Abstract
Inflammation plays an essential role in the initiation and progression of atherosclerosis, but its role in vascular repair after mechanical arterial injury (i.e., percutaneous transluminal coronary angioplasty, PTCA) is unknown. In animal models of vascular injury, leukocytes are recruited as a precursor to intimal thickening. Furthermore, markers of leukocyte activation — in particular, increased expression of the β2-integrin Mac-1 (αMβ2, or CD11b/CD18), which is responsible for firm leukocyte adhesion to platelets and fibrinogen on denuded vessels — predict restenosis after PTCA. To determine whether Mac-1–mediated leukocyte recruitment is causally related to neointimal formation, we subjected mice lacking Mac-1 to a novel form of mechanical carotid artery dilation and complete endothelial denudation. We now report that the selective absence of Mac-1 impairs transplatelet leukocyte migration into the vessel wall, reducing leukocyte accumulation over time. Diminished medial leukocyte accumulation was accompanied by markedly reduced neointimal thickening after vascular injury. These data establish a role for inflammation in neointimal thickening and suggest that leukocyte recruitment to mechanically injured arteries may prevent restenosis.
Introduction
Inflammation plays an essential role in the initiation and progression of atherosclerosis and in atherosclerotic plaque rupture that culminates in acute ischemic syndromes (1). Emerging experimental and clinical data indicate that leukocytes may be central to intimal growth after mechanical arterial injury. In animal models of vascular injury, leukocytes are recruited as a precursor to intimal thickening (2, 3). Quantitative immunohistochemical analysis of directional coronary atherectomy specimens from humans has shown that inflammatory cells are far more prevalent in restenotic lesions than in de novo lesions, and comprise substantial lesional volume (4). Moreover, in animal models, proliferation of vessel wall inflammatory cells is associated with intimal thickening (3), and blockade of the cell adhesion molecules that are important for leukocyte recruitment attenuates intimal growth (5, 6). However, the precise molecular mechanisms responsible for leukocyte recruitment to mechanically injured arteries that are devoid of endothelium, and the resultant effects of inflammation on vascular repair after PTCA are unknown.
Leukocyte recruitment in acute inflammation is mediated in part by the β2-integrin family of receptors, including LFA-1 (αLβ2, or CD11a/CD18), Mac-1 (αMβ2, or CD11b/CD18), and p150,95 (αXβ2, or CD11c/CD18). Mac-1, the primary fibrinogen receptor on leukocytes (7, 8), directly facilitates the adhesion and transmigration of leukocytes at sites of fibrin and platelet deposition in vitro (9); Mac-1 is upregulated locally and systemically after PTCA (10–12). We previously demonstrated that mAb-mediated blockade of Mac-1 reduces intimal thickening after angioplasty or stent implantation in rabbits (5). To study in isolation the role of Mac-1 in vascular repair, we developed a new murine model of mechanical injury designed to achieve complete endothelial denudation and controlled arterial stretching. We now report that the selective absence of Mac-1 markedly reduces neointimal thickening after vascular injury.
Methods
Arterial injury.
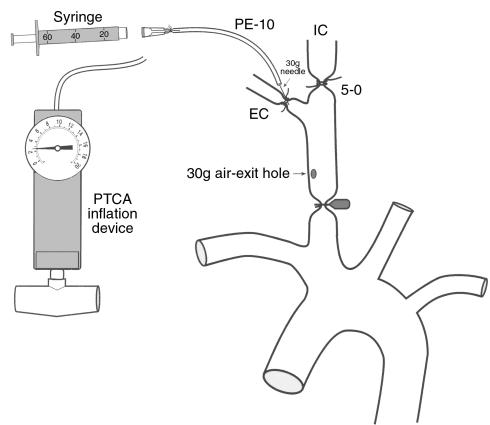
Wild-type C57BL/J6 (Mac-1+/+; n = 26) and Mac-1–deficient (Mac-1–/–; n = 29) male mice (8 weeks old, weighing approximately 25 g) were maintained on a standard diet and water ad libitum. Mac-1–deficient mice, generated as described previously (13), were backcrossed more than 6 generations on the C57BL/J6 strain. To rule out possible strain-specific, Mac-1–independent, genetic contribution to the phenotype, we repeated injury experiments in Mac-1–/– mice (n = 4) that were backcrossed 12 generations onto the C57BL/J6 background. Animals were anesthetized on day 0 using ketamine (80 mg/kg given intraperitoneally; Fort Dodge Laboratories Inc., Fort Dodge, Iowa, USA) and xylazine (5 mg/kg given intraperitoneally). All surgery was performed using sterile techniques with the aid of a dissecting microscope. To achieve complete endothelial denudation and controlled arterial stretching, we modified the air-drying model of Fishman et al. (Figure 1; ref. 14). The right carotid artery was surgically exposed and then isolated from the surrounding tissues. The proximal common and internal carotid arteries were occluded with microvascular clamps, and the external carotid artery was identified and ligated with 5-0 silk. An incision was made in the external carotid artery proximal to the ligature, and a 30-gauge needle connected to a saline-filled syringe with polyethylene tubing (PE-10; Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) was introduced. After gently irrigating the isolated common carotid segment with saline to remove blood, the 30-gauge needle was secured within the external carotid artery by placing a ligature around the artery proximal to the needle insertion site. The syringe was replaced with an angioplasty inflation device (Advanced Cardiovascular Systems/Guidant, Inc., Santa Clara, California, USA) and the isolated, saline-filled common carotid segment was dilated with 2.5 atmospheres of pressure for 30 seconds. The inflation device was then replaced with an air-filled 60-mL syringe, and a 30-gauge air-exit hole was made at the proximal end of the common carotid artery. Endothelial denudation was performed by air-drying the carotid artery for 3 minutes (20 mL/min). After air-drying, the artery was refilled with saline and the needle was removed. The external carotid artery was ligated proximal to the needle insertion site, and the clamps were removed to reestablish normal anterograde flow. A cotton-tipped applicator was applied to the air-exit hole in the common carotid artery to tamponade bleeding. The wound was irrigated with saline and the incision was closed with running 4-0 silk sutures. Topical antibiotic ointment was applied. All animals survived until the time of planned sacrifice without bleeding or infection. Animal care and procedures were reviewed and approved by the Harvard Medical School Standing Committee on Animals, and were performed in accordance with the guidelines of the American Association for Accreditation of Laboratory Animal Care and the National Institutes of Health (NIH).
Figure 1.
Schematic illustration of mechanical carotid artery dilation and complete endothelial denudation based on the air-drying rat model of Fishman et al. (14), as described in Methods. 30g, 30-gauge. EC, external carotid artery. IC, internal carotid artery.
Vascular tissue harvesting and analysis.
One day (Mac-1+/+: n = 6; Mac-1–/–: n = 6), 3 days (Mac-1+/+: n = 6; Mac-1–/–: n = 7), 7 days (Mac-1+/+: n = 3; Mac-1–/–: n = 3), or 28 days (Mac-1+/+: n = 11; Mac-1–/–: n = 9) after vascular injury, anesthesia was administered as above, the chest cavity was opened, and animals were sacrificed by right atrial exsanguination. A 22-gauge butterfly catheter was inserted into the left ventricle for in situ pressure perfusion at 100 mmHg with 0.9% saline for 1 minute, followed by fixation with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3) for 10 minutes. The right and left common carotid arteries were excised and immersed in buffered paraformaldehyde. Spleen and small intestine from 3 Mac-1+/+ and 3 Mac-1–/– animals were harvested as control tissues for immunohistochemistry. All animals received bromodeoxyuridine (BrdU) (50 mg/kg intraperitoneally) 18 hours and 1 hour before sacrifice.
Carotid arteries were embedded, and 2 cross-sections, cut 1 mm apart, were stained with hematoxylin and eosin and Verhoeff’s tissue elastin stain. A histologist blinded to animal genotype measured the lumen and the intimal and medial areas of each cross-sectional plane using a microscope equipped with a CCD camera interfaced to a computer running NIH Image version 1.60 software. Results from the 2 planes of each artery were averaged. For immunohistochemistry, standard avidin-biotin procedures for mouse CD45 (leukocyte common antigen; PharMingen, San Diego, California, USA), mouse neutrophil-specific marker (mAb 7/4; Harlan Bioproducts for Science Inc., Indianapolis, Indiana, USA), mouse macrophage-specific marker Mac-3 (mAb M3/84; PharMingen), BrdU (DAKO Corp., Carpinteria, California, USA), and smooth muscle cell (SMC) α-actin (DAKO Corp.) were used. Immunostained sections were quantified as percent positivity (number of immunostain-positive cells/total number of nuclei).
Additional animals (Mac-1+/+: n = 2; Mac-1–/–: n = 2) were prepared for transmission electron microscopy. Two mice were sacrificed without injury, and 2 others were sacrificed 1 day after injury. Vascular injury and tissue harvesting in these mice was performed as above, except that 2.5% glutaraldehyde with 2% paraformaldehyde in 0.1 M cacodylate buffer was used for perfusion and immersion fixation. Tissues were rinsed overnight in 0.1 M cacodylate buffer at 4°C and then dehydrated in graded ethanols. Tissues were then placed in propylene oxide and embedded in Poly/Bed 812 (Electron Microscopy Sciences, Fort Washington, Pennsylvania, USA). Planes were cut 2 μm thick for each vessel and then stained with toluidine blue stain. Thin sections (90 nm) were cut on an ultramicrotome fitted with a diamond knife and collected on carbon-coated, Formvar®-covered grids. Thin sections were stained with uranyl acetate and lead citrate, and observed and photographed in a JEOL 1200 EX transmission electron microscope. The operator was blinded to the presence of arterial injury and animal genotype.
Preparation of neutrophils and platelets.
Neutrophils were isolated from the bone marrow of Mac-1+/+ and Mac-1–/– mice by density separation using Neutrophil Isolation Medium (Cardinal Associates Inc., Santa Fe, New Mexico, USA) as described previously (15). Purified cells were stored in HBSS containing 2 mM CaCl2 and 2 mM MgCl2 supplemented with 10 mM HEPES and 0.5% BSA at pH 7.4. Platelet-rich plasma was prepared by centrifugation (at 120 g) of whole blood (anticoagulated with 13 mmol/L trisodium citrate) obtained from inferior vena cava puncture of wild-type mice. Platelets were washed and resuspended in HBSS. Platelets were counted on a Coulter cell counter (Coulter Electronics, Hialeah, Florida, USA); typical yield was 5 × 105 platelets/μL. Isolation of sufficient neutrophils and platelets required 3–4 Mac-1+/+ mice and 3–4 Mac-1–/– mice per migration experiment. Neutrophils and platelets were used within 2 hours of purification.
Neutrophil adhesion and transplatelet chemotaxis assays.
Neutrophil adhesion to and transmigration through surface-adherent platelets were investigated as described previously (9). For adhesion experiments, murine platelets (∼2.5 × 107) were added to 96-well microtiter plates coated with 100 μg/mL fibrinogen (preparation depleted of plasminogen, von Willebrand factor, and fibronectin; Enzyme Research Laboratories, South Bend, Indiana, USA). After 30 minutes of incubation at 37°C, microtiter wells were washed, and bound platelets were activated with 20 μM ADP. Neutrophils (1.5 × 105) were loaded with 1 μM 2′,7′-bis-(2-carboxyethyl)-5,6-carboxyfluorescein acetoxymethylester (BCECF AM; Molecular Probes Inc., Eugene, Oregon, USA), washed twice, and then added to each well and left for 60 minutes at 37°C in 5% CO2. Neutrophils were washed, and neutrophil adhesion was quantified as the percentage of total adherent cells by measuring the fluorescence of BCECF AM–loaded cells using a CytoFluor II fluorescence multiwell microplate reader (PerSeptive Biosystems, Framingham, Massachusetts, USA). Fluorescence of input neutrophils before washing served as a measure of total cell number.
For transmigration experiments, murine platelets (∼2.5 × 107) were bound to transwell polycarbonate membranes 6.5 mm in diameter with 3 μm pores (Corning Inc., Corning, New York, USA) that had been coated overnight with fibronectin (5 μg/mL) or fibrinogen (100 μg/mL). After 30 minutes of incubation at 37°C, the Transwells were centrifuged at 200 g for 2 minutes and then washed. Bound platelets were activated with 20 μM ADP. Neutrophils (2.5 × 105) were loaded with 1 μM BCECF AM, washed twice, and then added to each Transwell insert. Neutrophil chemotaxis was stimulated by placing the Transwells into 24-well tissue culture plates containing 300 ng/mL recombinant murine KC or IL-8 (R&D Systems, Inc., Minneapolis, Minnesota, USA) in a final volume of 600 μL RPMI 1640. After incubation for 1–2 hours at 37°C in 5% CO2, the inserts were removed, and transmigration was quantified as the percentage of total cells by measuring the fluorescence of BCECF AM–loaded neutrophils. A 1:20 dilution of input neutrophils added to a well that contained medium alone served as a measure of the number of input cells.
Statistics.
All data are presented as mean ± SEM unless otherwise indicated. Comparisons between groups used a unpaired Student’s t test. P values less than 0.05 were considered significant.
Results
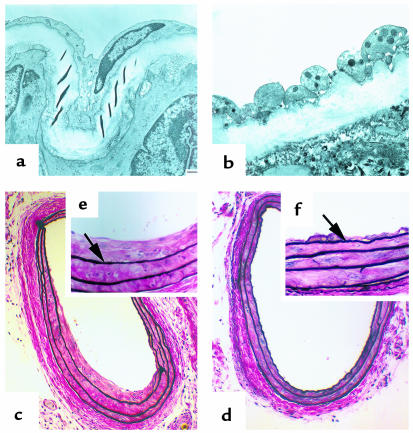
To determine whether Mac-1–mediated leukocyte recruitment is causally related to neointimal formation, we subjected mice lacking Mac-1 to a novel form of mechanical carotid artery dilation and complete endothelial denudation, based on the air-drying rat model of Fishman et al. (14) (Figure 1). In uninjured carotid arteries, a single endothelial cell layer overlies the internal elastic lamina (Figure 2a). Twenty-four hours after injury, transmission electron microscopy revealed that injured wild-type (Mac-1+/+) and Mac-1–deficient (Mac-1–/–) arteries were completely denuded of endothelium and lined with a platelet monolayer but were free of adherent leukocytes (Figure 2b). Cellular and nuclear debris from SMC injury and death were seen in the media. The extent of endothelial cell loss and medial injury was similar in Mac-1+/+ mice and Mac-1–/– mice (data not shown).
Figure 2.
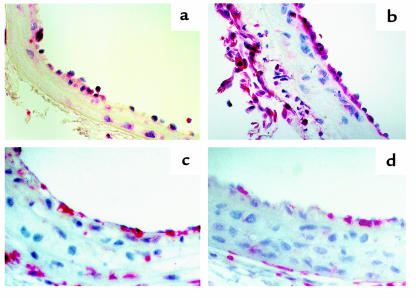
Photomicrographs of mouse carotid arteries after arterial dilation and endothelial denudation. (a and b) TEM from Mac-1+/+ mice. (a) Uninjured artery. (b) Artery 24 hours after injury (original magnification: ×3,000). (c–f) Verhoeff’s elastin stain 28 days after vascular injury. (c) Mac-1+/+ artery (×38); (d) Mac-1–/– artery (×38); (e) Mac-1+/+ artery (×150); (f) Mac-1–/– artery (×150). Neointima separates the internal elastic lamina (arrows) from the lumen.
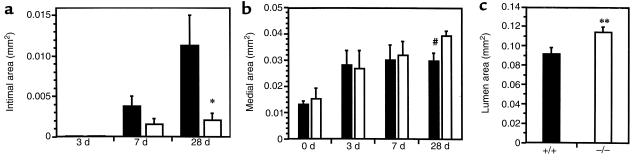
In Mac-1+/+ mice, the lumen was lined with leukocytes (i.e., CD45-positive cells) at 3 days. Intimal thickening began between 3 days and 7 days, and progressed significantly between 7 days (0.0038 ± 0.0012 mm2) and 28 days (0.0112 ± 0.0038 mm2) (Figure 2 and Figure 3a). In Mac-1–/– mice, intimal thickening was reduced 58% at 7 days and 80% at 28 days (P = 0.023), compared with wild-type mice (Figure 3a). Significant medial thickening was also observed from 0 days to 28 days after injury (Figure 3b), and was slightly greater in Mac-1–/– mice than in Mac-1+/+ mice at 28 days (P = 0.015). In Mac-1+/+ mice, the ratio of intima area to media area increased from 0.13 ± 0.03 at 7 days to 0.34 ± 0.13 at 28 days. In Mac-1–/– mice, this ratio was reduced 62% at 7 days and 85% at 28 days (P = 0.066). These carotid artery injury experiments were performed in Mac-1–/– mice backcrossed 6 generations on the C57BL/J6 strain. Selective absence of Mac-1 in mice backcrossed 12 generations onto the C57BL/J6 background reduced neointimal thickening by 71% at 28 days (P = 0.045) compared with wild-type mice. Furthermore, the reduction in neointimal thickening in these 12-generation Mac-1–/– backcrosses was indistinguishable from that observed in mice backcrossed 6 generations (0.0032 ± 0.0009 mm2 and 0.0021 ± 0.0008 mm2, respectively, at 28 days; P = 0.43), ruling out a possible strain-specific, Mac-1–independent, genetic contribution to the vascular repair phenotype observed in our experimental model.
Figure 3.
Bar graphs show intimal (a), medial (b), and lumen (c) cross-sectional area in Mac-1+/+ (solid bars) and Mac-1–/– (open bars) carotid arteries 0–28 days after injury. Absence of Mac-1 led to reduced intimal size and increased lumen area. Values are mean ± SEM. *P = 0.023 for Mac-1+/+ vs. Mac-1–/–; #P = 0.015 for Mac-1+/+ vs. Mac-1–/–; **P = 0.019 for Mac-1+/+ vs. Mac-1–/–.
Intimal and medial thickening were accompanied by progressive vessel enlargement or “positive remodeling” that was greater in Mac-1–/– mice than in Mac-1+/+ mice, as determined by external elastic lamina (EEL) radius measurements in uninjured vessels and vessels measured 28 days after injury (Mac-1+/+: 0.104 ± 0.015 mm to 0.204 ± 0.006 mm; Mac-1–/–: 0.138 ± 0.018 mm to 0.222 ± 0.005 mm; P = 0.07). Overall, reduced intimal thickening and greater EEL enlargement in Mac-1–/– mice resulted in significantly greater lumen area (0.114 ± 0.006 mm2) than in Mac-1+/+ mice (0.092 ± 0.006 mm2; P = 0.019) (Figure 3c).
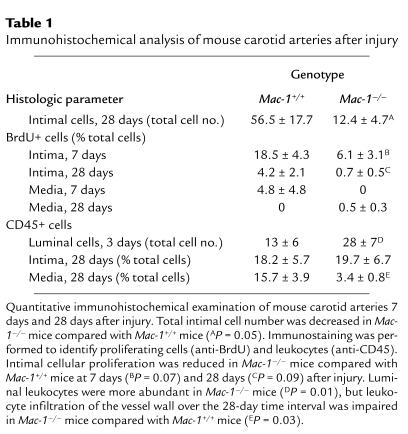
Total intimal cell number was reduced by 78% in Mac-1–/– mice (Table 1). Immunostaining for α-actin revealed that more than 80% of intimal cells were SMCs (data not shown). We assessed cellular proliferation by measuring incorporation of BrdU at the time of sacrifice. Substantial proliferation was observed in this model at 7 days (18.6% of intimal cells), and fell significantly by 28 days (4.2% of intimal cells) (Table 1). In Mac-1–/– mice, intimal cellular proliferation was reduced by 67% at 7 days, and by 83% at 28 days.
Table 1.
Immunohistochemical analysis of mouse carotid arteries after injury
Altered leukocyte accumulation within vessels was observed in Mac-1–/– mice. Luminally adherent leukocytes (CD45-positive cells) were sparse 1 day after injury, but had accumulated substantially by 3 days (Figure 4, a and b). Mac-1–/– mice had an increased number of leukocytes adhering to the lumen at 3 days, but 78% fewer leukocytes in the media than Mac-1+/+ mice had after 28 days (Table 1 and Figure 4, c and d). We expanded the CD45 analysis by immunostaining using cell-specific markers. There were 3.2-fold more luminal neutrophils (i.e., mAb 7/4–positive cells) in Mac-1–/– mice (n = 4) than in wild-type mice (n = 4) at 3 days (% total cells: 11.4 ± 5.7 vs. 3.6 ± 1.8, respectively). However, neutrophil accumulation within the media at 3 days was reduced by 67% in the absence of Mac-1 (Mac-1–/–: 13.9 ± 5.6% of total cells; Mac-1+/+: 41.7 ± 12.5% of total cells). Intimal and medial neutrophils were undetectable at 28 days after injury in both genotypes. Concordant with the CD45 staining, macrophages (Mac-3–positive cells) were reduced at 28 days in Mac-1–/– mice (n = 5) compared with wild-type (n = 4) mice (6.0 ± 2.1% vs. 13.8 ± 6.9%, respectively).
Figure 4.
Photomicrographs of mouse carotid arteries after arterial dilation and endothelial denudation. CD45 immunostaining (original magnification: ×100). (a) Mac-1+/+ artery 3 days after injury. (b) Mac-1–/– artery 3 days after injury. (c) Mac-1+/+ artery 28 days after injury. (d) Mac-1–/– artery 28 days after injury.
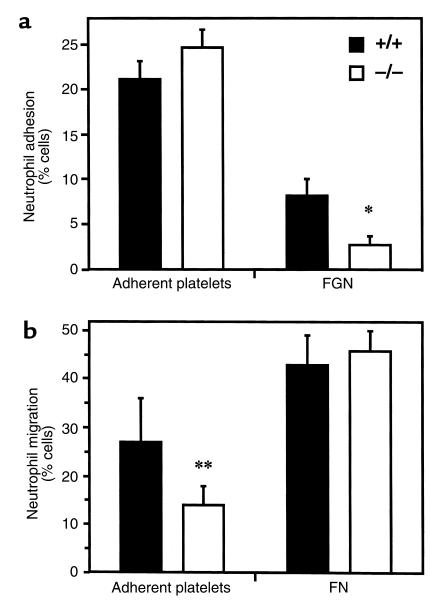
Transmission electron microscopy indicated that injured arteries were completely denuded of endothelium and were lined with a platelet monolayer (Figure 2b). Therefore, to further examine the potential mechanism for impairment of leukocyte infiltration into the vascular wall, we performed in vitro neutrophil adhesion and transplatelet chemotaxis assays, as described previously by Diacovo and coworkers (9), using murine platelets and bone marrow–derived neutrophils isolated from Mac-1+/+ and Mac-1–/– mice. Microtiter wells were coated with fibronectin- and von Willebrand factor–depleted fibrinogen to which platelets were bound and then activated. Adhesion to adherent platelets was similar in Mac-1+/+ (% adhesion = 21 ± 2) and Mac-1–/– (% adhesion = 25 ± 3; mean ± SD; P = NS) neutrophils (Figure 5a). In contrast, as we have described previously (13), adhesion to wells coated with fibrinogen alone without adherent platelets was impaired in Mac-1–/– neutrophils compared with wild-type neutrophils (P = 0.002).
Figure 5.
Neutrophil adhesion to and transmigration across platelets. Microtiter wells or Transwell insert membranes were coated either with fibrinogen and platelets or with the indicated extracellular matrix proteins alone, as described in Methods. Bar graphs show adhesion (a) and KC-stimulated transmigration (b) of bone marrow–derived neutrophils from Mac-1+/+ mice (solid bars) and Mac-1–/– mice (open bars). Values are mean ± SD of 3–5 separate experiments. *P = 0.002. **P = 0.047. FGN, fibrinogen; FN, fibronectin.
Preserved neutrophil adhesion to adherent platelets led us to hypothesize that Mac-1 deficiency was associated with an impairment in transplatelet neutrophil chemotaxis. For migration experiments, Transwell inserts were coated with fibrinogen to which platelets were bound and then activated. Recombinant murine KC or IL-8 stimulated the migration of Mac-1+/+ neutrophils across adherent platelets (% migration = 27 ± 9, mean ± SD) (Figure 5b). In Mac-1–/– neutrophils, transplatelet migration was reduced by 50% (% migration = 14 ± 4; P = 0.047). A similar migration defect (% migration Mac-1+/+neutrophils = 34%; % migration Mac-1–/– neutrophils = 19%; n = 2) was observed when platelets were bound to Transwell inserts coated with fibronectin, ruling out the possibility that impaired adhesion to fibrinogen contributed to a reduction in transplatelet migration. In contrast, β1-integrin–dependent migration across inserts coated with fibronectin alone without adherent platelets was indistinguishable in Mac-1+/+ (43 ± 6%) and Mac-1–/– (46 ± 4%; P = NS) neutrophils, ruling out a generalized chemotactic defect in Mac-1–/– neutrophils.
Discussion
Mac-1 and leukocyte recruitment after vascular injury.
We have shown that the absence of Mac-1 leads to decreased accumulation of leukocytes in the vessel wall and to a reduction in intimal proliferation and thickening after injury. We previously reported that mAb blockade of Mac-1 reduced intimal thickening in rabbits after iliac artery stent implantation (5). Our study using Mac-1–deficient mice provides rigorous and definitive evidence that Mac-1 is a determinant of neointimal thickening, and most importantly, reveals new information regarding the role of β2-integrins in leukocyte recruitment at sites of platelet and fibrin deposition.
The adhesion of leukocytes to and subsequent transmigration across the vascular endothelium has been studied in vitro and in vivo (16). Recruitment of circulating leukocytes to vascular endothelium requires multistep adhesive and signaling events involving selectin-mediated attachment and rolling, leukocyte activation, and integrin-mediated firm adhesion and diapedesis that results in the infiltration of inflammatory cells into the vessel wall (17). Most of our current understanding of the functions of Mac-1 is derived from the use of in vitro and in vivo blocking mAbs involving interactions between leukocytes and endothelial cells. Our prior rabbit study used an intact mAb that could have altered the process of restenosis through different mechanisms besides specific inhibition of the function of Mac-1. Although such studies have provided considerable insight into the roles of this integrin, there have been important limitations due to 3 factors. First, complete blocking is difficult, particularly for those integrins with mobilizable intracellular storage pools, such as Mac-1 (18). Second, antibodies binding to the surface of leukocytes can stimulate changes in cellular function and behavior (e.g., through activation of Fc receptors when IgG antibodies are used). Finally, because Mac-1 is closely associated with and influenced by other cell surface receptors, such as the urokinase receptor (19, 20), a mAb may cause steric interference with the associated receptor.
Prior studies with Mac-1–/– mice show a defect in chemoattractant-stimulated neutrophil adhesion to microvascular endothelium, but intact thioglycollate-induced neutrophil accumulation in the peritoneal cavity (13, 15). In contrast, there is little information on the molecular mechanisms that regulate the adhesion and subsequent trafficking of leukocytes to the vessel wall in the absence of the arterial endothelium. Our experiments, which used mice deficient in Mac-1 and did not require the use of mAbs, provide the first in vivo data indicating that Mac-1 is required for transplatelet leukocyte migration and vessel wall accumulation, and thereby influences neointimal thickening. Our data show that in the absence of endothelium, Mac-1 is not required for leukocytes to adhere to an injured artery that is covered with platelets and fibrinogen. Although adhesion is unaffected, deficiency of Mac-1 leads to decreased accumulation of leukocytes in the vessel wall over time. We performed in vitro adhesion and transplatelet chemotaxis experiments using murine platelets and bone marrow–derived neutrophils isolated from wild-type and Mac-1–deficient mice that support the in vivo experimental results. Whereas adhesion to adherent platelets was similar in Mac-1–deficient and wild-type neutrophils, transplatelet migration was reduced by 50% in the absence of Mac-1 (Figure 5b). Based on in vitro observations using mAbs, Diacovo and coworkers have proposed a model of leukocyte recruitment at sites of platelet and fibrin deposition in which Mac-1 is required for neutrophil diapedesis (9). Our study provides the first in vivo data supporting this model. Luminal leukocyte adhesion may require P-selectin, given the report of reduced numbers of luminally adherent leukocytes in injured arteries of P-selectin–deficient mice (21). In our study, leukocytes were sparse 1 day after injury, but were abundant in our model 3 days after injury. The reason for the delay in leukocyte recruitment is unknown, but it may reflect the time course of medial cell necrosis or chemoattractant expression.
Clinical studies have shown that Mac-1 expression is upregulated locally and systemically after PTCA (10–12), and is a marker of clinical restenosis (11). This study, in combination with our previous report using a mAbs to Mac-1 after angioplasty or stent implantation in rabbits (5), indicates that Mac-1–mediated leukocyte recruitment and function both play a pathophysiological role in neointimal thickening. By virtue of binding diverse ligands, including fibrinogen (7, 8), ICAM-1 (22), factor X (23), and C3bi (7), Mac-1 regulates important leukocyte functions, including adhesion, migration, proteolysis, phagocytosis, oxidative burst, and signaling (13, 15, 24, 25). Neutrophils and monocytes may contribute to neointimal thickening simply with their bulk within the intima (4), by generating injurious reactive oxygen intermediates (26), or with elaboration of growth and chemotactic factors (27, 28) or production of enzymes (e.g., matrix metalloproteinases or cathepsin S) capable of degrading extracellular constituents, thereby facilitating cell migration (29, 30).
Murine injury models.
Our study uses a new model of neointimal formation based on the air-drying rat carotid artery method (14). This mouse model shares much in common with widely studied and characterized experimental models of vascular injury, and takes advantage of the genetic diversity of the murine system. It is fundamentally different from previously reported murine models of arterial injury in its ability to reliably achieve both endothelial and medial injury using an endovascular approach that results in progressive intimal thickening over time. Prior murine models of neointimal thickening, including endovascular wire scraping (31), perivascular cuffing (32), ipsilateral carotid artery ligation (33), and electrically induced (34) or Rose Bengal/green light–induced (35) injury, are characterized by variable degrees of intimal and medial thickening, significant mural thrombus formation, or perivascular manipulation that may influence vessel wall inflammation.
Limitations of the study.
We have not directly focused on the adventitia as a source of infiltrating leukocytes. Although it may appear that there are more adventitial leukocytes in Mac-1–/– mice than in wild- type mice 3 days after injury (Figure 4), we have not observed overall differences in adventitial leukocyte recruitment that depend on the presence or absence of Mac-1. It is difficult to interpret the significance of adventitial leukocytes in a model that requires perivascular surgical manipulation. However, leukocytes may infiltrate the vessel wall from both the lumen and the adventitia. Our data suggest that Mac-1 is required for the medial accumulation of leukocytes at sites of platelet and fibrin deposition. Indeed, absence of Mac-1 is associated with a defect in transplatelet neutrophil migration induced by KCs (Figure 5). It is possible that adventitial leukocytes may parallel our observations of increased numbers of luminal leukocytes in Mac-1–/– mice, reflecting a general defect in transmigration.
Although our data show that antibody blockade of Mac-1 (5) or absence of Mac-1 each reduce neointimal thickening after experimental angioplasty or endovascular stent implantation, the relevance of these observations to clinical angioplasty is unknown.
Likewise, in this study, intimal and medial thickening were accompanied by progressive vessel enlargement that was greater in Mac-1–/– mice than in Mac-1+/+ mice, resulting in significantly greater lumen area in the Mac-1–/– mice (Figure 2c). This finding with the β2-integrin Mac-1 stands in contrast to negative remodeling or vessel shrinkage observed with blockade of β3-integrins expressed on SMCs and platelets (36). However, this differential effect on vessel wall remodeling is of uncertain clinical significance given the high rates of endovascular stenting in interventional cardiology practice.
Conclusion.
In summary, our study identifies Mac-1 as a molecular determinant of neointimal thickening after experimental arterial injury, and provides strong evidence for a direct effect of leukocytes, SMCs, and platelets in vascular repair. Mac-1’s participation in and promotion of vascular injury and repair are consistent with its recently reported role in glomerular injury in a murine model of glomerulonephritis (37). Inflammation is an orchestrated cellular response mediated by chemokines, cytokines, cell adhesion molecules, extracellular matrix proteins, and proteases. Further studies of vascular repair after injury in mice that are genetically devoid of these components, perhaps coupled with murine models of atherosclerosis (i.e., apoE- and LDL receptor–deficient mice) using a double knockout approach, will enable us to mechanistically explore vascular repair after mechanical injury in the setting of an atherosclerotic background, and to identify new targets for preventing clinical restenosis.
Acknowledgments
This work was supported in part by grants from the American Heart Association National Center (95004400 to C. Rogers and an Established Investigator Award to C.M. Ballantyne), the National Institutes of Health (HL-03104 to C. Rogers, GM/HL-49039 to E.R. Edelman, HL-42550 to C.M. Ballantyne, and HL-57506 to D.I. Simon), the Burroughs Wellcome Fund for Experimental Therapeutics, Durham, North Carolina, USA (E.R. Edelman), and the Whitaker Foundation, Rosslyn, Virginia, USA (E.R. Edelman). The authors gratefully acknowledge the expert technical assistance of Cindy Richmond.
Footnotes
This work was presented in part at the 71st Scientific Sessions of the American Heart Association, Dallas, Texas, USA, in November, 1998.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, et al. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 3.Rogers C, Welt FG, Karnovsky MJ, Edelman ER. Monocyte recruitment and neointimal hyperplasia in rabbits: coupled inhibitory effects of heparin. Arterioscler Thromb Vasc Biol. 1996;16:1312–1318. doi: 10.1161/01.atv.16.10.1312. [DOI] [PubMed] [Google Scholar]
- 4.Moreno PR, et al. Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina. Circulation. 1996;94:3098–3102. doi: 10.1161/01.cir.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 5.Rogers C, Edelman ER, Simon DI. A monoclonal antibody to the β2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci USA. 1998;95:10134–10139. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barron MK, Lake RS, Buda AJ, Tenaglia AN. Intimal hyperplasia after ballon injury is attenuated by blocking selectins. Circulation. 1997;96:3587–3592. doi: 10.1161/01.cir.96.10.3587. [DOI] [PubMed] [Google Scholar]
- 7.Wright SD, et al. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci USA. 1988;85:7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altieri DC, Bader R, Mannucci PM, Edgington TS. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J Cell Biol. 1988;107:1893–1900. doi: 10.1083/jcb.107.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 10.Inoue T, et al. Expression of polymorphonuclear leukocyte adhesion molecules and its clinical significance in patients treated with percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1996;28:1127–1133. doi: 10.1016/S0735-1097(96)00308-7. [DOI] [PubMed] [Google Scholar]
- 11.Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol. 1996;28:345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 12.Neumann FJ, Ott I, Gawaz M, Puchner G, Schomig A. Neutrophil and platelet activation at balloon-injured coronary artery plaque in patients undergoing angioplasty. J Am Coll Cardiol. 1996;27:819–824. doi: 10.1016/0735-1097(95)00563-3. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, et al. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1–deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman JA, Ryan GB, Karnovsky MJ. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975;32:339–351. [PubMed] [Google Scholar]
- 15.Coxon A, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 16.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 17.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: a multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 18.Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987;80:535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon DI, et al. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- 20.Sitrin RG, Todd RF, III, Albrecht E, Gyetko MR. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Invest. 1996;97:1942–1951. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Hoover JL, Simmons CA, Lindner V, Shebuski RJ. Remodeling and neointimal formation in the carotid artery of normal and P-selectin-deficient mice. Circulation. 1997;96:4333–4342. doi: 10.1161/01.cir.96.12.4333. [DOI] [PubMed] [Google Scholar]
- 22.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 23.Altieri DC, Morrissey JH, Edgington TS. Adhesive receptor Mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation cascade. Proc Natl Acad Sci USA. 1988;85:7426–7466. doi: 10.1073/pnas.85.20.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 25.Plow EF, Zhang L. A MAC-1 attack: integrin functions directly challenged in knockout mice. J Clin Invest. 1997;99:1145–1146. doi: 10.1172/JCI119267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peri G, Chiaffarino F, Bernasconi S, Padura IM, Mantovani A. Cytotoxicity of activated monocytes on endothelial cells. J Immunol. 1990;144:1444–1448. [PubMed] [Google Scholar]
- 27.Assoian RK, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glenn KC, Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981;25:603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- 29.Garbisa S, et al. Transient expression of type IV collagenolytic metalloproteinase produced by human mononuclear phagocytes. J Biol Chem. 1986;261:2369–2375. [PubMed] [Google Scholar]
- 30.Sukhova GK, Shi G-P, Simon DI, Chapman HA, Libby P. Expression of the elastinolytic cathepsins S and K in human atheroma and regulation of their production in smoth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 32.Moroi M, et al. Interaction of genetic deficiency of endotheial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet P, et al. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150:761–776. [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi S, Umemura K, Kondo K, Saniabadi AR, Nakashima M. Photochemically induced endothelial injury in the mouse as a screening model for inhibitors of vascular intimal thickening. Arterioscler Thromb Vasc Biol. 1998;18:1069–1078. doi: 10.1161/01.atv.18.7.1069. [DOI] [PubMed] [Google Scholar]
- 36.Deitch JS, et al. Effects of β3-integrin blockade (c7E3) on the response to angioplasty and intraarterial stenting in atherosclerotic nonhuman primates. Arterioscler Thromb Vasc Biol. 1998;18:1730–1737. doi: 10.1161/01.atv.18.11.1730. [DOI] [PubMed] [Google Scholar]
- 37.Tang T, et al. A role for Mac-1 (CD11b/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 defiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 1997;186:1853–1963. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]