for a total of 5 salivary cortisol samples (including the baseline sample). Following completion of the computerized stressors, participants completed self-report questionnaires including demographic information, the Multidimensional Personality Questionnaire-Negative Emotional Temperament (MPQ-NEM; Tellegen, 1985) to assess trait level vulnerability to negative affect, a widely used smoking history questionnaire measuring smoking characteristics including smoking status (i.e., never smoker, ex-smoker, regular smoker) and the number of cigarettes per day when smoking daily (Heatherton et al., 1989), and a drug use history questionnaire to assess past year frequency of drug use across drug classes (Babor and Del Boca, 1992). Patients received payment in the form of grocery store gift cards for participation in the study. Participant status was followed up with the administrative offices of the treatment center to determine the number of days they remained in treatment and whether they completed their entire contract duration or dropped out prematurely.
2.3. Computerized psychological stress tasks
Two computerized psychological stress tasks were used to induce distress. Both tasks include forced failure which is recommended for inducing a cortisol response (Dickerson and Kemeny, 2004). We used a modified version of the Paced Auditory Serial Addition Task (PASAT-C; Lejuez et al., 2003) which has been used in previous research with substance users and demonstrated an ability to increase participant distress levels (Brown et al., 2002; Daughters et al., 2005b, 2008). For this task, numbers were sequentially flashed on a computer screen, and participants were asked to add the currently presented number to the previously presented number before the subsequent number appeared on the screen. If the participant failed to add the numbers before the presentation of the next number or gave an incorrect response, an explosion sound would occur. As the task was designed to limit the role of mathematical skill, the presented numbers only ranged from 0 to 20, with no sum greater than 20. Participants were told that their score increased by one point with each correct answer and that incorrect answers or omissions would not affect their total score. The task consisted of three levels of increasing difficulty, with the latencies between number presentations titrated based upon the participants’ ability level. On the last level, the latency between number presentations is extremely short, making the task virtually impossible to complete successfully. During this final level, participants were informed that they could terminate exposure to the task at any time by clicking on a “Quit” button on the screen; however, an incentive to continue was provided such that they were told that the amount of money they would earn at the end of the session would depend upon their performance on the task.
We also used a computerized version of the Mirror Tracing Persistence Task (MTPT-C). As used previously with substance users to induce distress (Daughters et al., 2005a, 2008), participants were required to trace a red dot along the outline of a star using the computer mouse, with the mouse programmed to move the red dot in the reverse direction. Participants were told that their performance was measured based upon how far around the star they moved the red dot. If the participant moved the red dot outside of the outline of the star or if the participant stalled for more than 2 s, a loud buzz would sound and the red dot would return to the starting position. The outline of the star is extremely thin, making it virtually impossible to perform well on the task. Participants were told that they could end the task at any time by pressing any key on the computer, but that how well they did on the task would affect how much money they made.
The maximum duration of the final level was 5 min for both tasks, yet participants were not told the maximum duration prior to beginning the task. In line with previous studies using the PASAT-C and MTPT-C (Brown et al., 2002; Daughters et al., 2005a, 2008), we measured changes in distress using a four item scale consisting of self-reported anxiety, difficulty concentrating, irritability, and frustration, with each item independently rated on a 10-point Likert scale and a total score derived by summing the scores on each item. Reliability of this scale was good (α = .86).
2.4. Assessment of HPA axis response to stress
Salivary cortisol samples were used to assess HPA axis reactivity to the stress tasks. Salivary cortisol has been examined specifically in illicit drug users, and has been found to correlate significantly with plasma cortisol levels at multiple time points throughout the day, providing evidence for salivary cortisol as a reliable measure of HPA axis functioning in illicit drug users (Fox et al., 2006). Salivary cortisol was analyzed professionally off-site using salivary enzyme-immunoassay (EIA) technology, conducted by Salimetrics, LLC. Inter-assay coefficients of variation ranged from 5.5% to 7.3%.
2.5. Data analysis plan
Analyses were conducted with treatment dropout as the primary dependent variable. This variable was coded both categorically (dropout: yes/no) and continuously (days in treatment prior to dropout). Baseline demographic data, contract length, court mandated status, substance dependence and past year frequency of use, psychiatric comorbidity, self-report trait and state negative affect, and baseline cortisol readings were first examined using ANOVA and Chi-square analyses for their association with treatment dropout. If any of these variables were significantly associated with treatment dropout they were included as a covariate in subsequent analyses.
HPA axis response to stress was analyzed using both (1) a 2 (dropout, completer) × 5 (five cortisol assessments) repeated measures ANCOVA; and (2) calculating the peak change in cortisol from the baseline (pre-stress) reading to the four post-stressor assessment time points (Ramsay and Lewis, 2003). We also examined the unique effects of HPA axis response to stress on time to treatment dropout using Cox proportional hazards regression, a statistical method to examine the effects of continuous variables (i.e., peak change in cortisol) on event-based outcomes (i.e., treatment dropout) over a specified time period (i.e., days in treatment).
3. Results
3.1. Sample substance use history and psychiatric diagnoses
The most common substance use disorder among participants was past year crack/cocaine dependence (47.1%, n = 48), followed by alcohol (24.5%, n = 25), heroin (15.7%, n = 16), and/or cannabis (7.8%, n = 8) dependence. The majority of the sample reported being a current smoker (92.2%, n = 94) and smoking an average of 20.8 (SD = 11.1) cigarettes per day. Participants indicated past year weekly use of crack/cocaine (59.8%, n = 61), alcohol (49.0%, n = 50), heroin (16.7%, n = 17), marijuana (14.8%, n = 15), and/or PCP (6.9%, n = 7). A total of 47 individuals (36.2%) met DSM-IV criteria for an Axis I disorder other than substance dependence, including major depressive disorder (19.6%, n = 20), bipolar disorder (2.9%, n = 3), post-traumatic stress disorder (12.7%, n = 13), social phobia (10.8%, n = 11), generalized anxiety disorder (6.9%, n = 7), panic disorder without agoraphobia (2.0%, n = 2), and obsessive compulsive disorder (1.0%, n = 1). A total of 55 participants (53.9%) met criteria for borderline personality disorder and 54 (52.9%) met criteria for antisocial personality disorder.
3.2. Dropout status and potential covariates
Dropouts (n = 21; 20.6%) were defined as those individuals who left treatment before their contracted length of stay and completers (n = 81; 79.4%) were defined as individuals who finished their entire treatment. As displayed in Table 1, ANOVA and Chi-square analyses were used to determine differences between dropouts and completers on potential covariates. There was a significant group difference in self-reported trait levels of negative emotionality, with dropouts reporting significantly higher levels than treatment completers. There were no significant differences between dropouts and completers on demographic variables, contracted treatment length, court mandated status, trait negative affect, past year frequency or dependence on any drug class, or Axis I mood or anxiety disorders. Of note, group differences in ethnicity, panic disorder, and obsessive compulsive disorder were not analyzed due to group sample sizes of n = 0.
Table 1.
Group differences in demographic variables, psychiatric diagnoses, substance use, and self-report negative affect.
| Dropouts (n = 21) | Completers (n = 81) | Statistic | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 43.7 (SD = 11.6) | 41.5 (SD = 10.6) | F(1,101) = 0.69 | 0.41 |
| Gender (% male) | 71.4% | 83.9% | χ2(1) = 1.73 | 0.19 |
| Education | χ2(2) = 0.13 | 0.94 | ||
| % <HS education | 23.8% | 23.5% | ||
| % HS graduate or GED | 38.1% | 34.6% | ||
| % >HS graduate or GED | 38.1% | 42.0% | ||
| Income (% ≥$20,000) | 33.3% | 44.4% | χ2(1) = 0.84 | 0.36 |
| Contract Length | χ2(3) = 4.12 | 0.24 | ||
| 30 days | 47.6% | 46.9% | ||
| 60 days | 19.0% | 37.0% | ||
| 90 days | 4.8% | 2.5% | ||
| 180 days | 28.6% | 13.6% | ||
| Court mandated (% yes) | 38.1% | 60.5% | χ2(1) = 3.39 | 0.07 |
| Drug use | ||||
| Crack/cocaine dependence | 52.3% | 49.3% | χ2(1) = 0.06 | 0.81 |
| Crack/cocaine past year weekly use | 52.3% | 60.4% | χ2(1) = 0.45 | 0.50 |
| Heroin dependence | 28.6% | 14.8% | χ2(1) = 2.17 | 0.14 |
| Heroin, past year weekly use | 28.6% | 17.2% | χ2(1) = 1.35 | 0.25 |
| Marijuana dependencea | 9.5% | 9.9% | χ2(1) = 0.13 | 0.72 |
| Marijuana, past year weekly usea | 19.0% | 16.0% | χ2(1) = 0.00 | 1.00 |
| Alcohol dependence | 23.8% | 27.2% | χ2(1) = 0.10 | 0.76 |
| Alcohol, past year weekly use | 47.6% | 56.8% | χ2(1) = 0.57 | 0.45 |
| Current smoker | 90.5% | 92.6% | χ2(1) = 0.10 | 0.75 |
| Total # cigarettes per day | 23.7 (SD = 14.9) | 20.3 (SD = 10.5) | F(1,101) = 1.16 | 0.31 |
| Total # of dependence diagnoses | 1.10 (SD = 0.80) | 1.00 (SD = 0.89) | F(1,101) = 0.22 | 0.64 |
| Total # of drugs used weekly | 1.25 (SD = 1.18) | 1.28 (SD = 0.96) | F(1.101) = 0.02 | 0.90 |
| DSM-IV psychiatric comorbidity | ||||
| Major depressive disorder | 28.6% | 21.1% | χ2(1) = 0.77 | 0.38 |
| Post-traumatic stress disorder | 23.8% | 12.3% | χ2(1) = 1.75 | 0.19 |
| Social phobia | 23.8% | 9.9% | χ2(1) = 2.90 | 0.09 |
| Generalized anxiety disordera | 9.5% | 8.6% | χ2(1) = 0.09 | 0.76 |
| Total # mood and anxiety diagnoses | 0.89 (SD = 0.99) | 0.60 (SD = 0.94) | F(1,101) = 1.55 | 0.22 |
| Borderline personality disorder | 33.3% | 46.9% | χ2(1) = 0.76 | 0.38 |
| Antisocial personality disorder | 52.4% | 53.1% | χ2(1) = 0.00 | 0.95 |
| Self-report negative affect | ||||
| Trait negative emotionality | 19.2 (SD = 8.5) | 14.6 (SD = 8.1) | F(1,101) = 5.22 | 0.02 |
| State negative affect | 20.0 (SD = 7.7) | 17.5 (SD = 7.3) | F(1.101) = 1.94 | 0.17 |
Yates correction for continuity for 2 × 2 cell values < 5.
3.3. Validation of experimental manipulation
The average duration on the PASAT-C and MTPT-C was 702.9 s (SD = 113.8) and 197.0 s (SD = 109.1), respectively, with an average total time of 904.4 s (SD = 183.9) on both tasks, approximately 15 min. Task performance was calculated as the # of incorrect responses per second for the PASAT-C (M = 0.16, SD = 0.12) and # of errors per second for the MTPT-C (M = 4.8, SD = 3.2). Controlling for task performance, separate repeated measures ANOVAs indicated a significant increase in distress at the experimental administration of the PASAT-C [F(1, 101) = 4.50, p < .05] and MTPT-C [F(1, 101) = 10.84, p < .001], indicating that the tasks did indeed induce distress. As indicated in Table 2, there were no significant differences between dropouts and completers in task performance or changes in pre- to post-task distress.
Table 2.
Group differences in task performance, stress manipulation, and HPA axis response to stress.
| Dropouts (n = 21) | Completers (n = 81) | Statistic | p value | |
|---|---|---|---|---|
| Stress task performance | ||||
| PASAT-C skill (# incorrect/s) | 0.19 (SD = 0.15) | 0.17 (SD = 0.11) | F(1,101) = 0.38 | 0.54 |
| MTPT-C skill (# errors/s) | 5.3 (SD = 3.7) | 4.5 (SD = 3.2) | F(1,101) = 0.95 | 0.33 |
| PASAT-C stress manipulationa | F(1,100) = 0.05 | 0.83 | ||
| Pre-task negative affect | 2.8 (SD = 2.0) | 2.6 (SD = 1.9) | ||
| Post-task negative affect | 3.9 (SD = 2.9) | 3.8 (SD = 2.3) | ||
| MTPT-C stress manipulationa | F(1,100) = 1.63 | 0.21 | ||
| Pre-task negative affect | 3.1 (SD = 2.6) | 2.2 (SD = 2.2) | ||
| Post-task negative affect | 2.2 (SD = 2.2) | 3.2 (SD = 2.8) | ||
| HPA axis response to stressb | ||||
| Baseline | 0.11 (SD = 0.06) | 0.15 (SD = 0.14) | F(1,101) = 2.33 | 0.13 |
| 0 min post-stress exposure | 0.10 (SD = 0.05) | 0.12 (SD = 0.12) | F(1,101) = 0.73 | 0.39 |
| 10 min post-stress exposure | 0.10 (SD = 0.05) | 0.10 (SD = 0.07) | F(1,101) = 0.00 | 0.97 |
| 20 min post-stress exposure | 0.09 (SD = 0.04) | 0.10 (SD = 0.06) | F(1,101) = 0.13 | 0.72 |
| 30 min post-stress exposure | 0.10 (SD = 0.06) | 0.09 (SD = 0.07) | F(1,101) = 0.03 | 0.86 |
| Peak cortisol change from baselineb | −0.03 (SD = 0.04) | −0.08 (SD = 0.10) | F(1,101) = 5.34 | 0.02 |
Group (dropouts, completers) × time (pre-task, post-task) repeated measures ANOVA.
Salivary cortisol (ug/dL).
3.4. Dropout status and HPA axis response to stress
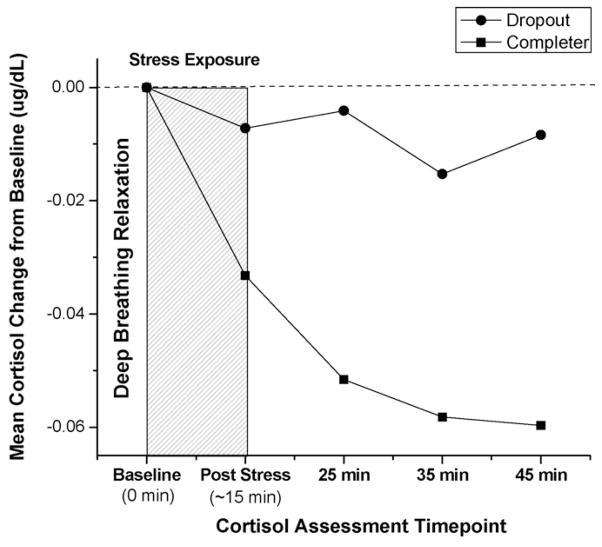
Analyses indicated significant group differences in HPA axis response to stress. As displayed in Table 2, treatment dropouts had a significantly higher peak cortisol response to stress compared to treatment completers. Of note, we used bivariate correlations to confirm that neither average # of cigarettes per day (r = .02, p > .05), total time on the stress tasks (r = .01, p = .91), or task performance (PASAT-C r = −.04, p > .05 MTPT-C r = −.02, p > .05) were associated with peak HPA axis response to stress. Second, controlling for time exposed to the stress tasks, repeated measures ANCOVA indicated a significant group × time interaction, with significantly higher salivary cortisol levels among the dropouts from the baseline cortisol assessment to the final post-stressor follow-up [F(1,100) = 4.21, p < .05]. Fig. 1 displays group differences in the mean salivary cortisol change from baseline (pre-stress) through the four post-stress assessment time points.
Fig. 1.
Differences between treatment dropouts and completers in mean salivary cortisol change in response to a psychological stress task.
3.5. Survival to treatment dropout
Cox proportional hazards survival analysis was used to examine the unique association between peak cortisol response to stress and total number of days in treatment. Variables that were significantly related to group status, namely trait negative emotionality, were entered into Step 1 of the model. Peak cortisol response to stress was entered in Step 2. Continuous independent variables were standardized using z scores to facilitate interpretation. Step 1 did not reach significance [χ2(1) = 2.63, p = .11], yet the final model was significant [χ2(2) = 6.8, p < .05], with the inclusion of peak cortisol response to stress in Step 2 providing a significant improvement in fit [Δχ2(1) = 9.8, p < .01]. In the final model, higher trait levels of negative emotionality (hazards ratio, 1.68; 95% confidence interval, 1.03–2.73; p < .05), and greater peak cortisol response to stress (hazards ratio, 4.09; 95% confidence interval, 1.44–11.6; p < .01) were associated with a shorter time to treatment dropout. In particular, for each additional unit increase in peak cortisol there was a four-fold increase in the likelihood of treatment dropout on any given day.
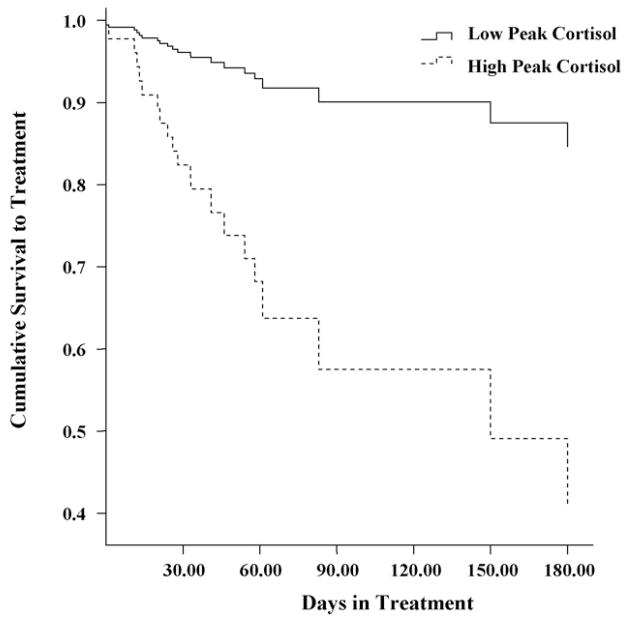
We also conducted a median split on peak cortisol response and ran an additional Cox proportional hazards survival analysis, replacing the continuous measure of peak cortisol response with the categorical variable (high peak = 1, low peak = 0). Similarly, the final model was significant [χ2(2) = 9.73, p < .01], with the inclusion of the categorical peak cortisol response to stress variable in Step 2 providing a significant improvement in fit [Δχ2(1) = 7.41, p < .01]. As illustrated in Fig. 2, patients in the high peak cortisol group evidenced a significantly greater likelihood of dropping out of treatment on any given day (hazard ratio, 0.26; p < .05; 95% confidence interval, 0.09–0.74).
Fig. 2.
Cumulative survival to treatment dropout among high vs. low peak cortisol response to a psychological stress task.
3.6. Secondary analyses
Although variation in contract length was not related to treatment dropout, and therefore not included as a covariate in our 180-day hazards model, we wanted to confirm that our findings across the entire possible 180 days of treatment were not a result of censoring participants at the end of their contract length, thereby reducing the available data for risk of dropout at the later time points. As such, we ran an additional Cox regression and censored all participants at 30 days to equate participants on total possible days in treatment. The final 30-day model was consistent with the 180-day model [χ2(2) = 6.31, p < .05], with the inclusion of the peak cortisol response to stress in Step 2 providing a significant improvement in fit [Δχ2(1) = 9.38, p < .01], and indicating that higher trait levels of negative emotionality (hazards ratio, 1.63; 95% confidence interval, 1.00–2.64; p < .05), and greater peak cortisol response to stress (hazards ratio, 3.98; 95% confidence interval, 1.44–11.0; p < .01) were associated with a shorter time to treatment dropout.
4. Discussion
The current findings indicate that substance dependent individuals who evidenced higher trait negative emotionality and higher peak HPA axis response to a psychological stressor upon entry into a residential facility were more likely to dropout of treatment prematurely. Further, survival analyses indicated that a higher HPA axis response to stress conferred a four-fold daily risk for dropout over the course of treatment.
A primary function of the HPA axis is to mediate the body’s response to stress through increases in glucocorticoid (cortisol) secretion. Results from the current study indicate that individuals who experience a heightened cortisol response to stress were significantly more likely to dropout of treatment. These findings are in line with recent conceptualizations of drug dependence which emphasize the establishment of a “negative affect” or psychologically distressed state during abstinence, potentially associated with neuroadaptive changes in brain stress and reward circuits (Kreek and Koob, 1998; Koob and Le Moal, 1997; Sinha, 2001). Specifically, chronic drug administration is associated with a decrease in activation of brain reward systems and an increase in opposing anti-reward brain stress circuits (including the HPA axis), which may reduce an individual’s ability to adapt or cope with additional stressors (Koob and Le Moal, 2008). In line with a negative reinforcement model of addiction, it is therefore hypothesized that individuals with a dysregulated HPA axis response to stress may be less able to cope with the commonly experienced stressors during the early stages of treatment. However, the current design did not measure the immediate effects of HPA axis response to stress on behavior and therefore future research aimed at capturing this causal relationship is needed.
The current findings are in line with existing literature on the relationship between HPA axis response to stress and substance use outcomes. Previous work indicates that substance dependent samples demonstrate a blunted HPA axis response to stress (Lovallo, 2007; Sinha et al., 2003, 2006), yet the relationship between HPA axis response to stress and substance use outcomes is mixed across drug classes. For example, smokers who relapse early have demonstrated a blunted HPA axis response to stress compared to successful quitters (al’Absi et al., 2005), whereas among illicit drug users an elevated HPA axis response to stress is associated with increased cocaine use following treatment (Sinha et al., 2006). Our findings are in line with this literature, such that overall our sample, which included current smokers and individuals who met criteria for dependence across a variety of drug classes, demonstrated a blunted HPA axis response to stress, yet the treatment dropout group evidenced an elevated cortisol response compared to treatment completers. Given literature indicating differential effects on HPA axis functioning across stimulants (Goeders, 2002) and depressants (Kreek et al., 2005), continued empirical attention aimed at disentangling the effects of nicotine, stimulants, and depressants on HPA axis response to stress is needed.
The current findings have significant clinical implications. Interventions aimed at improving treatment retention may be improved by focusing on enhancing one’s ability to persist through negative affective states during the early stages of abstinence. Acceptance-based behavioral treatments (e.g., Acceptance and Commitment Therapy, ACT (Hayes et al., 1999) or Dialectical Behavior Therapy, DBT (Linehan, 1993)) may be particularly useful in this regard. These treatments focus on increasing emotional acceptance stemming from the view that many maladaptive behaviors (e.g., drug use) are the result of unhealthy attempts to avoid or suppress thoughts, feelings, and/or bodily sensations, and recent evidence suggests that acceptance-based treatments may be useful in treating substance use (Hayes et al., 2004; Linehan et al., 1999; Linehan et al., 2002). For instance, a randomized control trial (RCT) comparing methadone maintenance to methadone maintenance plus 16 weeks of ACT indicated that the ACT group evidenced significantly less drug use during follow-up (Hayes et al., 2004). Similarly, among substance using populations, DBT has been found to significantly reduce the rates of premature treatment termination (Linehan et al., 1999), and significantly improve the rates of post-treatment abstinence (Linehan et al., 2002). In addition to empirically validated behavioral treatments aimed at tolerating negative affective states, pharmacological treatments that target stress-induced drug craving and normalization of the HPA axis during abstinence and distress states may also be of benefit. In particular, CRF antagonists and α2-adrenergic agonists decrease stress-induced reinstatement of cocaine seeking, and glucocorticoid antagonists decrease cocaine reinforcement in drug experienced laboratory animals (Erb et al., 2000; Goeders, 2002). The current findings support the need to test these stress ameliorating agents in humans to assess their efficacy in addressing early treatment dropout and reduction in stress-induced relapse in addictive disorders.
Additional limitations are of note. The number of treatment dropouts in the current study precluded an analysis of the effects of drug class or gender on the relationship between HPA axis response to stress and treatment retention. Further, although no differences were found on baseline (pre-stress) cortisol levels, future work examining cortisol on a non-stress control day will further corroborate these findings. Finally, although treatment retention is an established predictor of relapse outcomes, in the current study we did not assess relapse to substance use following treatment.
Despite these limitations, the current study provides important preliminary evidence that HPA axis response to stress at treatment entry predicts residential substance abuse treatment retention. The findings are in line with a growing literature indicating that a dysregulated HPA axis response to stress is associated with poor substance use outcomes, suggesting the need for future studies to expand on these findings by developing methods to accurately identify treatment seeking substance users with a dysregulated HPA axis response to stress, as well as behavioral and pharmacological treatments targeting this vulnerability.
Acknowledgments
Role of funding source
Funding for this study was partially supported by the following National Institute of Drug Abuse (NIDA) grants: R01-DA19405, R21-DA022741, P50-DA016556, and K02-DA017232.
We would like to thank C.W. Lejuez, Director of CAPER in the Department of Psychology at the University of Maryland for his support of this research; and staff at the Salvation Army Harbor Light Substance Abuse Treatment Center, Washington, DC, for their assistance with subject recruitment. Dr. Daughters had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Contributors
Stacey B. Daughters designed the study and wrote the protocol. Jessica M. Richards and Stephanie M. Gorka managed the literature searches and summaries of previous related work. Stacey B. Daughters wrote the first draft of the manuscript and undertook the statistical analysis. Rajita Sinha wrote portions of the manuscript and provided conceptual and statistical guidance. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflict of interest.
References
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- Babor BF, Del Boca FK. Just the facts: enhancing measurement of alcohol consumption using self-report methods. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc; 1992. pp. 3–30. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. J Abnorm Psychol. 2003;112 (3):448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;11 (1):180–185. [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial pre-frontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, Brown RA. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. J Abnorm Psychol. 2005a;114 (4):729–734. doi: 10.1037/0021-843X.114.4.729. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Kahler CW, Strong DR, Brown RA. Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychol Addict Behav. 2005b;19 (2):208–211. doi: 10.1037/0893-164X.19.2.208. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Sargeant MN, Gratz KL, Bornovalova MA, Lejuez CW. The relationship between distress tolerance and antisocial personality disorder among male residential treatment seeking inner-city substance users. J Personal Disord. 2008;22 (5):509–524. doi: 10.1521/pedi.2008.22.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130 (3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press, Inc; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) [Google Scholar]
- Fox HC, Wilker EH, Kreek MJ, Sinha R. Reliability of salivary cortisol assessments in cocaine dependent individuals. J Psychopharmacol. 2006;20 (5):650–655. doi: 10.1177/0269881106063474. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27 (1–2):13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Granger DA, Harmon AG, Hibel LC, Rumyantseva O. Measuring Salivary Cortisol in Studies of Child Development: Watch out—What Goes in May not Come out of Commonly Used Saliva Collection Devices: Penn State University. Department of Biobehavioral Health, Behavioral Endocrinology Laboratory; 2006. [Google Scholar]
- Hayes SC, Strosahl K, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. Guilford Press; New York: 1999. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Bissett R, Piasecki M, Batten SV, Byrd M, Gregg J. A preliminary trial of twelve-step facilitation and Acceptance and Commitment Therapy with polysubstance-abusing methadone-maintained opiate addicts. Behav Ther. 2004;35 (4):667–688. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert WS, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278 (5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain anti-reward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51 (1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. Behav Ther. 2003;26 (4):290–293. [Google Scholar]
- Linehan MM. Cognitive Behavioral Treatment of Borderline Personality Disorder. Guilford Press; New York: 1993. [Google Scholar]
- Linehan MM, Dimeff LA, Reynolds SK, Comtois KA, Welch SS, Heagerty P, Kivlahan DR. Dialectical behavior therapy versus comprehensive validation plus 12 steps for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67:13–26. doi: 10.1016/s0376-8716(02)00011-x. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Schmidt H, Dimeff LA, Craft CJ, Comtois KA. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addict. 1999;8 (4):279–292. doi: 10.1080/105504999305686. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Individual differences in response to stress and risk for addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Elsevier Academic Press; San Diego, CA: 2007. pp. 227–248. [Google Scholar]
- Piazza PV, Barrot M, Rouge-Pont F, Marinelli M, Maccari S, Abrous DN, Simon H, Le Moal M. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopamingergic transmission. Proc Natl Acad Sci. 1996;93 (26):15445–15450. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Derouche V, Rouge-Pont F, Le Moal M. Behavioral and biological factors associated with individual vulnerability to psychostimulant abuse. NIDA Res Monogr. 1998;169:105–133. [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormède P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavior response to stress. Child Dev. 2003;74 (2):456–464. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Miczek KA. Reinstatement toward a model of relapse. Psychopharmacology. 2003;168 (1–2):1–2. [Google Scholar]
- Simpson D, Joe GW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Psychol Addict Behav. 1997;11 (4):294–307. [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158 (4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and HPA axis responses predict cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek M. Hypothalamic-pituitary adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology. 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration. Results from the 2004 Treatment Episode Data Set. Office of Applied Studies; Rockville, MD: 2005a. (DASIS Series S-35, DHHS Publication No. SMA 06-4207) [Google Scholar]
- Substance Abuse Mental Health Services Administration. Overview of Findings from the 2004 National Survey on Drug Use and Health. Office of Applied Studies; Rockville, MD: 2005b. (NSDUH Series H-27, DHHS Publication No. SMA 05-4061) [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety with an emphasis on self-report. In: Tuma A, Master J, editors. Anxiety and the Anxiety Disorders. Erlbaum; Hillsdale, NJ: 1985. pp. 681–706. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scale. J Pers Soc Psychol. 1988;54 (6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]