Abstract
Hypertension is a major risk factor for cardiovascular disease and death. The “silent” rise of blood pressure that occurs over time is largely asymptomatic. However, its impact is deafening—causing and exacerbating cardiovascular disease, end-organ damage, and death. The present article addresses recent observations from human and animal studies that provide new insights into how the circadian clock regulates blood pressure, contributes to hypertension, and ultimately evolves vascular disease. Further, the molecular components of the circadian clock and their relationship with locomotor activity, metabolic control, fluid balance, and vascular resistance are discussed with an emphasis on how these novel, circadian clock-controlled mechanisms contribute to hypertension.
Keywords: blood pressure, vascular, obesity, Bmal1, diabetes
hypertension is a major risk factor for cardiovascular disease and death. The chronic elevation of blood pressure is a silent disorder in that its progression occurs largely asymptomatically. However, its impact is deafening—causing cardiovascular disease, end-organ damage, and death (44). This seemingly simple relationship between high blood pressure and cardiovascular disease that is heavily influenced by our behavior and what we eat (4, 9, 112) is also conditioned by the time of day. Indeed, circadian rhythm is a significant input into the regulation of blood pressure.
With each day, the human body experiences a reproducible rhythm in behavior, waking in the morning (or thereabouts) and sleeping in the evening—a circadian rhythm. This is a consequence of the brain “resting” and “waking” as evidenced by changes in electrical activity (26, 116). In the cardiovascular system, blood pressure exhibits a rhythm as well, as blood pressure rises in the daytime and falls at nighttime. Moreover, in human hypertension, there are significant deviations to this rhythm in blood pressure. Recent evidence suggests that the genetic components of the circadian clock exert a key and fundamental role in the regulation of blood pressure.
The circadian clock, the molecular basis of circadian rhythms, is an interlocking set of autoregulatory loops, regulated through transcriptional, translational, and posttranslational processes, to generate a 24-h period of oscillation (133). The core of this signaling pathway is a negative-feedback loop comprised of a positive limb of transcription factors (Bmal1, Clock, and Npas2) and a negative limb of regulatory proteins (Per1, Per2, Per3, Cry1, Cry2). Heterodimers between either Bmal1-Clock or Bmal1-Npas2 drive transcription of Period (Per) and Cryptochrome (Cry), which then feed back in a manner further regulated posttranslationally to inhibit the positive limb, resulting in rhythmic oscillation of all components [except for Clock which may oscillate in a tissue-specific manner (111, 130)]. In addition to these core clock genes, it is estimated that 5–10% of the transcriptome is under the control of the circadian clock (94) and rhythmic changes in the expression of these genes help to shape the function of organ systems. Such pervasive control is at least in part possible because the hands of the clock touch specialized promoters within all tissues, organs, and cells. Indeed, the same genetic components that comprise the central clock in the suprachiasmatic nucleus (SCN) are also found in peripheral clocks, including blood vessels (21, 111), liver (155), skeletal muscle (91, 94, 155), heart (127, 151), and kidneys (114). The present article addresses recent observations from human and animal studies that shed insight as to how the circadian clock regulates blood pressure, contributes to hypertension, and ultimately evolves to vascular disease.
RHYTHMS IN BLOOD PRESSURE AND CENTRAL ACTING SIGNALS
It is well established that blood pressure exhibits a circadian variation in mammals, including humans (93) and mice (83). In the absence of disease, there is a nighttime dip in blood pressure, a rise in the morning hours, followed again by a subsequent drop, in a cycle that occurs every 24 h. These cycles are inverted in nocturnal animals such as mice. With regard to the central clock, locomotor activity follows a circadian rhythm that originates in the SCN of the brain. The SCN is considered to be the master clock to all peripheral rhythms, and as such is also an important influence in the regulation of the blood pressure rhythm. Indeed, ablation of the SCN results in the loss of circadian variation of blood pressure (148). Behavioral aberrations can also significantly affect the circadian variation in blood pressure as has been recently shown in β1/β2 adrenergic receptor knockout (KO) mice (68), and also in humans with particular relevance to the morning surge (66, 78). As such, there are numerous candidate signals that exhibit a dual role in modulation of activity and blood pressure rhythms. At the core of blood pressure regulation is the brain medulla, which contains the vasomotor center that is the source of sympathetic and parasympathetic drive to the heart, kidneys, and vasculature. Recent studies have shown that in models where the circadian rhythm in blood pressure and activity is disrupted, catecholamine levels are perturbed (17, 87, 145). In hypertensive rats, sympatholytic drugs are also more effective at suppressing circadian variations in blood pressure than nonsympatholytic vasodilators (57). Similarly, central administration of cholinergic agonists can induce phase shifts in circadian activity (29), while a high-fat diet-induced ablation of rhythmic blood pressure in canines coincides with a reduction in type 2 muscarinic receptors in the heart (105). Although M2-receptor-deficient mice have no significant difference in single-time point assessments of blood pressure (76), studies that continuously assess blood pressure and its circadian variation are lacking.
Additional central clock-modifying peptides have been identified that may also transmit circadian cues to the vasculature. Vasoactive intestinal peptide (VIP) is known to condition central circadian clock function (1) and modulate vascular function (53), and its expression is reduced in hypertensive patients (37). Dysregulation of VIP even at the level of the central clock could impact blood pressure through effects on organs/tissues that are intrinsically important in blood pressure regulation, such as the vasculature (Fig. 1). Indeed, the receptor for VIP, VPAC2, which is also important in circadian function (5, 46), is located in the vascular wall (39). Another potential peptide mediator that is important in circadian rhythm is prokineticin (14, 82). Overexpression of the receptor for prokineticin-2 in the heart induces hypertrophy and capillary vessel leakage (138); however, whether it has a role in blood pressure regulation is unknown. Aside from peptides, a hormone shown to entrain the SCN is melatonin (85, 140). In patients with essential hypertension and impaired circadian variation in blood pressure, melatonin supplementation can improve the lowering of nocturnal blood pressure (117). However, the mechanism and targets of circadian blood pressure regulation, i.e., central or peripheral, remain obscure. Indeed melatonin receptors are found not just in the brain, but are also expressed in the cardiovascular system (27, 31, 143). Clearly, more studies are necessary to understand the contribution of central regulation of circadian variations in blood pressure.
Fig. 1.
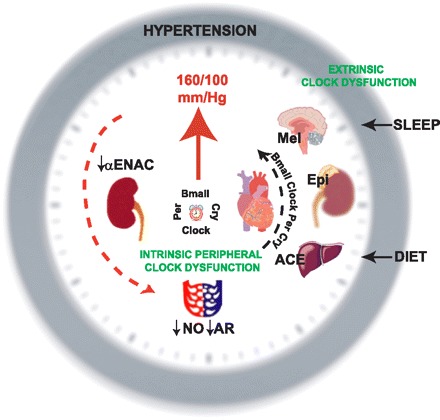
Counter “clockwise” productivity to hypertension. Behavioral disorders such as sleep apnea, shift work, or inadequate sleep impair central clock function, which may affect the release of hormones such as melatonin (Mel), which then impinge on blood pressure-regulating organs (heart, kidney, vasculature). Similarly, diet or other disturbances that originate from aberrations peripheral to the central nervous system may modify circadian clock function (depicted as distortion in the components Bmal1, Per, Cry, and Clock along black arrow) in peripheral tissues that are extrinsic to the organs/tissues important in the pressor response. These extrinsic peripheral tissues such as liver or the adrenal gland may affect release of pressor-modifying mediators [liver/angiotensin-converting enzyme; adrenal/epinephrine (Epi)], which then impinge on the pressure-regulating tissues. There may also be direct peripheral clock dysfunction in tissues such as heart, kidney, and vasculature that are intrinsically critical in blood pressure regulation by modulating the expression of ion channels (αENAC), paracrine signals (nitric oxide/NO), and receptors (adrenergic receptor/AR). An additional level of complexity is that hypertension itself alters circadian clock function in peripheral tissues (red dashed arrow) to induce a feedforward spiral of dysfunction.
BLOOD PRESSURE RHYTHMS: MORE THAN RUNNING AROUND THE CENTRAL CLOCK
The impetus underlying rhythmic changes in blood pressure is more than a consequence of changes in activity/locomotor/behavioral rhythms. In fact, there is considerable and growing evidence that rhythmic blood pressure may also be guided by circadian actions in the periphery. When studied over a full 24-h period, the relationship between locomotor and blood pressure rhythm varies during the course of the day, and this variation is not circadian (58). Moreover, in humans submitted to experimental conditions that force behavioral asynchrony (i.e., no locomotor rhythm), circadian rhythm in cardiovascular function persists (54). Thus there is significant evidence in humans that blood pressure rhythms can be uncoupled (be independent) from endogenous circadian rhythms of activity. These findings are analogous to the ability of food restriction in rodents to uncouple the peripheral (liver) clock from the central (SCN) (20, 125). Further evidence of uncoupling between blood pressure and activity is evident in transgenic, hypertensive rats that possess an extra copy of the renin gene [TGR(mREN2)27]. TGR(mREN2)27 rats develop an inverse blood pressure rhythm (inverse/reverse dipper), 6 wk after birth, transitioning from a blood pressure that peaks at night to a blood pressure that peaks in the day. Yet motor activity and rhythm in the TGR(mREN2)27 rats remain unperturbed (147). Thus regulation of rhythmic blood pressure is more than a surrogate of rhythmic locomotor activity driven by the SCN and may also be influenced by circadian clocks peripheral to the central nervous system. One emerging theme is that the genetic components that control circadian rhythm—the circadian clock—are critical in the control of circadian rhythm in blood pressure.
THE CIRCADIAN CLOCK IN BLOOD PRESSURE REGULATION
In mice, a role for core components of the circadian clock (Bmal1, Clock, Npas2, Per, and Cry) has now been established in the regulation of blood pressure (Table 1). In conditions of constant darkness, Cry1/Cry2 deficient mice (double knockout/DKO) are hypertensive in the daytime (a time when blood pressure falls in rodents) relative to wild-type mice (WT). This lack of a daytime or rest period dip abolishes the circadian variation in blood pressure, which rises in WT mice at night during the activity period but remains unchanged in the Cry deficient mice (90). Thus Cry deficient mice are hypertensive during the rest period and on average normotensive in the activity period. Targeted deletion of Bmal1 in mice (Bmal1-KO) also abolishes the circadian variation in blood pressure. However, this is due to a hypotensive phenotype during the activity period (17) that is distinct from the hypertensive period rest period phenotype observed in the Cry deficient mice. In contrast to global Bmal1 disruption (i.e., Bmal1-KO mice), the overall blood pressure rhythm remains intact in mice with endothelial cell (EC)-specific deletion of Bmal1 (EC-Bmal1-KO) (146). EC-Bmal1-KO mice do exhibit a reduction in blood pressure at discrete times within the activity phase, albeit the increment of hypotension is more modest than that observed in the global KOs and not present in the resting phase. Future studies are needed to address the role of Bmal1 within the more predominant vascular smooth muscle cell layer in the regulation of blood pressure rhythm. In addition, as disruption of endothelial cell clock function appears to leave smooth muscle cell clock function intact (146) (although if the converse is true is not known), more definitive answers as to the role of the vascular clock may emanate from approaches such as vascular cell-specific rescue in Bmal1-KO mice, comparable to studies by McDearmon et al. (92) where brain or peripheral tissue-specific rescue was implemented in mice with global Bmal1 disruption.
Table 1.
The circadian rhythm in blood pressure in circadian clock mutant mice
| Mouse Mutant | Light Cycle | Mean Arterial Blood Pressure, mmHg |
|||
|---|---|---|---|---|---|
| Mutant |
WT |
||||
| Night | Day | Night | Day | ||
| Cry1-Cry2-DKO (90) | DD | 90 | 92 | 86 | 80 |
| Bmal1-KO (17) | LD | 97 | 95 | 115 | 105 |
| Npas2-mut (17) | LD (DD) | 104 | 92 | 110 | 98 |
| Clock mut (17) | LD | ∼102 | ∼96 | 110 | 98 |
| Clock mut (Jcl/Icr) (120) | LD | ∼130 | ∼115 | ∼130 | ∼105 |
| Per2 mut (142) | LD | 105.9±1.9 | 116.5±2.1 | ||
Four distinct studies have examined blood pressure in mice with mutated circadian clocks (citations shown in parentheses). Shown are the average mean arterial pressures at night and day. Studies in Per2 mutant (mut) mice are single time point studies, but the hypotensive phenotype that was observed in all circadian clock mutant mice except for the Cry1-Cry2 double knockout (DKO) mice was still observed. Values for Clock mut mice were approximated from bar graphs in accompanying studies. WT, wild type; DD, constant darkness condition; LD, light-dark condition; KO, knockout.
Mice with mutation of Npas2 retain blood pressure rhythm, albeit the mice are also hypotensive. Clock mutant mice exhibit only a subtle dampening of blood pressure when conditioned in light-dark (LD) on either the C57Bl/6(17) or Jcl/Icr background (120), which may reflect the influence of the light cycle conditioning in these mice and the functional redundancy of Bmal1 to bind either Clock or NPAS2 (22, 23). Thus Bmal1-KO, EC-Bmal1-KO, Npas2 mutant, Clock mutant, and Per2 mutant mice exhibit lower blood pressures than corresponding WT mice, while Cry deficient and Clockmut (Jcl/Icr) mice exhibit higher blood pressures than their WT counterparts (Table 1). Aside from genetic strain, which may condition phenotype as has been observed with metabolic phenotypes in circadian mutant mice (102), another nuance to be considered is that most of the aforementioned blood pressure studies [aside from those in Cry deficient mice and Npas2 mutant mice] were conducted under standard LD conditions. Indeed, the locomotor impairment in circadian clock is variable depending on light cycle conditions, except for Bmal1-KO mice which are completely arrhythmic in either standard LD conditions or free-running conditions of constant darkness (DD). Mice with mutation in the other core circadian clock components only exhibit a difference in period, with the locomotor clock running faster or slower in LD, but the locomotor phenotype worsens in DD to arrhythmicity. Thus additional studies to examine blood pressure rhythm in LD versus DD may prove informative to reveal if blood pressure rhythm worsens in a manner comparable to locomotor function in free-running conditions or if in fact the blood pressure rhythm may be perturbed independent of light cycle-induced deterioration of behavioral rhythms. These studies also highlight the difficulty of identifying clock-dependent phenotypes and emphasize the need for multiple genetic models to counter the many hands of circadian clock.
In addition to these core circadian clock components, there are other mechanisms that may modulate the circadian clock to influence the circadian variation in blood pressure. Peroxisome proliferator-activated receptor-gamma (PPARγ) is one such mechanism. Mice with targeted deletion of PPARγ in the endothelium (EC-PPARγ-KO) exhibit a striking phenotypic resemblance to EC-Bmal1-KO mice (145, 146). EC-PPARγ-KO mice have lower blood pressures at night, like EC-Bmal1-KO mice, and normal blood pressures during the day, with rhythm remaining largely intact. In contrast, smooth muscle cell disrupted PPARγ-KO mice (SMC-PPARγ-KO mice) actually have higher blood pressure in the daytime and only trend to a lower blood pressure at night. Importantly, locomotor rhythm remains intact in EC and SMC-PPARγ mice (145), further evidence that circadian inputs into blood pressure regulation are not exclusively emanating from the central clock or activity rhythms. The ability of PPARγ to modulate blood pressure may arise from its ability to transactivate Bmal1, and as such corresponding loss of PPARγ in the aorta of both EC and SMC-PPARγ-KO mice leads to reduced expression of Bmal1, Npas2, Cry1, Cry2, and Per2.
That EC-Bmal1-KO and EC-PPARγ-KO mice retain blood pressure rhythm, albeit blunted, does not necessarily diminish a role for the endothelial clock in the regulation of blood pressure rhythm. The apparent disparity (i.e., rhythmic blood pressure in face of aberrant circadian clock expression) may, in part, stem from the known robustness of circadian rhythm to perturbation to individual clock components, which may in part occur through redundancy, networking (6), and also intercellular coupling (84) of the circadian clock mechanism. It should be added that evidence for intercellular coupling as a mechanism to mediate robustness of the circadian clock appears to be a phenomenon unique to SCN neurons and not apparent in fibroblastic cell populations (84). Indeed, vascular cells are “coupled” and communicate through gap junctions, paracrine mediators, and electrical/voltage impulses. However, whether these communications intimate robustness of the vascular circadian clock remains unknown. Moreover, additional studies remain necessary to assess circadian gene expression in the more abundant resistance vessels (as opposed to conduit vessels such as the aorta) that better reflect the basis of hypertension. The divergent blood pressure rhythms in EC vs. SMC PPARγ-disrupted mice further highlight the complexity involved in blood pressure regulation and respective involvement of the circadian clock.
METABOLIC CONTROL AND BLOOD PRESSURE
There is an intimate relationship between the circadian clock, metabolism, and obesity, one that is now known to also influence blood pressure. Indeed, obesity and metabolic dysfunction are major risk factors for the impaired control of blood pressure (24, 109). In rodent models of type II diabetes, mean blood pressure is mildly elevated (12, 103). While this in itself is a risk factor for cardiovascular diseases, the elevation in blood pressure is accompanied by changes in the circadian variation of blood pressure as demonstrated in experimental models of type II diabetes (db/db mice). Indeed, several independent observations have now shown that the daytime fall in blood pressure in mice is significantly blunted in db/db mice, suggesting that the mechanisms orchestrating the circadian timing of blood pressure are impaired (36, 121, 129). In humans, type II diabetes also dramatically increases the frequency of deficits in circadian blood pressure regulation and blunted dipping is observed in up to 75% of patients (18). This loss or reversal of the nocturnal dip in blood pressure is of great import and is associated with increased cardiovascular mortality (51, 55, 128). While type II diabetes comprises the vast majority of cases of diabetes, individuals with type I diabetes also exhibit blunted circadian regulation of blood pressure, and this correlates with declining renal function (86). Interestingly, experimental induction of type I diabetes in rats by streptozotocin alters the phase of oscillation of Bmal1, Clock, Cry1, Cry2, Per1, Per2, and Per3 in heart tissue (152). Clearly, more studies are necessary to understand the target organs and mechanisms by which diabetes compromises the circadian regulation of blood pressure.
Studies in animal models document that in addition to modifying blood pressure rhythms, type II diabetes also affects the circadian regulation of heart rate (36, 129) and locomotor activity (129). Other aspects of blood pressure control are also altered in animals with type II diabetes, including renal function (16), circulating hormones (33, 124), autonomic reflexes (119), and vascular (7, 25, 42, 59, 61) and sympathetic tone (36). However, the links between these variables and the impaired circadian regulation of blood pressure are also poorly understood. Another unknown is the relative contribution of the central clock versus local or peripheral clocks. Locomotor activity is frequently used as a surrogate for the activity of the central clock, and lesion of the SCN disrupts the rhythms of feeding, activity, and blood pressure (148). Indeed, mice with type II diabetes exhibit a gross reduction in locomotor activity that accompanies the impaired daily variations in blood pressure. However, the circadian rhythm of locomotor activity remains intact, suggesting that it is the morbidly obese phenotype that is the primary constraint on activity rather than an alteration of the central clock. These findings are supported by molecular evidence that expression of circadian clock genes is unaltered in the SCN of mice with type II diabetes (74). In contrast, the oscillation of circadian genes in the periphery, in isolated blood vessels, and the liver is impaired (74, 129). These results support the concept that type II diabetes promotes asynchrony of the peripheral and central circadian clocks and also that this asynchrony may contribute significantly to cardiovascular disease.
The aspects of the metabolic dysfunction that contribute to the asynchrony of circadian genes are poorly understood. Food intake rhythms are impaired in db/db mice (43), and the restricted timing of food intake has been shown to entrain peripheral clocks (20, 126) and normalize circadian clock expression in the liver (74). However, it is not yet known whether restricted feeding can improve the circadian timing of blood pressure in type II diabetes. Other approaches such as PPARγ agonists (2) and ACE inhibitors (19) have shown efficacy.
The link between type II diabetes and altered circadian timing is further compounded by the ability of the circadian clock itself to influence metabolism and ultimately obesity and diabetes. Clock mutant mice exhibit altered feeding patterns leading to obesity and insulin resistance (137). Furthermore, in both humans and rodents, polymorphisms of the Bmal1 gene are associated with increased incidence of type II diabetes and hypertension (150). While it is clear that the circadian clock participates in these processes, it should also be emphasized that these effects are subtle and require the complex interplay of other genes that can be modified by genetic background (30) and also that the individual clock genes may have different effects on blood pressure.
THE RHYTHM IN HUMAN HYPERTENSION AND CHRONOTHERAPY
The influence of circadian rhythm that occurs during normotension also presides during conditions of hypertension. In hypertension, there is a rise in blood pressure that is silent and chronic, inducing pathological processes of vascular remodeling, inflammation, and/or atherosclerosis to ultimately compromise end-organ perfusion. The rhythm and pattern of blood pressure oscillation can frequently be abnormal during hypertension, with patients presenting as nondippers (101), extreme dippers (62), and reverse dippers (63). Importantly perturbation to the amplitude and rhythm of blood pressure effectuates worsened cardiovascular and clinical outcome (134), a point whose importance is also highlighted in the 2003 JNC7 report (15). Moreover, the time at which the peak blood pressure occurs (acrophase), although less well characterized, may also have a significant impact on the secondary endpoints of disease. These variations in blood pressure are much more difficult to detect clinically and add stealth to an already silent disease.
The clinical relevance of the blood pressure rhythm was underscored in the Heart Outcomes Prevention Evaluation Study Investigators (HOPE trial), where the angiotensin-converting enzyme (ACE) inhibitor ramipril had significant effects on the reduction in the rates of death, myocardial infarction, and stroke (153). Surprisingly, the effect on lowering blood pressure during office visits (daytime) was not significant, misleadingly suggesting that the ACE inhibitor impacted cardiovascular outcomes independent of blood pressure. A subsequent substudy revealed ramipril did in fact lower blood pressure, but the effect was most prominent at night (131). Three important messages emerge from these studies. First, restoring the circadian variation and nighttime dip via antihypertensive therapy is important to cardiovascular outcome. Second, in addition to the silence of hypertension itself, there is the compounding factor of silence in circadian dysfunction with regard to blood pressure, especially if occurring at night. Third, hypertension may be more effectively treated by reducing blood pressure at a particular time of day.
The time at which antihypertensives are actually administered, chronotherapy, also impacts blood pressure control (79, 80). For example, in hypertensive patients with chronic renal failure, the calcium channel blocker (CCB) isradipine exhibits a greater blood pressure-lowering effect on nighttime blood pressure when given at 8 PM when compared with an 8 AM administration and under these conditions effectively restores the circadian blood pressure rhythm (107). Similarly, the administration of an α-adrenergic antagonist to patients with primary hypertension exhibits more pronounced effects on blood pressure control when administered before sleeping (47). Indeed, the restoration of circadian variability in blood pressure appears to be an important factor in the control of cardiovascular disease. Future studies are needed to determine if these aberrations in blood pressure rhythm stem from tissue-specific circadian clock dysfunctions, where experimental animal models of hypertension will be of utmost utility.
RAS IN EXPERIMENTAL HYPERTENSION
Circadian influences have also been explored in animal models of renin-angiotensin system (RAS)-induced hypertension that mimic an important manifestation of human hypertension. In addition to the TGR(mREN2)27 rat, which exhibits profound impairments in blood pressure rhythm as discussed earlier, the spontaneously hypertensive rat (SHR) [which has a mutation in a genetic locus in close proximity to the ACE gene (50, 56)] exhibits a blood pressure rhythm whose acrophase is shifted further toward the resting period (123). With regard to locomotor rhythm, SHRs also exhibit aberrant circadian function. Activity period in SHRs begins 1.5 h earlier than in WKY control rats, and their response to light cycle shifts is altered, perhaps stemming from a defect in vasoactive intestinal peptide (106) whose receptor VPAC is important in circadian locomotor function (46). Perhaps the most striking evidence in the SHR to implicate the circadian clock in the pathogenesis of hypertension is the identification of a single nucleotide polymorphism in the essential circadian clock component Bmal1 (150). Indeed, SHRs exhibit enhanced expression of Clock, Bmal1, and Per2 in heart tissue, albeit their rhythms are retained (98). Furthermore, SHRs exhibit enhanced amplitudes in the oscillation of renin, angiotensinogen, ACE, and angiotensin type 1a (AT1a) and type 2 (AT2) receptors in the heart (97). However, there are no data to examine either circadian clock or RAS oscillation in the vasculature or kidneys of SHRs, which are also relevant tissues in the control of blood pressure. Indeed, ANG II does have significant effects on the circadian clock in vascular cells. In cultured vascular smooth muscle cells, ANG II has potent effects on the circadian clock to induce synchronous circadian oscillation of Per2 and Bmal1 expression (99).
In contrast to the more subtle effects observed in the RAS-defective SHRs, chronic ANG II infusion by osmotic minipump in rats has more robust effects on circadian blood pressure (115). ANG II abolishes the circadian rhythm of arterial pressure in a sex-independent manner and further causes a modest reverse dipper phenotype in female rats, reminiscent of the response of TGR(mREN2)27 rats. With regard to effects on locomotor rhythm, although exogenous administration of ANG II directly to SCN brain slices does stimulate neuronal depolarization (10), behavioral rhythm remains intact in ANG II-infused mice in the face of the profound effects on blood pressure rhythm (115). These results again reinforce the concept that blood pressure regulation is more complex and cannot be explained solely by changes in activity.
Mice mutated in discrete components of the RAS signaling pathway largely retain the circadian variation in blood pressure, but impairments emerge during conditions of experimental hypertension. ACE-KO mice exhibit an intact blood pressure rhythm, but in response to a high salt diet, the amplitude of the rhythm is enhanced in ACE heterozygotes and blunted in ACE-KOs relative to WT mice (11). In AT1a receptor-KO mice, which also exhibit intact rhythmic blood pressure, fructose feeding also increased the difference in blood pressure between night- and daytime (32). Moreover, 5 days of high-salt diet abolishes the normal light-dark blood pressure rhythm in AT1a-KO mice (13). There is additional evidence to suggest that brain AT2 receptor may play a role in regulation of the circadian rhythm in blood pressure, whereby adenoviral transduction of the AT2 receptor in the rostral ventral lateral medulla of rats abolishes the circadian variation in blood pressure by blunting the nighttime spike (35). Thus an important concept is that the variation in blood pressure while appearing normal in the basal state may be disturbed by diet and other peripheral challenges aside from light cycle shifts. Additionally, hypertensive disease may worsen clock function, which may feed forward to further impair the pressor response.
THE CIRCADIAN CLOCK AND FLUID BALANCE
The kidney is the major organ for long-term blood pressure regulation and is also under circadian regulation. In nondipping individuals, the normal circadian pattern of sodium excretion is blunted in concert with blood pressure (28). Sodium restriction can convert nondippers into dippers, and alternatively sodium loading attenuates dipping, suggesting a vital role for the kidney and fluid volume in this process. (34, 49, 139). Thiazide diuretics which promote natriuresis can also restore the nighttime reduction in blood pressure (34). Further evidence for a role of the kidney comes from studies showing that loss of renal function following nephrectomy correlates with impaired circadian variation in blood pressure (38). Recent studies have begun to uncover putative molecular targets of the circadian clock in the kidney that account for the rhythmic changes in blood pressure. The Na+/H+ exchanger (NHE3) appears to be a bona fide target of the circadian clock as its expression in the kidney oscillates with a circadian rhythm and its promoter contains functional E-boxes that participate in the transactivation by Bmal1 and Clock (113). However, the loss of NHE3 in mice does not appear to affect the circadian regulation of blood pressure (100). Similarly, loss of the Na-2Cl-K cotransporter does not affect blood pressure rhythm (67). Interestingly, deficiency of carbonic anhydrase II in mice, which is important in sodium and bicarbonate reabsorption in the kidney, alters the circadian period of locomotor rhythm (64).
Perhaps most compelling are recent observations that describe a reciprocal relationship between the circadian clock component Per1 and sodium balance (41). In these studies, aldosterone, which has been shown to oscillate in plasma (120), stimulated Per1 expression in the kidney medulla in vivo and in vitro, further corroborated by reporter assay studies of transcriptional regulation of the Per1 promoter. In these studies, the renal epithelial sodium channel αENaC has emerged as a novel circadian target. Per1 regulated the expression of αENaC while its expression was altered in mice lacking functional Period genes and by Per1 knockdown. These studies further demonstrated that urinary sodium excretion was increased in Per-deficient mice. Interestingly, these observations are consistent with observations in Clock mutant mice, which have decreased water intake (120). These results suggest that the circadian clock may be important in control of sodium balance, while also acting as a sensor to sodium levels via aldosterone.
In addition, circadian changes in renal function may also be dependent on genes extrinsic to the kidney (i.e., liver), and indeed the expression of a large number of genes that affect renal function are known to oscillate in a circadian pattern including renin/angiotensin, kinins, AVP, and uroguanylin to name but a few (40, 52, 73, 118, 135). All of these factors are further influenced by age and salt load (69).
THE CIRCADIAN CLOCK AND PERIPHERAL VASCULAR RESISTANCE
Increased peripheral vascular resistance is a trademark and a significant target of therapy in hypertension. Indeed, vascular tone is known to exhibit a circadian variation (104). Catecholamines have largely been thought to in fact be responsible for the circadian variation in blood pressure, in part through effects to increase vascular resistance and cardiac output. Recent data in mice with disrupted or disturbed circadian clocks further support the role of catecholamines in blood pressure rhythm. Circadian clock deficient mice (Bmal1-KO and Npas2 mutant) (17) and EC-PPARγ-KO mice (which have reduced Bmal1) (145) have been demonstrated to have substantially reduced levels of norepinephrine and epinephrine in plasma at both night- and daytime, consistent with the hypotensive phenotype of the mice. Mice with disruption of chromogranin A, which produces the catecholamine inhibitory fragment catestatin (88), have complete impairment in circadian blood pressure rhythm and reduction in adrenal catecholamine levels (87). Independent observations suggest that these effects may not stem from the β-adrenergic receptor per se, as β1/β2-adrenergic-KO mice exhibit a normal blood pressure rhythm (68). That said, mice deficient in dopamine β-hydroxylase, the rate-limiting enzyme in catecholamine production, do have an impaired blood pressure rhythm (132), further evidence for epinephrine and norepinephrine in the circadian variation in blood pressure. Thus it may be that catecholamines mediate circadian changes in blood pressure through alpha-1 adrenergic receptors to control vascular resistance as opposed to control of cardiac contractility through the β-adrenergic receptors. This is supported by observations demonstrating that the pressor response to an α1-adrenergic receptor agonist is suppressed in Cry-deficient mice (90), alterations in the expression of α-adrenergic receptors, and our own observations in isolated aortic rings of Bmal1-KO mice (Anea CB and Rudic RD, unpublished observations). What is lacking, however, are studies that examine circadian clock function and signaling in resistance arterioles of the microvasculature, which are more relevant to blood pressure regulation.
Nitric oxide (NO) has also been implicated as a control mechanism for the circadian variation in blood pressure. NO and the ability of the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME) to increase blood pressure vary significantly with time. l-NAME also blunted the diurnal variation in blood pressure (149) and along with other NOS inhibitors was shown to modulate the function of the SCN (72). Additionally, the concentration of NO metabolites in the plasma also exhibits circadian variation (89). There are three isoforms of NOS, neuronal (nNOS), inducible (iNOS) and endothelial (eNOS), that are responsible for the generation of NO in higher mammals. Indeed, recent data suggest that eNOS signaling and endothelial function is impaired in mice with dysfunctional circadian clocks (3, 110, 144). eNOS expression is not modified in these mice, but there is evidence that posttranslational mechanisms regulating eNOS activity are compromised, consistent with observations demonstrating that eNOS activity exhibits a circadian variation (136), which may be a consequence of its phosphorylation state (3, 75, 109a, 144). Moreover, vascular disease is worsened in circadian clock mutant mice when challenged by arterial ligation or vascular injury (3). However, the absence of an effect on blood pressure rhythm per se in eNOS-KO mice (81, 141) suggests that circadian rhythms in blood pressure are either not mediated by this isoform or compensated for through other mechanisms, i.e., robustness of blood pressure rhythm. Similarly, the function of the SCN is unmodified in mice lacking nNOS, although it is not known whether nNOS contributes to circadian regulation of blood pressure (72). Although an unlikely contributor, there are no studies yet addressing whether iNOS can influence SCN or circadian blood pressure regulation.
Another mechanism that might account for a reduction in nocturnal pressure is a loss of endothelial function. That a circadian rhythm exists in the function of human blood vessels is long known (60). Indeed, endothelial function varies according to time of day (65), and this variation is altered in mice with mutated circadian clocks (3, 142), while the downstream effector response to NO, through guanylyl cyclase, remains intact (3, 142), consistent with observations in humans which demonstrate that the response to sodium nitroprusside does not vary according to time of day (104). In individuals with compromised endothelial function, this diurnal variation in blood vessel function is blunted (122). Forearm blood flow is also compromised in nondipping vs. dipping hypertensives (48). These findings raise important questions as to whether a loss of circadian rhythms causes endothelial dysfunction or if loss of endothelial function results in the disruption of circadian regulation. Indeed, hypertension is known to modify the circadian clock in the heart and vasculature (95, 98, 151). Recent studies support the former, as endothelial function is attenuated in mice with genetic disruption of the circadian clock (3, 142).
The increased production of superoxide is a powerful antagonist to the vasculoprotective actions of NO and a frequent cause of endothelial dysfunction in cardiovascular disease states. Blood vessels from hypertensive animals overproduce superoxide and accompanying ROS, which alters vascular tone and induces endothelial dysfunction (108). Increased production of ROS occurs in kidneys and spleen from Bmal1-KO mice (71). Moreover, the levels of superoxide production in brain and neutrophils have been shown to exhibit circadian variation (8, 96), but the source of the increased ROS is poorly characterized and thus it is not yet possible to determine if disruption of circadian rhythms promotes increased ROS production (Noxes, mitochondria) or reduced antioxidant defenses such as superoxide dismutase, glutathione peroxidase, catalase, or even heme oxygenase. Increased production of ROS can also disrupt the timing of the circadian clock (45, 154), suggesting a potential mechanism for stimuli that induce ROS production, such as high blood pressure or diabetes, to disrupt the local circadian clock and promote vascular disease.
It is evident that the regulation of the circadian clock impinges on multiple mechanisms involved in blood pressure regulation. The hands of the clock may directly touch on the genes and proteins involved in blood pressure regulation, i.e., through direct transcriptional control and posttranslational regulation. Perturbations to the circadian clock may occur in a silent manner perhaps influencing only peripheral clocks, i.e., nondipping hypertension or through central behavioral aberrations, i.e., sleep apnea, sleep duration, and shift work (Fig. 1). One possibility is that the genetic components of the circadian clock may act to directly activate or repress such mechanisms in tissues intrinsically important in blood pressure regulation or in tissues extrinsic to the pressor mechanism by release of mediators such as hormones or circulating peptides. Transcriptional activation by the positive limb of peripheral circadian clocks may transactivate genes important in vascular (111), cardiac (127), or renal function (70), and these functions can be antagonized by proteins of the negative limb. Future studies are needed to examine if there is an impact of ROS production in vascular function and blood pressure regulation in mice with a disrupted circadian clock as ROSs are established negative modulators (77). Moreover, it will be important to identify the putative signals that act as intermediaries to blood pressure and circadian control.
CONCLUSION
Circadian rhythms have long been known to be a characteristic feature of blood pressure regulation and hypertension. Recent evidence demonstrates that the genetic components of the circadian clock have a direct influence on the rhythmic oscillation of blood pressure. This may occur through the regulation of vasoactive agents, but also via the sensitivity of the response to these mediators. As the circadian clock impinges on the multiple mechanisms orchestrating blood pressure control including metabolic function, vascular function, and fluid balance, future studies are needed to examine the influence of the circadian clock on these downstream molecular mechanisms involved in hypertension, which may ultimately provide breakthroughs in our understanding and treatment of this silent killer.
REFERENCES
- 1. Albers HE, Liou SY, Stopa EG, Zoeller RT. Interaction of colocalized neuropeptides—functional significance in the circadian timing system. J Neurosci 11: 846–851, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, Umeno Y, Okada K, Wakasugi K, Yonemochi H, Eshima N, Saikawa T, Yoshimatsu H. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest 37: 709–714, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation 119: 1510–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336: 1117–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8: 476–483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol 7: e52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25: 1610–1616, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Baran D, Paduraru I, Saramet A, Petrescu E, Haulica I. Influence of light-dark cycle alteration on free radical level in rat CNS. Rom J Physiol 37: 23–38, 2000 [PubMed] [Google Scholar]
- 9. Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol Cell Physiol 232: C165–C173, 1977 [DOI] [PubMed] [Google Scholar]
- 10. Brown TM, McLachlan E, Piggins HD. Angiotensin II regulates the activity of mouse suprachiasmatic nuclei neurons. Neuroscience 154: 839–847, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Carlson SH, Oparil S, Chen YF, Wyss JM. Blood pressure and NaCl-sensitive hypertension are influenced by angiotensin-converting enzyme gene expression in transgenic mice. Hypertension 39: 214–218, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Oroszi TL, Morris M. Salt consumption increases blood pressure and abolishes the light/dark rhythm in angiotensin AT1a receptor deficient mice. Physiol Behav 88: 95–100, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417: 405–410, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Cohen MP, Clements RS, Hud E, Cohen JA, Ziyadeh FN. Evolution of renal function abnormalities in the db/db mouse that parallels the development of human diabetic nephropathy. Exp Nephrol 4: 166–171, 1996 [PubMed] [Google Scholar]
- 17. Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104: 3450–3455, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Czupryniak L, Pawlowski M, Saryusz-Wolska M, Loba J. Circadian blood pressure variation and antihypertensive medication adjustment in normoalbuminuric type 2 diabetes patients. Kidney Blood Press Res 30: 182–186, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Czupryniak L, Wisniewska-Jarosinska M, Drzewoski J. Trandolapril restores circadian blood pressure variation in normoalbuminuric normotensive Type 1 diabetic patients. J Diabetes Complications 15: 75–79, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens 27: 307–311, 2005 [PubMed] [Google Scholar]
- 22. Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50: 465–477, 2006 [DOI] [PubMed] [Google Scholar]
- 23. DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci 10: 543–545, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14: 173–194, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke 36: 342–347, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci 15: 3526–3538, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27: 101–110, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Dyer AR, Stamler R, Grimm R, Stamler J, Berman R, Gosch FC, Emidy LA, Elmer P, Fishman J, Van Heel N. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertension 10: 417–424, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Earnest DJ, Turek FW. Neurochemical basis for the photic control of circadian rhythms and seasonal reproductive cycles: role for acetylcholine. Proc Natl Acad Sci USA 82: 4277–4281, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol 16: 462–467, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ekmekcioglu C, Haslmayer P, Philipp C, Mehrabi MR, Glogar HD, Grimm M, Thalhammer T, Marktl W. 24-Hour variation in the expression of the mt1 melatonin receptor subtype in coronary arteries derived from patients with coronary heart disease. Chronobiol Int 18: 973–985, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Farah V, Elased KM, Morris M. Genetic and dietary interactions: role of angiotensin AT1a receptors in response to a high-fructose diet. Am J Physiol Heart Circ Physiol 293: H1083–H1089, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis 33: 29–35, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension 51: 521–527, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension 53: 387–392, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Goncharuk VD, van Heerikhuize J, Dai JP, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J Comp Neurol 431: 320–330, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Goto N, Uchida K, Morozumi K, Ueki T, Matsuoka S, Katayama A, Haba T, Tominaga Y, Fukuda M, Nakao A, Kimura G. Circadian blood pressure rhythm is disturbed by nephrectomy. Hypertens Res 28: 301–306, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Grant S, Lutz EM, McPhaden AR, Wadsworth RM. Location and function of VPAC(1), VPAC(2) and NPR-C receptors in VIP-induced vasodilation of porcine basilar arteries. J Cereb Blood Flow Metab 26: 58–67, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens 18: 955–961, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67: 723–735, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma-protein encoded by the obese gene. Science 269: 543–546, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 351: 1755–1762, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20: 921–962, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Harmar AJ, Marston HM, Shen SB, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109: 497–508, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Hermida RC, Calvo C, Ayala DE, Dominguez MJ, Covelo M, Fernandez JR, Fontao MJ, Lopez JE. Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol Int 21: 277–296, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Sasaki S, Goto C, Oshima T, Chayama K, Yoshizumi M. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and nondippers. J Am Coll Cardiol 40: 2039–2043, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Higashi Y, Oshima T, Ozono R, Nakano Y, Matsuura H, Kambe M, Kajiyama G. Nocturnal decline in blood pressure is attenuated by NaCl loading in salt-sensitive patients with essential hypertension : noninvasive 24-hour ambulatory blood pressure monitoring. Hypertension 30: 163–167, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Hilbert P, Lindpaintner K, Beckmann JS, Serikawa T, Soubrier F, Dubay C, Cartwright P, Degouyon B, Julier C, Takahasi S, Vincent M, Ganten D, Georges M, Lathrop GM. Chromosomal mapping of 2 genetic-loci associated with blood-pressure regulation in hereditary hypertensive rats. Nature 353: 521–529, 1991 [DOI] [PubMed] [Google Scholar]
- 51. Holl RW, Pavlovic M, Heinze E, Thon A. Circadian blood pressure during the early course of type 1 diabetes. Analysis of 1,011 ambulatory blood pressure recordings in 354 adolescents and young adults. Diabetes Care 22: 1151–1157, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Hurwitz S, Cohen RJ, Williams GH. Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol 96: 1406–1414, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Ignarro LJ, Byrns RE, Buga GM, Wood KS. Mechanisms of endothelium-dependent vascular smooth-muscle relaxation elicited by bradykinin and VIP. Am J Physiol Heart Circ Physiol 253: H1074–H1082, 1987 [DOI] [PubMed] [Google Scholar]
- 54. Ivanov P, Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci USA 104: 20702–20707, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Izzedine H, Launay-Vacher V, Deray G. Abnormal blood pressure circadian rhythm: a target organ damage? Int J Cardiol 107: 343–349, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Jacob HJ, Lindpaintner K, Lincoln SE, Kusumi K, Bunker RK, Mao YP, Ganten D, Dzau VJ, Lander ES. Genetic-mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell 67: 213–224, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Janssen BJ, Tyssen CM, Struyker-Boudier HA. Modification of circadian blood pressure and heart rate variability by five different antihypertensive agents in spontaneously hypertensive rats. J Cardiovasc Pharmacol 17: 494–503, 1991 [DOI] [PubMed] [Google Scholar]
- 58. Jones H, Atkinson G, Leary A, George K, Murphy M, Waterhouse J. Reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension 47: 778–784, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Kamata K, Kojima S. Characteristics of contractile responses of aorta to norepinephrine in db/db mice. Res Commun Mol Pathol Pharmacol 96: 319–328, 1997 [PubMed] [Google Scholar]
- 60. Kaneko M, Zechman FW, Smith RE. Circadian variation in human peripheral blood flow levels and exercise responses. J Appl Physiol 25: 109–114, 1968 [DOI] [PubMed] [Google Scholar]
- 61. Kanie N, Kamata K. Contractile responses in spontaneously diabetic mice. II. Effect of cholestyramine on enhanced contractile response of aorta to norepinephrine in C57BL/KsJ (db/db) mice. Gen Pharmacol 35: 319–323, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension 27: 130–135, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 38: 852–857, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Kernek KL, Trofatter JA, Mayeda AR, Lahiri DK, Hofstetter JR. A single copy of carbonic anhydrase 2 restores wild-type circadian period to carbonic anhydrase II-deficient mice. Behav Genet 36: 301–308, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Keskil Z, Gorgun CZ, Hodoglugil U, Zengil H. Twenty-four-hour variations in the sensitivity of rat aorta to vasoactive agents. Chronobiol Int 13: 465–475, 1996 [DOI] [PubMed] [Google Scholar]
- 66. Khoury AF, Sunderajan P, Kaplan NM. The early morning rise in blood pressure is related mainly to ambulation. Am J Hypertens 5: 339–344, 1992 [DOI] [PubMed] [Google Scholar]
- 67. Kim SM, Eisner C, Faulhaber-Walter R, Mizel D, Wall SM, Briggs JP, Schnermann J. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am J Physiol Renal Physiol 295: F1230–F1238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim SM, Huang Y, Qin Y, Mizel D, Schnermann J, Briggs JP. Persistence of circadian variation in arterial blood pressure in beta1/beta2-adrenergic receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 294: R1427–R1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kimura G. Kidney and circadian blood pressure rhythm. Hypertension 51: 827–828, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Kita Y, Shiozawa M, Jin W, Majewski RR, Besharse JC, Greene AS, Jacob HJ. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics 12: 55–65, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kriegsfeld LJ, Demas GE, Lee SE, Jr, Dawson TM, Dawson VL, Nelson RJ. Circadian locomotor analysis of male mice lacking the gene for neuronal nitric oxide synthase (nNOS−/−). J Biol Rhythms 14: 20–27, 1999 [DOI] [PubMed] [Google Scholar]
- 73. Kudo M, Yamazaki I, Suzuki T, Ebihara Y, Iwadate H, Kizuki K. Potential role of kallikrein in diurnal rhythms and perivascular distribution in rat pineal glands. Brain Res 797: 287–294, 1998 [DOI] [PubMed] [Google Scholar]
- 74. Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia 47: 1425–1436, 2004 [DOI] [PubMed] [Google Scholar]
- 75. Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, Miyauchi H, Kaneko S, Bradfield CA, FitzGerald GA, Komuro I. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res 102: 607–614, 2008 [DOI] [PubMed] [Google Scholar]
- 76. LaCroix C, Freeling J, Giles A, Wess J, Li YF. Deficiency of M2 muscarinic acetylcholine receptors increases susceptibility of ventricular function to chronic adrenergic stress. Am J Physiol Heart Circ Physiol 294: H810–H820, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95: 588–593, 1997 [DOI] [PubMed] [Google Scholar]
- 78. Leary AC, Struthers AD, Donnan PT, MacDonald TM, Murphy MB. The morning surge in blood pressure and heart rate is dependent on levels of physical activity after waking. J Hypertens 20: 865–870, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Lemmer B. Chronopharmacology of hypertension. Ann NY Acad Sci 783: 242–253, 1996 [DOI] [PubMed] [Google Scholar]
- 80. Lemmer B. The importance of circadian rhythms on drug response in hypertension and coronary heart disease–from mice and man. Pharmacol Ther 111: 629–651, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Lemmer B, Arraj M, Thomas M, Zuther P. eNOS-knock-out mice display a disturbed 24-h rhythm in heart rate but not in blood pressure. Am J Hypertens 17: S79–S79, 2004 [Google Scholar]
- 82. Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci 26: 11615–11623, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li P, Sur SH, Mistlberger RE, Morris M. Circadian blood pressure and heart rate rhythms in mice. Am J Physiol Regul Integr Comp Physiol 276: R500–R504, 1999 [DOI] [PubMed] [Google Scholar]
- 84. Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129: 605–616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19: 91–102, 1997 [DOI] [PubMed] [Google Scholar]
- 86. Lurbe A, Redon J, Pascual JM, Tacons J, Alvarez V, Batlle DC. Altered blood pressure during sleep in normotensive subjects with type I diabetes. Hypertension 21: 227–235, 1993 [DOI] [PubMed] [Google Scholar]
- 87. Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115: 1942–1952, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest 100: 1623–1633, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mastronardi CA, Yu WH, McCann SM. Resting and circadian release of nitric oxide is controlled by leptin in male rats. Proc Natl Acad Sci USA 99: 5721–5726, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol 566: 213–224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL, Wilsbacher LD, Song EJ, Hong HK, Bradfield CA, Takahashi JS. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314: 1304–1308, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Millarcraig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet 1: 795–797, 1978 [DOI] [PubMed] [Google Scholar]
- 94. Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104: 3342–3347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M. Alterations of circadian expressions of clock genes in Dahl salt-sensitive rats fed a high-salt diet. Hypertension 42: 189–194, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Muniain MA, Rodriguez MD, Romero A, Mata R, Vargas C, Naranjo A. Circadian variations in the superoxide production, enzyme release and neutrophil aggregation in patients with rheumatoid arthritis and controls. Rheumatology 30: 138–140, 1991 [DOI] [PubMed] [Google Scholar]
- 97. Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Iwasaki T. Augmented diurnal variations of the cardiac renin-angiotensin system in hypertensive rats. Hypertension 40: 827–833, 2002 [DOI] [PubMed] [Google Scholar]
- 98. Naito Y, Tsujino T, Kawasaki D, Okumura T, Morimoto S, Masai M, Sakoda T, Fujioka Y, Ohyanagi M, Iwasaki T. Circadian gene expression of clock genes and plasminogen activator inhibitor-1 in heart and aorta of spontaneously hypertensive and Wistar-Kyoto rats. J Hypertens 21: 1107–1115, 2003 [DOI] [PubMed] [Google Scholar]
- 99. Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 104: 1746–1748, 2001 [DOI] [PubMed] [Google Scholar]
- 100. Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol 288: R685–R691, 2005 [DOI] [PubMed] [Google Scholar]
- 101. O'Brien E, Sheridan J, O'Malley K. Dippers and nondippers. Lancet 2: 397, 1988 [DOI] [PubMed] [Google Scholar]
- 102. Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett 580: 127–130, 2006 [DOI] [PubMed] [Google Scholar]
- 103. Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 53: 381–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 325: 986–990, 1991 [DOI] [PubMed] [Google Scholar]
- 105. Pelat M, Verwaerde P, Merial C, Galitzky J, Berlan M, Montastruc JL, Senard JM. Impaired atrial M(2)-cholinoceptor function in obesity-related hypertension. Hypertension 34: 1066–1072, 1999 [DOI] [PubMed] [Google Scholar]
- 106. Peters RV, Zoeller RT, Hennessey AC, Stopa EG, Anderson G, Albers HE. The control of circadian-rhythms and the levels of vasoactive-intestinal-peptide messenger-RNA in the suprachiasmatic nucleus are altered in spontaneously hypertensive rats. Brain Res 639: 217–227, 1994 [DOI] [PubMed] [Google Scholar]
- 107. Portaluppi F, Vergnani L, Manfredini R, degli Uberti EC, Fersini C. Time-dependent effect of isradipine on the nocturnal hypertension in chronic renal failure. Am J Hypertens 8: 719–726, 1995 [DOI] [PubMed] [Google Scholar]
- 108. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. N Engl J Med 334: 374–381, 1996 [DOI] [PubMed] [Google Scholar]
- 109a. Rudic RD. Time is of the essence: vascular implications of the circadian clock. Circulation. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rudic RD, Curtis AM, Cheng Y, FitzGerald G. Peripheral clocks and the regulation of cardiovascular and metabolic function. Methods Enzymol 393: 524–539, 2005 [DOI] [PubMed] [Google Scholar]
- 111. Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112: 2716–2724, 2005 [DOI] [PubMed] [Google Scholar]
- 112. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344: 3–10, 2001 [DOI] [PubMed] [Google Scholar]
- 113. Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GTJ, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na+//H+ exchanger NHE3 in the kidney. Kidney Int 67: 1410–1419, 2005 [DOI] [PubMed] [Google Scholar]
- 114. Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, Ishida N. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem 273: 27039–27042, 1998 [DOI] [PubMed] [Google Scholar]
- 115. Sampson AK, Widdop RE, Denton KM. Sex-differences in circadian blood pressure variations in response to chronic angiotensin II infusion in rats. Clin Exp Pharmacol Physiol 35: 391–395, 2008 [DOI] [PubMed] [Google Scholar]
- 116. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263, 2005 [DOI] [PubMed] [Google Scholar]
- 117. Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43: 192–197, 2004 [DOI] [PubMed] [Google Scholar]
- 118. Scheving LA, Jin WH. Circadian regulation of uroguanylin and guanylin in the rat intestine. Am J Physiol Cell Physiol 277: C1177–C1183, 1999 [DOI] [PubMed] [Google Scholar]
- 119. Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543–H2549, 2007 [DOI] [PubMed] [Google Scholar]
- 120. Sei H, Oishi K, Chikahisa S, Kitaoka K, Takeda E, Ishida N. Diurnal amplitudes of arterial pressure and heart rate are dampened in Clock mutant mice and adrenalectomized mice. Endocrinology 149: 3576–3580, 2008 [DOI] [PubMed] [Google Scholar]
- 121. Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol 94: 648–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shaw JA, Chin-Dusting JPF, Kingwell BA, Dart AM. Diurnal variation in endothelium-dependent vasodilatation is not apparent in coronary artery disease. Circulation 103: 806–812, 2001 [DOI] [PubMed] [Google Scholar]
- 123. Shimamura T, Nakajima M, Iwasaki T, Hayasaki Y, Yonetani Y, Iwaki K. Analysis of circadian blood pressure rhythm and target-organ damage in stroke-prone spontaneously hypertensive rats. J Hypertens 17: 211–220, 1999 [DOI] [PubMed] [Google Scholar]
- 124. Sinha YN, Baxter SR, Larson BA, Vanderlaan WP. Levels of prolactin, growth hormone and insulin in genetically diabetic (db/db) mice. Proc Soc Exp Biol Med 161: 78–81, 1979 [DOI] [PubMed] [Google Scholar]
- 125. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001 [DOI] [PubMed] [Google Scholar]
- 126. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001 [DOI] [PubMed] [Google Scholar]
- 127. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002 [DOI] [PubMed] [Google Scholar]
- 128. Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 17: 360–364, 2000 [DOI] [PubMed] [Google Scholar]
- 129. Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol 295: H1634–H1641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila Period gene. Cell 90: 1003–1011, 1997 [DOI] [PubMed] [Google Scholar]
- 131. Svensson P, de Faire U, Sleight P, Yusuf S, Ostergren J. Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE Substudy. Hypertension 38: E28–E32, 2001 [DOI] [PubMed] [Google Scholar]
- 132. Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol 286: R108–R113, 2004 [DOI] [PubMed] [Google Scholar]
- 133. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764–775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Timio M, Venanzi S, Lolli S, Lippi G, Verdura C, Monarca C, Guerrini E. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol 43: 382–387, 1995 [PubMed] [Google Scholar]
- 135. Tominaga K, Shinohara K, Otori Y, Fukuhara C, Inouye ST. Circadian rhythms of vasopressin content in the suprachiasmatic nucleus of the rat. Neuroreport 3: 809–812, 1992 [DOI] [PubMed] [Google Scholar]
- 136. Tunctan B, Weigl Y, Dotan A, Peleg L, Zengil H, Ashkenazi I, Abacioglu N. Circadian variation of nitric oxide synthase activity in mouse tissue. Chronobiol Int 19: 393–404, 2002 [DOI] [PubMed] [Google Scholar]
- 137. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Urayama K, Dedeoglu DB, Guilini C, Frantz S, Ertl G, Messaddeq N, Nebigil CG. Transgenic myocardial overexpression of prokineticin receptor-2 (GPR73b) induces hypertrophy and capillary vessel leakage. Cardiovasc Res 81: 28–37, 2009 [DOI] [PubMed] [Google Scholar]
- 139. Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 96: 1859–1862, 1997 [DOI] [PubMed] [Google Scholar]
- 140. Van Reeth O, Olivares E, Zhang Y, Zee PC, Mocaer E, Defrance R, Turek FW. Comparative effects of a melatonin agonist on the circadian system in mice and Syrian hamsters. Brain Res 762: 185–194, 1997 [DOI] [PubMed] [Google Scholar]
- 141. Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549: 313–325, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 115: 2188–2195, 2007 [DOI] [PubMed] [Google Scholar]
- 143. Viswanathan M, Laitinen JT, Saavedra JM. Expression of melatonin receptors in arteries involved in thermoregulation. Proc Natl Acad Sci USA 87: 6200–6203, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, Liao JK. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation 118: 2166–2173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab 8: 482–491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 117: 2087–2095, 2008 [DOI] [PubMed] [Google Scholar]
- 147. Witte K, Lemmer B. Development of inverse circadian blood pressure pattern in transgenic hypertensive TGR(mREN2)27 rats. Chronobiol Int 16: 293–303, 1999 [DOI] [PubMed] [Google Scholar]
- 148. Witte K, Schnecko A, Buijs RM, van der Vliet J, Scalbert E, Delagrange P, Guardiola-Lemaitre B, Lemmer B. Effects of SCN lesions on circadian blood pressure rhythm in normotensive and transgenic hypertensive rats. Chronobiol Int 15: 135–145, 1998 [DOI] [PubMed] [Google Scholar]
- 149. Witte K, Schnecko A, Zuther P, Lemmer B. Contribution of the nitric oxide-guanylyl cyclase system to circadian regulation of blood pressure in normotensive Wistar-Kyoto rats. Cardiovasc Res 30: 682–688, 1995 [PubMed] [Google Scholar]
- 150. Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 104: 14412–14417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res 88: 1142–1150, 2001 [DOI] [PubMed] [Google Scholar]
- 152. Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol 34: 223–231, 2002 [DOI] [PubMed] [Google Scholar]
- 153. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 154. Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci USA 104: 15899–15904, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20: 1103–1110, 1998 [DOI] [PubMed] [Google Scholar]


