Abstract
The study examined the long-term outcome of cardiac stem cell transplantation in hearts with postinfarction left ventricular (LV) remodeling. Myocardial infarction (MI) was created by ligating the first and second diagonal branches of the left anterior descending coronary artery in miniature swines. Intramyocardial injections of 50 million LacZ-labeled bone marrow-derived multipotent progenitor cells (MPC) were performed in the periscar region (Cell, n = 7) immediately after MI, whereas, in control animals (Cont, n = 7), saline was injected. Functional outcome was assessed monthly for 4 mo with MRI and 31P-magnetic resonance spectroscopy. Engraftment was studied on histology, and gene chip (Affymetrix) array analysis was used to study differential expression of genes in the two groups. MPC treatment resulted in improvement of ejection fraction as early as 10 days after MI (Cell, 43.4 ± 5.1% vs. Cont, 32.2 ± 5.5%; P < 0.05). This improvement was seen each month and persisted to 4 mo (Cell, 51.2 ± 4.8% vs. Cont, 35.7 ± 5.0%; P < 0.05). PCr-to-ATP ratio (PCr/ATP) improved with MPC transplantation, which was most pronounced at high cardiac work states (subendocardial PCr/ATP was 1.70 ± 0.10 vs. 1.34 ± 0.14, P < 0.05). There was no significant difference in scar size (scar/LV area ∗ 100) at 10 days postinfarction. However, at 4 mo, there was a significant decrease in scar size in the Cell group (Cell, 4.6 ± 1.0% vs. Cont, 8.6 ± 2.4%; P < 0.05). No significant engraftment of MPC was observed. MPC transplantation was associated with a downregulation of mitochondrial oxidative enzymes and increased levels of myocyte enhancer factor 2a and zinc finger protein 91. In conclusion, MPC transplantation leads to long-term functional and bioenergetic improvement in a porcine model of postinfarction LV remodeling, despite no significant engraftment of stem cells in the heart. MPC transplantation reduces regional wall stresses and infarct size and mitigates the adverse effects of LV remodeling, as seen by a reduction in LV hypertrophy and LV dilatation, and is associated with differential expression of genes relating to metabolism and apoptosis.
Keywords: energetics, metabolism, heart failure, scar size
stem cell transplantation has emerged as a potential therapy for limiting postinfarction left ventricular (LV) remodeling and the consequent development of congestive heart failure in both animal (1, 13, 15, 33) and clinical (2, 3, 12, 24, 31) studies. Clinical studies have shown mixed results on LV function with stem cell therapy, with some showing benefit (3, 24, 31) and some no difference in LV ejection fraction (12, 14). In the bone marrow transfer to enhance ST-elevation infarct regeneration study, the treated group showed significant short-term increase of LV ejection fraction at 6 mo, but, interestingly, no difference between the two groups was present at 18 mo (16). Using a swine model of post-myocardial infarction (MI) LV remodeling, our laboratory recently reported that transplantation of bone marrow-derived multipotent progenitor cells (MPC) resulted in a significant improvement of cardiac function and correction of abnormal energy metabolism at 1 mo following transplantation (33). However, the long-term effect after MPC treatment still needs to be defined. Dai et al. (6) showed, in a rat MI model, that allogenic mesenchymal stem cell (MSC) treatment led to an improvement in LV function 4 wk after transplantation, but this improvement was lost at 6 mo. However, they did find stem cell engraftment as late as 6 mo and showed that these cells expressed markers that suggested endothelium and muscle phenotypes (6). Long-term studies of any stem cell type in large animal models of MI are relatively scarce.
The mechanisms underlying the beneficial effects of stem cell treatment are also not well defined. Our laboratory and others have demonstrated a very low engraftment rate and even lower cardiomyocyte differentiation rate a few weeks after bone marrow stem cell transplantation (11, 20, 33). Therefore, the direct contribution of regenerated myocytes to the improved cardiac contractile function appears less likely. Dzau's group have shown a significant protection of stressed cardiomyocytes from apoptosis and consequent reduction in infarct size by injection of concentrated cell culture medium that was obtained from bone marrow-derived MSCs with overexpression of Akt, or by injection of MSCs themselves into the myocardium at the time of acute MI (8, 9, 20). Thus the mechanisms underlying the long-term outcome after stem cell transplantation also require more elucidation.
The objectives of the present study are to determine the long-term functional and bioenergetic outcome after MPC transplantation in a clinically relevant porcine model of postinfaction LV remodeling and whether these effects are related to persistent long-term engraftment of MPC in the heart. We hypothesize that MPC transplantation leads to transient engraftment and secretion of factors that leads to long-term myocardial gene expression changes that contribute to the beneficial functional outcome.
MATERIALS AND METHODS
All experiments were performed in accordance with the animal use guidelines of the University of Minnesota, and the experimental protocol was approved by the University of Minnesota Research Animal Resources Committee. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85–23, revised 1985).
Study design.
The study design is depicted in Fig. 1. A total of 14 swine were randomized after MI to receive either 50 million stem cells (n = 7) or saline only (n = 7). The pigs were followed for a total of 4 mo after transplantation, with MRI occurring at baseline, 10 days, 1 mo, and then subsequently each month for 4 mo. At the end of the study, open-chest NMR spectroscopy was performed, and then, after death, histology and microarray analysis was done on the tissue samples.
Fig. 1.
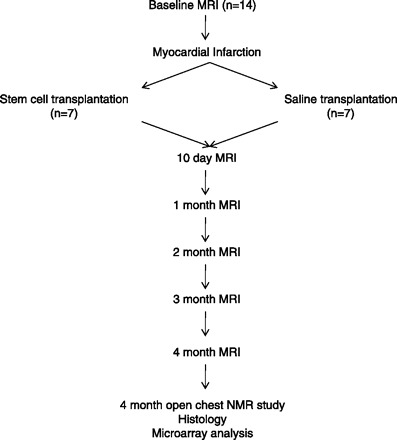
Study design. n, No. of pigs.
Cell culture.
Bone marrow-derived MPCs were expanded from one of the established swine multipotent adult progenitor cell lines generated in the laboratory of Drs. Verfaille and Zhang (33, 34), as described in detail in the appendix. The MPC cells have previously been shown to be able to differentiate to cells of different lineages (33). Cells used for transplantation were >80% viable.
Animal model and stem cell delivery method.
Sinclair miniswine (Sinclair Research Center) were used for this study. Sinclair pigs are research purpose bred and grow very slowly, which allowed us to follow these animals for the long term. These pigs have a shorter lifespan compared with humans, so 4 mo is equivalent to a longer follow-up in human years. The follow-up of 4 mo was also chosen because the bore size of the 4.7-T magnet for the magnetic resonance spectroscopy (MRS) studies limits us to perform these studies on larger animals. The postinfarction LV remodeling model was created as described before (10, 17). Briefly, female Sinclair miniswine (5 mo age, ∼15 kg) were anesthetized with pentobarbital (30 mg/kg iv), intubated, and ventilated with a respirator with supplemental oxygen. A left thoracotomy was performed, and the first and second diagonal branches of the left anterior descending artery were dissected free and permanently occluded with a ligature. The ischemic myocardial region became cyanotic in response to the coronary artery occlusion. MPCs were injected directly into five regions of the peri-infarct border zone (BZ) (10 million cells per location; 50 million total diluted in 2-ml saline). Injection sites were marked with a stitch to allow identification of the areas for histological studies. Animals in the placebo group received 2 ml of saline in five injections at similar BZ injection sites. Animals were observed in the open-chest state for 60 min. If ventricular fibrillation occurred, electrical defibrillation was performed immediately. The chest was then closed. Animals received standard postoperative care, including analgesia, until they ate normally and became active.
MRI methods.
MRI was performed on a 1.5-T clinical scanner (Siemens Sontata, Siemens Medical Systems, Islen, NJ) using a phased-array four-channel surface coil and ECG gating, as previously described in detail (40) (see appendix).
Spatially localized 31P-MRS technique.
Spatially localized 31P-NMR spectroscopy was performed using the rotating-frame experiment using adiabatic plane-rotation pulses for phase modulation (RAPP)-imaging-selected in vivo spectroscopy (ISIS)/Fourier series window (FSW) method (RAPP-ISIS/FSW), as previously described in details (18, 32, 36, 37) (See appendix).
1H-NMR spectroscopy technique.
1H-NMR methods have been previously reported in detail (5, 18) (See appendix).
Surgical preparation for open-chest MRS study.
Detailed surgical preparations for MRS study were published previously (10, 32, 33) and are described in detail in the appendix. The 31P and 1H coils were placed in the periscar region 1 cm away from the infarct site and adjacent to the injection sites.
MRS study protocol and hemodynamic measurements.
Hemodynamic measurements were acquired simultaneously with the NMR spectra. Aortic and LV pressures were measured using pressure transducers positioned at midchest level (32, 36, 37) and recorded on an eight-channel recorder. After all the baseline data were obtained, animals received combined dobutamine and dopamine infusion (20 μg·kg−1·min−1 each) to induce a very high cardiac work state. After waiting for ∼5 min after the initiation of catecholamine infusion, all of the spectroscopic and hemodynamic data were again measured under the high cardiac work states.
Tissue preparation.
Myocardial drill biopsies from BZ and remote zone were taken with a precooled biopsy drill and quickly (within 5 s) frozen in liquid nitrogen (18, 32, 36, 37) for subsequent microarray analysis. BZ tissue samples were taken at least 3 mm away from the edge of the infarct to avoid contamination with scar tissue. The remainder of the LV was sectioned in a bread-loaf manner into six transverse sections (∼10 mm in thickness) from apex to base. Even rings were subdivided into 12 circumferential specimens and fixed in zinc fixative (Anatech, Battle Creek, MI) for hematoxylin and eosin, immunofluorescence staining, and engraftment evaluation.
Engraftment evaluation.
Each of the 12 pieces of cardiac tissue of a short-axis ring were stained by X-gal (Invitrogen) and embedded in Tissue-Tek OCT (Fisher Scientific). Serial cryostat sections (10 μm in thickness) were obtained from the whole transverse even rings. Engrafted MPCs were identified by counting the X-gal-positive nuclei in every 10th serial section of the whole transverse ring and then multiplying by 10 to obtain the total number of engrafted MPCs per ring. The total number of engrafted MPCs equals twofold x-gal staining positive cells in all three even rings. The engraftment rate of transplanted MPCs was calculated by dividing the total number of engrafted MPCs by 50 × 106 (the number of MPCs injected) and multiplying by 100%.
RNA isolation.
Tissue was placed in TRIzol reagent and homogenized using a rotor-stator homogenizer. Homogenates were transferred to a Phase Lock Gel Heavy tube (Eppendorf), chloroform was added, and the mixture was centrifuged. Supernatants were collected, and isopropanol was added to precipitate the RNA. The RNA pellet was washed with 80% ethanol, then air-dried, before being dissolved in 100 μl of RNase-free H2O. RNA was further purified using the RNeasy Mini protocol, according to the manufacturer's directions (Qiagen). The integrity of each RNA sample was validated using an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA) to measure the ratio of 28s/18s ribosomal RNA.
Double-stranded cDNA synthesis.
Total RNA of 5 μg was used in the synthesis of double-stranded cDNA based on Affymetrix protocol with a T7-(dT)24 primer and 200 U/μl of SuperScript II RT. Second-strand synthesis was performed, according to the manufacturer's directions.
In vitro transcription-synthesis of biotin-labeled cRNA.
Biotin-labeled RNA targets were obtained by using the Enzo BioArray High Yield RNA Transcript Labeling Kit via in vitro transcription from bacteriophage T7 RNA polymerase promoters, according to the manufacturer's directions (Enzo). The cRNA was then fragmented, according to the Affymetrix protocol. Quality and fragmentation of cRNA was confirmed with gel electrophoresis.
Target hybridization and probe array wash and stain.
Hybridization cocktail for single standard probe array (Porcine Genome Array, Affymetrix) was made by mixing 15 μg of fragmented cRNA with control oligonucleotide B2, 20× eukaryotic hybridization controls, herring sperm DNA (10 mg/ml), acetylated BSA (50 mg/ml) 2× hybridization buffer, and RNase-free water. Hybridization was performed in our Affymetrix Core Facility.
Microarray data analysis.
Array data were normalized using GC-RMA method using gene data refiner software. Significance analysis of microarrays (28) was used to identify probes whose expression was modified following stem cell transplantation and showed a fold change >1.7 and a false discovery rate of <20%. Probes were mapped to human genes using the annotation described by Tsai et al. (27). Normalized and raw data are available at GEO under the entry GSE14643 (http://www.ncbi.nlm.nih.gov/geo/).
Data analysis.
Data were analyzed with repeated-measures ANOVA. A value of P < 0.05 was considered significant. The Bonferroni correction for significance level was used to take into account multiple comparisons. All statistical analyses were performed in Sigmastat version 3.5 (San Jose, CA). All results are presented as mean ± SD, unless otherwise specified. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the paper as written.
RESULTS
Anatomic data.
The anatomic data for the 14 swine are summarized in Table 1. MPC transplantation resulted in a significant decrease in LV hypertrophy (LVH), as reflected by a decrease in LV weight/body weight (2.86 ± 0.12 vs. 3.33 ± 0.16, P < 0.05) and right ventricular weight/body weight (1.03 ± 0.08 vs. 1.34 ± 0.10, P < 0.05) in the cell-treated pigs compared with the saline-treated pigs after 4 mo.
Table 1.
Anatomic data obtained 4 mo after MI
| n | BW, kg | LVW, g | RVW, g | LV/BW, g/kg | RV/BW, g/kg | |
|---|---|---|---|---|---|---|
| MI | 7 | 16.5 ± 3.7 | 54.2 ± 5.6 | 22.0 ± 3.9 | 3.33 ± 0.16 | 1.34 ± 0.10 |
| MI + cell | 7 | 14.3 ± 2.1 | 41.0 ± 6.0* | 14.5 ± 3.2* | 2.86 ± 0.12* | 1.03 ± 0.08* |
Values are means ± SD; n, no. of pigs. MI, myocardial infarction; BW, body weight; LVW, left ventricular weight; RVW, right ventricular weight.
P < 0.05 vs. MI.
Hemodynamic data.
The hemodynamic data at baseline and at increased workload are summarized in Table 2. Inotropic stimulation caused a significant increase in the heart rate, mean aortic pressure, and LV systolic pressure and, consequently, rate pressure product. However, there was no significant difference between the swine with cell treatment vs. swine without cell treatment.
Table 2.
Hemodynamic data obtained 4 mo after MI
| HR, beat/min | Mean AoP, mmHg | LVSP, mmHg | LVEDP, mmHg | RPP × 103, mmHg/min | |
|---|---|---|---|---|---|
| Baseline | |||||
| MI | 117 ± 19 | 80 ± 5 | 100 ± 16 | 7 ± 1 | 11.7 ± 2.4 |
| MI + cell | 110 ± 13 | 76 ± 8 | 97 ± 12 | 5 ± 1 | 10.6 ± 1.2 |
| Db + Dp | |||||
| MI | 150 ± 7* | 97 ± 7* | 155 ± 16* | 8 ± 2 | 23.3 ± 2.7* |
| MI + cell | 142 ± 14* | 101 ± 8* | 156 ± 21* | 5 ± 2 | 22.1 ± 3.3* |
Values are means ± SD. Db + Dp, dobutamine and dopamine (each 20 μg•kg−1•min−1 iv); HR, heart rate; Mean AoP, mean aortic pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; RPP, rate pressure product (HR × LVSP).
P < 0.05 vs. baseline.
Functional data.
The temporal changes in ejection fraction as measured by MRI are depicted in Fig. 2. There was no significant difference between the two groups at baseline. However, the cell-treated group showed a significant improvement in ejection fraction, which was seen as early as 10 days (43.4 ± 5.1 vs. 32.2 ± 5.5%, P < 0.05) after MPC transplantation and persisted up to 4 mo (51.2 ± 4.8 vs. 35.7 ± 5.0%, P < 0.05). In addition, the MPC treatment resulted in reduction of the LV dilatation seen at 4 mo after MI in the saline-treated animals, as depicted by significantly lower LV end-diastolic (16.9 ± 1.8 vs. 22.2 ± 4.8 ml, P < 0.05) and LV end-systolic volume (8.9 ± 2.3 vs. 14.1 ± 2.5 ml, P < 0.05) in the MPC-treated group at 4-mo follow-up (Table 3). The initial scar size was similar at 10 days in both groups; however, there was a significant decrease in scar size in the MPC-treated animals from 10 days to 4 mo (4.6 ± 1.0% at 4 mo vs. 8.3 ± 1.5% at 10 days, P < 0.05), which was also significantly smaller than the scar size in control animals at 4 mo (4.6 ± 1.0 vs. 8.6 ± 2.4%, P < 0.05) (Fig. 3).
Fig. 2.
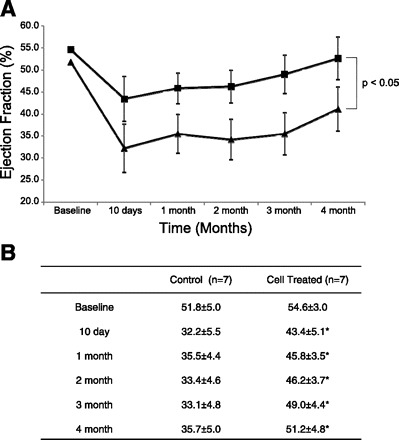
Long-term improvement in ventricular function after multipotent progenitor cell (MPC) transplantation. A: MPC transplantation leads to an improvement in ejection fraction as early as 10 days after cell transplantation that persists up to 4 mo (P < 0.05). ▲, Control; ■, cell treated. B: tabulated form of the ejection fraction data from 10-day, and 1-, 2-, 3-, and 4-mo MRI. Values are means ± SD; n, no. of pigs. *P < 0.05 vs. control.
Table 3.
Ventricular volumes
| LVEDV, ml |
LVESV, ml |
|||
|---|---|---|---|---|
| MI | MI + cell | MI | MI + cell | |
| Baseline | 16.4 ± 2.4 | 14.7 ± 2.9 | 7.9 ± 1.4 | 6.7 ± 1.4 |
| 10day | 15.8 ± 2.9 | 14.9 ± 3.1 | 10.7 ± 3.0 | 8.4 ± 1.7 |
| 1mo | 16.3 ± 2.5 | 16.7 ± 2.7 | 10.5 ± 1.9 | 9.0 ± 1.1 |
| 2mo | 17.4 ± 3.5 | 16.7 ± 1.5 | 11.5 ± 2.5 | 9.0 ± 0.2 |
| 3mo | 19.6 ± 6.3 | 16.2 ± 2.5 | 13.3 ± 5.3 | 8.2 ± 1.2 |
| 4mo | 22.2 ± 4.8 | 16.9 ± 1.8* | 14.1 ± 2.5 | 8.9 ± 2.3* |
Values are means ± SD. LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume.
P < 0.05 vs. MI.
Fig. 3.
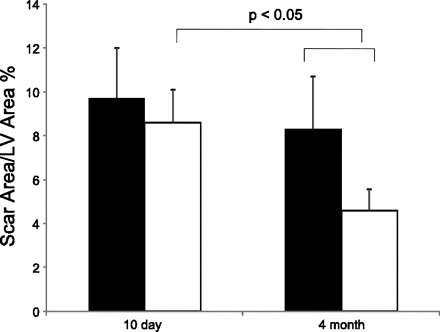
Long-term reduction in scar size after MPC transplantation. Solid bars, myocardial infarction; open bars, myocardial infarction + cell. LV, left ventricular. Values are means ± SD.
Myocardial high-energy phosphate and Pi levels.
Myocardial high-energy phosphate (HEP) and Pi levels are reflected by the PCr/ATP, as shown in Table 4. The subendocardial and subepicardial myocardial PCr/ATP are significantly higher at 4 mo in the pigs treated with MPC compared with those without treatment. After increased workload, the PCr/ATP decreased in both groups, but was still significantly higher in the cell-treated pigs. No deoxymyoglobin resonance peak was detected in either groups using 1H-MRS, which supports our previous findings that the bioenergetic abnormalities in hearts with postinfarction LV remodeling are not caused by a persistent myocardial ischemia (18).
Table 4.
Myocardial energetics 4 mo after MI
| Epi | Endo | |
|---|---|---|
| Baseline | ||
| MI | 1.94 ± 0.10 | 1.65 ± 0.15 |
| MI + cell | 2.17 ± 0.10* | 1.90 ± 0.14* |
| Db + Dp | ||
| MI | 1.62 ± 0.17† | 1.34 ± 0.14† |
| MI + cell | 1.91 ± 0.06*† | 1.70 ± 0.10*† |
Values are means ± SD. Epi, subepicardium; Endo, subendocardium.
P < 0.05 vs. MI.
P < 0.05 vs. baseline.
Cell engraftment.
There was no engraftment of MPC observed at 4 mo after transplantation in any of the cell-treated animals.
Microarray analysis.
As there was no long-term engraftment, it was postulated that the MPC engraft transiently after transplantation and exert a paracrine effect by secretion of factors that may lead to long-term gene expression changes that may ultimately lead to improved long-term outcome. Gene expression profiles were collected from seven MPC-treated animals and six control animals (one animal did not have quality RNA for array analysis) using the Affymetrix Porcine Gene chip array containing over 23,000 probe sets. Significance analysis of microarrays identified a number of genes that were differentially expressed in the heart of the MPC-treated animals (Fig. 4). There were 41 genes that were downregulated after MPC transplantation and 11 genes that were upregulated (Table 5). There was downregulation of several mitochondrial oxidative enzymes, including three different subunits of complex I of the respiratory chain, which were decreased by 0.59-, 0.58-, and 0.57-fold, respectively. The levels of zinc finger protein 91 [ZFP91; a member of the zinc finger family of proteins associated with ciliary neurotrophic factor (CNTF)] were increased by 3.62-fold. Similarly, myocyte enhancer factor 2a (MEF2a) was upregulated by 1.7-fold.
Fig. 4.
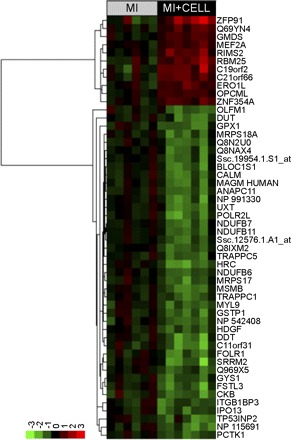
Heat map showing all differentially expressed transcripts found using Affymetrix array analyses of heart tissue, with and without exposure to stem cells. Expression levels are normalized to the average value of the untreated heart tissue and are log transformed. The names associated with each probe are the human names obtained from the annotation described in the materials and methods section. The probe names can be found in Table 5. Normalized and raw data are available at GEO under the entry GSE14643 (http://www.ncbi.nlm.nih.gov/geo/).
Table 5.
SAM table listing statistically significant genes that were differentially expressed after MPC transplantation
| Gene Name | Gene Description | Gene Cards Accession | Fold Change |
|---|---|---|---|
| Downregulated after MPC transplantation | |||
| Ssc.26278.1.S1_at | Importin 13 | IPO13 | 0.55 |
| Ssc.18764.1.A1_at | Integrin β1 binding protein 3 | ITGB1BP3 | 0.56 |
| Ssc.3563.1.S1_at | PCTAIRE protein kinase 1 | PCTK1 | 0.59 |
| Ssc.23233.1.S1_a_at | Transcribed locus, strongly similar to XP_536737.1 PREDICTED: similar to mitochondrial carrier protein MGC4399 (Canis familiaris) | NP_115691 | 0.49 |
| Ssc.16937.1.A1_at | Tumor protein p53 inducible nuclear protein 2 | TP53INP2 | 0.53 |
| Ssc.23233.1.S1_a_at | Glutathione peroxidase 1 | GPX1 | 0.49 |
| Ssc.16849.1.S1_at | Follistatin-like 3 (secreted glycoprotein) | FSTL3 | 0.48 |
| Ssc.2329.1.A1_at | Glycogen synthase 1 (muscle) | GYS1 | 0.57 |
| Ssc.9914.1.A1_at | Creatine kinase, brain | CKB | 0.52 |
| Ssc.23508.1.S1_at | Endoplasmic reticulum-golgi intermediate compartment (ERGIC) 1 | Q969X5 | 0.57 |
| Ssc.13255.1.A1_at | Serine/arginine repetitive matrix 2 | SRRM2 | 0.45 |
| Ssc.11779.1.S1_at | Folate receptor 1 (adult) | FOLR1 | 0.52 |
| Ssc.21553.1.S1_at | Hepatoma-derived growth factor (high-mobility group protein 1-like) | HDGF | 0.57 |
| Ssc.1819.1.S1_at | Trafficking protein particle complex 1 | TRAPPC1 | 0.58 |
| Ssc.22694.1.S1_at | NADH dehydrogenase (ubiquinone) 1β subcomplex, 6, 17 kDa | NDUFB6 | 0.59 |
| Ssc.5886.1.S1_at | Mitochondrial ribosomal protein S17 | MRPS17 | 0.56 |
| Ssc.25389.1.S1_at | Histidine rich calcium binding protein | HRC | 0.43 |
| Ssc.9125.1.S1_at | Myosin, light chain 9, regulatory | MYL9 | 0.54 |
| Ssc.12999.1.S1_at | Glutathione S-transferase pi 1 | GSTP1 | 0.53 |
| Ssc.12207.1.A1_at | Transcribed locus, weakly similar to XP_425282.1 PREDICTED: similar to MGC68763 protein (Gallus gallus) | NP_542408 | 0.57 |
| Ssc.14392.1.A1_at | Microseminoprotein, β | MSMB | 0.51 |
| Ssc.2339.1.S1_at | d-dopachrome tautomerase | DDT | 0.48 |
| Ssc.2514.1.S1_at | Chromosome 11, open-reading frame 31 | C11orf31 | 0.46 |
| Ssc.2447.1.A1_at | Chromosome 17, open-reading frame 49 | Q8IXM2 | 0.56 |
| Ssc.12576.1.A1_at | Transcribed locus, strongly similar to XP_548156.1 PREDICTED: similar to CG3420-PA (Canis familiaris) | 0.58 | |
| Ssc.23542.1.A1_at | NADH dehydrogenase (ubiquinone) 1 β subcomplex, 11, 17.3 kDa | NDUFB11 | 0.58 |
| Ssc.26100.1.S1_at | NADH dehydrogenase (ubiquinone) 1 β subcomplex, 7, 18 kDa | NDUFB7 | 0.57 |
| Ssc.27967.1.A1_at | Polymerase (RNA) II (DNA directed) polypeptide L, 7.6 kDa | POLR2L | 0.48 |
| Ssc.21753.1.S1_at | Ubiquitously-expressed transcript | UXT | 0.52 |
| Ssc.3197.1.S1_at | Transcribed locus, moderately similar to XP_533944.1 PREDICTED: similar to QIL1 (Canis familiaris) | NP_991330 | 0.51 |
| Ssc.2261.1.S1_at | APC11 anaphase promoting complex subunit 11 homolog (yeast) | ANAPC11 | 0.56 |
| Ssc.24290.1.S1_at | Biogenesis of lysosomal organelles complex-1, subunit 1 | BLOC1S1 | 0.53 |
| Ssc.3675.1.A1_at | Calmodulin 2 (phosphorylase kinase, δ) | CALM2 | 0.54 |
| Ssc.21117.1.A1_at | Transcribed locus, strongly similar to XP_213214.2 PREDICTED: similar to mitochondria-associated granulocyte macrophage CSF signaling molecule (Rattus norvegicus) | MAGM_HUMAN | 0.55 |
| Ssc.7580.1.A1_at | Chromosome 17, open-reading frame 61 | Q8N2U0 | 0.56 |
| Ssc.19954.1.S1_at | Transcribed locus, moderately similar to NP_060694.1 hypothetical protein FLJ10803 (Homo sapiens) | 0.57 | |
| Ssc.17618.1.S1_at | Clone Clu_6860.scr.msk.p1.Contig4, mRNA sequence | Q8NAX4 | 0.56 |
| Ssc.19477.1.A1_at | Trafficking protein particle complex 5 | TRAPPC5 | 0.58 |
| Ssc.5035.1.S1_at | Mitochondrial ribosomal protein S18A | MRPS18A | 0.56 |
| Ssc.19928.1.S1_a_at | Deoxyuridine triphosphatase | DUT | 0.54 |
| Ssc.14361.1.A1_at | Olfactomedin 1 | OLFM1 | 0.56 |
| Upregulated after MPC transplantation | |||
| Ssc.24458.1.A1_at | KIAA1429 | Q69YN4 | 1.83 |
| Ssc.10473.1.A1_at | Zinc finger protein 91 homolog (mouse) | ZFP91 | 3.62 |
| Ssc.8896.1.A1_at | GDP-mannose 4,6-dehydratase | GMDS | 1.76 |
| Ssc.21898.1.S1_at | ERO1-like (S. cerevisiae) | ERO1L | 1.86 |
| Ssc.25201.1.A1_at | Regulating synaptic membrane exocytosis 2 | RIMS2 | 2.09 |
| Ssc.29206.1.A1_at | Chromosome 19, open-reading frame 2 | C19orf2 | 2.56 |
| Ssc.13545.1.A1_at | Chromosome 21, open-reading frame 66 | C21orf66 | 2.15 |
| Ssc.18307.1.A1_at | RNA binding motif protein 25 | RBM25 | 2.38 |
| Ssc.12654.2.A1_at | Myocyte enhancer factor 2A | MEF2A | 1.70 |
| Ssc.8247.1.A1_at | Opioid binding protein/cell adhesion molecule-like | OPCML | 1.71 |
| Ssc.27217.2.A1_at | Zinc finger protein 354A | ZNF354A | 1.84 |
SAM, Significance Analysis of Microarrays. Normalized and raw data are available at GEO under the entry GSE14643 (http://www.ncbi.nlm.nih.gov/geo/).
DISCUSSION
The present study demonstrates that MPC transplantation leads to long-term functional and bioenergetic improvement in a clinically relevant porcine model of postinfarction LV remodeling, despite no significant engraftment of MPCs at 4 mo in the heart. Stem cell transplantation resulted in an overall improved long-term remodeling of the LV following MI, with a decrease in LVH and amelioration of LV dilatation. This was associated with a reduction in scar size at 4 mo. MPC transplantation also resulted in long-term differential expression of genes, which included a downregulation of mitochondrial oxidative enzymes and upregulation of MEF2a and ZFP91.
Long-term improvement in ventricular function.
The present study unequivocally demonstrates that MPC transplantation leads to long-term improvement in ventricular function in a large preclinical animal model. This is an important finding, as clinical studies have shown mixed outcomes after stem cell transplantation, with some showing benefit (3, 24, 31), and some no difference in LV ejection fraction (12, 14). In the bone marrow transfer to enhance ST-elevation infarct regeneration study, the treated group showed significant short-term increase of LV ejection fraction at 6 mo, but, interestingly, no difference between the two groups was present at 18 mo (16). Similarly, in a rat model of MI, allogenic MSC treatment led to an improvement in LV function 4 wk after transplantation, but this improvement was lost at 6 mo (6). In the present study, MPC transplantation also mitigated the adverse long-term effects of ventricular remodeling. This was seen as a reduction in LVH (Table 1) and LV dilatation (Table 3) in the MPC-treated animals.
Myocardial energetics.
Postinfarction LV remodeling is associated with decreased HEPs, PCr/ATP, and expression of several proteins crucial to oxidative ATP production, and these abnormalities are most severe in the BZ (10). Our study confirmed these reports and showed that these abnormalities are worse in the subendocardial layers and further worsened during catecholamine stimulation-induced high-cardiac work state (Table 4). Previously, our laboratory has shown improvement in myocardial energetics 1 mo following MPC transplantation, which is related to reduction in wall stress and consequent improvement in the mismatch of energy delivery and demand (33). In the present study, we demonstrate that this improvement in myocardial energetics persists up to 4 mo after transplantation (Table 4).
In hearts with cardiac hypertrophy, we have previously reported that myocardial PCr/ATP is significantly decreased, which is linearly related to the severity of hypertrophy and LV dysfunction and is independent from a persistent myocardial ischemia (35–37, 39). These LVH hearts are also associated with a significant lower myocardial total creatine level (36–39), which, in principle, can cause the reduction of the myocardial creatine phosphate level. In the heart, creatine is not synthesized in myocytes and is taken up by the cell via the creatine transporters against a significant concentration gradient (25, 29). The reduction of myocardial total creatine content in the LVH hearts was found to be caused by the decrease of myocardial creatine transporter (25, 29). In the present study, we do not have direct evidence on whether the improvement of the PCr/ATP is accompanied by the increase of myocardial total creatine content or creatine transporter protein expression.
Lack of long-term engraftment of MPCs.
The functional and bioenergetic improvement occurred, despite no long-term engraftment of stem cells in pig hearts at 4 mo. Our laboratory has previously shown that there is a very low engraftment rate and even lower cardiomyocyte differentiation rate 1 mo following MPC transplantation (33). Thus it seems that there is transient engraftment of MPCs in the myocardium, which decreases with time and is not present at 4 mo. This conclusion is supported by other groups that have found transient engraftment of MSCs with very little differentiation as early as 3 days after transplantation in a mice model of MI (20). In contrast, Amado et al. have shown that MSC transplantation in a porcine model of MI led to engraftment of these cells at 2 mo, which was associated with a decrease in scar size and improvement in LV function (1). In a rat model of MI, MSC engraftment was seen up to 6 mo follow-up (6). These cells expressed muscle-specific markers but did not fully differentiate into a cardiac phenotype. In another study, intravenous injection of human MSC in a mice MI model resulted in functional improvement with no engraftment in the heart after 3 wk (11). These data support the conclusion of the present study that host myocardial structural (Fig. 3) and genomic expression changes (Table 5) are associated with the observed chamber functional improvements (Fig. 2). Our laboratory has also previously shown an increase in vascular density in the BZ 1 mo following cell transplantation (30).
Effect of cell transplantation on infarct size.
Sequential MRI functional assessment allowed us to conclude that the infarct size in swine treated with MPC decreased from 1 wk to 4-mo interval, while the scar sizes in swine without MPC treatment were maintained throughout the follow-up (Fig. 3). This reduction in scar size with stem cell treatment has been seen previously in other reports (1, 9). The beneficial effects of MPC transplantation could be related to MPC differentiation into cardiomyocytes (23) or mobilization of endogenous progenitors to the injury site by paracrine effects (9). As we did not observe any engraftment of MPCs at 4 mo, the functional and bioenergetic improvements are likely secondary to paracrine effects that may include sparing of native cardiomyocytes from apoptosis.
Downregulation of mitochondrial oxidative enzymes.
MPC transplantation was associated with downregulation of several different subunits of the respiratory chain (Table 5). The LV dysfunction of failing hearts is associated with the oxidative stress caused by the excess of reactive oxygen species production in the cytoplasm and the electron transport chain of mitochondria in myocytes (26). In the present study, the observed reduction of several different subunits of the respiratory chain oxidative enzyme (Table 5) in hearts with the MPC transplantation may result in the reduction of the oxidative stress, which could in turn, reduce the myocardial apoptosis that we have observed recently using the same cell type for the transplantation and same animal model (30).
Our laboratory has previously reported that LV bulging at infarct zone was accompanied by a significant increase of regional LV wall stress at the infarct zone and peri-infarct BZ (10). This particular increase of regional wall stresses and its associated severe bioenergetic abnormality were significantly ameliorated by the MPC transplantation (7, 33). In the present study, we reasoned that the decrease of LV bulging at LV scar area resulted in reduction of the regional wall stress and energy demand, which, in turn, resulted in the differential expression of several different subunits of the respiratory chain enzymes (Table 5).
Increased levels of ZFP91.
ZFP91 is a member of the zinc finger family of proteins, and its expression was increased by 3.62-fold. Apart from the monocistronic transcript originating from this locus, a cotranscribed variant composed of ZFP91 and CNTF sequence has been identified. CNTF promotes survival and decreases apoptosis in neurons and islet cells and has also been shown to reverse cardiac hypertrophy in mice (21, 22).
Upregulation of MEF2a.
The expression of MEF2a was increased by 1.7-fold in our present study. MEF2a is the predominant MEF2 gene product expressed in postnatal cardiac muscle. MEFs play an important role in myogenesis (4). MEF2a is also important for maintaining appropriate mitochondrial content and cyto-architectural integrity in the postnatal heart (19). We postulate that MPC treatment may cause an activation of endogenous cardiac stem cells, and MEF2a may play a role in their differentiation into cardiomyocytes.
In summary, the present study demonstrates that MPC transplantation leads to long-term functional and bioenergetic improvement in a porcine model of postinfarction LV remodeling, despite no significant engraftment of stem cells in the heart. MPC transplantation also mitigates the adverse effects of LV remodeling, as seen by a reduction in LVH and LV dilatation. These beneficial effects are accompanied by a significant reduction in infarct size. It was also shown that MPC transplantation results in differential expression of certain genes, including mitochondrial oxidative enzymes, ZFP91, and MEF2a, which may play a role in long-term functional improvement after transplantation.
APPENDIX
Cell culture.
Bone-marrow derived MPCs were expanded from one of the established swine multipotent adult progenitor cell lines generated as described previously in detail (34). MPCs at population doubling 80 (approximately population doubling 70) were transduced with a LacZ retrovirus (RV) supernatant [LacZ RV supernatants were generated by cotransfection of pVSV-G retroviral vector (BD Biosciences) and pCL-MFG-LacZ RV packaging vector (Imgenex) using the calcium phosphate method (BD Bioscience, K2051-1) in GP-293 cell line (BD Bioscience)]. Large-scale expanded and LacZ-transduced cells expressed low to nondetectable Oct-4 mRNA. These MPCs have previously been shown to differentiate along different cell lineages (33).
The transduced swine cells were expanded using the following methods. Cells were cultured in 60% DMEM-low glucose/40% MCDB-201 supplemented with 1× insulin transferrin selenium, 0.5× linoleic acid-BSA, 0.1 mM l-ascorbic acid 2-phosphate, 100 U penicillin, 1,000 U streptomycin, 50 nM dexamethasone, and 2% prescreened FBS with 10 ng/ml each human PDGF-BB and human EGF and passaged every 2–3 days through removal of the growth media and addition of 5 ml 0.25% trypsin per T150 flask. After the flask was rocked to coat the bottom of the flask with trypsin, the cells were incubated at room temperature for 1 min. Each flask was tapped lightly, and 1 ml of FBS was then added. Cells were removed, and cells were combined into a 50-ml conical tube. Flasks were rinsed with 10-ml PBS, and this volume was added to cells. Cells were pelleted through centrifugation at 400 g for 5 min, and, after removal of the supernatant, the cells were resuspended in 1 ml of complete medium and counted using a hemacytometer. Flasks were routinely seeded at 500 cells/cm2 in flasks coated with 10 ng/ml fibronectin A with 10 ml media per T150 flask. After an initial placement of the flasks at 37°C, 5.5% CO2 overnight, the media was removed and replaced with fresh media, and cells were incubated another 2 days before passaging.
MPCs were harvested, centrifuged, and resuspended at 10 million cells/1 ml cryopreservation solution that consists of 70% growth media, 20% FBS, and 10% DMSO in 1 ml cryovials. Cells were frozen at ∼1°C/min to −80°C and transferred into liquid nitrogen for long-term storage until implantation. To thaw MPCs before grafting, basal media (expansion media without PDGF-BB, EGF, FBS, and penicillin/streptomycin) was prewarmed to 37°C, and 9 ml of the warmed media were placed into a 15-ml conical centrifuge tube. Vials of frozen MPC were thawed in a 37°C water bath for ∼1 min, after which cells were transferred to the prewarmed medium. The cell number was counted to reach 50 million by hematocytometer and Trypan blue was used to check cell viability during the counting. MPC were washed by 2× PBS, spun down at 400 g for 5 min, and resuspended in 2 ml saline. A small fraction of cells were replated in a T-150 flask to check the cell viability and percentage of LacZ-positive cells. Cells used for transplantation were >80% viable.
MRI methods.
MRI was performed on a 1.5-T clinical scanner (Siemens Sontata, Siemens Medical Systems, Islen, NJ) using a phased-array four-channel surface coil and ECG gating. Animals were anesthetized with 1% isoflurane and positioned in a supine position within the scanner. The protocol consisted of 1) localizing scouts to identify the long- and short-axis of the heart; 2) short- and long-axis cine for the measurement of global cardiac function; and 3) delayed contrast-enhancement for the assessment of scar size. Steady-state free precession “True-FISP” cine imaging used the following MR parameters: repetition time (TR) = 3.1 ms, echo time (TE) = 1.6 ms, flip angle = 79°, matrix size = 256 × 120, field of view = 340 mm × 265 mm, slice thickness = 6 mm (4 mm gap between slices), and 16–20 phases were acquired across the cardiac cycle. Global function and regional wall thickness data were computed from the short-axis cine images using MASS (Medis Medical Imaging Systems, Leiden, The Netherlands) for the manual segmentation of the endocardial and epicardial surfaces at both end-diastole and end-systole from base to apex. Short-axis turboFLASH imaging, from base to apex, used TR = 16 ms, TE = 4 ms, inversion time ∼ 220 ms, flip angle = 30°, matrix size = 256 × 148, field of view = 320 mm × 185 mm, slice thickness = 6 mm (0 mm gap between slices), and two signal averages. The appropriate inversion time was chosen to adequately null the signal intensity of normal myocardium. Infarct size was calculated from the delayed contrast-enhanced images using MASS to manually segment regions of nonviable tissue. Infarct size was calculated as the ratio of the total scar area to the total LV area.
Spatially localized 31P-MRS technique.
Magnetic field shimming to improve the magnetic field homogeneity of the heart was achieved by the “auto-shim” scheme (Varian). Spatially localized 31P-NMR spectroscopy was performed using the RAPP-ISIS/FSW method (18, 32, 36, 37), which is the rotating-frame experiment using the adiabatic plane-RAPP-ISIS/FSW method. Detailed experiments documenting voxel profiles, voxel volumes, and spatial resolution attained by this method have been published previously. In this application of RAPP-ISIS/FSW, the signal origin was first restricted to a 12 × 12-mm two-dimensional column perpendicular to the LV wall. The signal was later localized into three well-resolved and five partially resolved layers along the column and, hence, across the LV wall. Localization along the column was based on B1 phase encoding and employed a nine-term FSW, as previously describe (18, 32, 36, 37). The phase-encoded data were used to generate a voxel or a “window” that can be shifted arbitrarily by post-data-acquisition processing along the phase-encode direction (in this case, perpendicular to the surface coil and thus the heart wall); consequently, voxels were generated at different distances or “depths” from the outer LV wall. However, we normally present five voxels centered about 45, 60, 90, 120, and 135° phase angles, as previously described. There is absolutely no overlap between the 135° voxel (corresponding to the subepicardium) and the 45° voxel (corresponding to the subendocardium). Whole wall spectra were obtained with the ISIS technique, defining a column 12 × 12 mm2 perpendicular to the heart wall. The calibration of spectroscopic parameters was facilitated by placing a polyethylene capillary filled with 15 μl of 3 M/l phosphonoacetic acid into the inner diameter of the surface coil. This phosphonoacetic acid standard was used only for calculating the 90° pulse length of the RAPP-ISIS method (18, 32, 36, 37). The position of the voxels relative to the coil was set according to the B1 strength at the coil center, which was experimentally determined in each case by measuring the 90° pulse length for the phosphonoacetic acid standard contained in the reference capillary at the coil center. NMR data acquisition was gated to the cardiac and respiratory cycles using the cardiac cycle as the master clock to drive both the respirator and the spectrometer, as previously described (18, 32, 36, 37). The surface coil was constructed from a single-turn copper wire, 25 mm in diameter, with each side of the coil leads soldered to a 33-pF capacitor. Complete transmural data sets were obtained in 10-min time blocks using a repetition time of 6–7 s to allow for full relaxation for ATP and Pi and ∼95% relaxation of the PCr resonance (18, 32, 36, 37). The ratios of PCr to ATP (PCr/ATP) were calculated for each transmurally differentiated spectra set, as previously described (18, 32, 36, 37). All resonance intensities were quantified using integration routines provided by the SISCO software.
1H-NMR spectroscopy technique.
1H-NMR methods have been previously reported in detail (5, 18). In brief, radio-frequency transmission and signal detection were performed with the dually tuned 28-mm-diameter surface coil. A single-pulse-collection sequence with a frequency selective Gaussian excitation pulse (1 ms) was used to selectively excite the N-δ proton resonance signal of the proximal histidine in deoxymyoglobin (Mb-δ). This technique provided sufficient water suppression due to the large chemical shift difference between water and Mb-δ (>14 kHz). The NMR signal was optimized by adjusting the radio-frequency pulse power using the water signal as a reference. A short TR (25 ms) was used due to the short T1 of Mb-δ. Each spectrum was acquired in 5 min (10,000 free induction decays). Although the short T1 of Mb-δ and fast acquisition prevent gating to the cardiac cycle, the signal loss due to motion is negligible compared with the inherently broad line width of the Mb-δ peak.
Surgical preparation for open-chest MRS study.
Detailed surgical preparations for MRS study were published previously (10, 32, 33). Briefly, animals were anesthetized with pentobarbital (30 mg/kg followed by a 4 mg·kg−1·h−1 iv), intubated, and ventilated with a respirator and supplemental oxygen. Arterial blood gases were maintained within the physiological range by adjustments of the ventilator settings and oxygen flow. Heparin-filled polyvinyl chloride catheters (3.0 mm outer diameter) were inserted into the ascending aorta and inferior vena cava. A sternotomy was performed, and the heart was suspended in a pericardial cradle. A third heparin-filled catheter was introduced into the LV through the apical dimple and secured with a purse-string suture. A 25-mm-diameter NMR surface coil was sutured onto the anterior wall of the LV adjacent to the infarct region. The pericardial cradle was then released, and the heart was allowed to assume its normal position in the chest. The surface coil leads were connected to a balanced-tuned external circuit, and the animals were positioned within the magnet. Hearts with postinfarction LV remodeling are very sensitive to alterations of loading conditions of the LV, and that subtle differences in LV end-diastolic pressure or LV systolic pressure could affect myocardial contractile performance measurements. To compensate for insensible fluid loss in the open-chest studies, we routinely administer saline at rate of 1 ml/min iv during the entire experimental data acquisition. Ventilation rate, volume, and inspired oxygen content were adjusted to maintain physiological values for arterial Po2, Pco2, and pH. Aortic and LV pressures were monitored continuously throughout the study. Hemodynamic measurements were acquired simultaneously with the 1H- and 31P-MR spectra.
GRANTS
This work was supported by US Public Health Service Grants HL50470, HL61353, and HL67828. M. N. Jameel was supported by American Heart Association Greater Midwest Predoctoral Award 0810015Z.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 102: 11474–11479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 355: 1222–1232, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 106: 3009–3017, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol 14: 167–196, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chen W, Cho Y, Merkle H, Ye Y, Zhang Y, Gong G, Zhang J, Ugurbil K. In vitro and in vivo studies of 1H NMR visibility to detect deoxyhemoglobin and deoxymyoglobin signals in myocardium. Magn Reson Med 42: 1–5, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation 112: 214–223, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol 293: H1772–H1780, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, Swingen C, Zhang G, Feygin J, Ochiai K, Bransford TL, From AH, Bache RJ, Zhang J. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol 291: H648–H657, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun 354: 700–706, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367: 113–121, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA 102: 8966–8971, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 355: 1199–1209, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9: 1195–1201, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113: 1287–1294, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Murakami Y, Zhang J, Eijgelshoven MH, Chen W, Carlyle WC, Zhang Y, Gong G, Bache RJ. Myocardial creatine kinase kinetics in hearts with postinfarction left ventricular remodeling. Am J Physiol Heart Circ Physiol 276: H892–H900, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Murakami Y, Zhang Y, Cho YK, Mansoor AM, Chung JK, Chu C, Francis G, Ugurbil K, Bache RJ, From AH, Jerosch-Herold M, Wilke N, Zhang J. Myocardial oxygenation during high work states in hearts with postinfarction remodeling. Circulation 99: 942–948, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Naya FJ, Black BL, Wu H, Bassel-Duby R, Richardson JA, Hill JA, Olson EN. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med 8: 1303–1309, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 14: 840–850, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Raju SV, Zheng M, Schuleri KH, Phan AC, Bedja D, Saraiva RM, Yiginer O, Vandegaer K, Gabrielson KL, O'Donnell CP, Berkowitz DE, Barouch LA, Hare JM. Activation of the cardiac ciliary neurotrophic factor receptor reverses left ventricular hypertrophy in leptin-deficient and leptin-resistant obesity. Proc Natl Acad Sci USA 103: 4222–4227, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rezende LF, Stoppiglia LF, Souza KL, Negro A, Langone F, Boschero AC. Ciliary neurotrophic factor promotes survival of neonatal rat islets via the BCL-2 anti-apoptotic pathway. J Endocrinol 195: 157–165, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA 104: 17783–17788, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355: 1210–1221, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Ten Hove M, Chan S, Lygate C, Monfared M, Boehm E, Hulbert K, Watkins H, Clarke K, Neubauer S. Mechanisms of creatine depletion in chronically failing rat heart. J Mol Cell Cardiol 38: 309–313, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Trachtenberg BH, Hare JM. Biomarkers of oxidative stress in heart failure. Heart Fail Clin 5: 561–577, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Tsai S, Cassady JP, Freking BA, Nonneman DJ, Rohrer GA, Piedrahita JA. Annotation of the Affymetrix porcine genome microarray. Anim Genet 37: 423–424, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, Dawson D, Hulbert K, Zhang W, Zhang MH, Watkins H, Clarke K, Neubauer S. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: insights from creatine transporter-overexpressing transgenic mice. Circulation 112: 3131–3139, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Jameel MN, Li Q, Mansoor A, Qiang X, Swingen C, Panetta C, Zhang J. Stem cells for myocardial repair with use of a transarterial catheter. Circulation 120: S238–S246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364: 141–148, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation 103: 1570–1576, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 115: 1866–1875, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O'Brien TD, Zhang J, Verfaillie C. Multipotent adult progenitor cells from swine bone marrow. Stem Cells 24: 2355–2366, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, Merkle H, Ugurbil K, From AH, Bache RJ. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation 92: 1274–1283, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Zhang J, McDonald KM. Bioenergetic consequences of left ventricular remodeling. Circulation 92: 1011–1019, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Zhang J, Merkle H, Hendrich K, Garwood M, From AH, Ugurbil K, Bache RJ. Bioenergetic abnormalities associated with severe left ventricular hypertrophy. J Clin Invest 92: 993–1003, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang J, Murakami Y, Zhang Y, Cho YK, Ye Y, Gong G, Bache RJ, Ugurbil K, From AH. Oxygen delivery does not limit cardiac performance during high work states. Am J Physiol Heart Circ Physiol 277: H50–H57, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Zhang J, Toher C, Erhard M, Zhang Y, Ugurbil K, Bache RJ, Lange T, Homans DC. Relationships between myocardial bioenergetic and left ventricular function in hearts with volume-overload hypertrophy. Circulation 96: 334–343, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Zhang J, Wilke N, Wang Y, Zhang Y, Wang C, Eijgelshoven MH, Cho YK, Murakami Y, Ugurbil K, Bache RJ, From AH. Functional and bioenergetic consequences of postinfarction left ventricular remodeling in a new porcine model. MRI and 31 P-MRS study. Circulation 94: 1089–1100, 1996 [DOI] [PubMed] [Google Scholar]


