Abstract
Congenital disorders of glycosylation (CDG), formerly known as carbohydrate-deficient glycoprotein syndromes, lead to diseases with variable clinical pictures. We report the delineation of a novel type of CDG identified in 2 children presenting with severe developmental delay, seizures, and dysmorphic features. We detected hypoglycosylation on serum transferrin and cerebrospinal fluid β-trace protein. Lipid-linked oligosaccharides in the endoplasmic reticulum of patient fibroblasts showed an accumulation of the dolichyl pyrophosphate Man5GlcNAc2 structure, compatible with the reduced dolichol-phosphate-mannose synthase (DolP-Man synthase) activity detected in these patients. Accordingly, 2 mutant alleles of the DolP-Man synthase DPM1 gene, 1 with a 274C>G transversion, the other with a 628delC deletion, were detected in both siblings. Complementation analysis using DPM1-null murine Thy1-deficient cells confirmed the detrimental effect of both mutations on the enzymatic activity. Furthermore, mannose supplementation failed to improve the glycosylation status of DPM1-deficient fibroblast cells, thus precluding a possible therapeutic application of mannose in the patients. Because DPM1 deficiency, like other subtypes of CDG-I, impairs the assembly of N-glycans, this novel glycosylation defect was named CDG-Ie.
Introduction
The detection of hypoglycosylation of proteins based on rapid assays such as isoelectric focusing of serum transferrin has led to the identification of several novel defects of N-linked glycosylation. The related diseases have been classified as congenital disorders of glycosylation (CDG), formerly known as carbohydrate-deficient glycoprotein syndromes (1, 2). In keeping with the importance of oligosaccharides for glycoprotein functions (3), defective glycosylation leads to various clinical presentations, such as dysmorphism, encephalopathy, coagulation disorders, and other organ dysfunctions.
To date, 5 types of CDG have been characterized at the biochemical and genetic level. The currently established subtypes of CDG-I are caused by deficiencies in various compounds. CDG-Ia is caused by a defect in the PMM2 gene and a resulting deficiency in phosphomannomutase (4). The PMI gene is responsible for CDG-Ib and its characteristic deficiency in phosphomannose isomerase (5, 6). CDG-Ic is linked to the ALG6 gene and a deficiency in Man9GlcNAc2-PP-Dol–dependent α1,3-glucosyltransferase (PP-Dol = dolichyl pyrophosphate) (7–9), and CDG-Id is caused by a defect in ALG3 and a resulting deficiency in Man5GlcNAc2-PP-Dol–dependent α1,3-mannosyltransferase (10). All of these defects lead to incomplete biosynthesis of lipid-linked Glc3Man9GlcNAc2 in the endoplasmic reticulum (ER) and subsequent hypoglycosylation of nascent proteins. By contrast, deficiency of the Golgi-localized N-acetylglucosaminyltransferase-II enzyme in CDG-IIa leads to defective maturation of complex-type N-linked glycans (11).
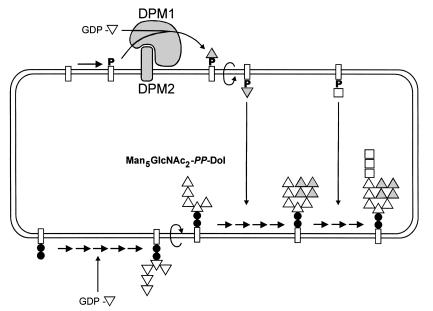
The assembly of lipid-linked oligosaccharides takes place at the membrane of the ER. First, Man5GlcNAc2-PP-Dol is assembled at the cytoplasmic side of the membrane; UDP-GlcNAc and GDP-Man serve as sugar donors. After the flipping of the oligomannose core into the luminal side of the ER, 4 mannose residues and 3 glucose residues are added using dolichol-phosphate-mannose (DolP-Man) and dolichol-phosphate-glucose (DolP-Glc), respectively, as donor substrates (Figure 1) (12). DolP-Man is synthesized from GDP-Man and DolP by DolP-Man synthase. The human enzyme consists of 2 subunits encoded by 2 genes, DPM1 (13, 14) and DPM2 (15). Cell lines with impaired DolP-Man synthase activity exhibit altered N-linked protein glycosylation; glycosylphosphatidylinositol-anchor (GPI-anchor) biosynthesis (16) and C-linked protein mannosylation (17) are also affected. Herein we describe the identification of DolP-Man synthase deficiency caused by mutations in the DPM1 gene as the primary cause for a novel type of CDG, which we call CDG-Ie.
Figure 1.
Assembly of the oligomannose core in the ER. Mannose (triangles) is first incorporated into dolichol-bound oligosaccharides (rectangles) from the GDP-Man donor substrate. After flipping into the luminal side, Man5GlcNAc2-PP-Dol is further decorated with 4 mannoses provided by DolP-Man. The formation of DolP-Man from GDP-Man is catalyzed by the DolP-Man synthase enzyme, which is constituted of a catalytic subunit (Dpm1) and a membrane-anchored subunit (Dpm2). Once fully mannosylated, the core receives 3 glucose residues (squares) before being transferred by the oligosaccharyltransferase complex onto nascent glycoproteins. Filled circles, GlcNAc.
Methods
Patient cells.
Primary fibroblasts isolated from a skin biopsy were grown in DMEM/F12 with high glucose levels (Life Technologies Inc., Paisley, Scotland) supplemented with 10% FCS. Alternatively, cells were cultured in medium supplemented with 5 mM α-D-mannose (Sigma-Aldrich Co., Buchs, Switzerland) for 15 days.
PMM and PMI assays.
PMM and PMI activities were measured in enucleated fibroblast cell lysates using the assays of Van Schaftingen and Jaeken (18).
Isoelectric focusing and Western blot.
Isoelectric focusing separation of serum transferrin was performed as described previously (19). Proteins from 100-μL cerebrospinal fluid samples were precipitated with ethanol and then resuspended in Laemmli buffer (20). Proteins were separated under reducing conditions by SDS-PAGE. After transfer to nitrocellulose, β-trace protein was detected using an anti–β-trace protein monoclonal antibody (provided by H.S. Conradt, Gesellschaft für Biotechnologische Forschung mblt, Braunschweig, Germany) (21).
Analysis of lipid-linked oligosaccharides.
Fibroblast cells grown to 90% confluence on 530-cm2 plates were washed with PBS and then incubated for 90 minutes at 37°C in minimal Eagle’s medium (Life Technologies Inc.) supplemented with 5% dialyzed FCS. After this initial incubation period, cells were labeled by the addition of 175 μCi [3H]mannose (Amersham Pharmacia Biotech, Freiburg, Germany) for 1 hour at 37°C. Cells were then washed twice with ice-cold PBS and scraped off in 10 mL methanol and 0.1 mM Tris, pH 7.4 (8:3 vol/vol). After the addition of 10.9 mL chloroform and extensive mixing of the samples, cells were collected by centrifugation at 5,300 g for 5 minutes. Lipid-linked oligosaccharides were extracted as described previously (8) and were analyzed by HPLC (22).
DolP-Man synthase assay.
Fibroblasts grown on a 530-cm2 plate were trypsinized and washed twice in ice-cold PBS. Cells were lysed on ice in 50 mM HEPES (pH 7.4), 25 mM KCl, 5 mM MgCl2, 5 mM MnCl2, and 0.2% Triton X-100 for 15 minutes; nuclei were removed by centrifugation. DolP-Man synthase activity was assayed using 30 μL of cell lysate in 100 μL of the same buffer, with the addition of 40 μg/mL DolP (Sigma-Aldrich Co.) and 17 μM GDP-[14C]mannose (Amersham Pharmacia Biotech). Reaction mixtures were incubated for 0, 5, and 15 minutes at 37°C. DolP-Man was isolated by organic extraction (23), and the radioactivity was measured in a beta counter (Beckman Coulter Inc., Fullerton, California, USA).
RT-PCR.
Extraction of total RNA from patient and control fibroblasts (2 × 107) was performed by the procedure of Chomczynski and Sacchi (24). Eight micrograms of total RNA and 100 units of Moloney murine leukemia virus reverse transcriptase (New England Biolabs Inc., Beverly, Massachusetts, USA) were used for reverse transcription. The reaction mixture (100 μL total volume) was incubated for 2 hours at 37°C. DPM1 cDNA (13) was amplified by PCR using the primer pair 5′-CATGGCCTCCTTGGAAGTCAG-3′ and 5′-ACCAGGCTTCTTTCATGTTTAACC-3′, with 8 μL reverse transcription product as template. The cycling conditions were 35 cycles at 94°C for 45 seconds, 56°C for 30 seconds, and 72°C for 1 minute, preceded by 6 cycles performed at higher annealing temperatures (twice at 65°C, twice at 63°C, twice at 60°C).
Genomic PCR.
The human DPM1 cDNA sequence (GenBank accession D86198) was used to screen the available databases using the basic local alignment search tool (BLAST) algorithm (25). One human genomic sequence from a BAC contig on chromosome 20q13 (accession AL334553) was found to include the DPM1 gene. Exon and intron boundaries were determined by alignment of the genomic sequence with the cDNA sequence. The regions including exons 3 and 8 of the human DPM1 gene were amplified with the primers 5′-TGCTTTAAAGTTCTAACAAGTGCAA-3′ and 5′- AGCAGGTGTGAGGGGTTAGA-3′, and 5′-ACCAATGGCCAGTGAAAGTT-3′ and 5′-AAACCTTACTGCTCCTTTACCA-3′, respectively.
DNA sequencing.
PCR fragments were purified using GENECLEAN (Bio 101 Inc., Carlsbad, California, USA) or QIAquick (QIAGEN Gmblt, Hilden, Germany) DNA purification kits. Sequencing was performed by a combination of cycle sequencing using the ThermoSequenase fluorescent-labeled primer cycle sequencing kit with 7-deaza dGTP, and solid phase sequencing using the AutoRead Sequencing Kit (both from Amersham Pharmacia Biotech). The sequences were analyzed on an ALF DNA sequencer (Amersham Pharmacia Biotech).
Cloning of human DPM1 cDNA and site-directed mutagenesis.
DPM1 cDNA was amplified by PCR as described above using 100 ng of the human T-cell cDNA library as template (26), and 4 units of Klentaq polymerase (Sigma-Aldrich Co.). The resulting 840-bp fragment was subcloned into the SmaI site of pBluescript II SK+ (Stratagene, La Jolla, California, USA). Site-directed mutagenesis was performed using 100 ng of the resulting plasmid (pBS-DPM1wt) as template, and 4 units of Klentaq polymerase. The primers applied to introduce the 274C>G mutation were 5′-CATGGCCTCCTTGGAAGTCAG-3′, 5′-CCCAACTTTTTCTCTCCTGGTCTTAG-3′, 5′-TCTAAGACCAGGAGAGAAAAAGTTG-3′, and 5′-ACCAGGCTTCTTTCATGTTTAACC-3′. The primers used to introduce the 628delC deletion were 5′-CATGGCCTCCTTGGAAGTCAG-3′, 5′-TCTGAAGACGTAGCCTTTAGAAACAC-3′, 5′-TCTTCAGATGGAGATGATTGTTCG-3′, and 5′-ACCAGGCTTCTTTCATGTTTAACC-3′. Twenty PCR cycles were carried out at 94°C for 45 seconds, 52°C for 30 seconds, and 68°C for 60 seconds. The PCR products were gel purified, and the corresponding fragments were mixed and used as template for a second PCR that used the full-length primers to obtain the 2 DPM1 fragments with the designed mutations. The accuracy of the obtained fragments was confirmed by sequencing. The DPM1 cDNA sequence and the 2 mutant variants were directionally subcloned into the BamHI-XhoI sites of the mammalian expression vector pcDNA3.1+ (Invitrogen, Corp. Carlsbad, California, USA).
Transfection of BW5147 cells.
The mouse BW5147 Thy1-null cells of complementation class E (Thy1-E cells; 27) were generously provided by Robert Hyman (The Salk Institute, San Diego, California, USA), and were cultured in DMEM supplemented with 10% FCS. For transfection, 20 μg of plasmid DNA was mixed with 107 cells resuspended in cold PBS. Cells were electroporated with a Gene Pulser (Bio-Rad, Hercules, California, USA) set at 250 V and 960 μF. After a 10-minute resting period on ice, cells were resuspended in cell medium and incubated at 37°C for 3 days.
Flow cytometry.
FITC-labeled antibodies to mouse Thy1.1 and human CD59 were purchased from PharMingen (San Diego, California, USA). Before addition of anti-Thy1.1 antibody, BW5147 cells were incubated with anti-CD16/CD32 antibody for 10 minutes on ice to block Fc receptor–mediated antibody binding. Cells were washed twice with PBS and 1% FCS and then incubated with diluted (1:100) antibodies for 15 minutes on ice. After rinsing cells once in PBS and 1% FCS, fluorescence was analyzed on a FACScan flow cytometer equipped with CellQUEST software (Becton Dickinson, Franklin Lakes, New Jersey, USA).
Results
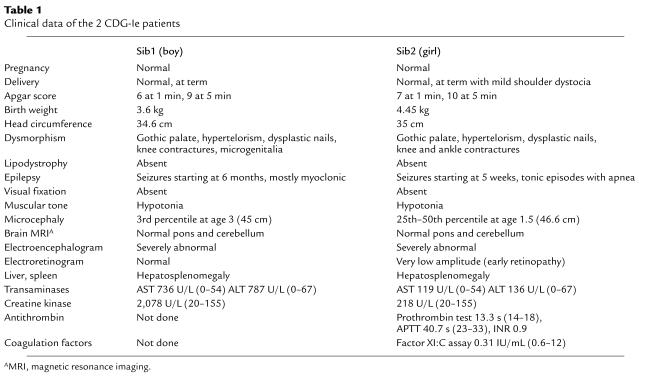
Two siblings (a boy 3 years and 4 months of age, and his younger sister aged 19 months) were hospitalized after repeated seizure episodes. They were born at term after an uneventful pregnancy. In both children, weight, length, and head circumference was normal at birth, but microcephaly developed in early childhood. Hypertelorism, gothic palate, small hands with dysplastic nails, and knee contractures were observed. Notably, nipples were not inverted as is found in CDG type Ia. Because of a failure to thrive, the younger child received nasogastric feeding. Her early childhood was complicated by recurrent infections. Severe epilepsy started in the girl at the age of 5 weeks, and in her brother at the age of 6 months. Both children were hypotonic and showed a severe global developmental delay. There was no visual fixation, and they were unable to interact socially. Electroencephalogram was severely abnormal with bilateral epileptiform changes. Magnetic resonance imaging of the brain revealed widening of the frontotemporal lobe and the ventricular system but normal pons and cerebellum. A very low flash electroretinogram suggested early retinopathy in the baby girl. Both children showed recurrent moderately elevated transaminases and creatine kinase, and signs of mild coagulopathy (Table 1).
Table 1.
Clinical data of the 2 CDG-Ie patients
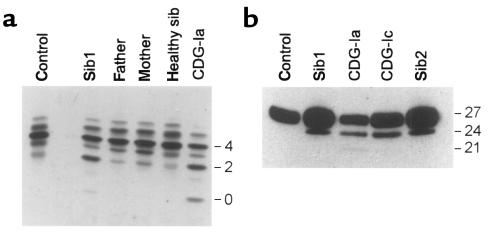
Isoelectric focusing of serum transferrin showed decreased tetrasialotransferrin and increased disialotransferrin and asialotransferrin levels, suggesting a possible lack of entire N-glycan chains (Figure 2a). The hypoglycosylation phenotype was also detected in cerebrospinal fluid, as shown by the presence of hypoglycosylated β-trace protein (Figure 2b). These patterns were reminiscent of the distribution observed in CDG types Ia, Ib, and Ic.
Figure 2.
Isoelectric focusing and Western blotting. (a) Serum transferrin was separated by isoelectric focusing. The anode is at the top. The number of negative charges is indicated at the right. (b) β-trace protein was analyzed by SDS-PAGE followed by Western blotting. Molecular weights (in kDa) are given at the right. (Sib1 and sib2 are described in Table 1.)
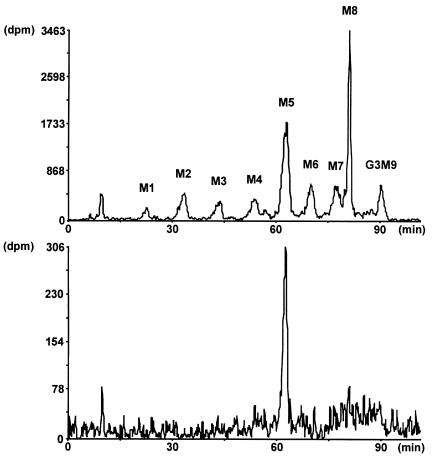
However, PMM and PMI activity levels were found to be normal in the patients’ fibroblasts (data not shown). We investigated the profile of dolichol-bound oligosaccharides in the ER to detect a possible defect in the assembly of the oligomannose core. This analysis revealed an accumulation of the Man5GlcNAc2-PP-Dol precursor structure in both patients (Figure 3). This accumulation was indicative of decreased availability of DolP-Man, the substrate for mannosyltransferases that act in the lumen of the ER (see Figure 1). Alternatively, a block at the level of the Man5GlcNAc2-PP-Dol–dependent mannosyltransferase would yield a phenotype similar to that observed in the Saccharomyces cerevisiae alg3 yeast mutant, which lacks this mannosyltransferase activity (28, 29).
Figure 3.
Lipid-linked oligosaccharide profile in a CDG-Ie patient. [3H]oligosaccharides were separated by HPLC after labeling CDG fibroblasts with [3H]mannose for 1 hour. The top profile shows the retention times of yeast-derived oligosaccharide standards from Man1GlcNAc2 (M1) to Glc3Man9GlcNAc2 (G3M9). The bottom profile shows the Man5GlcNAc2 peak accumulating in the fibroblasts from a CDG-Ie patient.
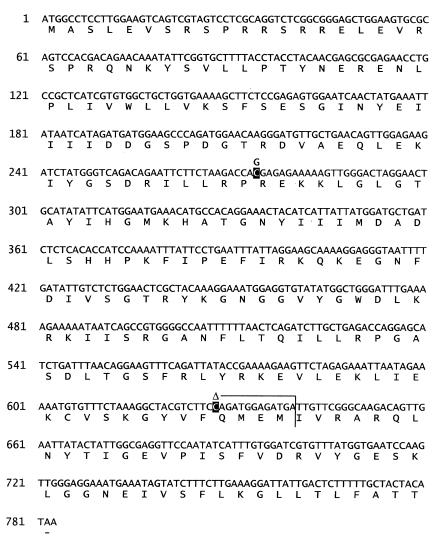
The coding sequences of the 3 candidate genes (DPM1, DPM2, and the ALG3 mannosyltransferase gene) were investigated in the patients. Whereas human DPM1 and DPM2 cDNA have been cloned previously, the human gene orthologous to the ALG3 mannosyltransferase gene was not known as such, although its cDNA had been isolated and specified as encoding a Not56-like protein (GenBank accession Y09022). The sequences of the DPM2 and ALG3 mannosyltransferase cDNA of the patients revealed no abnormality (not shown). However, based on cDNA analysis, 2 mutant DPM1 alleles were identified in the patients. The 2 siblings and their mother were found to be heterozygous for a C→G transversion at position 274, resulting in arginine-to-glycine substitution (R92G). The 2 children were also heterozygous for an allele with a C deletion at position 628 that was also detected in the father. The mutation resulted in a premature stop of the translation at position 640 (codon 213) (Figure 4).
Figure 4.
Two mutant alleles of the DPM1 gene in CDG-Ie patients. The positions of the 274C>G and 628delC mutations are marked in black on the human DPM1 cDNA sequence. The early termination of DPM1 translation caused by the 628delC-mediated frameshift is shown as a vertical line at position 640 of the cDNA.
Comparison of Dpm1 protein sequences from several species indicated that the arginine residue at position 92 was semiconserved. This was found in Caenorhabditis briggsiae and Schizosaccharomyces pombe, but not in Saccharomyces cerevisiae, Trypanosoma brucei, or Ustilago maydis (14). It is worth noting that the species in which the R92 residue is conserved contain a class of DolP-Man synthase constituted of 2 subunits, Dpm1 and Dpm2.
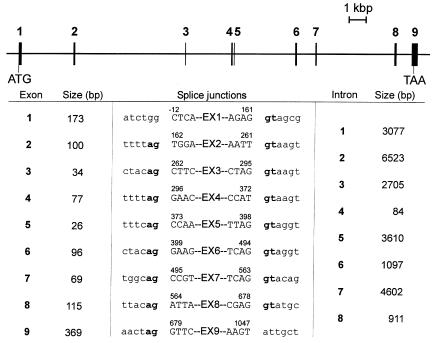
The genomic structure of the DPM1 gene was elucidated to confirm the presence of the mutations at the genomic level. The genomic organization of DPM1 had been only partially characterized, with the first 3 exons revealed (13). To complete the task, we first searched GenBank for possible genomic sequences that would include the DPM1 gene. In fact, a contig sequence (accession AL334553) on chromosome 20q13 was found to contain the entire DPM1 gene, which spans 23.6 kbp. The DPM1 gene was split in 9 exons (Figure 5). Examination of the exon-intron boundaries showed that the 274C>G mutation was in exon 3 and the 628delC deletion was in the middle of exon 8, thereby excluding possible splicing artifacts. Sequencing of exons 3 and 8 in the CDG patients and their parents confirmed the mutations found at the cDNA level (data not shown).
Figure 5.
Genomic organization of the human DPM1 gene. Exons are numbered and represented as filled rectangles. Introns are indicated as solid lines. The positions of initiation and termination of translation are symbolized by ATG and TAA, respectively. The sizes of the exons and introns are shown proportionally. The bottom panel shows the sizes and boundaries of the DPM1 exons and introns.
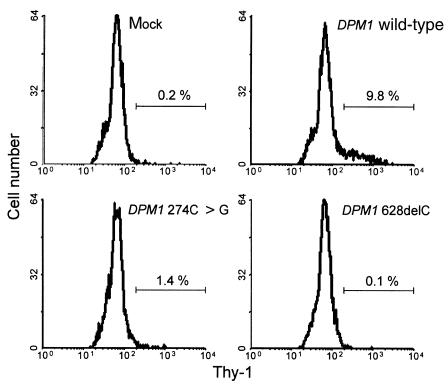
DolP-Man synthase activity was measured in fibroblasts from the CDG patients and from healthy control subjects. An activity of 11.8 pmol/min per mg protein was found in control cells, and a residual activity of 0.7 pmol/min per mg protein was detected in the CDG cells. Although DolP-Man synthase activity in the CDG fibroblasts was decreased to 6% of the normal level, the remaining activity was significantly above the detection limit of the assay, indicating that the 2 mutations identified in the DPM1 gene of CDG patients did not completely inactivate the enzyme. To determine the impact of each mutation on DolP-Man synthase activity, we engineered DPM1 expression vectors that expressed either the wild-type cDNA, the 274C>G mutation, or the 628delC deletion. The constructs were transfected into mouse BW5147 Thy1-E cells (27), which are deficient in DolP-Man synthase activity due to inactive Dpm1 enzyme (13). Restoration of Thy1 expression was analyzed by flow cytometry 3 days after transfection. Cells transfected with a construct expressing wild-type DPM1 reacted positively for Thy1 staining, whereas cells transfected with an empty vector remained Thy1-negative (Figure 6). A small population of Thy1-positive cells was detected after transfection with the DPM1 gene containing the 274C>G substitution, indicating that this mutation does not abolish DolP-Man activity. By contrast, the transfection of Thy1-E cells with the vector including the DPM1 gene with the 628delC deletion failed to restore Thy1 expression at all (Figure 6); this is compatible with the observation that a deletion of the 24 COOH-terminal amino acids also abolished DolP-Man synthase activity (15).
Figure 6.
Complementation of Thy1-E cells. BW5147 Thy1-null cells, transfected with DPM1 expression vectors, were analyzed for Thy1 staining by flow cytometry. Each panel shows the fluorescence histogram obtained for cells transfected with a mock vector, the human DPM1wild-type cDNA, the human DPM1 274C>G cDNA, and the DPM1 628delC cDNA. The region marked with the horizontal bar denotes the percentage of Thy1-positive cells.
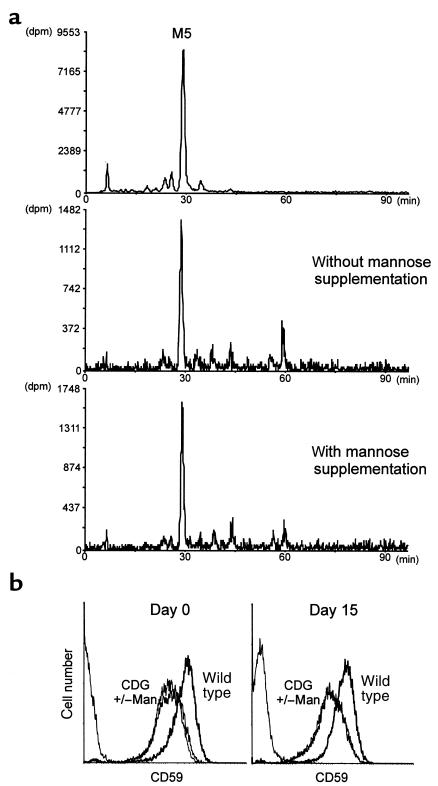
Depletion of GDP-Man caused by deficiencies in PMM and PMI, and the resulting protein hypoglycosylation can be improved in vitro by supplementing with 1–2 mM mannose in the cell culture medium (30, 31). To evaluate whether the DolP-Man synthase deficiency detected in CDG patients can be overcome by mannose addition, we cultured the CDG fibroblasts in the presence or absence of 5 mM α-D-mannose for a period of 15 days. The dolichol-bound oligosaccharides were then analyzed in both cell populations. At the same time, we analyzed the levels of the GPI-anchored protein CD59 at the surface of CDG fibroblasts before and after mannose supplementation. Identical lipid-linked oligosaccharide profiles were detected in cells cultured with or without the addition of mannose (Figure 7a). Similarly, after mannose supplementation, the CD59 levels measured on DPM1-deficient CDG fibroblasts remained unchanged, at 40% of the amount normally found on fibroblast cells (Figure 7b).
Figure 7.
Mannose supplementation in CDG fibroblasts. Lipid-linked oligosaccharide profiles (a) and CD59 levels (b) were analyzed in CDG cells cultured for 15 days in the presence of 5 mM mannose. The top panel shows the HPLC elution profile of the Man5GlcNAc2 standard (M5) derived from the Saccharomyces cerevisiae alg3 yeast mutant cells.
Discussion
We have identified a novel type of CDG caused by a deficiency in DolP-Man synthase activity. The shortage of DolP-Man affects the biosynthesis of the lipid-linked Glc3Man9GlcNAc2 substrate for N-linked protein glycosylation, and results in a suboptimal N-glycosylation of nascent glycoproteins and suboptimal biosynthesis of GPI-anchored proteins. Because DolP-Man deficiency causes hypoglycosylation of proteins similar to that encountered in CDG-I, we propose to classify this novel ER-associated defect as CDG-Ie. Clinically, apart from the psychomotor retardation and the marked hypotonia, these patients are quite different from CDG-Ia patients. By contrast, CDG-Ie patients show striking similarities with the patients reported by Stibler et al. and defined as CDG type IV (32). The molecular basis of the defect underlying the glycosylation deficiency in these type IV patients has not been disclosed yet, but we speculate that it may be either identical to the defect presented here or otherwise related to an alteration at the level of DolP-Man availability or incorporation into oligosaccharides.
DolP-Man serves as donor substrate in the process of N-linked protein glycosylation, GPI-core biosynthesis, and C-glycosylation of proteins (17). Therefore, a limitation in the DolP-Man pool can possibly affect the formation of different types of glycoproteins. Indeed, we detected decreased levels of the GPI-anchored CD59 protein at the surface of CDG-Ie fibroblasts. Similarly, the shortage of mannose-1-phosphate caused by both PMM and PMI deficiencies may also affect the same pathways, because GDP-Man is the substrate for DolP-Man synthesis. However, analysis of CD59 levels on fibroblasts of several CDG-Ia and -Ib patients revealed normal levels (data not shown). Because GDP-Man not only serves as a substrate for DolP-Man synthesis, the differences in the biochemical and clinical representation of CDG-Ia and CDG-Ib vs. CDG-Ie can be understood on this basis. In addition, it is important to note that all mutations that cause the clinical picture of CDG are missense mutations allowing for some residual activity of the affected protein. The extent of this residual activity strongly affects the clinical picture of the CDG patients.
In this respect it is interesting to note that in this first described case of CDG-Ie, the combination of a severe deletion with a missense mutation is observed: the deletion 628delC causes a complete inactivation of the protein (Figure 6) (15), whereas 274C>G seems to be a mild mutation. It may well be that, analogous to the PMM2 mutations in CDG-Ia (33), homozygosity for the severe 628delC mutation is lethal. This fits with the observation of a relatively mild mutation in combination with a deletion, yielding an inactive enzyme. It is possible that homozygosity for the 274C>G mutation may leave enough DolP-Man synthase activity to prevent a shortage of DolP-Man with pathological consequences.
The form of CDG caused by PMI deficiency (CDG-Ib) can be successfully treated with oral mannose (5). Because DolP-Man synthase activity was not completely lost in the CDG-Ie fibroblasts, we thought that an elevation of the cellular mannose pool might force the defective enzyme to produce more DolP-Man. However, our data showed that the addition of mannose had no effect on the hypoglycosylation phenotype of CDG-Ie cells. It is probable that an excess of intracellular mannose does not lead to the high pool of GDP-Man required for increasing the synthesis of DolP-Man. It is possible that a conversion of GDP-Man to GDP-fucose (34) lowers the GDP-Man pool in mannose-fed cells. In any case, considering the severe symptomatology, it is unlikely that supplementation therapy would improve the condition of CDG-Ie patients.
Acknowledgments
We thank Emile van Schaftingen for measuring the PMM and PMI activity in fibroblast cells, Bob Hyman and Andreas Conzelmann for kindly providing us with BW5147 cells, and Bea Berger for assisting with cell cultivation. This work was supported by the Swiss National Science Foundation (grant 3100-46836.96 to E.G. Berger and T. Hennet, and grants 3100-040350.94 and 31-57082.99 to M. Aebi) and by grant G.0243.98 from the Fund for Scientific Research–Flanders to G. Matthijs.
Footnotes
Patricie Burda’s present address is: Howard Hughes Medical Institute, Division of Cellular and Molecular Medicine, University of California–San Diego, 9500 Gilman Drive, La Jolla, California 92093, USA.
Timo Imbach and Barbara Schenk contributed equally to this work.
References
- 1.Jaeken J, Carchon H, Stibler H. The carbohydrate-deficient glycoprotein syndromes: pre-Golgi and Golgi disorders? Glycobiology. 1993;3:423–428. doi: 10.1093/glycob/3.5.423. [DOI] [PubMed] [Google Scholar]
- 2.Krasnewich D, Gahl WA. Carbohydrate-deficient glycoprotein syndrome. Adv Pediatr. 1997;44:109–140. [PubMed] [Google Scholar]
- 3.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthijs G, et al. Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13, in carbohydrate-deficient glycoprotein type I syndrome (Jaeken syndrome) Nat Genet. 1997;16:88–92. doi: 10.1038/ng0597-88. [DOI] [PubMed] [Google Scholar]
- 5.Niehues R, et al. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest. 1998;101:1414–1420. doi: 10.1172/JCI2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeken J, et al. Phosphomannose isomerase deficiency: a carbohydrate-deficient glycoprotein syndrome with hepatic-intestinal presentation. Am J Hum Genet. 1998;62:1535–1539. doi: 10.1086/301873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imbach T, et al. A mutation in the human ortholog of the Saccharomyces cerevisiae ALG6 gene causes carbohydrate-deficient glycoprotein syndrome type-Ic. Proc Natl Acad Sci USA. 1999;96:6982–6987. doi: 10.1073/pnas.96.12.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burda P, et al. A novel carbohydrate-deficient glycoprotein syndrome characterized by a deficiency in glucosylation of the dolichol-linked oligosaccharide. J Clin Invest. 1998;102:647–652. doi: 10.1172/JCI2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Körner C, et al. Carbohydrate-deficient glycoprotein syndrome type V: deficiency of dolichyl-P-Glc:Man9GlcNAc2-PP-dolichyl glucosyltransferase. Proc Natl Acad Sci USA. 1998;95:13200–13205. doi: 10.1073/pnas.95.22.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Körner C, et al. Carbohydrate deficient glycoprotein syndrome type IV: deficiency of dolichyl-P-Man: Man(5)GlcNAc(2)-PP-dolichyl mannosyltransferase. EMBO J. 1999;18:6818–6822. doi: 10.1093/emboj/18.23.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan J, Dunn J, Jaeken J, Schachter H. Mutations in the MGAT2 gene controlling complex N-glycan synthesis cause carbohydrate-deficient glycoprotein syndrome type II, an autosomal recessive disease with defective brain development. Am J Hum Genet. 1996;59:810–817. [PMC free article] [PubMed] [Google Scholar]
- 12.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 13.Tomita S, et al. A homologue of Saccharomyces cerevisiae Dpm1p is not sufficient for synthesis of dolichol-phosphate-mannose in mammalian cells. J Biol Chem. 1998;273:9249–9254. doi: 10.1074/jbc.273.15.9249. [DOI] [PubMed] [Google Scholar]
- 14.Colussi PA, Taron CH, Mack JC, Orlean P. Human and Saccharomyces cerevisiae dolichol phosphate mannose synthases represent two classes of the enzyme, but both function in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1997;94:7873–7878. doi: 10.1073/pnas.94.15.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda Y, Tomita S, Watanabe R, Ohishi K, Kinoshita T. DPM2 regulates biosynthesis of dolichol phosphate-mannose in mammalian cells: correct subcellular localization and stabilization of DPM1, and binding of dolichol phosphate. EMBO J. 1998;17:4920–4929. doi: 10.1093/emboj/17.17.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama E, et al. Identification of defects in glycosylphosphatidylinositol anchor biosynthesis in the Thy-1 expression mutants. J Biol Chem. 1991;266:12119–12122. [PubMed] [Google Scholar]
- 17.Doucey MA, Hess D, Cacan R, Hofsteenge J. Protein C-mannosylation is enzyme-catalysed and uses dolichyl-phosphate-mannose as a precursor. Mol Biol Cell. 1998;9:291–300. doi: 10.1091/mbc.9.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Schaftingen E, Jaeken J. Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett. 1995;377:318–320. doi: 10.1016/0014-5793(95)01357-1. [DOI] [PubMed] [Google Scholar]
- 19.de Jong G, van Eijk HG. Microheterogeneity of human serum transferrin: a biological phenomenon studied by isoelectric focusing in immobilized pH gradients. Electrophoresis. 1988;9:589–598. doi: 10.1002/elps.1150090921. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Grünewald S, et al. β-Trace protein in human cerebrospinal fluid: a diagnostic marker for N-glycosylation defects in brain. Biochim Biophys Acta. 1999;1455:54–60. doi: 10.1016/s0925-4439(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 22.Zufferey R, et al. STT3, a highly conserved protein required for yeast oligosaccharyl transferase activity in vivo. EMBO J. 1995;14:4949–4960. doi: 10.1002/j.1460-2075.1995.tb00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLachlan KR, Krag SS. Three enzymes involved in oligosaccharide-lipid assembly in Chinese hamster ovary cells differ in lipid substrate preference. J Lipid Res. 1994;35:1861–1868. [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Altschul SF, Boguski MS, Gish W, Wootton JC. Issues in searching molecular sequence databases. Nat Genet. 1994;6:119–129. doi: 10.1038/ng0294-119. [DOI] [PubMed] [Google Scholar]
- 26.Hennet T, et al. Genomic cloning and expression of three murine UDP-galactose: β-N-acetylglucosamine β1,3-galactosyltransferase genes. J Biol Chem. 1998;273:58–65. doi: 10.1074/jbc.273.1.58. [DOI] [PubMed] [Google Scholar]
- 27.Trowbridge IS, Hyman R. Abnormal lipid-linked oligosaccharides in class E Thy-1-negative mutant lymphomas. Cell. 1979;17:503–508. doi: 10.1016/0092-8674(79)90258-7. [DOI] [PubMed] [Google Scholar]
- 28.Aebi M, Gassenhuber J, Domdey H, te Heesen S. Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae. Glycobiology. 1996;6:439–444. doi: 10.1093/glycob/6.4.439. [DOI] [PubMed] [Google Scholar]
- 29.Huffaker TC, Robbins PW. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci USA. 1983;80:7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panneerselvam K, Freeze HH. Mannose corrects altered N-glycosylation in carbohydrate-deficient glycoprotein syndrome fibroblasts. J Clin Invest. 1996;97:1478–1487. doi: 10.1172/JCI118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Körner C, Lehle L, von Figura K. Carbohydrate-deficient glycoprotein syndrome type 1: correction of the glycosylation defect by deprivation of glucose or supplementation of mannose. Glycoconj J. 1998;15:499–505. doi: 10.1023/a:1006939104442. [DOI] [PubMed] [Google Scholar]
- 32.Stibler H, Stephani U, Kutsch U. Carbohydrate-deficient glycoprotein syndrome: a fourth subtype. Neuropediatrics. 1995;26:235–237. doi: 10.1055/s-2007-979762. [DOI] [PubMed] [Google Scholar]
- 33.Pirard M, et al. Effect of mutations found in carbohydrate-deficient glycoprotein syndrome type IA on the activity of phosphomannomutase 2. FEBS Lett. 1999;452:319–322. doi: 10.1016/s0014-5793(99)00673-0. [DOI] [PubMed] [Google Scholar]
- 34.Sturla L, et al. Expression, purification and characterization of GDP-D-mannose 4,6-dehydratase from Escherichia coli. FEBS Lett. 1997;412:126–130. doi: 10.1016/s0014-5793(97)00762-x. [DOI] [PubMed] [Google Scholar]