Background: Ceramide synthases N-acylate (dihydro-)sphingosine to (dihydro-)ceramide in mammals.
Results: Enzymatically inactive ceramide synthase 6 in mice (CerS6KO) results in an altered sphingolipid metabolism and behavioral abnormalities.
Conclusion: Catalytically active CerS6 is necessary to maintain sphingolipid homeostasis in mice.
Significance: The CerS6KO mouse reveals for the first time the metabolic and physiological consequences of CerS6 inactivation.
Keywords: Brain, Ceramide, Glycerophospholipid, Kidney, Mass Spectrometry (MS), CerS6, Lass6, Ceramide Synthase Activity, Sphingomyelin
Abstract
The N-acyl chain length of ceramides is determined by the specificity of different ceramide synthases (CerS). The CerS family in mammals consists of six members with different substrate specificities and expression patterns. We have generated and characterized a mouse line harboring an enzymatically inactive ceramide synthase 6 (CerS6KO) gene and lacz reporter cDNA coding for β-galactosidase directed by the CerS6 promoter. These mice display a decrease in C16:0 containing sphingolipids. Relative to wild type tissues the amount of C16:0 containing sphingomyelin in kidney is ∼35%, whereas we find a reduction of C16:0 ceramide content in the small intestine to about 25%. The CerS6KO mice show behavioral abnormalities including a clasping abnormality of their hind limbs and a habituation deficit. LacZ reporter expression in the brain reveals CerS6 expression in hippocampus, cortex, and the Purkinje cell layer of the cerebellum. Using newly developed antibodies that specifically recognize the CerS6 protein we show that the endogenous CerS6 protein is N-glycosylated and expressed in several tissues of mice, mainly kidney, small and large intestine, and brain.
Introduction
Complex sphingolipids contain a ceramide backbone, which consists of a long chain sphingoid base and an N-fatty acyl residue of varying chain length. The acylation of the sphingoid base is catalyzed by ceramide synthase (CerS)2 proteins, which in mammals constitute a protein family consisting of 6 members termed CerS1–6. In the de novo pathway CerS catalyze the acylation of dihydrosphingosine to dihydroceramide (1). After degradation of glycosphingolipids or hydrolysis of sphingomyelin, ceramide can be hydrolyzed by ceramidases and the sphingosine released can be reacylated by CerS to yield ceramide in the salvage pathway (2, 3). All CerS harbor at least five transmembrane domains and are suggested to be located in the endoplasmic reticulum (4, 5). They differ regarding their tissue distribution as well as their acyl chain length specificity (6, 7). The lag1 domain, which is shared by all CerS, is located within the TLC (tram-lag-CLN8) domain in the center of the protein and is part of the active center of the enzymes (8). Two histidine residues within this domain are essential for ceramide synthase activity (9, 10). Another common motif is the homeobox-like domain in the N-terminal region of CerS2–6 (11). Only the CerS1 protein lacks this motif and the function of this domain remains as yet unclear (11).
The first described ceramide synthase was CerS1 (12), which is mainly expressed in the brain and preferably uses C18:0 acyl residues for acylation of (dihydro-) sphingosine (6). CerS1-deficient mice exhibit cerebellar ataxia and Purkinje cell degeneration (13, 14). CerS2 is the best examined CerS with a fatty acid specificity of C20–C24 (6, 15) and a very broad tissue distribution. CerS2-deficient mice show severe phenotypic abnormalities (15–17), e.g. hepatocarcinoma, myelin sheath defects, and behavioral deficits. CerS3, which is mostly expressed in testis and skin, produces ceramides with mainly ultra-long chain fatty acid residues (up to C34:0 (7, 18)). Deletion of this gene in mice leads to early lethality due to transepidermal water loss (19). CerS4 is a nearly ubiquitously expressed protein responsible for the synthesis of C18-C22 (dihydro-) ceramides (6). The closest relative of the CerS6 protein is CerS5. These two enzymes share their substrate specificity for C16:0 acyl-CoA and are similar in their expression pattern (6, 20). Additionally CerS5 and CerS6 show a very high extent of amino acid sequence identity.
Although CerS6 has a broad tissue distribution, its mRNA transcript level is rather low (6, 7). Recent findings indicate a possible role of CerS6 and its main product, C16:0 ceramide, in autoimmune diseases, such as multiple sclerosis (21), whereas other studies suggest a role in the regulation of apoptosis in head and neck squamous cell carcinoma (22).
In the present study we have generated and analyzed transgenic (CerS6KO) mice expressing an enzymatically inactive CerS6 protein to investigate the function of the CerS6 protein in sphingolipid metabolism. Using quadrupole time-of-flight mass spectrometry (MS) we show that C16:0 containing sphingolipid levels are decreased in most tissues investigated. Furthermore, we have generated new CerS6-specific antibodies to study the expression pattern of this protein. With these antibodies we could determine the specific expression of the CerS6 protein in podocytes of the kidney. Behavioral analyses of CerS6KO mice revealed explorative abnormalities in a novel environment including a behavioral habituation deficit and a clasping phenotype of the hind limbs.
EXPERIMENTAL PROCEDURES
Cloning of the CerS6KO-vector
We amplified parts of intron 6 (5 kb upstream of exon 7) of the murine Cers6 gene by PCR using primers with restriction site overhangs (5′ = PspOMI, 3′ = XhoI). Additionally we amplified 440 bp of intron 6 and the first 9 bp of exon 7 with the splice acceptor site, introducing 5′ an XhoI and 3′ an EcoRV restriction site. Furthermore, we integrated an in-frame stop codon downstream of the 9 bp of exon 7. By overlap extension-PCR we fused these two PCR products together separated by the integrated XhoI restriction site. Next we integrated this product using the inserted restrictions sites (PspOMI and EcoRV) together with the 3′ homology region generated via PCR (last 9 bp of exon 7 and first 1.7 kb of intron 7 including the splice donor site and additional restriction sites: 5′-EcoRV and 3′-XbaI) into the pBlueScript vector. This construct was cloned into the pDTA-vector, which contained a MC1-diphtheria toxin A (DTA)-negative selection cassette (23). A lacz reporter gene with an internal ribosomal entry site was integrated downstream of the inserted stop codon. Then we inserted the frt-flanked neomycin resistance cassette driven by the SV40 promoter upstream of the 3′ homologous region. This vector then served as a retrieval vector in a gap repair cloning step on a BAC (bMQ463F1, Sanger Institute, United Kingdom) to increase the size of the 5′ homologous region (24). For this purpose we used the integrated restriction site XhoI and extended the 5′ homology region to >5 kb. Functionality of the frt-sites was analyzed by transforming the final CerS6KO-vector into Escherichia coli bacteria expressing the Flp-recombinase (25). Finally the correct cloning of the CerS6KO-vector was verified by sequencing (GATC, Konstanz, Germany) and restriction site analyses.
Screening of ES Cell Clones
The CerS6KO-vector was linearized with NotI, quantified, and ∼350 μg of DNA were electroporated (0.8 kV, 3 μF, Gene Pulser, Bio-Rad) into HM1 embryonic stem (ES) cells (26). The ES cells were then selected using 350 μg/ml of G418 (Invitrogen). Positive clones were tested via PCR (primers: ES forward, CTG GTT GCT GAC TAA TTG AGA TGC; ES reverse, CCA ATA GTG TGT AAG TGC TGT GGA) for correct insertion of the 3′ homology region and in addition by Southern blot hybridization (3′ probe, 5′ probe, and internal probe). DNA of the relevant clones was incubated with the restriction enzymes EcoRV (internal probe) or NsiI (5′ external probe). The DNA was then separated by agarose gel electrophoresis, blotted onto Hybond N+ membranes (Amersham Biosciences), and fixed on the membrane by cross-linking with UV light. The membrane was hybridized (2 h, 68 °C) with the radioactively labeled probes ([α-32P]dCTP, Amersham Biosciences) in Quick Hyb Solution (Stratagene, La Jolla, CA). The external probe was synthesized by PCR using the primers 5′ HR forward, AGA AGC TGG AGA AGC CAT TGC; and 5′ HR reverse, TTT CCC TCT CTT CCT CTT CCC ,resulting in a 541-bp fragment, whereas the internal probe was generated by restriction of CerS6KO-vector DNA with EcoRI and SacI leading to a 1063-bp fragment of the lacz reporter gene (data not shown).
Generation and Genotyping of CerS6-deficient Mice
Clones positive in the ES cell PCR as well as in the corresponding Southern blot hybridization were injected into C57BL/6 blastocysts as described earlier (27). The chimeric offspring were then mated with C57BL/6 mice. The resulting animals were genotyped by PCR analyses of tail DNA to distinguish between the three possible genotypes by three primer-PCR analyses (CerS6–1, CTA TCA GCT GTC GCT TAG TGG G; CerS6–2, TTC TGG AAT AGC TCA GAG GCC G; CerS6–3, GAG GAA ACA TGA CAC AGC AGG G). The resulting fragments for cers6-WT-locus, 508 bp; cers6-neo-locus, 925 bp; and cers6-KO-locus, 616 bp, were separated by agarose gel electrophoresis. After breeding with Flp-recombinase expressing mice (leading to mice without the neomycin cassette), the animals were backcrossed with C57BL/6 mice up to at least 87.5% C57BL/6 background. To prove the correct homologous recombination additional Southern blot analyses were performed as described above.
Animals were kept under standard housing conditions, a 12-h light/dark cycle and food and water ad libitum. The experiments performed with the animals were in accordance with the guidelines of the corresponding local and state authorities. If not mentioned otherwise, for tissue preparation animals were anesthetized and transcardially perfused with phosphate-buffered saline as previously described (28).
Ceramide Synthase Activity Assay
Ceramide synthase activity was measured with protein extracted from small intestine, large intestine, kidney, thymus, forebrain, and cerebellum of 15-week-old animals using NBD (nitrobenzoxadiazol)-labeled sphinganine as substrate, as described in Ref. 29. The fluorescence of the formed ceramides was detected using a Decon Science Tec documentation system (Hohengandern, Germany), equipped with a Q-Imaging Rolera MGi Plus EMCCD camera (Surrey, Canada). The software used for detection was Gel-Pro Analyzer (Rockville, MD) and the pixel intensity was measured with the program Fiji (München, Germany).
Generation of Custom-designed CerS6-specific Antibodies and Immunoblotting
The primary CerS6 antibodies were raised in rabbits against the C-terminal peptide CDDEDSEPPGKKPH of the mouse CerS6 protein by Pineda Antibody Service (Berlin, Germany) and then affinity purified with this peptide in our laboratory.
Various tissues of CerS6KO and wild type mice were dissected, frozen on dry ice, and homogenized with a Precellys Homogenizer (Peqlab Biotechnologie GmbH, Erlangen, Germany) in 1 ml of homogenization buffer (10 mm sodium phosphate, 150 mm NaCl, 2 mm EDTA, 50 mm NaF, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1 mm phenylmethanesulfonyl fluoride (PMSF), and a protease inhibitor mixture (Roche Applied Science)). After centrifugation for 5 min, protein amounts were determined by BCA assay (Sigma). Thirty μg of each protein lysate in urea buffer (0.04 m Tris-HCl, 9 m urea, 5% SDS, 1 mm EDTA, 0.01% (w/v) bromphenol blue, 2-mercaptoethanol 5% (v/v), pH 6–8) were separated in a 10% gel by SDS-PAGE. The proteins were blotted at 100 V for 1 h onto nitrocellulose membrane (Hybond-ECL, GE Healthcare) in transfer buffer (20 mm Tris base, 150 mm glycine, 20% methanol). After blocking the membrane in TBS-T containing 5% milk powder overnight at 4 °C, it was washed three times for 5 min. The custom-designed primary antibodies against CerS6 were incubated with the membrane at a concentration of 1:100 for 4 h at room temperature (RT) followed by three washing steps. After 1 h of incubation with horseradish peroxidase (HRP)-conjugated antibodies (1:10,000, Dianova, Hamburg, Germany) and 5 min with the SuperSignal West Pico Chemiluminescence detection kit (Pierce) the membranes were developed using VersaDoc Imaging System. For deglycosylation we used N-glycosidase F from New England Biolabs (Ipswich, MA) following the instructions of the manufacturer for 20 μg of glycoprotein.
Immunofluorescence Analysis
Cryosections of frozen tissues (12 μm, Leica CM3050 S, Wetzlar, Germany) were mounted on Superfrost plus slides (Menzel-Gläser, Braunschweig, Germany), fixed with 100% methanol for 20 min at −20 °C. After three washing steps with buffer (50 mm Tris, 1.5% NaCl, 0.3% Triton X-100, pH 7.6), sections were incubated first with blocking solution (washing buffer, 5% normal goat serum) for 30 min at RT and afterward with primary polyclonal affinity purified CerS6 antibodies (1:100, overnight). The secondary Alexa 488-labeled antibodies (Invitrogen) were incubated for 1 h at RT. Sections were then mounted with Mounting Medium (Dako, Glostrup, Denmark).
Immunoperoxidase Light and Electron Microscopy
CerS6KO and control littermates (6 months of age) were anesthetized and transcardially perfused with 4% paraformaldehyde as previously described (30). Kidneys and brains were removed, the latter adjusted in plexiglass frames, embedded in 2% agarose in PBS, and cut into 2-mm frontal brain blocks. Blocks containing the hippocampus, cerebellum, or the kidney were cut into 50-μm vibratome sections. CerS6 peroxidase immunohistochemical analyses (1:3,000) were performed on every fifth section either for light or electron microscopy as published (31). For identification of the hippocampal subregions and integrity of cerebellar Purkinje cells, polyclonal Calbindin antibodies (1:10,000; CB38, Swant, Bellinzona, Switzerland) were used for immunostaining every fifth vibratome section. Adjacent sections were labeled with parvalbumin antibodies (1:3000, PV25, Swant). Light microscopic sections were mounted on Superfrost Plus slides (Menzel-Gläser, Braunschweig, Germany), air-dried, quickly dehydrated and coverslipped. For electron microscopy, immunostained sections were flat-embedded in Araldite (Serva, Heidelberg, Germany) as described (31). Some brain and kidney sections were embedded without immunostaining as morphological reference.
Alternating semi- (0.8 μm) and ultrathin (100 nm) sections were cut. Every second semithin section was counterstained with toluidine blue. Ultrathin sections were contrasted with uranyl acetate and lead citrate (30, 31).
β-Galactosidase Staining
Brains of adult mice were frozen on dry ice, embedded in Tissue-Tek OCT (Sakura Finetek, Heppenheim, Germany), and prepared for vibratome sectioning (50 μm, Leica VT1200 S, Wetzlar, Germany). Slices were fixed with 0.2% glutaraldehyde in 100 mm Na2HPO4, 100 mm NaH2PO4, 1.25 mm MgCl2, 2 mm EGTA, pH 7.4, for 5 min at RT. After rinsing three times with the same buffer containing additional 0.01% deoxycholate and 0.2 Nonidet P-40, the sections were incubated with substrate buffer (same as above with 5 mm K3[Fe(CN)6], 5 mm K4[Fe(CN)6], and 80 mg/ml of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) overnight at 37 °C. Then the slices were rinsed with buffer and bi-destilled water, air dried, and mounted with Entellan (Merck Millipore, Darmstadt, Germany).
Lipid Extraction and Tandem Mass Spectrometry
Lipid extraction was performed according to Refs. 14 and 15. Sphingolipids were dissolved in methanol, chloroform, 300 mm ammonium acetate (665:300:35, v/v/v) (32) and internal standards were added (14). Glycerophospholipids were measured as described (32, 33). Briefly, glycerophospholipids were dissolved in the same solvent and the following internal standards added: di14:0-phosphatidylcholine (PC), di20:0-PC, di14:0-phosphatidylethanolamine (PE), di20:0-PE, di14:0-phosphatidylglycerol (PG), di20:0-PG, di14:0-phosphatidic acid (PA), di20:0-PA di14:0-phosphatidylserine (PS), di20:0-PS (Avanti Polar Lipids). 34:0-Phosphatidylinositol (PI) was used as internal standard after removing the double bonds by hydrogenation of soybean PI (Larodan). All lipids were measured via direct infusion nanospray mass spectrometry (Agilent 6530 Accurate-Mass quadrupole time-of-flight LC/MS). The sample was infused using an HPLC infusion chip at a flow rate of 0.5 μl/min in methanol, chloroform, 300 mm ammonium acetate (665:300:35; v/v/v) (32). Lipids were measured after positive ionization as proton or ammonia adducts with all instrumental parameters as previously described (34). Data were corrected for isotopic overlap, and nanomole values were calculated using a linear regression of the two internal standards.
Thin Layer Chromatography
HPTLC analyses were performed to examine the amounts of acidic lipids. To this end the desalted lipid samples were dissolved in chloroform/methanol/water (2:1:0.1; v/v/v) and aliquots corresponding to equal amounts of protein per lane were applied onto HPTLC plates (20 × 20-cm Silica Gel 60 plates, Merck). The HPTLC plates were developed in a vertical chamber with chloroform, methanol, 0.22% aqueous calcium chloride (11:9:2; v/v/v). After separation, the plates were briefly immersed into an aqueous 10% copper sulfate and 8% phosphoric acid solution. Staining of the plates was achieved by heating to 180 °C for about 5 min.
Behavioral Experiments
Ten male CerS6KO mice (18.8 ± 1.2 weeks old) and 10 wild type littermates (15.7 ± 1.1 weeks old) were used for the behavioral experiments. An additional set of 10 male CerS6KO mice (25 ± 2 weeks old) and 10 wild type littermates (26.5 ± 1.6 weeks old) were used for the hind limb experiments. Animals were housed individually in Techniplast blue label macrolone cages (42.5 × 26.5 × 18 cm) with metal covers and sawdust bedding. They had free access to water and standard diet. Animals were maintained in a closed ventilated room under constant temperature and humidity conditions. A 12-h light/dark cycle with lights on between 7 a.m. to 7 p.m. was used.
Hind Limb Clasping Test
To quantify the duration of clasping of the hind limbs, mice were picked up by their tail for 15 s and the time spent in a clasping position was scored (0 = negative, no clasping; 1 = positive, clasping (35, 36)). A positive score was recorded if the hind limbs remained in close contact with the body, whereas other movements were not regarded as hind limb clasping. The minimum duration of clasping position that would qualify a positive score was predetermined as 1 s.
Horizontal Wire Test
Mice were subjected to the horizontal wire test according to a previously described procedure (37). Briefly, 6-month-old CerS6KO and wild type mice were lifted up by their tail and allowed to grasp in the middle of a horizontally clamped wire (1 meter) that connected two platforms. The animals were allowed to reach one of the platforms and climb onto it. Each animal was subjected twice to the horizontal wire test (with an inter-trial interval of 30–45 min) and the performance of the animal was scored by an observer blind to the genotype by using a recently developed scoring system (37). Additionally, the time spent on the horizontal wire was measured for each trial. The trial was terminated when an animal reached one of the platforms or a total duration of 120 s had elapsed.
Open Field Behavioral Habituation
To examine exploratory behaviors in a novel environment and behavioral habituation after short (2 h) and long (24 h) retention intervals, the mice were given 3 trials in the open field. Mice exposed to a novel environment show explorative behaviors in terms of increased locomotion and rearing activity. When mice were repeatedly placed into the same open field, a progressive reduction in these exploratory behaviors became evident, suggesting that the initially novel environment had become familiar (38). The open field was a rectangular chamber (50 × 50 × 40 cm) made of gray polyvinylchloride. A CCD video camera was mounted 50 cm above the maze. The open field was illuminated by diffuse white light with an intensity of 5 lux at the center of the apparatus. The digitized image of the path of the animal was analyzed with a semiautomated tracing device (EthoVision, Noldus, the Netherlands). After each trial, the apparatus was cleaned with water containing 75% ethanol.
The behavioral parameters registered during 3 min sessions were: (i) locomotion, which is the distance (cm) an animal moved; (ii) the frequency of rearings on hind limbs; (iii) mean running speed (cm/s); (iv) the total duration (s) of immobility (or freezing) defined as the cessation of body movements except those accompanied by breathing; (v) the total duration of body movements (larger than those associated with, e.g. breathing); and (vi) the frequency of rotational behavior (the number of clockwise and counter-clockwise 360° rotations made by the animal).
One-trial Object Recognition
It is well known that mice readily approach and explore novel objects. Exploration of novel objects leaves a memory trace that includes information on the physical characteristics of the objects as well as their spatial and temporal context. Animals exposed to a familiar and a novel object spend more time exploring the novel object (39).
The novel object exploration test consisted of a sample trial and a test trial. The test was performed in the open field apparatus described above. In the sample trial each mouse was allowed to freely explore the open field, which contained two copies of a novel object for 3 min. A delay of 60 min was interposed between the sample trial and the test trial. During the test trial the mouse was exposed to a third copy of the familiar object and a novel object. The objects were placed into randomly selected corners that were diagonally opposed to each other. The corner in which the novel object was placed was randomly selected.
Two plastic objects that differed in terms of color, shape, height (18 and 25 cm), and surface texture were used during the sample and test trial. The time spent exploring the objects (seconds), the frequency of object contacts were scored online by using the manually defined behaviors option of the Noldus tracking software (Ethovision XT 3.0, Noldus, The Netherlands). Exploration of an object was assumed when the mouse approached the object with its nose or forepaws having either a physical contact with the object or staying at a distance of less than 2 cm away from the object.
The proportion of time exploring the novel object, relative to the total time spent exploring both objects, was taken as a measure of object recognition: tnovel/(tnovel + tfamiliar). Novel object recognition index values higher than 0.5 suggest a preference for the novel object, values close to 0.5 would suggest no recognition, whereas values well below 0.5 suggest a preference for the familiar object.
Anxiety-like Behavior in the Elevated Plus Maze
To probe whether unconditioned fear was affected differentially by CerS6 deficiency, the animals were subjected to the elevated plus-maze (40). It consisted of two open arms (29 × 5 × 15 cm) and two arms with transparent side walls (29 × 5 ×15 cm) with an open roof, arranged around a central platform (5 × 5 cm), so that the two arms of each type were opposite to each other. The maze was elevated to a height of 40 cm. The recording, digitized tracking, masking noise, and illumination conditions were the same as for the open field experiment. After each trial, the apparatus was cleaned with water containing 75% ethanol solution. The mice were placed on the central platform of the maze facing one of the walled arms, and were observed for 5 min, during which the number of entries into and time spent (second) in the open and walled arms were recorded.
The proportion of time spent in and number of entries into the open arms, relative to the total time spent in and number of entries to both walled and open arms, was taken as measures of anxiety-like behavior: t(f)open/(t(f)open + t(f)walled). The lower the value of the anxiety index score the more anxiety-like behavior is exhibited by the animal.
Statistical Analysis
Behavioral habituation data were analyzed by means of repeated measures analysis of variance. The p values were considered to be significant when p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
RESULTS
Generation of Ceramide Synthase 6-deficient Mice
The catalytically active domain of CerS6 is encoded by DNA sequences starting in exon 6 and ending in exon 8. Two functionally essential histidine residues are located within the coding region of exon 7 (10). We decided to minimize the impact on the genomic locus, since deletion of the whole coding region including the introns might lead to unintended loss of possible regulatory sequences. Thus we deleted 111 bp of the coding region of the catalytically active exon 7 including the two above mentioned histidine residues and instead integrated a stop codon after the first 9 bp of exon 7.
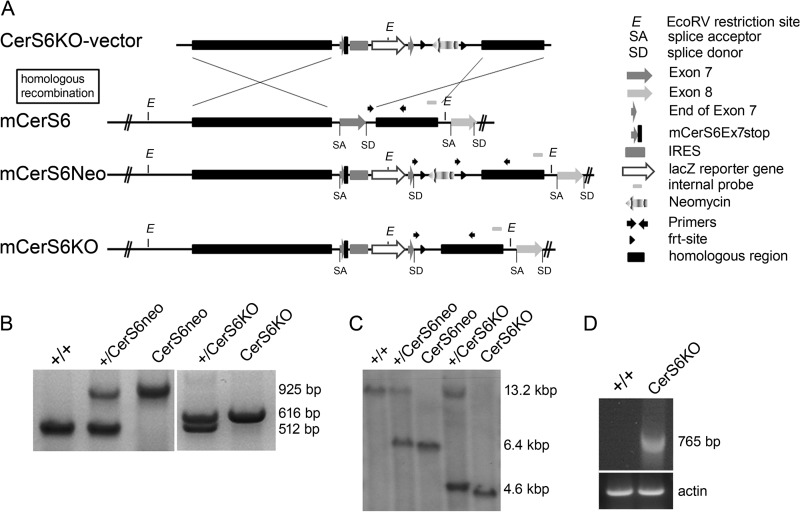
Therefore we used the non-conditional vector shown in Fig. 1A, which consists of the splice acceptor site of intron 6, the first 9 base pairs of the coding region of exon 7 and the integrated stop codon, followed by an internal ribosomal entry site, the lacz reporter cDNA, the last 9 base pairs of exon 7, and the splice donor site of intron 7 resulting in a truncated enzymatically inactive CerS6 protein. This cassette is localized upstream of the frt-flanked neomycin resistance cDNA, under control of the SV40 promoter. The whole construct is flanked by a 5-kb 5′ homology region and a 1.6-kb 3′ homology region for homologous recombination into the genomic locus.
FIGURE 1.
Generation of transgenic mice harboring an enzymatically inactive ceramide synthase 6 gene (CerS6KO). A, homologous recombination scheme of the CerS6KO-vector with the Cers6 wild type gene (+/+) leads to mCerS6Neo. The CerS6KO-vector is flanked by two homologous regions (5′ HR and 3′ HR). The stop codon and the internal ribosomal entry site (IRES) and the lacz reporter gene are integrated between the first 9 and the last 9 bp of the catalytically active exon 7. The splice acceptor site (SA) in intron 6 and the splice donor site (SD) in intron 7 remain in position. The coding region of the neomycin resistance gene is flanked by two flippase recognition target (frt) sites. Flippase (Flp)-mediated recombination then leads to the mCerS6KO genomic locus. B, genotyping PCR analysis of the five possible genotypes (+/+; +/CerS6Neo; CerS6Neo; +/CerS6KO; CerS6KO). The CerS6-specific primers indicated by black arrowheads differ between the three genotypes: WT, 512 bp; Neo, 925 bp; KO, 616 bp. C, Southern blot analysis of EcoRV digested genomic DNA of liver hybridized with an internal probe. The three possible fragment sizes are: WT, 13.2 kb; Neo, 6.4 kb; KO, 4.6 kb. D, PCR analysis of brain cDNA of WT and CerS6KO mice. One primer binds in the coding region of the lacz-reporter gene, the second primer at the C-terminal end of the coding region of exon 10.
The vector was transfected via electroporation into HM1 mouse embryonic stem cells (26). After homologous recombination, G418-resistant clones were tested via PCR and Southern blot analyses (data not shown). ES cells with a correctly recombined cers6 locus were then injected into mouse blastocysts to yield germ-line chimeras. The animals were cross-bred with C57BL/6 mice to obtain heterozygous (+/CerS6Neo) offspring. Breeding with Flp-recombinase expressing mice yielded animals lacking the neomycin resistance cassette (CerS6KO). The investigated mice had a C57BL/6 genetic background of at least 87.5%. Mice were genotyped by PCR (Fig. 1B) and Southern blot analyses (Fig. 1C). RT-PCR using primers that bind in the coding region of the lacz reporter gene and in exon 10 of Cers6 showed expression of the Cers6-lacz mRNA in CerS6KO mice (Fig. 1D). With PCR analyses using primers that bind in the first and the last exon we also found a splice variant in the CerS6KO status in which exon 7 and the internal ribosomal entry site-lacz were completely missing (data not shown).
Ceramide Synthase Activity Assay
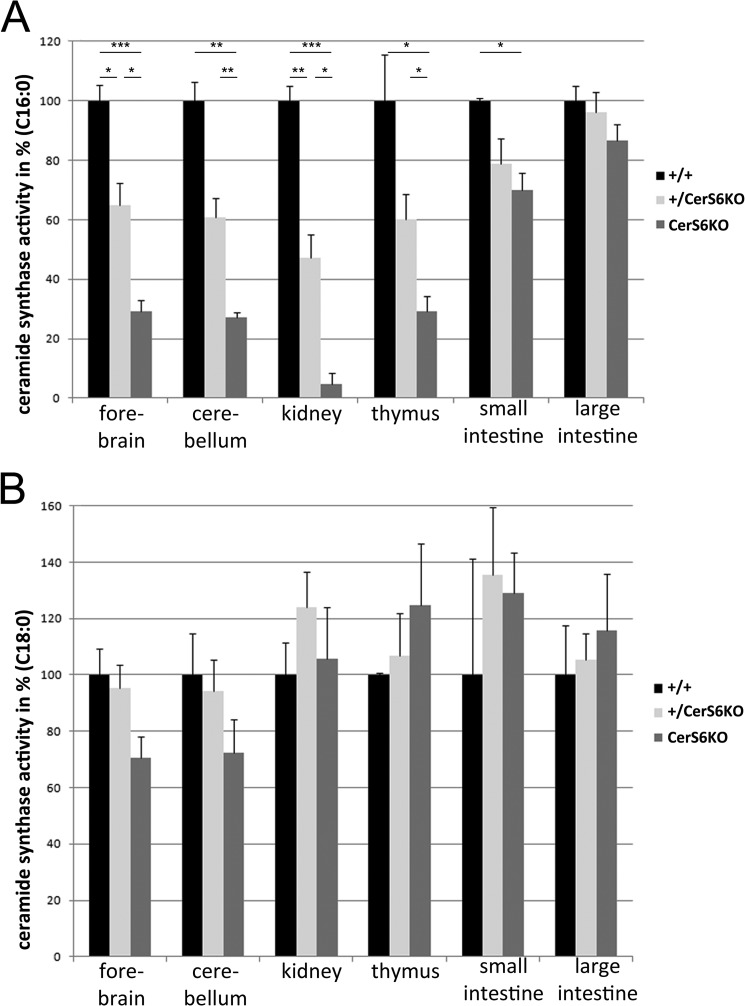
The CerS activity was determined in wild type, heterozygous (+/CerS6KO), and knock-out animals (CerS6KO), with lysates of different tissues from 15-week-old mice. C16:0 and C18:0 acyl-CoAs were applied as substrates for ceramide synthase activity assays to study the impact of CerS6 deficiency on the activity with the preferred substrate (C16:0 acyl-CoA) and with one non-preferred substrate (C18:0 acyl-CoA). We found strong decreases (<40% residual activity as compared with wild type) for the CerS6-specific substrate C16:0 acyl-CoA in forebrain, cerebellum, thymus, and kidney, whereas the activity was less severely reduced in small intestine (∼75% of wild type activity) and was not significantly altered in the large intestine (Fig. 2A). Heterozygous mice also displayed a reduction of activity in comparison to their wild type littermates although the effect was less pronounced (about 50% reduction) as compared with CerS6KO mice. We also tested C18:0 acyl-CoA but found no significant alterations in the activity toward this substrate (Fig. 2B).
FIGURE 2.
Ceramide synthase (CerS) activity assay. A, activity toward palmitoyl-CoA in homogenates of forebrain, cerebellum, and thymus of 15-week-old ceramide synthase 6 knock-out (CerS6KO) mice (n = 3 for each group) was significantly reduced to 30% in comparison to their wild type (+/+) and heterozygous (+/CerS6KO) littermates. The reduction was stronger in the kidney (10% residual activity), whereas the activity was slightly diminished in small intestine (70%) and no alterations were observed in large intestine. Activity in heterozygous mice was approximately half in comparison to wild type and CerS6KO animals (forebrain, cerebellum, and thymus, ∼60%; kidney, 50%; and small intestine, 80%). B, activity toward stearoyl-CoA was not significantly altered in any tissue of the three different genotypes. The data are mean ± S.E., * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
Ceramide Synthase 6 Protein Expression Pattern and Glycosylation Status
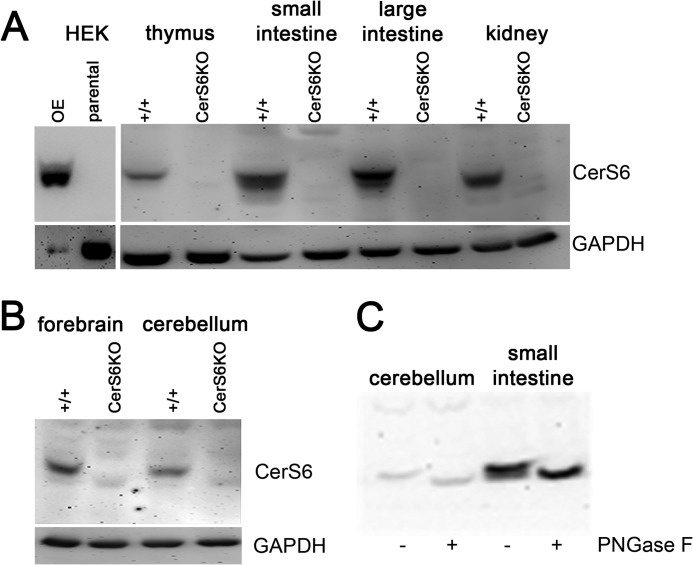
None of the tested commercially available antibodies (Anti-Lass6 pAB, Abnova Taiwan Corporation; LASS6 Antibody, ProSci Inc., Poway, CA) could differentiate between wild type and CerS6KO protein lysate at the beginning of our study. Thus we decided to generate specific CerS6 antibodies. For this purpose we used the C terminally located peptide CDDEDSEPPGKKPH of the mouse CerS6 protein. The predicted molecular mass of about 45 kDa could be verified by immunoblotting with the newly generated antibodies. Furthermore, these antibodies were able to discriminate between wild type and knock-out tissue. As a control we cloned a CerS6 overexpressing vector and transiently transfected it into HEK293T cells. Homogenates of these cells were used as a positive control for the antibodies, whereas we additionally included a parental HEK293T control that did not overexpress CerS6 (Fig. 3). Earlier studies indicated that cers6 mRNA is expressed in most mouse tissues in different amounts (6, 7). Immunoblot analysis using the new CerS6 antibodies showed a proportional correlation between the reported mRNA levels and the amount of CerS6 protein in different tissues, suggesting that CerS enzyme levels are mainly regulated at the transcriptional level. Especially in kidney, and small and large intestine we found a strong expression, whereas the signal in thymus was rather weak (Fig. 3A). We also detected CerS6 protein in the cerebellum and forebrain (Fig. 3B) of wild type animals. There were no specific bands of the corresponding molecular mass in the CerS6KO tissue. The splice variant with the missing exon 7 was not detected in immunoblot analyses with our antibodies. Therefore we conclude that this truncated form is not translated or is rapidly degraded, for example, due to misfolding.
FIGURE 3.
Immunoblot analyses of different tissues. A, the newly generated antibodies recognized ceramide synthase 6 (CerS6) protein in wild type (+/+) tissue and in HEK (CerS6−) overexpressing cell lysate but not in ceramide synthase 6 knock-out (CerS6KO) tissue or in HEK parental control homogenate. The CerS6 protein is expressed in most tested tissues in different amounts at the expected size of about 45 kDa. Strong expression was observed in kidney and large and small intestine. B, expression of CerS6 in cerebellum and forebrain of +/+ but not in CerS6KO tissue. C, the endogenous form of CerS6 is N-glycosylated in the small intestine and the cerebellum. Experiments were repeated at least 5 times with similar results.
To investigate the glycosylation state of the protein, we performed deglycosylation experiments with N-glycosidase F using lysates of the cerebellum and the small intestine and we found that the endogenous protein is N-glycosylated in these tissues (Fig. 3C), most likely at the N terminus as described previously using transfected HEK 293T cells (6).
Horizontal Wire Test
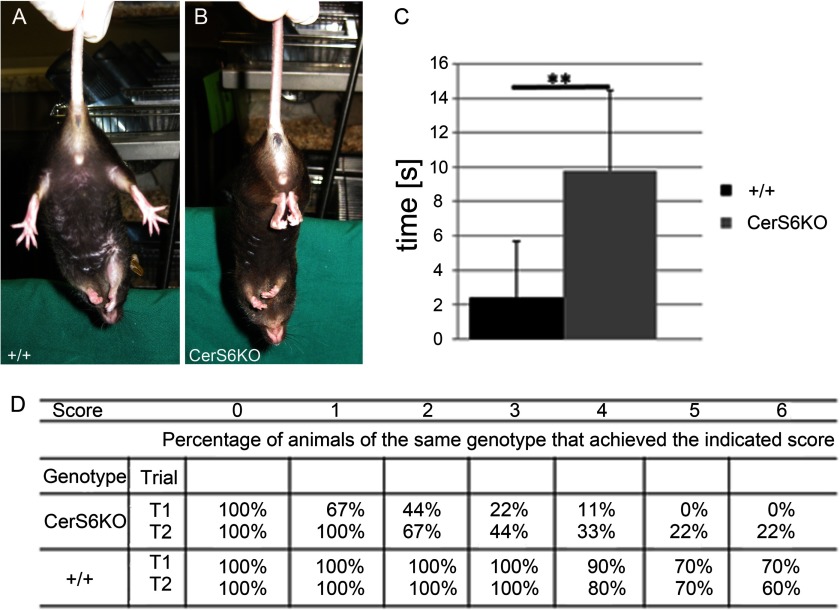
Behavioral analyses revealed several abnormalities in CerS6KO mice as compared with their wild type littermates. When lifted up by their tail, CerS6KO animals showed a clasping phenotype of their hind limbs of different duration (Fig. 4, A and B) that was absent in the wild type mice. The CerS6KO animals displayed a significantly increased clasping time compared with wild type controls (Fig. 4C). Accordingly, the performance of Cers6KO mice in the horizontal wire test differed relative to wild type mice. For instance, the performance scores during trial 1 (p < 0.0001; Student's t test for independent groups) and trial 2 (p < 0.05) were significantly smaller in CerS6KO relative to wild type mice. Accordingly, there was also a genotype difference in the percentage of animals successfully completing the horizontal wire test (see Fig. 4D). Altogether these data suggest impaired neuromotoric function in CerS6KO mice.
FIGURE 4.
Clasping phenotype and horizontal wire examination. A and B, enzymatically inactive ceramide synthase 6 mice (CerS6KO) show a clasping abnormality of their hind limbs when picked up by their tail. C, the duration of this clasping was significantly increased during a 15-s (s) trial. D, results of the horizontal wire test in CerS6KO and wild type (+/+) mice during two trials (T1, T2). Animals were analyzed according to a sensitive scoring system that ranges from 0 to 6. Score 0: unable to grasp the wire with the forepaws and hold on for at least 3 s. Score 1: able to grasp the wire with forepaws and to hold on for 4–9 s. Score 2: able to grasp the wire with forepaws and hold on for 10 s or more. Score 3: able to grasp the wire with both fore- and hind paws and to hold on for 10 s or more. Score 4: achieved a score of 3 and is able to move at least 15 cm toward one of the two platforms. Score 5: achieved a score of 4 and is able to reach one of the two platforms but fail to climb on it. Score 6: achieved a score of 5 and is able to climb onto the platform. Data displayed are mean ± S.E. (n = 10), ** = p < 0.01.
Open Field Behavioral Habituation
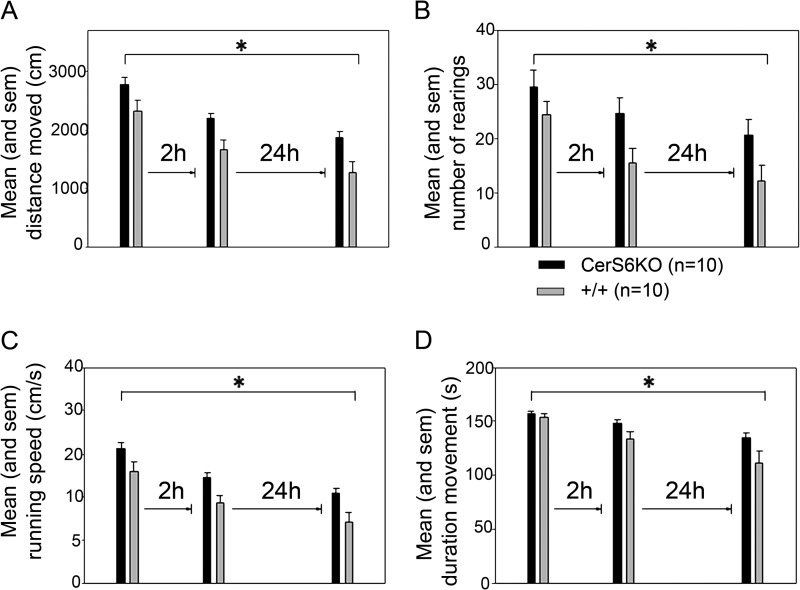
Another behavioral abnormality we observed was that CerS6KO animals appeared to be more agitated and nervous than their control littermates. Thus we performed the open field behavioral habituation test. The animals (irrespective of genotype) showed significant decreases in locomotion (F(2,36) = 32.380, p < 0.001, mean effect of trials, repeated measures analysis of variance, Fig. 5A), the frequency of rearings (F(2,36) = 10.023, p < 0.001, Fig. 5B), the mean running speed (F(2,36) = 31.866, p < 0.001, Fig. 5C), and the total duration of movement (F(2,36) = 18.919, p < 0.001, Fig. 5D), but increases in the total duration of immobility (F(2,36) = 18.389, p < 0.001) across the 3 trials in the open field. There was no change in rotational behavior across the 3 trials (p > 0.05). The CerS6KO mice showed significantly increased levels of locomotion (F(1,18) = 10.176, p = 0.005, main effect of genotype), rearing activity (F(1,18) = 5.693, p = 0.028), mean running speed (F(1,18) = 10.384, p = 0.005), and the total duration of movement (F(1,18) = 6.496, p = 0.020) as compared with wild type controls. There were no significant genotype effects on the parameters immobility and rotational behavior (p values > 0.05). Furthermore, no significant genotype × trials interactions were found for any of the parameters analyzed (P > 0.05). These results suggest that CerS6KO mice exhibited significantly higher levels of agitation and exploratory activity in the open field than the wild type controls.
FIGURE 5.
Open field exploration and habituation. A, mean distance moved (in cm) of ceramide synthase 6 knock-out (CerS6KO) and wild type (+/+) mice on the indicated trials. B, mean number of rears of CerS6KO and +/+ mice on the indicated trials. C, mean running speed (cm/s) of CerS6KO mice and their +/+ controls on the indicated trials. D, total time of movement (s) of CerS6KO and +/+ mice on the indicated trials. *, p < 0.05. All experiments were performed with n = 10 for each group.
We next analyzed whether the CerS6KO mice showed significant behavioral habituation after repeated exposures to the open field. Single trial analysis indicated that on trial 1 CerS6KO and wild type mice displayed similar levels of exploratory activity in terms of locomotion, rearings, running speed, total duration of movement, or immobility and frequency of rotational behavior (p values > 0.05, t test for independent groups). These results suggest that exposure to a novel environment induces similar levels of exploratory activity in CerS6KO as compared with control mice.
During trial 2 when the animals were re-exposed to the open field after a short retention interval of 2 h, the CerS6KO mice displayed significantly increased levels of locomotion (T(18) = −2.802, p = 0.012), rearings (T(18) = −2.215, p = 0.040), and running speed (T(18) = −2.861, p = 0.010) relative to the wild type controls. There were no significant differences between the groups regarding the time spent in a moving or immobile state and rotational behavior (P > 0.05). From this pattern of results we conclude that CerS6KO mice show impaired behavioral habituation to novel spatial environments.
On trial 3, after a long retention interval of 24 h, the CerS6KO mice again exhibited increased levels of exploratory activity. CerS6KO mice showed significantly increased locomotion (T(18) = −2.597, p = 0.018) and running speed (T(18) = −2.576, p = 0.019), relative to the controls. In line with this hyperactive phenotype, the duration of immobility was significantly decreased in the CerS6KO mice (T(18) = 2.122, p = 0.048) relative to controls. These results suggest that the behavioral habituation deficit of CerS6KO mice is present after both short and long retention intervals and suggests an inability to either generate and/or maintain a cognitive spatial map. Analyses within each group demonstrated that both CerS6KO and wild type mice showed significant reductions in locomotion (+/+: T(9) = 3.547, p = 0.006; CerS6KO: T(9) = 3.044, p = 0.014, t test for dependent samples), running speed (+/+: T(9) = 3.470, p = 0.007; CerS6KO: T(9) = 2.992, p = 0.015), and the total duration of movement (+/+: T(9) = 3.466, p = 0.007; CerS6KO: T(9) = 3.122, p = 0.012) on trial 2 as compared with trial 1.
However, only the wild type mice also showed significant reductions in the number of rearings (T(9) = 3.510, p = 0.007) and an increase in the total duration of immobility (T(9) = −2.748, p = 0.023) on trial 2 as relative to trial 1, whereas there was no significant change for these parameters between trials in the within the group of CerS6KO mice (P > 0.05). Furthermore, there was no significant change in rotational behavior between trials 1 and 2 for any group (P > 0.05).
Although there was no significant change in exploratory activity of the CerS6 wild type mice between trials 2 and 3 (P > 0.05) (possibly due to a floor effect), the CerS6KO mice still showed a significant change in locomotion (T(9) = 2.937, p = 0.017), mean running speed (T(9) = 3.136, p = 0.012), and the total duration of movement (T(9) = 3.175, p = 0.011) and immobility (T(9) = −2.864, p = 0.019), but not in terms of rearing behavior (p > 0.05). These results suggest that CerS6KO mice display a deficit in behavioral habituation to a novel environment and require at least 2 exposures to a novel environment to display significant reductions at least in terms of locomotoric behavior, whereas there was still no significant reduction in rearing behavior. The latter is known as a more sensitive measure of spatial information gathering than the amount of locomotion displayed. Altogether, these results suggest impairment in the encoding, consolidation, and/or retrieval of spatial information in the CerS6KO mice.
One-trial Object Recognition
We investigated whether CerS6KO mice are able to recognize a familiar object after a delay of 60 min. The total time spent exploring the two objects and the number of object contacts during the sample or test trials were not significantly different between groups (P > 0.05, t test for independent samples, supplemental Table S1). Furthermore, there was no significant difference between the groups in respect of the novel object recognition index or regarding the number of contacts and the time spent exploring the novel or familiar object during the test trial (P > 0.05, data not shown). These results suggest that the motivation to explore novel objects as well as the ability to recognize familiar objects after a delay of 60 min is not affected in the CerS6KO mice.
Anxiety-like Behavior in the Elevated Plus-maze
There were no significant differences between the groups in terms of the number of entries and the time spent in the open or walled arms or the anxiety index scores (P > 0.05, supplemental Table S1). These results suggest that CerS6 deficiency in the mouse has no impact on unconditioned anxiety in the elevated plus-maze.
lacZ Staining and Histological Analyses
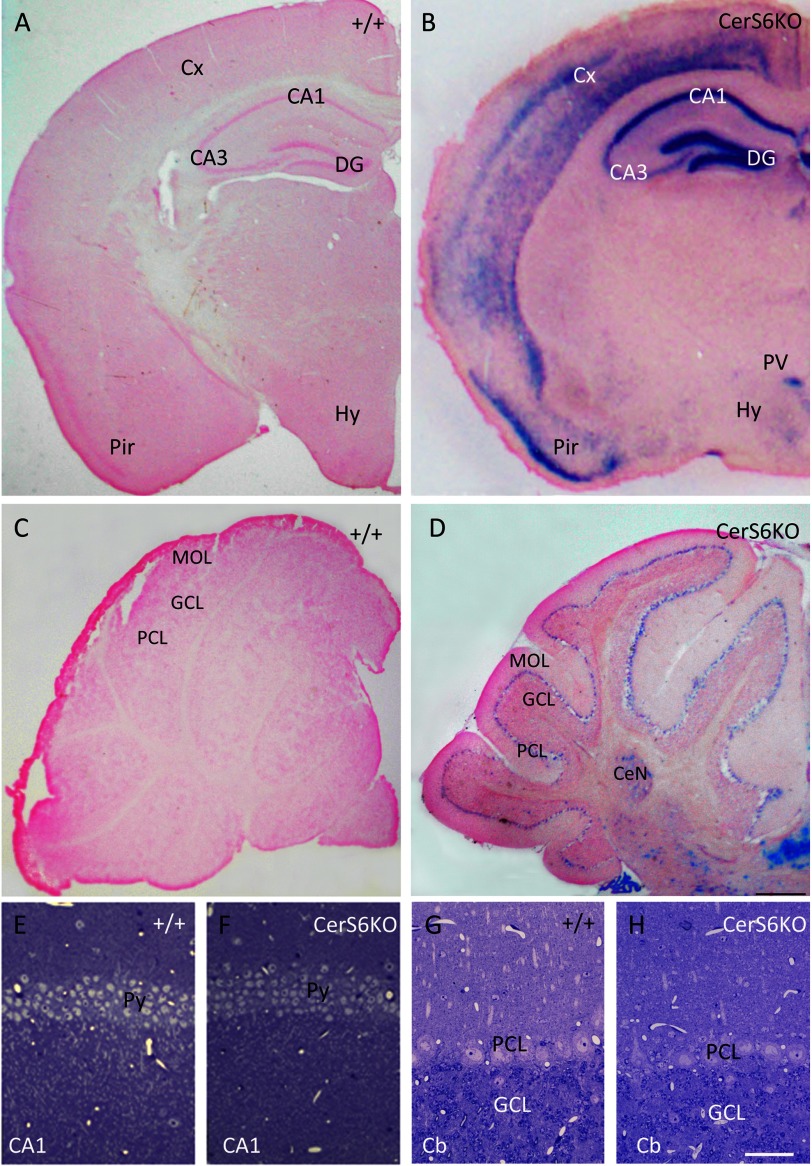
Because the behavioral data suggested involvement of brain functions we investigated CerS6 localization in selected brain areas. The physiologically defined place cells are located in the hippocampus. Therefore, we investigated CerS6 expression in this region. We used forebrains and cerebella of wild type animals as negative controls (Fig. 6, A and C). Interestingly, lacZ staining in CerS6KO mice revealed strong CerS6 expression in the hippocampus, most prominent in the CA1 area and dentate gyrus (Fig. 6B), whereas in the CA3 area only light activity was found. Furthermore, lacZ was also moderately present in several layers of the cortex and the paraventricular hypothalamic nucleus (Fig. 6B). Of note the pyramidal cell layer of the piriform cortex showed strong lacZ activity. These findings were in line with in situ hybridization data, previously performed on brain sections of adult C57BL/6 wild type mice (41). Unfortunately our newly generated CerS6 antibodies were not sensitive enough to recognize specific immunofluorescence or peroxidase signals in all brain areas investigated and could therefore not be used for immunohistochemical analyses of wild type and CerS6KO mice (data not shown). However, to elucidate if the behavioral changes in CerS6KO mice might reflect morphological changes in brain areas with high Cers6 gene expression, we additionally performed histological analyses with special focus on the hippocampus. Semithin sections revealed no obvious morphological differences in neurons of the CA1 regions between wild type (Fig. 6E) and CerS6KO littermates (Fig. 6F). Additionally we also checked the CA3 region and dentate gyrus but found no differences in morphological structure (data not shown). Calbindin, which is expressed in hippocampal subregions and parvalbumin, which is necessary for hippocampal network synchronization were used as immunohistochemical markers. Differences were neither found with calbindin nor parvalbumin immunohistochemical analyses between both genotypes (data not shown). Immunoblots regarding myelin basic protein and myelin-associated glycoprotein also yielded no significant alterations (data not shown).
FIGURE 6.
LacZ staining (A–D) and histological analyses (E–H) of ceramide synthase 6-deficient (CerS6KO) and wild type (+/+) brain. Coronal vibratome sections (50 μm) show prominent lacZ staining in the hippocampus of the CerS6KO mouse (B), mostly expressed in the CA1 area and the dentate gyrus (DG) and layer II of the piriform cortex (Pir). Moderate lacZ expression is also detected in neocortical layers (Cx) and the paraventricular nucleus (PV) of the hypothalamus (Hy). D, the cerebellum displays distinct lacZ staining in the Purkinje cell layer (PCL), not observed in the molecular cell layer (MOL). Some lacZ staining is also detected in the granule cell layer (GCL), the cerebellar nuclei (CeN), and the choroid plexus. Sections of other +/+ forebrain areas at the level of the hippocampus (A) lack lacZ staining. Histological analysis of toluidine blue-stained semithin sections (0.8 μm) of hippocampal CA1 (F) and cerebellar cortex (Cb in H) shows regular morphology in the CerS6KO and age-matched +/+ littermates (E and G); CA3, CA3 area of Ammon's horn; Py, pyramidal cells; bar in D for A–D = 500 μm; bar in H for E–H = 50 μm. Staining was performed three times with similar results.
In the cerebellum of CerS6KO mice, lacZ staining was moderately visible in the Purkinje cell layer and showed a spot-like distribution in the granular cell layer (Fig. 6D). Morphological investigations of the cerebellar cortex yielded no differences between wild type and CerS6KO mice neurons (Fig. 6, G and H).
However, we did observe alterations in a different cell population of the brain, the microglia. In the hippocampus we detected a slightly increased density in microglia cells indicating a mild affection of this area. Initial electron microscopic analysis showed signs of degeneration in the respective areas (data not shown).
Analyses of Lipid Composition
Because the behavioral phenotype of the CerS6KO mice could not be clearly linked to obvious morphological changes of neurons so far, we searched for alterations in the lipid composition of the cerebellum and forebrain. Furthermore, we measured the lipid composition of kidney, thymus, small intestine, and large intestine, where CerS6 is strongly expressed.
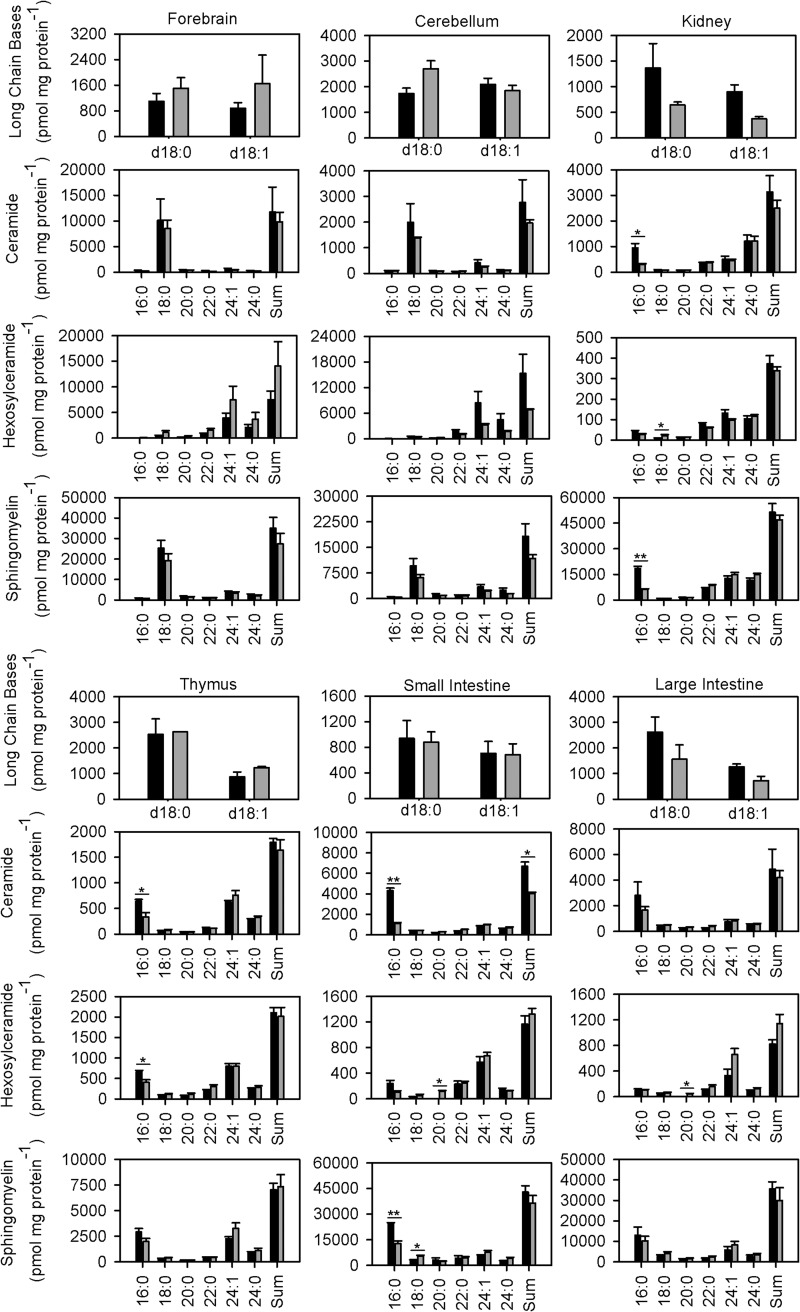
We examined long chain bases, ceramide, hexosylceramide, sphingomyelin, and glycerophospholipid levels in tissues of 9-week-old mice. We found no obvious differences in the amount of long chain bases in CerS6KO mice compared with their wild type controls (Fig. 7). C16:0-ceramide levels were significantly decreased in three of the investigated tissues from CerS6KO animals compared with controls. There was a decrease in the C16:0 ceramide content in small intestine (to 25%), thymus (to 50%), and kidney (to 35%). The strong decrease in small intestine was accompanied with a loss of total ceramide to about 60%. The amounts of the other ceramide species remained similar in kidney, thymus, and small intestine. We only found low amounts of C16:0-ceramide in the brain (forebrain and cerebellum).
FIGURE 7.
Quantitative measurement of neutral sphingolipids in different tissues via quadrupole time-of-flight MS. The long chain base levels remained unaltered in wild type versus CerS6KO mouse tissue. C16:0 ceramide levels in kidney, thymus, and small intestine were strongly reduced in contrast to large intestine. Only small amounts of C16:0 ceramide were found in brain. The C16:0 sphingomyelin levels were significantly altered in kidney and small intestine. Long chain hexosylceramides were increased in kidney and small and large intestine, whereas no significant alterations could be observed in the cerebellum or forebrain. In the thymus the levels of C16:0 containing hexosylceramides were significantly reduced. Measurements were performed with +/+ (n = 4; black columns) and CerS6KO (n = 3; gray columns) animals (9 weeks of age). The data are mean ± S.E., *** = p < 0.001; ** = p < 0.01; * = p < 0.05.
In accordance with the low C16:0-ceramide contents, only very low levels of C16:0 hexosylceramides could be detected in forebrain and cerebellum of wild type and CerS6KO animals. In small intestine, large intestine, and kidney the amounts of C16:0 hexosylceramides remained unaltered, whereas the thymus showed a significant reduction by about 40% (Fig. 7). We found significant increases in the contents of some molecular species of hexosylceramides in the (small and large) intestines (C20:0 hexosylceramide) and kidney (C18:0 hexosylceramide).
In small intestine and kidney we observed a highly significant reduction of C16:0-containing sphingomyelin (to ∼50% in small intestine and ∼35% in kidney). In small intestine we found a small but significant increase in C18:0 containing sphingomyelin, whereas we noticed no alterations in the amount of sphingomyelin with other chain lengths in kidney. Neither in forebrain nor in cerebellum could we detect any significant alterations in sphingolipid content. Thus to investigate the causes of the behavioral phenotype we analyzed the effect of CerS6 deficiency on the total amounts of complex glycosphingolipids. Therefore, we performed TLC analyses on lipid extracts from forebrain and cerebellum of wild type versus knock-out mice but found no differences (supplemental Fig. S1).
Because it is possible that the reduction in ceramide synthesis affects the acyl-CoA pool and thus alters glycerophospholipid synthesis in CerS6KO mice, we analyzed the glycerophospholipids including phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylethanolamine (PE), and their ether lipid forms (ePC, ePS, and ePE) by tandem mass spectrometry in all tissues. We found no changes in whole glycerophospholipid contents with the exception of PS, which was significantly increased in the forebrain of CerS6KO mice (supplemental Fig. S2). These data show that deficiency in CerS6 results in specific changes in the composition of neutral sphingolipids (ceramide, sphingomyelin, and hexosylceramide), whereas the amounts of glycerophospholipids remained mainly unchanged.
Cell Type-specific Expression of Ceramide Synthase 6 in the Kidney
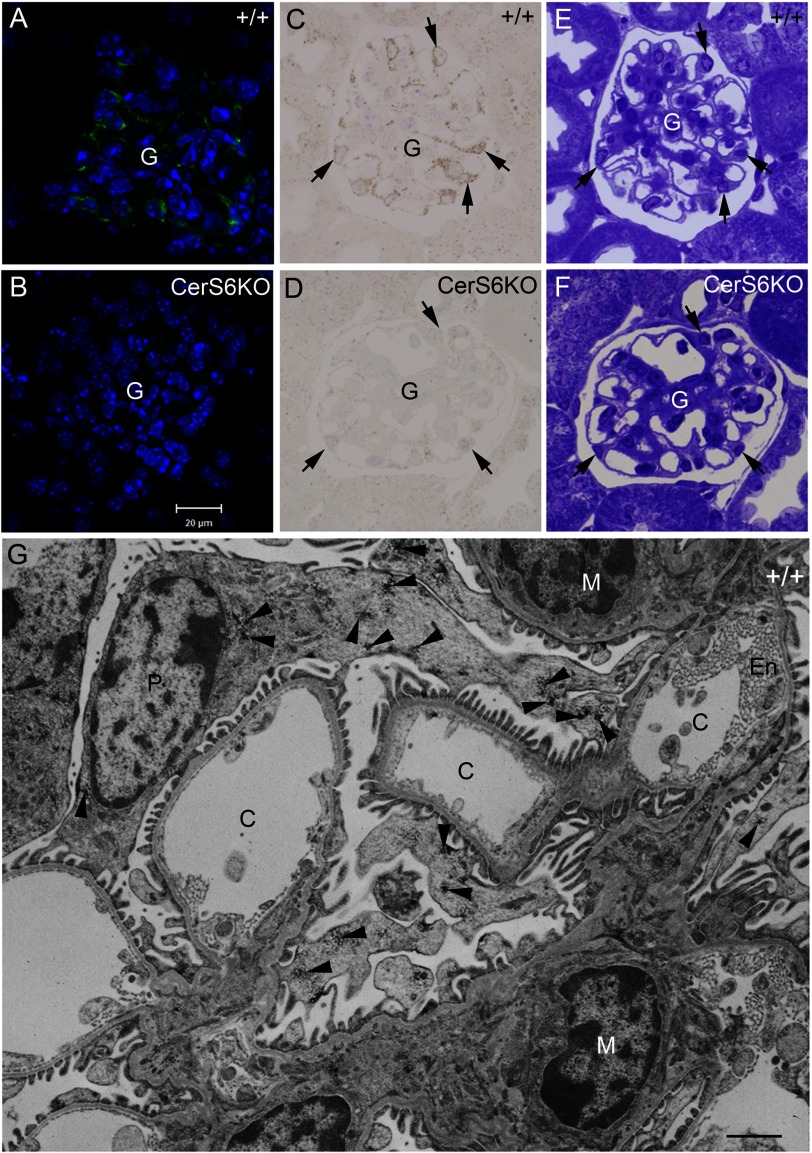
Because the kidney shows a high level of CerS6 protein expression in immunoblot analysis, we investigated the cell type-specific localization of the CerS6 protein in this tissue. LacZ staining of CerS6KO mice revealed strong expression in the glomeruli (data not shown). Therefore we performed fluorescence immunohistochemical analyses, which showed specific signals for CerS6 in the podocyte somata and processes of wild type glomerula, whereas the nuclei were spared (Fig. 8A). Semithin sections of peroxidase-immunostained vibratome sections confirmed CerS6-positive podocytes (Fig. 8C), which were clearly identified in the adjacent toluidine blue-stained section (Fig. 8E). The corresponding sections of CerS6KO mice (Fig. 8, B, D, and F) revealed no CerS6 immunoreactivity (Fig. 8, B and C) and no obvious morphological abnormality (Fig. 8E). Electron microscopy showed abundant patch-like CerS6 immunoreactive signals in podocytes including their processes contacting the blood-urine barrier, whereas the mesangial and capillary endothelial cells lack CerS6 immunoreactive signals (Fig. 8G).
FIGURE 8.
CerS6 immunofluorescence (A and B) and peroxidase staining (C–G) in the kidney of wild type (+/+) and CerS6-deficient (CerS6KO) mice. CerS6 is specifically expressed in +/+ glomeruli as documented by fluorescence immunohistochemical analyses (A) on 12-μm cryosections counterstained with DAPI and 0.8-μm semithin sections (C) of embedded peroxidase immunostained vibratome sections, both of which show CerS6 immunoreaction in podocytes (arrows). The corresponding areas of CerS6KO mice show no immunoreactivity (B and D). The adjacent toluidine blue-stained semithin sections revealed normal glomerular morphology in +/+ (E) and CerS6KO mice (F). G, electron microscopic analysis of CerS6 reactivity in the glomerulus shows a patch-like reaction product (arrowheads) in the cytoplasm and the processes of the podocytes, not observed in the mesangium (M) or in the endothelium cells (En) of the capillaries (C). Bar in B for A–E = 20 μm; bar in G = 1 μm. Experiments were performed five times with similar results.
DISCUSSION
To study the biochemical and physiological function of CerS6 we have generated an enzymatically inactive CerS6 mouse line. The genomic locus of the mouse Cers6 gene is about 320 kb long and includes 10 exons. Because of this rather large size the deletion of all exons and introns of the Cers6 gene might cause artificial phenotypic abnormalities due to deletion of regulatory elements in the corresponding introns (42) or the 3′ untranslated region (UTR). Thus, instead of deleting the whole coding region we chose a minor modification and integrated a stop codon in exon 7. Additionally we deleted parts of the coding region of exon 7 including the 2 histidine residues described to be essential for catalytic activity of CerS proteins (9). The main product of CerS6, C16:0-ceramide, has been described to play an important yet contradictory role in cell fate. Some studies characterized it as anti-apoptotic (22), whereas in others it was suggested to have a pro-apoptotic role (43, 44). A recent investigation demonstrated that increases in the amount of CerS6 protein and its main product C16:0-ceramide are involved in the onset of experimental autoimmune encephalomyelitis where CerS6 mediates the generation of iNOS and TNF-α (21). Because the functional relevance of C16:0-ceramide is not fully understood, the CerS6KO mice could serve as a biological tool to decipher the onset of this disease (21). In the present study we show that inactivation of CerS6 leads to a 70% diminished incorporation of palmitoyl-CoA into C16:0-ceramide in forebrain, cerebellum, and thymus as revealed by the ceramide synthase activity assay. The incorporation was even stronger reduced in kidney (by 90%) but less severely in small intestine (by 30%). No significant alteration could be measured in large intestine in extracts of 15-week-old mice. Interestingly heterozygous mice also showed an altered ceramide synthase activity with palmitoyl-CoA in the activity assay, leading to the conclusion that one allele is not sufficient to maintain the wild type status. Using a non-CerS6-specific substrate like stearoyl-CoA we found no significant alterations in the activity assay in any of the tested tissues.
In addition we show that inactivation of the CerS6 protein leads to a decrease in C16:0 containing sphingolipid species in nearly all of the CerS6KO tissues investigated. We found no significant reduction in the C16:0 ceramide content in large intestine, which is in accordance with the activity assay, although we noticed a strong expression of CerS6 protein by immunoblot analyses. We detected only minor amounts of C16:0 ceramide in the brain of the mice, which is in line with previous publications (14). In small intestine, the total ceramide level was significantly reduced in contrast to the other investigated tissues. Because C16:0 ceramide makes up for about 65% of total ceramide in small intestine, a decrease to 25% of the C16:0 ceramide content in CerS6KO mice is expected to have a large impact on total ceramide content. The reduction in C16:0 containing neutral sphingolipids in most tissues, was surprising, considering possible complementary effects by CerS5 (45), which shows a similar tissue-dependent expression pattern and acyl-CoA specificity (6). A possible explanation could be that these two enzymes are not expressed in the same cell type.
Next we analyzed the glycerophospholipid content in forebrain and cerebellum, because it is possible that changes in ceramide synthase activity have an impact on the acyl-CoA pool and thus on glycerophospholipid synthesis. However, no differences in glycerophospholipids were found, except for a significant increase in phosphatidylserine in forebrain. At present, we cannot exclude the possibility that this change contributes to the behavioral alterations.
As yet two ceramide synthase-deficient mouse lines have been described that exhibit behavioral abnormalities (13–16). In CerS2-deficient mice a decrease of myelin basic protein could be observed in the brain, whereas in the cerebellum and forebrain of CerS1-deficient mice the myelin-associated glycoprotein content was reduced. We measured both protein contents in wild type and CerS6KO mice but found no differences.
The observed clasping abnormality of CerS6KO mice is commonly seen in mouse models of neurodegenerative disorders (46). In combination with the inferior performance on the horizontal wire test we conclude that these mice are likely to have impaired motoneuronal functions. Mice suffering from a loss of glycosylceramide synthase (GlcCer-synthase) in the nervous system display a similar behavioral phenotype as the enzymatically inactive CerS6 mice (47). However, the GlcCer synthase knock-out mice showed a significant decrease in the number of Purkinje cells and a reduction in complex glycosphingolipids, which we did not detect in CerS6KO mice. Furthermore, mice with a loss of saposin B, an activity enhancer for several glycosphingolipid hydrolases also display hind limb clasping among other phenotypic abnormalities (48).
Although the morphology of the cerebellum appeared normal, impaired function of the cerebellum in CerS6KO mice cannot be excluded. However, at least some of the observed behavioral abnormalities cannot be explained by altered signal processing in the cerebellum alone. CerS6KO mice reacted more agitated and nervous than their wild type controls and they showed behavioral abnormalities in open field investigations. There we observed that mice harboring the enzymatically inactive CerS6 protein exhibited impaired behavioral habituation toward a novel environment, suggesting an impaired ability to encode and maintain spatial information, because the motivation to explore novel objects and the unconditioned anxiety remained unaltered. It is known that hippocampal place cells fire in relationship to the location in the environment of the animal (49). Therefore it is possible that the impaired behavioral habituation of CerS6KO mice is related to a mild hippocampal alteration that may affect the activity and function of place cells. It has also been shown that inhibition of neutral sphingomyelinase 2 (nSMase2), which catalyzes the hydrolysis of sphingomyelin to ceramide, plays a role in spatial and episodic-like memory in mice (50). In this case the inhibition of nSM2 leads to decreased levels of ceramide and increased levels of sphingomyelin except for C16:0 containing sphingomyelin, which was decreased in all measured brain regions.
We speculate that minor alterations in the sphingolipid content of forebrain as well as cerebellum taken together might lead to the observed phenotypes. Thus if C16:0 containing sphingolipids (all species included) are decreased and cannot be elevated under possible pathological conditions, e.g. in the case of experimental autoimmune encephalomyelitis, this might also lead to pathophysiological phenotypes. For example, it has been shown that not an overall loss in lipid mass but a decrease in sphingolipid and gain in phospholipid content takes place in the active phase of multiple sclerosis (51). Additionally in CerS6KO mice we observed slight morphologic alterations including an increased density in microglia cells and minor signs of degeneration in glial structures between normal appearing neurons in the hippocampus based on initial electron microscopical investigations. We confirmed the expected neuronal expression pattern of CerS6 by analysis of the lacz reporter gene expression. However, detailed studies of certain brain areas, possibly under different conditions are necessary to prove if this might be the cause for the behavioral abnormalities.
In CerS2-deficient mice, the decrease in very long chain (C22:0-C24:0) ceramides was accompanied by an increase in C16:0-ceramides (15, 16). The same observation was made in MCF-7 cells carrying a siRNA construct for down-regulation of CerS2 expression (45). These results lead to the conclusion that in CerS2KO mice, C16:0 ceramides increase in compensation for the decrease of C22:0-C24:0 ceramides (15, 16). We found a significant increase of C18:0 sphingomyelin in small intestine (along with a decrease in C16:0-ceramide) and significant increases of C18:0 hexosylceramide in kidney, and C20:0 hexosylceramide in small and large intestine. In analogy to lipid changes in the CerS2KO animals, the moderate increases in C18:0, C20:0 sphingolipids in CerS6KO mice might be a complementary effect due to the increased incorporation of sphingosine in C18:0-C20:0 sphingolipids because of the block in ceramide synthase 6 activity. Furthermore, it is possible that other ceramide synthases might be up-regulated as recently shown with siRNA experiments in a human breast adenocarcinoma cell line (45).
With the newly generated CerS6 antibodies we determined CerS6 protein levels in various tissues. Our results are in line with findings based on mRNA studies of other groups (6, 7) suggesting that Cers6 gene expression is to a major extent regulated at the transcriptional level. We also found that the CerS6 protein is endogenously N-glycosylated thus confirming previous results on exogenous CerS6 expression in transfected cells (6). Additionally we investigated the localization of the CerS6 protein in the kidney and detected specific signals in podocytes, i.e. the glomerular cells, which are important components of the blood-urine barrier, whereas other glomerular cells lack CerS6 immunoreactivity.
The CerS6KO mice represent a new biological tool to investigate the role of the C16:0 N-acyl membrane anchor of ceramide in mice. It has recently been shown that CerS6 is part of a signaling pathway leading to experimental autoimmune encephalomyelitis (21). These investigations suggest a special role of at least the main product C16:0-ceramide in the onset of autoimmune diseases. It is likely that the biological function of CerS6 and CerS5 are different, although the expression pattern and the substrate specificity of these enzymes are rather similar. Further characterization of the mice with enzymatically inactive CerS6 protein may therefore help to unravel the mechanisms in the onset of experimental autoimmune encephalomyelitis.
Acknowledgments
The excellent technical assistance of Hans-Werner Habbes (Bochum) and Marlen Löbbecke-Schumacher (Bochum) is gratefully acknowledged. We thank Christine Siegmund (Bonn) for blastocyst injections and Prof. Maarten Egmond (University of Utrecht) for synthesis and purification of the immunogenic CerS6 peptide.
This work was supported by the German Research Foundation through Collaborative Research Centre Grants SFB-645 projects B2 (to K. W.), B5 (to M. E.), and Z4 (to P. D.), and DE1149/6-1 (to E. D.).

This article contains supplemental Figs. S1 and S2 and Table S1.
- CerS
- ceramide synthase
- ES
- embryonic stem
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PA
- phosphatidic acid
- PS
- phosphatidylserine
- PI
- phosphatidylinositol.
REFERENCES
- 1. Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling. Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 2. Kitatani K., Idkowiak-Baldys J., Hannun Y. A. (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. (1994) Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 180, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Echten G., Sandhoff K. (1993) Ganglioside metabolism. Enzymology, topology, and regulation. J. Biol. Chem. 268, 5341–5344 [PubMed] [Google Scholar]
- 5. Stiban J., Tidhar R., Futerman A. H. (2010) Ceramide synthases. Roles in cell physiology and signaling. Adv. Exp. Med. Biol. 688, 60–71 [DOI] [PubMed] [Google Scholar]
- 6. Mizutani Y., Kihara A., Igarashi Y. (2005) Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laviad E. L., Albee L., Pankova-Kholmyansky I., Epstein S., Park H., Merrill A. H., Jr., Futerman A. H. (2008) Characterization of ceramide synthase 2. Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 283, 5677–5684 [DOI] [PubMed] [Google Scholar]
- 8. Winter E., Ponting C. P. (2002) TRAM, LAG1 and CLN8. Members of a novel family of lipid-sensing domains? Trends Biochem. Sci. 27, 381–383 [DOI] [PubMed] [Google Scholar]
- 9. Tidhar R., Ben-Dor S., Wang E., Kelly S., Merrill A. H., Jr., Futerman A. H. (2012) Acyl chain specificity of ceramide synthases is determined within a region of 150 residues in the Tram-Lag-CLN8 (TLC) domain. J. Biol. Chem. 287, 3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spassieva S., Seo J. G., Jiang J. C., Bielawski J., Alvarez-Vasquez F., Jazwinski S. M., Hannun Y. A., Obeid L. M. (2006) Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J. Biol. Chem. 281, 33931–33938 [DOI] [PubMed] [Google Scholar]
- 11. Venkataraman K., Futerman A. H. (2002) Do longevity assurance genes containing Hox domains regulate cell development via ceramide synthesis? FEBS Lett. 528, 3–4 [DOI] [PubMed] [Google Scholar]
- 12. Lee S. J. (1991) Expression of growth/differentiation factor 1 in the nervous system: conservation of a bicistronic structure. Proc. Natl. Acad. Sci. U.S.A. 88, 4250–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao L., Spassieva S. D., Jucius T. J., Shultz L. D., Shick H. E., Macklin W. B., Hannun Y. A., Obeid L. M., Ackerman S. L. (2011) A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet. 7, e1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ginkel C., Hartmann D., Vom Dorp K., Zlomuzica A., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Rabionet M., Dere E., Doermann P., Sandhoff K., Willecke K. (2012) Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. J. Biol. Chem. 287, 41888–41902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imgrund S., Hartmann D., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Gieselmann V., Sandhoff K., Willecke K. (2009) Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 284, 33549–33560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brügger B., Sachsenheimer T., Wieland F., Prieto M., Merrill A. H., Jr., Futerman A. H. (2010) A critical role for ceramide synthase 2 in liver homeostasis. I. Alterations in lipid metabolic pathways. J. Biol. Chem. 285, 10902–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pewzner-Jung Y., Brenner O., Braun S., Laviad E. L., Ben-Dor S., Feldmesser E., Horn-Saban S., Amann-Zalcenstein D., Raanan C., Berkutzki T., Erez-Roman R., Ben-David O., Levy M., Holzman D., Park H., Nyska A., Merrill A. H., Jr., Futerman A. H. (2010) A critical role for ceramide synthase 2 in liver homeostasis. II. Insights into molecular changes leading to hepatopathy. J. Biol. Chem. 285, 10911–10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mizutani Y., Kihara A., Igarashi Y. (2006) LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem. J. 398, 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jennemann R., Rabionet M., Gorgas K., Epstein S., Dalpke A., Rothermel U., Bayerle A., van der Hoeven F., Imgrund S., Kirsch J., Nickel W., Willecke K., Riezman H., Gröne H. J., Sandhoff R. (2012) Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 21, 586–608 [DOI] [PubMed] [Google Scholar]
- 20. Levy M., Futerman A. H. (2010) Mammalian ceramide synthases. IUBMB Life 62, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schiffmann S., Ferreiros N., Birod K., Eberle M., Schreiber Y., Pfeilschifter W., Ziemann U., Pierre S., Scholich K., Grösch S., Geisslinger G. (2012) Ceramide synthase 6 plays a critical role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 188, 5723–5733 [DOI] [PubMed] [Google Scholar]
- 22. Senkal C. E., Ponnusamy S., Bielawski J., Hannun Y. A., Ogretmen B. (2010) Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 24, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gierl M. S., Karoulias N., Wende H., Strehle M., Birchmeier C. (2006) The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 20, 2465–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee E. C., Yu D., Martinez de Velasco J., Tessarollo L., Swing D. A., Court D. L., Jenkins N. A., Copeland N. G. (2001) A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73, 56–65 [DOI] [PubMed] [Google Scholar]
- 25. Buchholz F., Angrand P. O., Stewart A. F. (1996) A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res. 24, 3118–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magin T. M., McWhir J., Melton D. W. (1992) A new mouse embryonic stem cell line with good germ line contribution and gene targeting frequency. Nucleic Acids Res. 20, 3795–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wells D. (1993) Production of chimeras derived from murine embryonic stem cells. Methods Mol. Biol. 18, 217–237 [DOI] [PubMed] [Google Scholar]
- 28. Requardt R. P., Kaczmarczyk L., Dublin P., Wallraff-Beck A., Mikeska T., Degen J., Waha A., Steinhäuser C., Willecke K., Theis M. (2009) Quality control of astrocyte-directed Cre transgenic mice. The benefits of a direct link between loss of gene expression and reporter activation. Glia 57, 680–692 [DOI] [PubMed] [Google Scholar]
- 29. Kim H. J., Qiao Q., Toop H. D., Morris J. C., Don A. S. (2012) A fluorescent assay for ceramide synthase activity. J. Lipid Res. 53, 1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrasch-Parwez E., Habbes H. W., Weickert S., Löbbecke-Schumacher M., Striedinger K., Wieczorek S., Dermietzel R., Epplen J. T. (2004) Fine-structural analysis and connexin expression in the retina of a transgenic model of Huntington's disease. J. Comp. Neurol. 479, 181–197 [DOI] [PubMed] [Google Scholar]
- 31. Petrasch-Parwez E., Nguyen H. P., Löbbecke-Schumacher M., Habbes H. W., Wieczorek S., Riess O., Andres K. H., Dermietzel R., Von Hörsten S. (2007) Cellular and subcellular localization of Huntingtin (corrected) aggregates in the brain of a rat transgenic for Huntington disease. J. Comp. Neurol. 501, 716–730 [DOI] [PubMed] [Google Scholar]
- 32. Welti R., Li W., Li M., Sang Y., Biesiada H., Zhou H. E., Rajashekar C. B., Williams T. D., Wang X. (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 277, 31994–32002 [DOI] [PubMed] [Google Scholar]
- 33. Brügger B., Erben G., Sandhoff R., Wieland F. T., Lehmann W. D. (1997) Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 94, 2339–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wewer V., Dombrink I., vom Dorp K., Dörmann P. (2011) Quantification of sterol lipids in plants by quadrupole time-of-flight mass spectrometry. J. Lipid Res. 52, 1039–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Homma D., Sumi-Ichinose C., Tokuoka H., Ikemoto K., Nomura T., Kondo K., Katoh S., Ichinose H. (2011) Partial biopterin deficiency disturbs postnatal development of the dopaminergic system in the brain. J. Biol. Chem. 286, 1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gantois I., Fang K., Jiang L., Babovic D., Lawrence A. J., Ferreri V., Teper Y., Jupp B., Ziebell J., Morganti-Kossmann C. M., O'Brien T. J., Nally R., Schütz G., Waddington J., Egan G. F., Drago J. (2007) Ablation of D1 dopamine receptor-expressing cells generates mice with seizures, dystonia, hyperactivity, and impaired oral behavior. Proc. Natl. Acad. Sci. U.S.A. 104, 4182–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zlomuzica A., Tress O., Binder S., Rovira C., Willecke K., Dere E. (2012) Changes in object recognition and anxiety-like behaviour in mice expressing a cx47 mutation that causes pelizaeus-merzbacher-like disease. Dev. Neurosci. 34, 277–287 [DOI] [PubMed] [Google Scholar]
- 38. Dere E., De Souza Silva M. A., Topic B., Fiorillo C., Li J. S., Sadile A. G., Frisch C., Huston J. P. (2002) Aged endothelial nitric-oxide synthase knockout mice exhibit higher mortality concomitant with impaired open-field habituation and alterations in forebrain neurotransmitter levels. Genes Brain Behav. 1, 204–213 [DOI] [PubMed] [Google Scholar]
- 39. Dere E., Huston J. P., De Souza Silva M. A. (2007) The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 31, 673–704 [DOI] [PubMed] [Google Scholar]
- 40. Dere E., Topic B., De Souza Silva M. A., Srejic M., Frisch C., Buddenberg T., Huston J. P. (2002) The graded anxiety test. A novel test of murine unconditioned anxiety based on the principles of the elevated plus-maze and light-dark test. J. Neurosci. Methods 122, 65–73 [DOI] [PubMed] [Google Scholar]
- 41. Becker I., Wang-Eckhardt L., Yaghootfam A., Gieselmann V., Eckhardt M. (2008) Differential expression of (dihydro)ceramide synthases in mouse brain. Oligodendrocyte-specific expression of CerS2/Lass2. Histochem. Cell Biol. 129, 233–241 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez A., Griffiths-Jones S., Ashurst J. L., Bradley A. (2004) Identification of mammalian microRNA host genes and transcription units. Genome Res. 14, 1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grösch S., Schiffmann S., Geisslinger G. (2012) Chain length-specific properties of ceramides. Prog. Lipid Res. 51, 50–62 [DOI] [PubMed] [Google Scholar]
- 44. Thomas R. L., Jr., Matsko C. M., Lotze M. T., Amoscato A. A. (1999) Mass spectrometric identification of increased C16 ceramide levels during apoptosis. J. Biol. Chem. 274, 30580–30588 [DOI] [PubMed] [Google Scholar]
- 45. Mullen T. D., Spassieva S., Jenkins R. W., Kitatani K., Bielawski J., Hannun Y. A., Obeid L. M. (2011) Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J. Lipid Res. 52, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cuellar T. L., Davis T. H., Nelson P. T., Loeb G. B., Harfe B. D., Ullian E., McManus M. T. (2008) Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 105, 5614–5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamashita T., Allende M. L., Kalkofen D. N., Werth N., Sandhoff K., Proia R. L. (2005) Conditional LoxP-flanked glucosylceramide synthase allele controlling glycosphingolipid synthesis. Genesis 43, 175–180 [DOI] [PubMed] [Google Scholar]
- 48. Sun Y., Witte D. P., Ran H., Zamzow M., Barnes S., Cheng H., Han X., Williams M. T., Skelton M. R., Vorhees C. V., Grabowski G. A. (2008) Neurological deficits and glycosphingolipid accumulation in saposin B deficient mice. Hum. Mol. Genet. 17, 2345–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Keefe J., Dostrovsky J. (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 [DOI] [PubMed] [Google Scholar]
- 50. Tabatadze N., Savonenko A., Song H., Bandaru V. V., Chu M., Haughey N. J. (2010) Inhibition of neutral sphingomyelinase-2 perturbs brain sphingolipid balance and spatial memory in mice. J. Neurosci. Res. 88, 2940–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wheeler D., Bandaru V. V., Calabresi P. A., Nath A., Haughey N. J. (2008) A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 131, 3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]