Abstract
Roux-en-Y gastric bypass (RYGB) surgery has been shown to decrease consummatory responsiveness of rats to high sucrose concentrations, and genetic deletion of glucagon-like peptide-1 receptors (GLP-1R) has been shown to decrease consummatory responsiveness of mice to low-sucrose concentrations. Here we assessed the effects of RYGB and pharmacological GLP-1R modulation on sucrose licking by chow-fed rats in a brief-access test that assessed consummatory and appetitive behaviors. Rats were tested while fasted presurgically and postsurgically and while nondeprived postsurgically and 5 h after intraperitoneal injections with the GLP-1R antagonist exendin-3(9–39) (30 μg/kg), agonist exendin-4 (1 μg/kg), and vehicle in 30-min sessions during which a sucrose concentration series (0.01–1.0 M) was presented in 10-s trials. Other rats were tested postsurgically or 15 min after peptide or vehicle injection while fasted and while nondeprived. Independent of food-deprivation state, sucrose experience, or GLP-1R modulation, RYGB rats took 1.5–3× as many trials as sham-operated rats, indicating increased appetitive behavior. Under nondeprived conditions, RYGB rats with presurgical sucrose experience licked more to sucrose relative to water compared with sham-operated rats. Exendin-4 and exendin-3(9–39) impacted 0.3 M sucrose intake in a one-bottle test, but never interacted with surgical group to affect brief-access responding. Unlike prior reports in both clearly obese and relatively leaner rats given RYGB and in GLP-1R knockout mice, we found that neither RYGB nor GLP-1R blockade decreased consummatory responsiveness to sucrose in our less obese chow-fed rats. Collectively, these results highlight the fact that changes in taste-driven motivated behavior to sucrose after RYGB and/or GLP-1R modulation are very model and measure dependent.
Keywords: gustatory, bariatric surgery, sugar, type 2 diabetes mellitus, anorexigenic peptides
roux-en-y gastric bypass (rygb) surgery is currently considered a successful long-term treatment option for morbid obesity and can cause remission of type 2 diabetes (38, 49, 59). In this procedure, the majority of the stomach and the proximal intestine are surgically isolated from contact with food, and the remaining small gastric pouch is anastamosed to the jejunum (Fig. 1). The residual stomach and proximal small intestine are reattached to the jejunum distal to its junction with the gastric pouch. The weight loss and relatively swift improvement of glycemic status that follow RYGB are thought to be due, in large part, to behavioral and neuroendocrine changes.
Fig. 1.
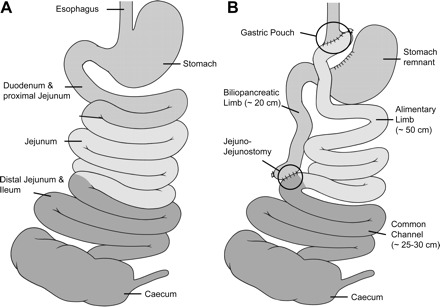
Schematic illustration of the small bowel anatomy before (A) and after (B) Roux-en-Y gastric bypass (RYGB) operation.
That RYGB decreases caloric intake in patients has been consistently shown across many studies (7, 9, 26, 29, 58) and has been reported to continue up to 15 yr after the operation (48, 49). It is not clear, however, if patients only eat less in general, or if they also choose different types of foods to eat after RYGB. Some RYGB patients have reported, via food diaries and food-frequency questionnaires, that they eat fewer sweet or fatty foods (7, 26, 58), but the interpretation as to the extent to which the avoidance is due to learned aversions, decreased motivation for food, or to changes in the sense of taste differs across studies. For example, some patients after RYGB claim that sweet foods taste sweeter, and they accordingly change their food selections (14). In contrast, other patients hedonically rate a series of sucrose concentrations similarly before and after the operation (13). Still other patients indicate that they continue to regard high-calorie foods as delicious, but avoid these foods because of tolerability and/or lack of interest (22).
Some investigators have used animal models to explore the effect of RYGB on sucrose responsiveness by using high-fat-diet-fed obese (as determined by body composition analysis and leptin levels) rats (46), or rats with genetic manipulations that result in increased adiposity (21). Shin et al. (46) reported that high-fat-diet-fed obese rats licked at a lower rate to very low-sucrose concentrations (those near detection threshold; see Refs. 8, 54, 60) and at a higher rate to high-sucrose concentrations during 10-s trials in a brief-access test compared with age-, but not body-weight-matched, chow-fed rats with significantly lower adiposity and leptin levels. The concentration-response function seen in the leaner chow-fed rats was rescued in high-fat-diet-fed obese rats when they were tested after RYGB. Similar effects at high, but not low, sucrose concentrations were seen in cholecystokinin-1 receptor-deficient Otsuka Long-Evans Tokushima Fatty (OLETF) rats after RYGB when they were compared with sham-operated OLETF rats that were pair-fed based on the intake of RYGB rats (21).
Similar to Hajnal et al. (21), Tichansky et al. (62) found that chow-fed rats with no genetic manipulation that received RYGB licked at lower rates to high, but similar rates to low, sucrose concentrations in a brief-access test compared with sham-operated rats (although note that these sham-operated rats were not pair fed to the intake of RYGB rats as in Ref. 21; also, adiposity and leptin levels were not reported in this study). Also, using a previously established model in chow-fed rats (10–13, 30–32), Bueter et al. (13) reported that RYGB rats showed less preference for sucrose across a range of concentrations in a 24-h two-bottle test, but this effect was partially attenuated by presurgical exposure to sucrose. Although these studies all used different models and methods, they all suggest that the affective component of sucrose taste may be blunted, at least at higher concentrations, along with the body weight loss that accompanies RYGB surgery; however, the mechanisms underlying such changes in taste-guided behavior remain to be elucidated.
The changes in responsiveness to, and preference for, sucrose seen after RYGB in rats, and, potentially, food selection in humans, may stem from how the surgery alters the hormonal milieu. RYGB increases postprandial release of peptide-YY and glucagon-like peptide-1 (GLP-1), hormones released by the gut in the presence of nutrients (Refs. 6, 30, 31, 33; for reviews, see Refs. 39, 41, 67); this has also been seen in a rat model of RYGB (e.g., Refs. 11, 13, 32). Systemic administration of the GLP-1-receptor agonist exendin-4 (Ex4) decreases intake of food and sucrose solution (3, 5, 6, 35, 68, 72, 74), and this effect is blocked by the GLP-1-receptor antagonist exendin-3(9–39) (Ex9) (3, 68, 73, 74), which, under certain conditions, independently increases food intake (Refs. 73, 74; although see Refs. 37, 43). GLP-1 is also expressed in taste receptor cells on the tongue, and its receptors are present on afferent gustatory nerve fibers innervating taste buds (47). Mice with genetic deletion of the GLP-1 receptor show decreased responsiveness to low, but not high, concentrations of sucrose and low concentrations of the nonnutritive sweetener, sucralose, compared with wild-type mice in a brief-access taste test (47). This suggests that alterations in GLP-1 levels and/or GLP-1-receptor activity could be responsible for any observed effect of RYGB on affective taste processing. Accordingly, sucrose responsiveness in rats could be altered by pharmacological modulation of GLP-1 receptors independent of RYGB surgery and/or exaggerated or nullified after RYGB.
Here, we sought to recapitulate the blunted behavioral responsiveness to high concentrations of sucrose seen after RYGB in other rat models in a brief-access test designed to assess both the appetitive and consummatory aspects of taste-guided affective behavior. We also explored the independent and possible interactive effects of pharmacological GLP-1-receptor modulation on affective responsiveness to sucrose in rats with and without RYGB. In our version of the brief-access test, water and each sucrose concentration were presented in randomized blocks of 10-s trials, and a given trial began only when contact was made with the stimulus. Therefore, licking during each trial was measured only in instances in which the stimulus contacted taste receptors; as such, licks served as a measure of consummatory responsiveness. The total number of trials initiated across each session served as a complementary measure of taste-motivated behavior indicative of overall appetitive responsiveness (i.e., approach) associated with the opportunity to engage the reinforcing stimulus.
MATERIALS AND METHODS
General Methods
Subjects.
Male Sprague-Dawley rats (Charles River) were used in all of the experiments. All rats were housed individually in conventional polycarbonate tub cages in vivarium rooms where temperature, humidity, and lighting (12-h lights on; 12-h lights off) were controlled automatically. The rats did not receive high-fat diets at any time, and, except when otherwise noted, rats received ad libitum access to standard maintenance chow (PMI 5001, LabDiets, St. Louis, MO) and reverse-osmosis deionized water. Body weight was measured daily, but we performed no direct measures of adiposity. All procedures were approved by the Animal Care and Use Committee of Florida State University.
Test stimuli.
Test stimuli consisted of reverse-osmosis deionized water and sucrose solutions (BDH Chemicals, West Chester, PA). All solutions used were prepared daily and presented at room temperature.
Peptides.
The GLP-1-receptor antagonist Ex9 and agonist Ex4 (California Peptide Research, Napa, CA) were dissolved in phosphate-buffered saline. Solutions of the necessary concentrations were prepared en masse and frozen in aliquots; the amount needed for injection each day was thawed that morning and injected at room temperature. A washout period of at least 24 h occurred between each peptide administration.
Because there is some evidence that central administration of GLP-1 in rats can serve as an unconditioned stimulus in taste-aversion learning (e.g., Refs. 27, 45, 61), and patients receiving peripheral injections of the GLP-1 analog exenatide (Byetta) have reported nausea and malaise as side effects (e.g., Refs. 2, 75), we chose the highest doses, particularly of Ex4 (1 μg/kg; Ex9: 30 μg/kg), that appear to produce consistent effects on feeding, without the threat of nonspecific effects on behavior that could interfere with our testing protocol.
Brief-access apparatus and procedure.
The rats were trained and tested in a lickometer known as a Davis rig (Davis MS-160, DiLog Instruments; see Ref. 50). We used a brief-access procedure (see Ref. 51) in which a rat was placed in the test chamber and allowed access to solutions during a 30-min session. In a series of sessions, water was presented from a stationary spout, water from different tubes was presented in 10-s trials, or a concentration series of sucrose was presented individually in 10-s trials. Access to taste stimuli was available to the rat via spouts attached to small glass tubes that held solutions. The tubes and spouts were seated in a horizontal array on a computer-controlled motorized rack and were positioned individually behind a slot, covered by a shutter, in the front panel of the test chamber. During sessions in which the stimuli were presented in successive trials, the shutter moved to uncover the access slot, and a trial was initiated with the first lick, after which the solution was available for 10 s. Upon completion of the trial, the shutter was closed over the access slot, the rack was moved, and a different tube was placed into position. After an intertrial interval of 7.5 s, the shutter was again opened, and the rat was given the opportunity to initiate another trial. If the rat did not sample a presented stimulus, the rack would not advance, and the unsampled stimulus would stay in place for the remainder of the session. Presentation order of the stimuli was randomized without replacement in blocks of trials, which means that, once the rat had sampled all of the concentrations in one block of seven trials, another block would begin, and trials would cycle once again through all of the concentrations, which were presented in random order within each block.
In general, to train the animals to sample in the Davis rigs, water bottles were removed from the rats' cages ∼23 h before a session in which water was presented from one spout that remained stationary. A session with these parameters was repeated the following day. On the next 2 days, sessions were conducted once daily in which water was presented in multiple bottles across successive trials. On the 5th day during which the rats were water restricted, water and the sucrose concentration series (0.01, 0.03, 0.06, 0.1, 0.3, 1.0 M) were presented in trials. Water was returned to the rats ∼30 min after this final training session. While water restricted, the body weights of the rats were monitored daily with a contingency in place that any rat falling below 85% of its prerestriction body weight would receive 10 ml supplemental water 30 min after completion of its session. After training, the rats were tested once daily in sessions in which the sucrose concentration series was presented in trials, either after they had been ∼23 h fasted or had been provided with ad libitum access to food (i.e., nondeprived).
Data analysis.
Our measures for each phase of the studies in which brief-access performance was measured consisted of the following: 1) the number of licks of water taken and interlick interval (ILI) during the 2nd day of testing during which water was presented from a stationary spout; 2) the lick score, defined as the mean number of licks taken per trial of each sucrose concentration less the mean number of licks taken per trial of water during testing with the sucrose concentration series; and 3) the number of trials initiated by the rats during each session. The individual ILIs, which, under constant test conditions, are relatively invariant in rodents (15, 64, 69, 71) and, ultimately, place a limit on the maximal lick rate, were filtered to include those between 50 and 250 ms in duration, because values <50 ms were considered double licks, and those values >250 ms were considered pauses between licking bursts (17); the individual ILIs meeting the criterion were averaged across session. Only rats that had initiated at least two trials of each concentration across the days that they were tested in each phase were included in the statistical analysis of lick score. This was done to increase the veracity of our estimate of concentration-dependent responsiveness and minimize the influence of potential spurious values. However, all rats were included in the analysis of trials initiated because, even if an animal did not engage a sufficient number of trials to form a reliable estimate of consummatory responsiveness across the concentration range, the number of trials initiated is still an accurate reflection of the animal's general appetitive tone.
The average lick score of each surgical group in each condition was used to fit the three-parameter logistic function:
| (1) |
in which x is sucrose concentration, a is asymptotic lick response adjusted for water, b is slope, and c is log10 concentration at the inflection point (EC50).
ANOVA was used to compare data from sham-operated and RYGB-operated groups, which were between-subject factors, and, during certain phases, injection conditions and sucrose concentration, which were within-subject factors. When two-way ANOVA revealed significant effects, t-tests and/or one-way ANOVA were conducted to compare the appropriate conditions; the resulting P values were Bonferroni corrected. An α of 0.05 was used throughout the experiments as the statistical rejection criterion.
Experiment 1: Effects of RYGB and Pharmacological GLP-1-receptor Manipulations on Brief-access Responding to Sucrose and Water
Age and body weight of subjects.
In this experiment, the rats weighed, on average, 340 g and were ∼2.5 mo old on arrival and 540 g and ∼4 mo old at the time of surgery (Fig. 2).
Fig. 2.
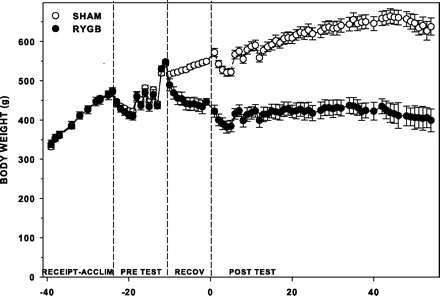
Mean (±SE) absolute body weight of sham and RYGB rats (n = 7 per surgical group) at receipt and through acclimation (Receipt-Acclim), during presurgical training and testing (Pre Test), the first 10 days after surgery (Recov), and throughout postsurgical testing (Post Test). Slight changes in weight during testing can be attributed to food and water manipulations, which are noted in Table 1.
Peptide doses and time course.
During portions of testing in which the effects of pharmacological manipulations of GLP-1 receptors were assessed, all of the rats were injected with Ex9 (30 μg/kg ip), Ex4 (1 μg/kg ip), and vehicle (1 ml/kg ip) 5 h before testing. The doses and time course of the peptides were chosen based on our unpublished pilot work that showed a 220% increase and 17% decrease in chow intake of rats (n = 6 per peptide group) 5 h after injection of Ex9 and Ex4, respectively, and the published work of others that showed similar effects (72–74).
Presurgical brief-access training and testing.
During training (Table 1), provision of supplemental water was necessary for two rats. The rats were fasted and tested with sucrose every other day for 1 wk (Table 1).
Table 1.
Training and testing protocol for experiments 1 and 2
| Condition | Deprivation State | Stimuli a | No. of Test Sessions | Injection b | Days in Experiment 1c | Days in Experiment 2c |
|---|---|---|---|---|---|---|
| Presurgery d | Water restricted e | Water: stationary | 2 | None | −23, −22 | |
| Water: trials | 2 | −21, −20 | ||||
| Sucrose | 1 | −19 | ||||
| Fasted e | Sucrose | 3 | −17, −15, −13 | |||
| Postsurgery | Water restricted e | Water: stationary | 2 | None | 2, 3 | 2, 3 |
| Water: trials | 2 | 4, 5 | 4, 5 | |||
| Sucrose | 1 | 6 | 6 | |||
| Fasted e | Sucrose | 2 | 9, 13 | 9, 17 | ||
| Nondeprived | Sucrose | 2 | 17, 21 | 33, 35 | ||
| Postsurgery pharmacological GLP-1-receptor manipulations d | Nondeprived f | Sucrose | 2 | Vehicle | 31, 33 or 32, 34 | |
| Sucrose | 2 | Ex9 | 35, 37 or 36, 38 | |||
| Sucrose | 2 | Ex4 | 39, 41 or 40, 42 | |||
| Sucrose | 2 | Vehicle | 43, 45 or 44, 46 | |||
| Water restricted f | Water: stationary | 1 | Vehicle | 49 or 50 | ||
| Water: stationary | 1 | Ex9 | 51 or 52 | |||
| Water: stationary | 1 | Ex4 | 53 or 54 |
GLP-1, glucagon-like peptide-1; Ex4, exendin-4; Ex9, exendin-3(9-39).
Presented in 30-min sessions in a Davis rig lickometer.
Injections were performed 5 h before session.
Because of different postoperative recovery periods for each rat, day 0 reflects the start of postsurgical testing, and negative numbers are counted backward from the 10th day of recovery after surgery (See Figs. 2 and 5).
Only performed in experiment 1.
Food or water removed from cage ∼23 h before session.
Food was removed at time of injection.
Surgery.
Rats were assigned to either RYGB or sham-operated (sham) surgery groups such that the following parameters were matched: licks of water taken on the 2nd day of training when water was presented continuously, licks and trials of sucrose taken during presurgical testing, and body weight.
Food was removed from the rats overnight before surgery. Anesthesia was induced in a chamber filled with 5% isoflurane in oxygen (1 l/min). Each rat was shaved from sternum to pelvis, and the area disinfected with a betadine scrub. The rat was then placed in supine position on an isothermal heating pad and positioned in a nose cone that maintained anesthesia (2–4% isoflurane in 1 l/min oxygen) for the duration of the surgery. All surgeries were conducted by one surgeon (M. Bueter), as previously described (10–13, 32). Briefly, for the RYGB procedure (Fig. 1), which lasted ∼70 min, the small bowel was transected 20 cm distal to the pylorus of the stomach. The portion of the intestine caudal to the transection was anastomosed to a small section of gastric mucosa (∼4–5 mm), which was separated from the stomach close to the gastroesophageal junction. The portion of the intestine anterior to the transection, continuous with the remaining portion of the stomach, was anastomosed to the ileum ∼25–30 cm from the cecum. For sham operations, which took ∼30 min, an anterior gastrotomy and a jejunotomy with subsequent closures were performed. The abdominal wall and the skin were closed in layers after both operations.
Immediately following surgery, each rat was injected subcutaneously with an antibiotic (gentamycin; 8 mg/kg), an analgesic (ketoralac; 2 mg/kg), and 5 ml of saline and was placed on an isothermal pad in a polycarbonate cage until fully recovered from anesthesia, at which time the animal was returned to its home cage. The rats were allowed at least 10 days to recover before further testing; body weight was monitored daily during this time. For the first 3 days after surgery, each rat was injected with analgesic and antibiotic and was given wet mash (1 part powdered chow and 2 parts water), which was presented fresh daily. Normal chow pellets were returned ∼24 h after surgery.
Across 7 days, RYGB surgery was performed on 23 rats and sham surgery on 15 rats. Postsurgical complications that we observed after RYGB included excessive salivation, difficult respiration, lethargy, accumulation of fluid under the incision site, and soft feces. Of the 23 RYGB rats, 1 died during surgery, 5 died or were euthanized 1–4 days after surgery, and 2 were euthanized 10–12 days after surgery. No complications or deaths were seen after sham surgery. Of the 15 surviving RYGB rats, 7 were used in this study, along with 7 of the 15 sham rats; the remaining rats were used in a study not presented here.
Postsurgical brief-access testing.
To assess the effect of RYGB and sham operations on intake during sessions in which water was presented continuously and to refamiliarize the rats with the Davis rigs, the training procedure used presurgically was repeated (Table 1). During this time, we also assessed effects on the mean ILI. Rats were allowed access to water for 48 h before the next phase of testing, during which time they fully gained back the body weight they had lost while on the postsurgical water-restriction schedule.
To examine if RYGB and sham surgery impacts trial initiation and licking responses to sucrose, the testing procedure that was performed presurgically was repeated postsurgically. However, a 72-h period of refeeding was allowed between fasts, during which time the body weights of the RYGB rats rose to prefast levels. Thus only two test sessions, one on Monday and the other on Friday, were conducted. Rats were also tested with sucrose while nondeprived. These testing sessions under nondeprived conditions took place on Tuesday and Saturday to remain consistent with the procedure used postsurgically to test the rats while they were fasted (Table 1).
Postsurgical brief-access testing with pharmacological GLP-1-receptor manipulations.
The rats were tested once a day for a total of two sessions each after injection of the peptides active at the GLP-1 receptor, starting with the antagonist Ex9. Two vehicle test sessions were conducted both before and after the test series during which Ex4 and Ex9 were injected (Table 1). Food was removed from the rats at the time of injection, which was 5 h before the tests, and returned 30 min after completion of the sessions. Because of the delay between injection and testing, the rats were divided into two squads, with one tested on Mondays and Wednesdays, and the other on Tuesdays and Thursdays for a total of 4 wk. Sham and RYGB rats were represented equally in both of the squads.
After testing with sucrose, the effects of pharmacological GLP-1-receptor manipulation on the intake and ILI of water-restricted rats licking water from a stationary spout were assessed. Water bottles were removed from the rats ∼23 h before each test session and returned 30 min after the completion of each session. The peptides and vehicle were administered as described above for the sucrose tests, except that only one test session was conducted for each peptide and for vehicle, the latter of which was performed before testing with the peptides (Table 1). The rats were tested in two squads, with one tested on Monday, Wednesday, and Friday, and the other on Tuesday, Thursday, and Saturday across 1 wk.
Data analysis.
Differences in average licks and ILI during water testing between sham and RYGB were analyzed separately for the presurgical, postsurgical, and injection conditions. Differences in average sucrose lick score and number of sucrose trials initiated between sham and RYGB were analyzed separately for the presurgical fasted, postsurgical fasted, postsurgical nondeprived conditions, and injection conditions. Because of computer error, total lick data of one of the RYGB rats postsurgically were lost, and so presurgical values from that rat were also not included in analysis. One sham rat did not take enough trials to meet our inclusion criterion, and so its lick score data were excluded from all conditions.
Experiment 2: Effect of RYGB on Brief-access Responding to Sucrose and Water in Rats Without Presurgical Sucrose Testing Experience
Age and body weight of subjects.
Twenty male Sprague-Dawley rats that weighed, on average, 335 g and were ∼2.5 mo old at receipt and 467 g and ∼3.5 mo old at the time of surgery were used in this experiment (see Fig. 5).
Fig. 5.
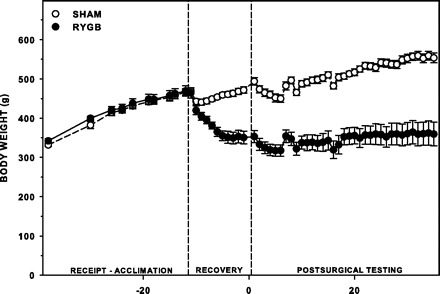
Mean (±SE) absolute body weight of sham rats (n = 9) and RYGB rats (n = 4–6) at receipt and during acclimation, for the first 10 days after surgery (Recovery), and throughout postsurgical testing. Slight changes in weight during testing can be attributed to food and water manipulations, which are noted in Table 1.
Surgery.
Across 7 days, the RYGB operation described in experiment 1 was performed on 10 rats, and the sham operation was performed on 9 rats. The groups were established such that average body weight did not statistically differ between them. Two RYGB rats were euthanized before the start of testing (one 11 days after surgery, and one 23 days after surgery), both due to continual weight loss and marked lethargy. No postoperative complications were seen in the sham group.
Postsurgical brief-access training and testing.
After at least 10 days of recovery from surgery, the rats were trained as described (Table 1). Following training, two sucrose test sessions separated by 6 days were conducted while the rats were fasted (Table 1). Two RYGB rats were euthanized due to marked lethargy before the second day of testing while fasted, and so their data were removed from the entire study, reducing the overall sample size of the RYGB group to six. Approximately 3 wk later, we also performed two sessions of testing, which were separated by 48 h, while the rats were nondeprived (Table 1). Two RYGB rats were not tested during this phase, because they did not completely recover the body weight they had lost during the fasted testing conditions; thus the subject size during tests in which food had not been removed from the rats was reduced to four.
Data analysis.
Differences in the average licks of water taken and ILI during water testing between sham and RYGB were analyzed postsurgically, and sucrose lick score and number of sucrose trials initiated were analyzed separately for the postsurgical fasted and postsurgical nondeprived conditions. Due to machine failure, two sham rats were not tested on the 2nd day of nondeprived testing, but their performance on the single session was sufficient to meet the criterion for inclusion. A different sham rat did not take enough trials across the sessions to be included in lick score analysis.
Experiment 3: Assessment of Shorter Time Course of Pharmacological GLP-1-receptor Manipulations on Brief-access Responding to, and Intake of, Sucrose in Unoperated Rats
Subjects.
Sixteen male Sprague-Dawley rats that weighed, on average, 250 g and were ∼2 mo of age at the beginning of testing were used.
Peptide dose and time course.
The doses of Ex4 and Ex9 listed in experiment 1 were used, but they were injected 15 min before presentation of water and/or sucrose solution.
Brief-access training and testing.
The rats were trained to lick water as described, with the exception that, between the first set of training sessions in which water was presented continuously and the second set in which water was presented in trials, we measured the effect of Ex4 or Ex9 (and vehicle) on licks and ILI. After training in which water was presented in trials, we measured the effect of the peptides on number of trials initiated and licks taken during water trials. The details of these tests are presented in Table 2.
Table 2.
Experiment 3 protocol for training and testing
| Deprivation State | Test Procedure | Stimuli | Injection* | No. of Test Sessions |
|---|---|---|---|---|
| Water restricted† | 1-Bottle intake in Davis rig | Water | None | 2 |
| Vehicle | 1 | |||
| Exendin | 1 | |||
| Brief access trials in Davis rig | Water | None | 2 | |
| Vehicle | 1 | |||
| Exendin | 1 | |||
| Sucrose array | None | 1 | ||
| Fasted† | Brief access trials in Davis rig | Sucrose array | Vehicle | 2 |
| Exendin | 2 | |||
| Vehicle | 2 | |||
| Nondeprived | Brief access trials in Davis rig | Sucrose array | Vehicle | 2 |
| Exendin | 2 | |||
| Vehicle | 2 | |||
| Fasted† | 1-Bottle intake in home cage | 0.3 M sucrose | Vehicle | 2 |
| Exendin | 2 | |||
| Vehicle | 2 | |||
| Vehicle + Vehicle‡ | 2 | |||
| Ex9 + Ex4‡ | 2 | |||
| Vehicle + Vehicle‡ | 2 |
One group (n = 8) received only Ex4, and the other (n = 8) Ex9, and both groups were injected with vehicle; injections were administered 15 min before the start of the session.
Food or water was removed from cage ∼23 h before session.
Vehicle or Ex9 was injected 15 min before vehicle or Ex4 injection. Only the group of rats that were previously injected with Ex4 were used in this phase.
To assess the effect of Ex4 and Ex9 on concentration-dependent licking to sucrose, the rats were tested every other day for a total of two sessions after injection with their assigned peptide. A total of four vehicle test sessions were conducted: two before and two after the peptide tests (Table 2). This testing series was performed first while the rats were fasted and then repeated when the rats were nondeprived.
Home-cage intake of 0.3 M sucrose.
Food was removed from the rats ∼23 h before injection with Ex9 or Ex4, which was administered according to their assigned peptide group; water was removed at the time of injection. Fifteen minutes after injection, a 100-ml bottle with a curved sipper spout that was filled with 0.3 M sucrose solution was presented in the position normally occupied by the water bottle. Sucrose intake was measured at 0.5 and 1 h after presentation. The bottle was removed at the last time point, and the maintenance water bottle replaced. Food was returned to the rats ∼1 h after removal of the sucrose. Two test days with the peptides and 2 test days with vehicle, both before and after peptide testing, were performed (Table 2), with a day off in between each test that served as a period of refeeding for the rats and washout of the peptide.
We also measured the extent to which the dose of Ex9 that we selected was able to block the effect of our chosen Ex4 concentration. We used only those rats that had previous experience with Ex4. Food was removed from the rats ∼23 h before injection with Ex9; water was removed at the time of this injection. Fifteen minutes after Ex9 injection, the rats were injected with Ex4. Fifteen minutes after that, a bottle of 0.3 M sucrose solution was presented. Measures were taken at 0.25, 0.5, and 1 h after presentation. Food and water were returned to the rats as described above. Two test days with the antagonist challenge of the agonist, and 2 test days with two vehicle injections (one injection for each peptide), both before and after peptide testing, were performed, with a day off in between each test (Table 2).
Data analysis.
Differences in the average number of licks of water taken, ILI, and the number of trials initiated during water testing between each peptide group were analyzed with injection condition (peptide vs. vehicle) as the within-subject factor. Sucrose lick score and number of sucrose trials initiated under vehicle and peptide conditions for each group were analyzed separately for the postsurgical fasted and nondeprived conditions. All rats in both groups met the criterion for lick score inclusion when tested while fasted, but, when tested while ad libitum fed, only five rats in the Ex4 group and four in the Ex9 group took enough trials in both injection conditions to be included in lick score analysis.
An average total intake of 0.3 M sucrose at each time point was calculated for each rat for the two peptide sessions and four vehicle sessions, and also for the two antagonist challenge sessions and their corresponding four vehicle sessions. The average intake of each peptide group was compared with their own intake during vehicle conditions at each time point by one-way ANOVA.
RESULTS
Experiment 1: Effect of RYGB and Pharmacological GLP-1-receptor Manipulations on Brief-access Responding to Sucrose and Water
Body weight change after RYGB.
After RYGB, the rats lost an average of 67 g during the 10- to 20-day recovery period before the start of postsurgical experimental procedures, whereas sham-operated rats gained an average of 33 g (Fig. 2). By the end of the experiment, sham rats weighed ∼220 g more than RYGB rats. No other measures of adiposity were obtained.
Brief-access responding to water and sucrose before and after RYGB.
Before surgery, no differences between the rats identified for RYGB or sham operations were seen in licks of water taken during sessions in which water was presented continuously or licks of sucrose taken relative to water in sessions during which the sucrose concentration series was presented in 10-s trials (i.e., lick scores; Fig. 3A). There were also no differences postsurgically when the rats were tested while water-deprived (Table 3) or while fasted (Table 4, Fig. 3C). However, when tested while nondeprived, rats with RYGB had overall higher lick scores than sham rats (Table 4, Fig. 3E). RYGB rats also initiated over three times as many trials as sham rats when tested nondeprived [F(1,12) = 40.752, P < 0.001; Fig. 3F] and 2.5 times as many when tested while fasted [F(1,12) = 29.04, P < 0.001; Fig. 3D]. This suggests that, in conditions during which taste would be the main factor motivating licking, our model of RYGB increased consummatory behavior to sucrose during brief-access tests in rats. In addition, the difference in trials initiated between the groups implies that, independent of food-deprivation state, our model of RYGB increased appetitive behavior toward sucrose in a brief-access test in rats.
Fig. 3.
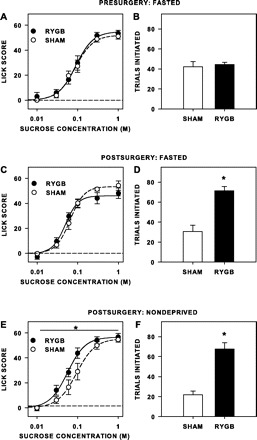
Mean (±SE) lick scores (A, C, and E) and number of trials initiated to sucrose (B, D, and F) during brief-access tests of rats when tested presurgically while fasted (A and B), when tested postsurgically while fasted (C and D), and when tested postsurgically while nondeprived (E and F). Before surgery, rats assigned to sham and RYGB groups (n = 7 per group) did not significantly differ in lick scores or trials initiated. After surgery and when tested while fasted, RYGB rats took significantly more trials than sham rats, but RYGB rats did not have lick scores different than that of sham rats (n = 6/7). When tested while nondeprived, RYGB rats had higher overall lick scores to sucrose than sham rats (n = 6/7), as well as taking more trials. *Statistical significance (P ≤ 0.05).
Table 3.
Mean ± SE and statistical comparison of licks and ILI of RYGB and sham rats to water during postsurgical testing in experiments 1 and 2
| Experiment | Measure | RYGB | Sham | One-Way ANOVA |
|---|---|---|---|---|
| 1 | Licks, no. | 2,926.2 ± 436.3 | 3,370.4 ± 188.5 | F(1,11) = 0.974, P = 0.345 |
| ILI, ms | 160.8 ± 5.4 | 163.4 ± 5.5 | F(1,11) = 0.120, P = 0.736 | |
| 2 | Licks, no. | 2,742.4 ± 169.3 | 3,026.8 ± 202.1 | F(1,15) = 1.131, P = 0.304 |
| ILI, ms | 163.3 ± 4.9 | 158.5 ± 4.2 | F(1,15) = 0.573, P = 0.461 |
Values are means ± SE. ILI, interlick interval; RYGB, Roux-en-Y gastric bypass.
Table 4.
Two-way repeated-measures ANOVA values of sucrose lick score comparisons in experiments 1 and 2
| Expiment | Condition | Surgery | Concentration | Interaction |
|---|---|---|---|---|
| 1 | Presurgery: fasted | F(1,11) = 0.208, P = 0.657 | F(5,55) = 158.721, P < 0.001 | F(5,55) = 0.459, P = 0.805 |
| Postsurgery: fasted | F(1,11) = 0.251, P = 0.627 | F(5,55) = 102.829, P < 0.001 | F(5,55) = 1.393, P = 0.241 | |
| Postsurgery: nondeprived | F(1,11) = 5.462, P = 0.039 | F(5,55) = 81.178, P < 0.001 | F(5,55) = 1.367, P = 0.251 | |
| 2 | Postsurgery: fasted | F(1,13) = 0.021, P = 0.887 | F(5,65) = 95.859, P < 0.001 | F(5,65) = 0.832, P = 0.532 |
| Postsurgery: nondeprived | F(1,7) = 0.06, P = 0.813 | F(5,35) = 37.673, P < 0.001 | F(5,35) = 1.234, P = 0.314 |
P values in bold represent significant differences.
Brief-access responding to water and sucrose after RYGB and GLP-1-receptor peptides.
There was no effect of surgery on licks of water taken [F(1,11) = 0.009, P = 0.928; Table 5] during water testing while the RYGB and sham rats were water-deprived and injected with Ex9, Ex4, and vehicle, nor was there a surgery × injection interaction [F(2,22) = 2.489, P = 0.106]. There was, however, a significant main effect of the pharmacological GLP-1-receptor manipulations on licks of water taken [F(2,22) = 37.234, P < 0.001]. Post hoc testing revealed an overall effect of Ex4 relative to vehicle on licks of water taken [F(1,12) = 47.817, P < 0.001], but no overall effect of Ex9 [F(1,12) = 1.305, P = 0.552]. Similarly, no effect of surgery was seen on the average ILI [F(1,11) = 0.557, P = 0.471; Table 5], nor was there a surgery × injection interaction [F(2,22) = 0.259, P = 0.774]. There was, however, a significant main effect of the pharmacological GLP-1-receptor manipulations on ILI [F(2,22) = 4.652, P = 0.021], but no overall effect of Ex4 [F(1,12) = 5.306, P = 0.08] or Ex9 [F(1,12) = 0.487, P = 0.998] was revealed when each peptide was compared individually with vehicle. Thus, while administration of a potent GLP-1-receptor agonist, at a dose that has been shown to decrease food intake, decreased water intake, this decrease was not seen in RYGB rats whose endogenous levels of GLP-1 presumably increased after surgery; likewise, the dose used of the GLP-1-receptor antagonist Ex9 had no effect on water licking by RYGB rats, which performed similarly to sham rats.
Table 5.
Mean ± SE licks and ILI of RYGB and sham rats to water during stationary water testing following pharmacological GLP-1-receptor manipulations
| Surgery | Injection Condition | ILI, ms | Total Licks, no. |
|---|---|---|---|
| RYGB | Vehicle | 166.4 ± 5.3 | 2,309.3 ± 200.9 |
| Ex9 | 166.5 ± 7.8 | 2,294.0 ± 338.0 | |
| Ex4 | 177.5 ± 6.7 | 656.1 ± 261.1 | |
| Sham | Vehicle | 159.2 ± 2.5 | 2,621.2 ± 379.6 |
| Ex9 | 161.6 ± 3.2 | 2,552.8 ± 456.6 | |
| Ex4 | 165.5 ± 3.6 | 1,308.5 ± 523.9 |
Values are means ± SE.
There was no significant effect of surgery on sucrose lick scores after pharmacological GLP-1-receptor manipulations [F(1,11) = 0.783, P = 0.395; Fig. 4A], nor did surgery interact with injection condition [F(2,22) = 0.444, P = 0.647], concentration [F(5,55) = 0.292, P = 0.915], or both [F(10,110) = 1.385, P = 0.197]. There was, however, a main effect of injection condition [F(2,22) = 3.964, P = 0.034], but post hoc tests that compared each peptide to vehicle revealed no overall effect of either peptide [Ex4: F(1,12) = 4.413, P = 0.114; Ex9: F(1,12) = 0.063, P ≥ 0.99], or significant interaction with concentration [Ex4: F(5,60) = 2.553, P = 0.074; Ex9: F(5,60) = 1.748, P = 0.276]. As seen previously, RYGB rats initiated more trials to sucrose than did sham rats [F(1,11) = 41.621, P < 0.001], but neither a significant independent [F(2,24) = 3.191, P = 0.059] nor interactive effect [F(2,24) = 2.705, P = 0.087] of the peptides was revealed in our study (Fig. 4B). These findings suggest that additional GLP-1-receptor activity via pharmacological antagonism with this dose of Ex4 does not significantly further increase consummatory licking or appetitive trial initiation in RYGB rats, nor does it recapitulate in sham rats the increase in approach or lick responsiveness toward sucrose seen in RYGB rats when tested nondeprived. Likewise, the findings also suggest that pharmacological antagonism of GLP-1 receptors in rats after RYGB was not sufficient to reduce the number of trials initiated to the level seen in sham rats. Because the increased consummatory behavior that was seen when RYGB rats were tested when nondeprived was not observed under vehicle conditions, it could not be tested whether Ex9 would block this effect. It should be noted, though, that, because food had been removed at the time of injection 5 h before testing, the rats were not in a completely nondeprived state during the tests. Although we expect that the impact of this short period of lack of food access was minimal because it occurred during the light phase, which is when food intake is normally lower compared with the dark phase, this difference from the original nondeprived testing protocol, along with potential stress caused by the injection, does not allow direct comparison of these two tests.
Fig. 4.
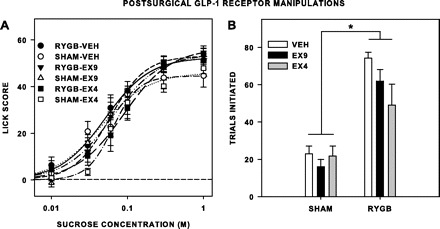
Mean (±SE) lick scores (A) and number of trials initiated to sucrose (B) by rats given RYGB or sham surgery (n = 7 per group) and injected intraperitoneally on separate brief-access testing series with the glucagon-like peptide-1 (GLP-1) receptor antagonist exendin-3(9–39) (Ex9; 30 μg/kg), agonist exendin-4 (Ex4; 1 μg/kg), and vehicle (Veh; 1 ml/kg) 5 h before the test session. Food was removed from the rats at the time of injection. A: there were no differences in lick scores between sham rats (n = 6/7) and RYGB rats, no significant effect of either peptide relative to Veh, and no significant interaction of the peptides with surgical condition overall or at any concentration. B: RYGB rats initiated more trials than sham rats, and the peptides had no independent or interactive impact on this effect. *Statistical significance (P ≤ 0.05).
Experiment 2: Effect of RYGB on Brief-access Responding to Sucrose and Water in Rats Without Presurgical Sucrose Experience
Body weight change.
Rats after RYGB lost an average of 111 g during the 10- to 20-day recovery period before the start of postsurgical experimental procedures, whereas sham-operated rats gained, on average, 24 g during recovery (Fig. 5). By the end of the experiment, sham rats weighed ∼190 g more than RYGB rats. No other measures of adiposity were obtained.
Brief-access responding to water and sucrose after RYGB.
After RYGB, rats that had no presurgical brief-access or sucrose experience did not differ in a statistically significant way from sham rats in terms of licks of water taken or ILI during training while rats were water deprived (Table 3), nor were any significant effects of surgery seen on lick scores to sucrose, when rats were tested either while fasted, or while nondeprived (Table 4; Figs. 6, A and C). Similar to experiment 1, however, in either deprivation state, RYGB rats took significantly more trials than sham rats [fasted: F(1,13) = 17.693, P = 0.001; nondeprived: F(1,9) = 55.269, P < 0.001; Fig. 6, B and D]. Thus, as in experiment 1, the rats did not decrease their lick responsiveness to sucrose, even though they had no presurgical experience with the taste stimulus. We did not, however, replicate the increased lick responsiveness after RYGB observed in experiment 1 when rats were tested while nondeprived, so this phenomenon either could be contingent on prior sucrose exposure, or may not be particularly robust. We did replicate, however, in both states, that RYGB increased appetitive taste-guided behavior in that RYGB rats with no presurgical sucrose experience took about three times as many trials as sham rats when tested while nondeprived and almost twice as many trials when tested while fasted.
Fig. 6.
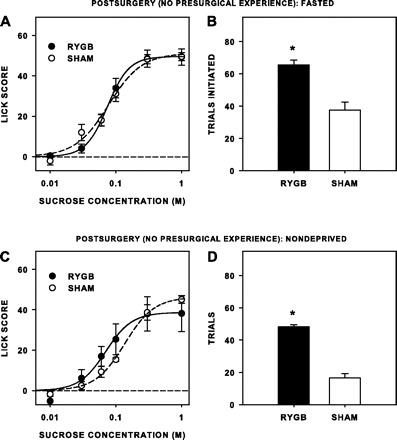
Mean (±SE) lick scores (A and C) and number of trials initiated to sucrose (B and D) during brief-access tests of rats when tested postsurgically while fasted (A and B) and when tested postsurgically while nondeprived (C and D). After surgery and when tested while fasted, RYGB rats (n = 6) took significantly more trials than sham rats (n = 9), but RYGB rats did not have lick scores different from that of sham rats. When tested while nondeprived, RYGB rats (n = 4) took more trials than sham rats (n = 8/9), but did not have statistically different lick scores. *Statistical significance (P ≤ 0.05).
Experiment 3: Assessment of a Shorter Time Course of Pharmacological GLP-1-receptor Manipulations on Brief-access Responding to, and Intake of, Sucrose in Unoperated Rats
Brief-access responding to sucrose 15 min after injection of Ex4 or Ex9.
Ex4 decreased the number of licks of water taken by water-restricted rats when water was presented continuously, which is similar to its effect when injected 5 h before the test (Table 6). Furthermore, Ex4 decreased the number of trials initiated and the average number of licks per trial taken by water-restricted rats when water was presented in trials (Table 6). The peptide also increased ILI (Table 6), suggesting that increasing GLP-1-receptor activity may directly or indirectly influence activity in the putative central-pattern generator (see Refs. 64, 71), in addition to decreasing water intake. Ex9 did not affect licks of water taken, ILI, or average licks taken per trial. It did, however, significantly decrease the number of water trials taken by water-restricted rats, but the effect was relatively minor. This suggests that decreasing GLP-1-receptor activity via pharmacological antagonism has little effect on water intake under our test conditions.
Table 6.
Licks, ILI, and trials taken to water by unoperated rats after pharmacological GLP-1-receptor manipulations
| Group | Measure | Total Licks, no. | ILI, ms | No. of Trials | Licks Per Trial, no. |
|---|---|---|---|---|---|
| Ex4 | Vehicle | 3,201.4 ± 253.5 | 159.7 ± 3.5 | 52.8 ± 3.7 | 59.0 ± 1.4 |
| Ex4 | 2,114.6 ± 308.1 | 178.7 ± 8.0 | 33.6 ± 4.5 | 49.1 ± 3.9 | |
| ANOVA | F(1,7) = 12.629, P = 0.009 | F(1,7) = 7.678, P = 0.028 | F(1,7) = 10.393, P = 0.015 | F(1,7) = 8.887, P = 0.020 | |
| Ex9 | Vehicle | 3,220.5 ± 174.3 | 159.3 ± 3.4 | 53.0 ± 2.4 | 59.0 ± 2.3 |
| Ex9 | 3,137.4 ± 135.4 | 159.6 ± 3.8 | 44.9 ± 1.7 | 59.7 ± 2.0 | |
| ANOVA | F(1,7) = 0.147, P = 0.712 | F(1,7) = 0.053, P = 0.825 | F(1,7) = 6.342, P = 0.040 | F(1,7) = 0.371, P = 0.561 |
Values are means ± SE. P values in bold represent significant differences.
Neither Ex4 nor Ex9, at the doses used, had an effect on concentration-dependent responding to sucrose (Table 7, Figs. 7 and 8) when the rats were tested while fasted. Although Ex9 significantly increased overall responsiveness to sucrose while rats were nondeprived, the magnitude of this effect was clearly small (Fig. 7C). Ex4 had no effect on sucrose licking when the rats were tested while either fasted or nondeprived (Fig. 8, A and C), but the peptide did decrease trials taken when the rats were tested while nondeprived [F(1,7) = 9.299, P = 0.019; Fig. 8D], but not when they were tested while fasted [F(1,7) = 0.478, P = 0.512; Fig. 8B]. Ex9 had no significant effect on trials initiated when rats were tested either fasted [F(1,7) = 0.062, P = 0.811, Fig. 7B] or nondeprived [F(1,7) = 4.355, P = 0.075; Fig. 7D]. Although the peptides had some effects on lick responsiveness and trial initiation to sucrose when injected 15 min rather than 5 h before testing, they did not mimic the effects seen by RYGB, especially with respect to trial initiation.
Table 7.
Comparison of lick scores during brief access testing with sucrose of rats 15 min after pharmacological GLP-1-receptor modulation
| Deprivation State | Group | Injection | Concentration | Interaction |
|---|---|---|---|---|
| Fasted | Ex4 | F(1,7) = 0.402, P = 0.546 | F(5,35) = 239.941, P < 0.001 | F(5,35) = 0.428, P = 0.826 |
| Ex9 | F(1,7) = 0.502, P = 0.501 | F(5,35) = 138.051, P < 0.001 | F(5,35) = 0.311, P = 0.903 | |
| Nondeprived | Ex4 | F(1,4) = 1.337, P = 0.312 | F(5,20) = 59.736, P < 0.001 | F(5,20) = 1.350, P = 0.284 |
| Ex9 | F(1,3) = 20.977, P = 0.020 | F(5,15) = 65.251, P < 0.001 | F(5,15) = 0.946, P = 0.480 |
P values in bold represent significant differences.
Fig. 7.
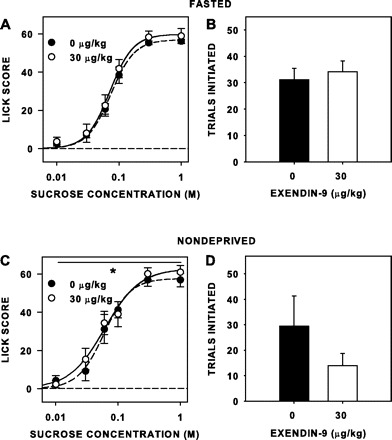
Mean (±SE) lick scores (A and C) and number of trials initiated to sucrose (B and D) of unoperated rats injected intraperitoneally with 30 μg/kg Ex9 and Veh 15 min before separate brief-access sessions, while either fasted (n = 8; A and B) or nondeprived (n = 4/8; C and D). Ex9 slightly but significantly increased overall sucrose lick scores of nondeprived rats. *Statistical significance (P ≤ 0.05).
Fig. 8.
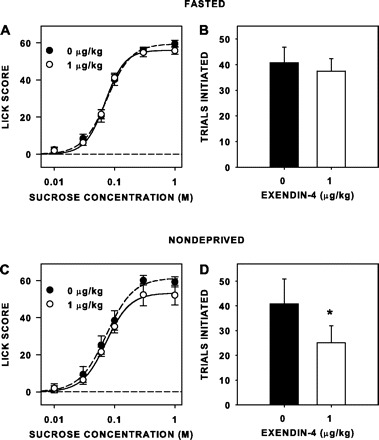
Mean (±SE) lick scores (A and C) and number of trials initiated to sucrose (B and D) of unoperated rats injected intraperitoneally with 1 μg/kg Ex4 and Veh 15 min before separate brief-access sessions, while either fasted (n = 8; A and B) or nondeprived (n = 5/8; C and D). Ex4 decreased trials initiated to sucrose by nondeprived rats. *Statistical significance (P ≤ 0.05).
Intake of 0.3 M sucrose after GLP-1-receptor peptides.
Ex4 significantly decreased intake of 0.3 M sucrose in fasted rats 30 min and 1 h after presentation (Table 8). Ex9 had no effect on sucrose intake at either time point (Table 8). Ex9 when injected 15 min before Ex4 did not completely block the hypophagic effect of Ex4 (Table 8), but the percent reduction in sucrose intake by rats when injected with Ex4 alone compared with vehicle was cut roughly by one-half by the antagonist challenge compared with vehicle at both 30 min [F(1,7) = 8.942, P = 0.02] and 1 h [F(1,7) = 8.912, P = 0.02] (Fig. 9). Thus we were able to demonstrate that our preparation of the peptides affected sucrose intake during the same time frame in which brief-access testing occurred. Furthermore, we showed that injection of Ex9, before Ex4 administration, decreased the agonist's hypophagic effect, indicating that the impact of Ex4 on sucrose intake was mediated, at least in part, by a GLP-1-receptor-specific action.
Table 8.
Analysis of home-cage 0.3 M sucrose intake of unoperated rats after administration of the GLP-1-receptor antagonist Ex9 or agonist Ex4 alone, or after Ex9 challenge of Ex4
| Group | Measure | 0.25 h | 0.5 h | 1 h |
|---|---|---|---|---|
| Ex4 | Vehicle | NA | 23.2 ± 1.7 | 26.3 ± 1.9 |
| Ex4 | 12.5 ± 2.1 | 16.1 ± 2.4 | ||
| ANOVA | F(1,7) = 49.206, P < 0.001 | F(1,7) = 49.560, P < 0.001 | ||
| Ex9 | Vehicle | NA | 21.0 ± 1.5 | 21.6 ± 1.4 |
| Ex9 | 24.6 ± 1.2 | 27.2 ± 1.1 | ||
| ANOVA | F(1,7) = 0.107, P = 0.753 | F(1,7) = 4.068, P = 0.084 | ||
| Ex9 + Ex4 | Vehicle + Vehicle | 23.6 ± 1.6 | 29.2 ± 2.4 | 31.5 ± 2.6 |
| Ex9 + Ex4 | 18.1 ± 2.3 | 21.3 ± 3.3 | 25.1 ± 3.5 | |
| ANOVA | F(1,7) = 17.488, P = 0.004 | F(1,7) = 44.842, P < 0.001 | F(1,7) = 31.114, P = 0.001 |
Values are means ± SE in ml. P values in bold represent significant differences.
Fig. 9.
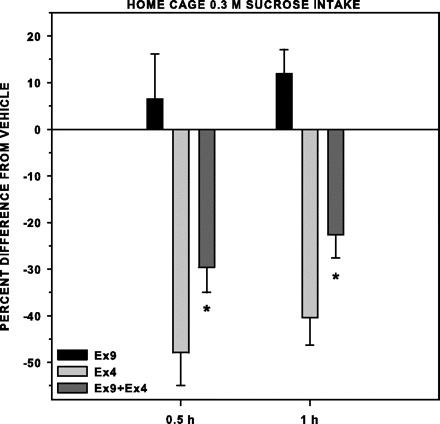
Mean (±SE) difference in 0.3 M sucrose intake expressed as a percentage from Veh (raw data presented in Table 8) by fasted rats (n = 8 per group) injected intraperitoneally with either Ex9 or Ex4 15 min before access during a one-bottle, home-cage test; the rats injected with Ex4 were also on a separate occasion injected with Ex9, 15 min later injected with Ex4, and then 15 min later given 0.3 M sucrose access. Ex4 alone significantly decreased 0.3 M sucrose intake at 0.5 and 1 h after sucrose presentation. Ex9 when injected 15 min before Ex4 significantly decreased this effect. *Statistical significance (P ≤ 0.05).
DISCUSSION
Effects of RYGB on Responsiveness to Sucrose in a Brief-access Test
To our surprise and in contrast to three reports in the literature, which used a variety of rat models of RYGB (21, 46, 62), in two experiments using our model of RYGB in rats that did not have high-fat diets at any time, we did not see an attenuation of licking responses to a concentration series of sucrose solutions in a brief-access taste test. If anything, in our hands, RYGB increased sucrose licking overall across concentrations during trials when the rats that had been tested with sucrose presurgically were tested postsurgically while nondeprived. Collectively these data suggest that our model of RYGB in rats that were chow fed promotes little change in consummatory responses of rats for sucrose, at least in our version of the short-term, brief-access taste test. This type of test is designed to limit the ability of an animal to associate postingestive events arising from the mixing of ingested fluids with a specific concentration during a trial because of the very limited duration of access, which is on the order of seconds, and the randomized nature of stimulus presentation. Consummatory behavior is defined as the final act of the appetitive chain that is typically stimulated by contact of the goal object with receptors. In the brief-access test, upon initiation of the trial with the first lick, the taste stimulus comes into direct contact with orosensory receptors, triggering further oromotor responses. Accordingly, once a very brief trial is engaged, the lick rate (e.g., licks produced in the 10-s trial) can be considered to be largely driven by consummatory processes and reflects the relative affective value of the solution. In general, the concentration-response function was unaltered by RYGB, with the exception of a 0.28 log10 leftward shift relative to sham-operated controls in experiment 1 when animals were tested in a nondeprived state. Thus, in our hands, when the surgery had an effect on consummatory responsiveness to sucrose, it was an increase, and the expression of this change was not consistently observed throughout all testing conditions.
This is not to say that there was only a minor effect of the RYGB surgery on motivated behavior toward sucrose in the brief-access test. We observed, on average, a threefold increase in the number of trials initiated after RYGB, independent of the deprivation state in which the rats were tested or whether the rats were given presurgical sucrose experience. An increase in the total number of trials initiated is indicative of an RYGB-induced facilitation of overall appetitive behavior, which is defined as behavior that brings the animal to the goal object (e.g., foraging, approaching a drinking spout). The increase in the number of trials initiated, coupled with either a lack of change or even an increase in concentration-dependent licking by rats after RYGB, means that these rats actually ingested more sucrose than the sham controls under these test conditions. For example, when the rats in experiment 1 were tested while nondeprived, we estimated from lick data that the RYGB rats drank, on average, ∼13 ml across all concentrations, including water, amounting to ∼6 kcal, while sham rats drank ∼3 ml or ∼2 kcal.
Our results differ with those of several recent studies (21, 46, 61), which, while employing a variety of models of RYGB, all showed that, after RYGB, rats licked less to high concentrations of sucrose. A close comparison of our work with these other reports suggests that differences in the diet used before and after surgery, the level of adiposity and metabolic state of the animals, the exact nature of the surgical intervention, and/or the behavioral methodology employed may be at the root of the disparity in the results. Our rats received normal chow at all times and were not exposed to a high-fat diet. Moreover, the surgical procedure for producing RYGB differs from those used in all of the other studies in how much stomach remains contiguous with the jejunum in the alimentary limb. In our rat model of RYGB, a very small gastric pouch, consisting of <5% of the original stomach volume, is fashioned (Fig. 1); in other models, 10–20% of the stomach remains. Thus, in our preparation, food moves directly into the jejunum rather than being diluted in the pouch by other foods and fluids and then slowly transported into the jejunum (e.g., Ref. 57). These differences in the restrictive nature of the stomach remnant have, in some cases, been shown to affect weight loss in humans (Refs. 42, 44; but see Refs. 11, 40, 63), and the transit time to the intestine may impact ingestive bouts of sucrose by rats. It should be noted, though, that the type of gastric tissue (e.g., muscular, glandular) that remains in contact with food in the surgical preparations differs anatomically between the rat and human stomach (24), and the effect of these differences on food intake has not, to our knowledge, been explored. Finally, while less interference with the stomach would presumably cause less damage to the vagus nerve, the extent to which vagal afferents are damaged by these various procedures remains to be explicitly tested (11).
Although differences in the geometry of the gut remodeling could be contributing to the lack of consistency between our results and those of others, an equally possible source is some fundamental variations in the design of the brief-access test itself. For example, in experiment 1, our animals had three presurgical test sessions, allowing us to measure baseline performance and counterbalance the groups while familiarizing the rats with the sucrose stimuli and their availability from the test apparatus. Although in all of the other studies the animals had presurgical exposure to solid or liquid diets that consisted of a higher percentage of sugar than chow, in none of these studies were the rats tested presurgically, and thus they were not explicitly exposed to the procedure and sucrose solutions before postsurgical testing. Because there is evidence that presurgical two-bottle preference testing with a series of sucrose concentrations can significantly attenuate the effectiveness of RYGB to decrease sucrose preference (13), we tested rats after RYGB that were not presurgically exposed to sucrose (experiment 2). Once again, we saw no evidence of decreased consummatory responsiveness to sucrose after RYGB, and we replicated the RYGB-induced increase in appetitive behavior seen previously in the form of increased trials.
The procedural parameters of our brief-access test differed from those used by others (21, 46, 62) in that animals could initiate as many trials as possible, presented in randomized blocks, during the 30-min session. We chose to do this because 1) it increased the reliability of our estimate of consummatory responding; 2) it provided an assessment of the effect of RYGB on appetitive behavior; and 3) this procedure has been shown to be sensitive to manipulations of the gustatory system (47, 52–55, 65). Tichansky et al. (62) also allowed rats to initiate as many trials as possible in 30 min, while Shin et al. (46) presented two series of ascending concentrations, and Hajnal et al. (21) limited presentations to five or six at each concentration. Nevertheless, the fact that, in our study, licking increased monotonically as concentration was raised suggests that, unlike short-term, single-bottle intake tests, which generally produce an inverted-U concentration-response function, satiation did not influence relative responding to the various concentrations.
Our brief-access procedure also required that the rats sample the taste stimulus before another one was presented; as such, a measure of consummatory responsiveness was made every time a stimulus was presented. In the Hajnal et al. (21) and Shin et al. (46) studies, each concentration was present for 10 s, during which the animals could lick or not, and then the next concentration was moved into place. Although this standardizes the amount of access time that each rat has to each stimulus, it also means that a rat could have trials in which the stimulus was never sampled or sampled late in the access period, which could have had a disproportionate effect on the nature of the curves, especially considering the limited number of trials during which each concentration was presented, and that the animals were receiving pure sucrose solution for the first time ever. Tichansky et al. (62) also used a limited hold, at which point a new concentration was presented, although it was set at 120 s.
The final major procedural consideration deals with the relative body weight and adiposity, as well as the resulting metabolic state, between groups of animals at the time of surgery and testing. Interestingly, Hajnal et al. (21) found that RYGB-operated, chow-fed OLETF rats licked high concentrations of sucrose significantly less than did sham-operated OLETF rats, but that there was no such effect of RYGB observed in Long Evans Tokashima Otsuka rats compared with their sham-operated controls, which are leaner than OLETF rats. Such an outcome supports the possibility that body composition at the time of surgery might be a critical factor in whether RYGB affects consummatory responsiveness to sucrose. Because we did not measure body composition or metabolic parameters such as leptin levels or glucose tolerance, we cannot conclude that our animals had adiposity levels similar to those of Hajnal et al. (21), Shin et al, (46), or Tichansky et al. (62), and so the role of this factor in RYGB-induced effects on taste-guided behavior in our model remains to be established. Until recently, RYGB has only been carried out in humans with a body mass index (BMI) ≥ 35 kg/m2 (38), but new initiatives have been taken to perform the surgery in individuals who are not categorized as obese (e.g., BMI = 27 kg/m2; BMI ≥ 30 kg/m2 is considered obese) with the specific intent to treat type 2 diabetes (25). It would be interesting to see if differences in taste perceptions would differ after surgery between these groups of bariatric candidates.
Whether some or all of the parametric differences in the designs of these experiments involving the use of the brief-access test led to the differences between our results and those of these other studies remains to be resolved. Nevertheless, it must be emphasized that RYGB surgery in rats clearly does not lead unequivocally to a decrease in motivated licking of high-sucrose concentrations. Indeed, under our conditions, appetitive responses in the form of trial initiation were remarkably augmented. This latter result receives some support from a linear runway experiment conducted by Shin et al. (46) in which, during the initial testing trials, high-fat-diet-fed obese rats that received RYGB and chow-fed leaner sham-operated controls reached the goal box significantly sooner than high-fat-diet-fed obese sham-operated rats when access to a small amount (2 g) of Fruit Loops (a sweet-tasting cereal to humans) was used as the reinforcer. Although this result could, in part, be explained by the large body weight difference between the high-fat-diet-fed obese sham-operated rats and the leaner RYGB and chow-fed, sham-operated groups, creating differences in the work demand of the task, the finding that an appetitive response toward a putatively sweet-tasting stimulus appears to be enhanced by RYGB is, nonetheless, in agreement with the RYGB-induced increase in the number of sucrose trials initiated in our study. Therefore, it appears that the ingestive response to sucrose, and perhaps sweet-tasting stimuli in general, is not universally decreased by RYGB, but, rather, may vary as a function of the parameters of the surgical manipulation itself, the environmental context (i.e., test conditions) in which the sucrose is offered, and/or the level of adiposity and metabolic state of the animal at the time of surgery and testing.
The Effects of GLP-1-receptor Modulation on Responsiveness to Sucrose in the Brief-access Test
Because postprandial GLP-1 levels are elevated in humans after RYGB (e.g., Refs. 6, 30, 32, 33) and rats show increased baseline and postprandial levels of the peptide after similar surgery (e.g., Refs. 10, 11, 31), we reasoned that sham rats injected with the GLP-1-receptor agonist Ex4 might take as many trials of sucrose as RYGB rats, and that RYGB rats injected with Ex9 might reduce the number of trials that they initiate to the level of sham controls. However, injection of Ex4 and Ex9, at doses and time courses that have been shown to affect food and sucrose intake (Refs. 72–74, unpublished data), did not affect sucrose trial initiation of RYGB or sham rats in our version of the brief-access test to the degree that we saw with the surgical manipulations alone. We also hypothesized that injection of Ex4 might increase lick scores in sham rats, and that Ex9 injection might decrease it in both groups, similar to that seen in GLP-1-receptor knockout mice (47), but we saw no significant effect relative to vehicle of either peptide when injected 5 h before tests.
Although the 5-h delay between injection and test was chosen on the basis of the effectiveness of these peptides to influence food intake (i.e., Refs. 73, 74, unpublished data), it is possible that their time course of action on gustatory processes is more rapid. Accordingly, in intact rats, we examined the effects of these peptides on responding to sucrose in the brief-access test when they were injected 15 min before the start of the test during both fasted and nondeprived states. As when injecting RYGB- and sham-operated rats with Ex4 and Ex9, we hypothesized that Ex4 would increase trials and possibly lick score, and that Ex9 would decrease lick score and possibly decrease trials. In contrast to our expectations, under our testing conditions, Ex9 resulted in a slight, albeit statistically significant, increase in lick rate for sucrose when the rats were tested while nondeprived, with no effect on trials. This is different from findings using GLP-1-receptor knockout mice (47), but it should be noted that a lower concentration series of sucrose was presented to the knockout mice than what was used in our study (0.001–0.1 M compared with 0.01–1.0 M), and, at 0.4 M sucrose, lick responses of GLP-1-receptor knockout mice were not significantly different from those observed in wild-type mice. Ex4 caused a significant drop in the number of trials initiated, but only when the rats were tested while nondeprived, and had no effect on lick score. To functionally confirm the potency of the peptides, we injected rats with Ex4 15 min before a one-bottle 0.3 M sucrose intake test and showed that it decreased their intake by >40%, and that this effect was partially blocked by administration of Ex9 15 min before (similar to Ref. 68). These results suggest that exogenous modulation of GLP-1-receptor signaling via the peptide doses and time courses used here has little effect on brief-access responding for sucrose, and that GLP-1-receptor activity is not sufficient to explain the effects of RYGB during our tests.
One of the most interesting effects of pharmacological GLP-1-receptor modulation that we observed was that Ex4 injected 15 min before testing increased the time between lick onsets (i.e., increased ILI). Although the change, ∼20 ms, would only account for a difference of 7 licks from 62 in a 10-s trial in which the rat was licking without pause, it could have a greater impact over a longer time scale of ingestion. The effect of a variety of age, environmental, and surgical manipulations on ILI is typically mild (e.g., Refs. 23, 36, 53, 56, 70); changes seen occasionally in this parameter are rarely of the magnitude seen here and typically occur with drugs that produce overt motor disturbances (e.g., Refs. 19, 20, 34), which were not observed in this study with Ex4. That the ILI was affected to such a notable degree by Ex4 suggests that GLP-1-receptor activation might directly or indirectly act on premotor or motoneurons in the brain stem that control oromotor function (see Refs. 64, 71). This interpretation is also consistent with a study by Aja et al. (1), in which the ILI-increasing effect of central administration of cocaine- and amphetamine-related transcript was blocked by central administration of Ex9.
Perspectives and Significance
In these studies, rats that were only exposed to normal chow diets and given our model of RYGB increased appetitive behavior and, under some conditions, increased consummatory behavior toward a concentration series of sucrose solutions. Our results in rats seem to be at odds with reports of decreased preference for sweet-tasting foods and beverages in human bypass patients. As noted in the literature, however, there are reasons other than potential changes in the sensory nature or unconditioned hedonic value of sweets as to why patients would avoid some such foods and fluids (e.g., learning from postingestive consequences, nutritional counseling). Moreover, although patients eat fewer total calories after RYGB, their selection from the macronutrient categories either remains stable or returns to preoperative levels within 9 mo (4, 7, 9, 16, 26, 29, 66). Because in virtually all studies intake is assessed through dietary recall in RYGB patients, it remains to be directly established if the macronutrient intake or actual food selections within macronutrient categories are altered after surgery. It would be useful to complement the measures currently used to assess food preference and intake in RYGB patients with others that quantify actual food choices and ingestive behavior itself to provide a full characterization of the nature of surgically induced changes in feeding (e.g., Refs. 18, 28), as well as to provide an objective bridge to behavioral findings from parallel experiments in animal models.
GRANTS
This work was supported in part by National Institute on Deafness and Other Communication Disorders National Research Service Award F32DC010517-01 to C. M. Mathes, Deutsche Forschungsgemeinschaft BU 2430/1-1 and 1-2 to M. Bueter, National Science Foundation Graduate Research Fellowship to K. R. Smith, and the National Institutes of Health Research Clinician Scientist Award to C. W. le Roux.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M.M., M.B., T.A.L., C.W.L.R., and A.C.S. conception and design of research; C.M.M., M.B., and K.R.S. performed experiments; C.M.M. analyzed data; C.M.M. and A.C.S. interpreted results of experiments; C.M.M. and M.B. prepared figures; C.M.M. drafted manuscript; C.M.M., M.B., K.R.S., T.A.L., C.W.L.R., and A.C.S. edited and revised manuscript; C.M.M., M.B., K.R.S., T.A.L., C.W.L.R., and A.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ginger Blonde, Jillian Gregson, Drew Basset, and Julia Comer for technical assistance during portions of the behavioral experiments, and Dr. Diana Williams for advice regarding Ex4 and Ex9.
Present address of M. Bueter: Department of Visceral and Transplantation Surgery, University Hospital Zurich, Zurich, Switzerland.
REFERENCES
- 1. Aja S, Schwartz GJ, Kuhar MJ, Moran TH. Intracerebroventricular CART peptide reduces ingestive behavior and alters licking microstructure. Am J Physiol Regul Integr Comp Physiol 280: R1613–R1619, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, Trautmann ME. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 29: 2333–2348, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Barrera JG, D'Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 58: 2820–2827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bavaresco M, Simara P, Lima TP, Salgado W, Jr, Ceneviva R, Dos Santos JE, Nonino-Borges CB. Nutritional course of patients submitted to bariatric surgery. Obes Surg 20: 716–21, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bojanowska E, Nowak A. Interactions between leptin and exendin-4, a glucagon-like peptide-1 agonist, in the regulation of food intake in the rat. J Physiol Pharmacol 58: 349–360, 2007 [PubMed] [Google Scholar]
- 6. Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93: 210–215, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Brolin RL, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and roux-en-y gastric bypass. Ann Surg 220: 782–790, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brosvic GM, Slotnick BM. Absolute and intensity-difference taste thresholds in the rat: evaluation of an automated multi-channel gustometer. Physiol Behav 38: 711–717, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Brown EK, Settle EA, Van Rij AM. Food intake patterns of gastric bypass patients. J Am Diet Assoc 80: 437–443, 1982 [PubMed] [Google Scholar]
- 10. Bueter M, Ashrafian H, Frankel AH, Tam FW, Unwin RJ, le Roux CW. Sodium and water handling after gastric bypass surgery in a rat model. Surg Obes Relat Dis 7: 68–73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, Lutz T, Le Roux CW. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 20: 616–622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, Sharkey KA, Lutz TA, Le Roux CW. Gastric bypass increases energy expenditure in rats. Gastroenterology 138: 1845–1853, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 104: 709–721, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 95: 666–670, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Corbit JD, Luschei ES. Invariance of the rat's rate of drinking. J Comp Physiol Psychol 60: 119–125, 1969 [DOI] [PubMed] [Google Scholar]
- 16. Coughlin K, Bell RM, Bivins BA, Wrobel S, Griffen WO. Preoperative and postoperative assessment of nutrient intakes in patients who have undergone gastric bypass surgery. Arch Surg 118: 813–816, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992 [PubMed] [Google Scholar]
- 18. Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol Behav 35: 617–622, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Fowler SC, Mortell C. Low doses of haloperidol interfere with rat tongue extensions during licking: a quantitative analysis. Behav Neurosci 106: 386–395, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Gramling SE, Fowler SC. Some effects of pimozide and of shifts in sucrose concentration on lick rate, duration, and interlick interval. Pharmacol Biochem Behav 25: 219–222, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 299: G967–G979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes 5: 457–464, 1981 [PubMed] [Google Scholar]
- 23. Hernandez-Mesa N, Mamedov Z, Bures J. Operant control of the pattern of licking in rats. Exp Brain Res 58: 117–124, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Kararil TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16: 351–380, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Kashyap SR, Bhatt DL, Schauer PR, STAMPEDE investigators. Bariatric surgery vs. advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently trial (STAMPEDE). Diabetes Obes Metab 12: 452–454, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr 52: 87–92, 1990 [DOI] [PubMed] [Google Scholar]
- 27. Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klein DA, Schebendach JE, Gershkovich M, Smith GP, Walsh BT. Modified sham feeding of sweet solutions in women with anorexia nervosa. Physiol Behav 101: 132–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kruseman M, Leimgruber A, Zumbach F, Golay A. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc 110: 527–534, 2010 [DOI] [PubMed] [Google Scholar]
- 30. le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJ. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg 252: 50–56, 2010 [DOI] [PubMed] [Google Scholar]
- 32. le Roux CW, Bueter M, Theis N, Werling M, Ashradian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301: R1057–R1066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after roux-en-y gastric bypass. Ann Surg 246: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Lydall ES, Gilmour G, Dwyer DM. Analysis of licking microstructure provides no evidence for a reduction in reward value following acute or sub-chronic phencyclidine administration. Psychopharmacology (Berl) 209: 153–162, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes 30: 1332–1340, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Mamedov Z, Bures J. Sensory feedback modulates the central pacemaker of licking in rats. Neurosci Lett 45: 1–6, 1984 [DOI] [PubMed] [Google Scholar]
- 37. Nowak A, Bojanowska E. Effects of peripheral or central GLP-1 receptor blockade on leptin-induced suppression of appetite. J Physiol Pharmacol 59: 501–510, 2008 [PubMed] [Google Scholar]
- 38. O'Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol 25: 1358–1365, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Ochner CN, Gibson C, Carnell S, Dambkowski C, Geliebter A. The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity. Physiol Behav 100: 549–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Connor EA, Carlin AM. Lack of correlation between variation in small-volume gastric pouch size and weight loss after laparoscopic roux-en-y gastric bypass. Surg Obes Relat Dis 4: 399–403, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Pouranaras DJ, le Roux CW. Obesity, gut hormones, and bariatric surgery. World J Surg 33: 1983–1988, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Roberts K, Duffy A, Kaufman J, Burrell M, Dziura J, Bell R. Size matters: gastric pouch size correlates with weight loss after laparoscopic roux-en-y gastric bypass. Surg Endosc 21: 1397–1402, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav 100: 291–296, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Scruggs DM, Cowan GS, Klesges L, Defibaugh N, Walker R, Kuyper B, Hiler ML. Weight loss and caloric intake after regular and extended gastric bypass. Obes Surg 3: 233–238, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35: 642–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin Y, Martin B, Golden E, Doston C, Maudsley S, Kim W, Jang H, Mattson M, Drucker D, Egan J, Munger S. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106: 455–463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H, Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Smith JC. The history of the “Davis Rig”. Appetite 36: 93–98, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Spector AC. Psychophysical evaluation of taste function in nonhuman animals. In: Handbook of Olfaction and Gustation, edited by Doty RL. New York: Dekker, 2003, p. 861–879 [Google Scholar]
- 52. Spector AC, Grill HJ, Norgren R. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol Behav 53: 277–283, 1993 [DOI] [PubMed] [Google Scholar]
- 53. Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transaction on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 100: 1096–1109, 1996 [PubMed] [Google Scholar]
- 54. Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci 109: 939–954, 1995 [PubMed] [Google Scholar]
- 55. Spector AC, Travers SP, Norgren R. Taste receptors on the anterior tongue and nasoincisor ducts of rats contribute synergistically to behavioral responses to sucrose. Behav Neurosci 107: 694–702, 1993 [DOI] [PubMed] [Google Scholar]
- 56. Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered orolingual motor function: relationships with nigrostriatal neurochemical measures. Neurobiol Aging 24: 259–266, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Stylopolous N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 17: 1839–1847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sugerman HJ, Starkey JV, Birkenhauser R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg 205: 613–622, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes 33: S28–S32, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Thaw AK, Smith JC. Conditioned suppression as a method of detecting taste thresholds in the rat. Chem Senses 17: 211–223, 1992 [Google Scholar]
- 61. Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusions of GLP-1, but not leptin, produces conditioned taste aversion in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 62. Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc 25: 1176–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Topart P, Becouarn G, Ritz P. Pouch size after gastric bypass does not correlate with weight loss outcome. Obes Surg 21: 1350–1354, 2011 [DOI] [PubMed] [Google Scholar]
- 64. Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanics of licking. Neurosci Biobehav Rev 21: 631–647, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855–R865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Trostler N, Mann A, Zilberbush N, Avinoach E, Charuzi II. Weight loss and food intake 18 months following vertical banded gastroplasty or gastric bypass for severe obesity. Obes Surg 5: 39–51, 1995 [DOI] [PubMed] [Google Scholar]
- 67. Vincent RP, Le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 69: 173–179, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Washington MC, Raboin SJ, Thompson W, Larsen CJ, Sayegh AI. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Res 1344: 124–133, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Weijnen JAWM. The recording of licking behavior. In: Drinking Behavior, edited by Weijnen JAWM, Mendelson J. New York: Plenum, 1977, p. 93–114 [Google Scholar]
- 70. Weijnen JAWM. Licking behavior in the rat: measurement and situational control of licking frequency. Neurosci Biobehav Rev 22: 751–760, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Weisenfeld ZB, Halpern B, Tapper DN. Licking behavior: evidence of hypoglossal oscillator. Science 196: 1122–1124, 1977 [DOI] [PubMed] [Google Scholar]
- 72. Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55: 3387–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav 103: 557–564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 31: 1511–1523, 2009 [DOI] [PubMed] [Google Scholar]


