Abstract
Background
We tested the hypotheses that adolescents who show elevated reward region responsivity are at increased risk for initial onset of overweight/obesity and substance use, which is important because there have been no such prospective tests of the reward surfeit model of these motivated behaviors.
Methods
One hundred sixty-two adolescents (mean age = 15.3 ± 1.06 years) with healthy weights (mean body mass index = 20.8 ± 1.90) completed functional magnetic resonance imaging paradigms that assessed neural activation in response to receipt and anticipated receipt of palatable food and monetary reward; body fat and substance use were assessed at baseline and 1-year follow-up.
Results
Elevated caudate (r = .31, p < .001) and putamen (r = .28, p < .001) response to monetary reward predicted substance use onset over 1-year follow-up, but reward circuitry responsivity did not predict future overweight/obesity onset. Adolescents who reported substance use versus abstinence at baseline also showed less caudate (r = –.31, p < .001) response to monetary reward.
Discussion
Results show that hyper-responsivity of reward circuitry increases risk for future substance use onset, providing novel support for the reward surfeit model. Results also imply that even a limited substance use history was associated with reduced reward region responsivity, extending results from studies that compared substance-dependent individuals with healthy control subjects and suggesting that substance use downregulates reward circuitry. However, aberrant reward region responsivity did not predict initial unhealthy weight gain.
Keywords: fMRI, prospective, reward sensitivity, substance use, weight gain
Theorists posit that individuals with less responsive reward circuitry are more likely to overeat and use psychoactive substances, because they are compensating for a reward deficit (1). Both of these behaviors cause dopamine (DA) signaling and activation in the striatum and other mesolimbic regions (2,3). In apparent support of the reward deficit theory, obese versus lean rats have lower basal DA levels, ex vivo striatal DA release, and D2 receptor availability (4–6); and obese versus lean humans show less striatal D2 receptor availability (7–9) and weaker striatal activation in response to palatable food intake (10–13). Echoing these findings, substance-using versus non-using rats show less striatal D2 receptor availability and sensitivity (14–17), and humans with versus without various substance use disorders show lower striatal D2 receptor availability and sensitivity (18–20), less DA release from stimulant drug use (21), and less ventral striatal response to anticipated monetary reward (22,23).
However, overeating and substance use seem to downregulate reward circuitry signaling. Women who gained weight over 6 months showed less striatal response to milkshake receipt relative to baseline and women who did not gain weight (24), converging with evidence that pigs randomized to a weight gain intervention versus a stable weight control condition showed less resting striatal activation (25). Other experiments indicate that both overeating (26) and intake of high-fat/sugar food versus isocaloric intake of a low-fat/sugar diet results in downregulation of striatal D1 and D2 receptors in rats (27). Likewise, animal experiments show that substance use reduces striatal D2 receptors (16,17), sensitivity of reward circuitry (14,15), D2 receptor sensitivity, and basal DA transmission (28,29).
Other theorists posit that individuals with more responsive reward circuitry are at greater risk for overeating and substance use (30). In apparent support of the reward surfeit model, obese versus lean humans show greater activation of striatal and other mesolimbic regions and report greater cravings in response to palatable food pictures (31–34) and cues that signal impending palatable food receipt (13,35). Similarly, individuals with versus without various substance use disorders show greater activation of reward processing regions and report greater craving in response to substance use images (36–39).
Yet this hyper-responsivity of reward regions to food and substance use cues might be a consequence of conditioned associations between food/drug reward and cues that predict these rewards rather than an initial vulnerability factor. Rodent studies indicate that firing of DA neurons initially occurs in response to intake of high-fat/high-sugar food and substance use but that firing shifts to cues that predict impending receipt of that food and substances after repeated pairing of food and substance use reward with the predictive cues (40,41), implying that this conditioning process leads to increased reward region responsivity to food and substance use cues.
Because it is unclear whether hypo- or hyper-responsivity of reward regions is an initial vulnerability factor or a consequence of overeating and substance use, it is vital to conduct prospective studies on neural vulnerability factors that predict future weight gain and substance use onset. Low striatal response to intake (12) and images of high-fat/sugar foods (33) predicted future weight gain for young women with a TaqIA A1 allele, a genotype associated with lower D2 striatal receptor availability and striatal resting metabolism (42,43), implying that individuals who show less reward region responsivity gain weight if they are at genetic risk for reduced DA signaling in reward circuitry. In contrast, elevated amygdala response to high-fat/sugar food olfactory cues (44), nucleus accumbens response to high-fat/sugar food images (45), and orbitofrontal cortex response to cues signaling impending high-fat/sugar food image presentation (46) predicted future weight gain. Furthermore, greater dorsal striatum and orbitofrontal cortex response to unhealthy food images predicted future weight gain for individuals with a TaqIA A2/A2 allele (33), which is associated with higher striatal D2 receptor availability and striatal resting metabolism (42,43).
Collectively, prospective data suggest that both elevated responsivity of reward regions to food images/cues and blunted responsivity to food cues and food receipt for humans at genetic risk for reduced DA signaling capacity increases risk for future weight gain. Yet, because some participants in these studies were overweight, a history of overeating in a subset of participants might have driven these prospective effects. Another gap in the literature is that no research has tested whether hyper- or hypo-responsive reward circuitry predicts future substance use onset or escalation. Thus, we initiated a large prospective study to test whether individual differences in reward region responsivity predicted overweight/obesity onset among initially healthy-weight adolescents and substance use onset among initially abstinent adolescents. We investigated reward region response to a natural unconditioned reward (high-fat/sugar food) and a conditioned reward (money) and investigated reward region response to both receipt and anticipated receipt of food and monetary reward, to provide a comprehensive assessment of reward region responsivity. We also compared neural responsivity of reward regions for adolescents who were in a healthy weight range or overweight/obese at baseline and for those who reported substance use versus abstinence at baseline, to determine whether even a limited history of overeating and substance use was associated with reduced reward region responsivity.
Methods and Materials
Participants
Participants were 82 female and 80 male adolescents (mean age = 15.3 ± 1.1 years; mean body mass index [BMI] = 20.8 ± 1.9; 4% Hispanic, 1% Native-American, 1% Asian/Pacific Islander, 76% European-American, and 18% mixed racial heritage) recruited in a medium-sized town in the Western US via advertisements and flyers. Exclusion criteria were a BMI <18 or >25, current use of psychoactive medications or drugs more than once weekly, pregnancy, head injury with a loss of consciousness, significant cognitive impairment, major medical problems, or current Axis I psychiatric disorder (Supplement 1).
Measures
Body Fat Percentage
Air displacement plethysmography was used to assess percent body fat of participants at baseline and 1-year follow-up with the Bod Pod S/T (COSMED, Pavona di Albano, Italy) with recommended procedures and age/sex-appropriate equations (47). Body density was calculated as body mass (assessed by direct weighing) divided by body volume. Body fat percentage estimates show test-retest reliability (r = .92–.99) and correlate with dual energy x-ray absorptiometry and hydrostatic weighing estimates (r = .98–.99) (48). To determine healthy weight, overweight, and obesity at each assessment, age- and sex-adjusted body fat percentiles were used, where >85th percentile was considered overweight and >95th percentile was considered obese, which are cutoffs associated with elevated weight-related morbidity and mortality (49).
Substance Use
Substance use was assessed with items measuring the frequency of use during the past year of beer/wine/wine coolers, hard liquor, cigarettes, marijuana, stimulants, downers, inhalants, and hallucinogens. This scale has shown internal consistency (mean α = .86), test-retest reliability (mean r = .86), and predictive validity for future increases in substance abuse symptoms (50). In the current study, Cronbach's α = .81, verifying it was appropriate to aggregate across various substances. Furthermore, by 1-year follow-up, 64% of adolescents reporting substance use reported using more than one drug category.
Functional Magnetic Resonance Imaging Paradigms
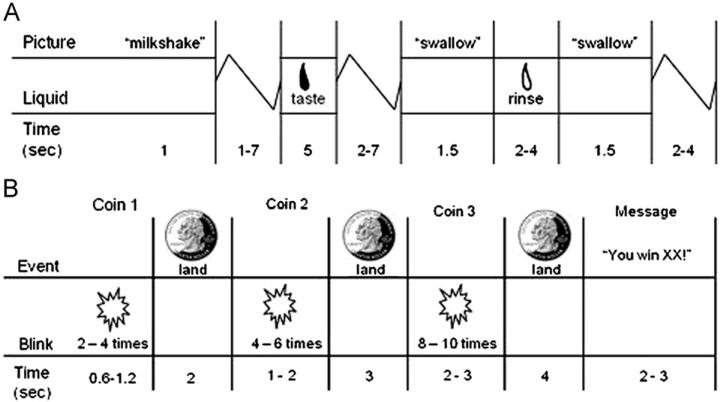
On the scan day, participants were asked to consume their regular meals but to refrain from eating or drinking caffeinated beverages for 5 hours preceding their scan. The food reward paradigm (Figure 1A) (12) assessed response to receipt and anticipated receipt of palatable food. Stimuli were two images (glasses of milkshake and water) that signaled impending delivery of either .5 mL of chocolate milkshake or tasteless solution, respectively. On 40% of the trials the taste was not delivered after the cue to allow investigation of the neural response to anticipation of a taste that was not confounded with actual receipt of the taste (unpaired trials). There were 30 repeats of both milkshake receipt and tasteless solution receipt and 20 repeats of both the unpaired milkshake cue and the unpaired tasteless solution cue. Tastes were delivered with programmable syringe pumps. Syringes filled with milkshake and tasteless solution were connected via Tygon tubing to a manifold that fit into the mouths of participants and delivered the taste to a consistent tongue segment. Participants were instructed to swallow when they saw the “swallow” cue.
Figure 1.
Example of timing and ordering of presentation of (A) pictures and beverages during the food reward paradigm and of (B) presentation of images and notification of monetary reward during the monetary reward paradigm.
The second functional magnetic resonance imaging (fMRI) paradigm assessed activation in response to receipt and anticipated receipt of monetary reward (Figure 1B). First a coin on the left side of the screen alternates between blinking heads (H) and tails (T) 2–4 times for 300 msec/blink and then “lands” on either H or T. After 2 sec, a second coin blinks 4–6 times before landing on H or T. After 3 sec, a third coin blinks 8–10 times before landing on H or T. After the presentation of the coins, a message appeared saying whether or not the subject won (“You win $3” or “You don't win”). In total, there were 20 win events (HHH or T T T displays), 30 win anticipation events (HH or T T displays), and 30 reward-neutral events (when a single H or T was displayed).
We included an operant behavioral task that assesses individual differences in food and monetary reinforcement (51) to validate the fMRI paradigms. Reward-related activation (caudate, r = .30) in response to milkshake receipt correlated with how much participants worked for energy-dense snacks. Activation in regions involved in attention (inferior parietal lobe, r = .42; anterior cingulate cortex, r = .32), visual processing (cuneus, r = .38), and reward (mid insula, r = .31) in response to monetary reward receipt or anticipated receipt correlated with how much participants worked for money (n = 162). These data suggest that both paradigms are valid measures of individual differences in food and monetary reward sensitivity.
Activation in response to food receipt was assessed by contrasting blood oxygen level–dependent (BOLD) signal during receipt of milkshake versus tasteless solution; activation in response to anticipated food receipt was assessed by contrasting BOLD signal during presentation of the unpaired milkshake cue versus unpaired tasteless solution cue; activation in response to monetary reward was assessed by contrasting BOLD signal when a participant “won” (HHH or T T T displays) versus a reward-neutral coin display (the time the first coin stopped blinking, which conveyed no information about possible monetary reward); activation in response to anticipated monetary reward was assessed by contrasting BOLD activation during presentation of the display signaling a potential win (i.e., an HH or a T T display) versus the reward-neutral coin display. Condition-specific effects at each voxel were estimated with general linear models. Vectors of the onsets for each event of interest were entered into a design matrix so that event-related responses could be modeled by the canonical hemodynamic response function. A 128-sec high-pass filter removed low-frequency noise and slow signal drift.
Individual level maps were constructed for comparison of activation within each participant for the four contrasts on the second level (e.g., win–reward-neutral coin display). To assess differences between participants showing overweight/obesity onset versus those who remained at a healthy weight, we conducted mixed, between-/within-subjects 2 × 2 analyses of variance of the four contrasts of interest. A similar model compared adolescents who reported substance use at 1-year follow-up but not at baseline versus adolescents who reported abstinence at both assessments. We also conducted mixed between/within-subject 2 × 2 analyses of variance to compare participants: 1) overweight/obese at baseline versus those with a healthy weight; and 2) those reporting substance use at baseline versus those reporting abstinence.
Whole brain analyses were conducted after the sample-specific gray matter mask was applied. An overall significance level of p < .05 corrected for multiple comparisons across the masked whole brain was calculated. This was accomplished by first estimating the inherent smoothness of the masked functional data with the three-dimensional full-width-at-half-maximal module in AFNI (52). This smoothness was then used in 10,000 Monte Carlo simulations of random noise at 3 mm3 through the gray matter masked data with the 3DClustSim module of AFNI (52,53). Results from these simulations indicated activity surviving a threshold of p < .001, with a cluster (k) ≥ 12 considered significant corrected for multiple comparisons. Effect sizes (r) were derived from the Z values (Z/√N). We confirmed that effects were not driven by influential outliers.
Results
Thirteen participants were overweight/obese at baseline (mean percent body fat = 27.9 ± 5.3), 17 transitioned from a healthy weight to overweight (n = 12) or obese (n = 5) at 1-year follow-up (mean percent body fat change = 5.6 ± 3.1), and 123 remained in a healthy range from baseline to 1-year follow-up (mean percent body fat change = –.3 ± 3.2), on the basis of body fat percentile. At baseline, 50 participants reported substance use (mean = 2.98 ± 3.0; 88% beer/wine/wine coolers, 56% hard liquor, 22% cigarettes, 30% marijuana, 0% stimulants, 2% downers, 0% inhalants, and 4% hallucinogens), and 111 participants reported abstinence. At 1-year, 25 participants reported substance-use onset (total onset substance use = 2.96 ± 2.6; 76% beer/wine/wine coolers, 60% hard liquor, 12% cigarettes, 28% marijuana, 0% stimulants, 0% downers, 0% inhalants, and 0% hallucinogens), and 80 participants reported abstinence at both baseline and 1 year. For those who reported baseline substance use or substance use onset, the degree of use reflects using three types of alcohol or drugs a “few times” in the past year, report using one type of alcohol or drugs 1–3 times/month over the last year, or some other intermediate combination, reflecting moderate use levels for this developmental period. Additional details are reported in Supplement 1.
Relation of Neural Responsivity to Onset of Overweight/Obesity Over 1-Year Follow-Up
Participants showing future overweight/obesity onset relative to those remaining at a healthy weight exhibited less activity in the middle temporal gyrus extending into the lateral occipital gyrus (r = –.31) in response to milkshake receipt > tasteless receipt (Table 1) and less activity in the precuneus (r = –.34) when anticipating milkshake > anticipating tasteless at baseline (Table 1). No significant effects were observed for responsivity to winning or anticipating winning money. A TaqIA A1 status did not moderate the relation between neural response to receipt or anticipated receipt of milkshake or monetary reward in the prediction of overweight/obesity onset (see Stice et al. [12] for details with regard to genotyping procedures).
Table 1.
Between-Group Comparisons for Adolescents Who Showed Overweight/Obesity Onset Versus Continued Healthy Weight and Substance Use Onset Versus Continued Abstinence
| Contrast and Region | k | Z Value | MNI Coordinates | r |
|---|---|---|---|---|
| Overweight/Obesity Onset vs. Persistent Healthy Weight | ||||
| Milkshake receipt > tasteless receipt | ||||
| Middle temporal gyrus | 36 | –3.63 | –45, –55, 10 | –.31 |
| –3.50 | –39, –79, 13 | –.30 | ||
| –3.27 | –48, –70, 16 | –.28 | ||
| Anticipated milkshake > anticipated tasteless | ||||
| Precuneus | 19 | –3.97 | –15, –76, 46 | –.34 |
| –3.49 | –21, –64, 40 | –.29 | ||
| Substance Use Onset vs. Persistent Abstinence | ||||
| Winning > neutral | ||||
| Caudate | 17 | 3.93 | –9, 8, 1 | .31 |
| Putamen | 12 | 3.60 | 21, 11, 1 | .28 |
Between-group comparisons for adolescents showing overweight/obesity onset (n = 17) versus a healthy weight at both baseline and 1-year follow-up (n = 118) and comparisons for adolescents who reported substance use onset (n = 25) versus abstinence at both baseline and 1-year follow-up (n = 80). For all contrasts, activated regions, number of contiguous voxels (k), Z values, and coordinates within the Montreal Neurological Institute (MNI) coordinate system are displayed. Peaks within the regions were considered significant at k ≥ 12, p < .05, whole brain corrected for multiple comparisons.
Relation of Neural Responsivity to Baseline Overweight/Obesity
Overweight/obese relative to healthy-weight participants at baseline showed greater activity in the right hippocampus (r = .29) and mid cingulate (r = .36) in response to milkshake receipt > tasteless receipt (Table 2). No significant differences were observed between baseline overweight/obesity and healthy weight when anticipating milkshake. In response to the monetary reward > reward neutral display and the anticipated monetary reward > reward neutral display overweight/obesity participants showed greater activity in the fusiform gyrus (win: r = .41; anticipated win: r = .39) (Table 2).
Table 2.
Between-Group Comparisons for Overweight Versus Healthy Weight and Substance-Using Versus Abstinent Adolescents
| Contrast and Region | k | Z Value | MNI Coordinates | r |
|---|---|---|---|---|
| Baseline Overweight vs. Healthy Weight | ||||
| Milkshake receipt > tasteless receipt | ||||
| Mid cingulate | 14 | 4.56 | 15, –22, 49 | .36 |
| Hippocampus | 13 | 3.75 | 33, –43, –8 | .29 |
| Winning > neutral | ||||
| Fusiform gyrus | 49 | 5.26 | –27, –58, –20 | .41 |
| Anticipating winning > neutral | ||||
| Fusiform gyrus | 83 | 4.96 | –27, –61, –20 | .39 |
| 3.21 | –24, –76, –8 | .25 | ||
| Baseline Substance Use vs. Abstinent | ||||
| Winning > neutral | ||||
| Caudate | 35 | –3.98 | 21, –10, 31 | –.31 |
Between-group comparisons for overweight (n = 13) versus healthy weight (n = 149) and substance using (n = 51) versus abstinent (n = 111) adolescents at baseline. For all contrasts, activated regions, number of contiguous voxels (k), Z values, and coordinates within the Montreal Neurological Institute (MNI) coordinate system are displayed. Peaks within the regions were considered significant at k ≥ 12, p < .05, whole brain corrected for multiple comparisons.
Relation of Neural Responsivity to Substance-Use Onset Over 1-Year Follow-Up
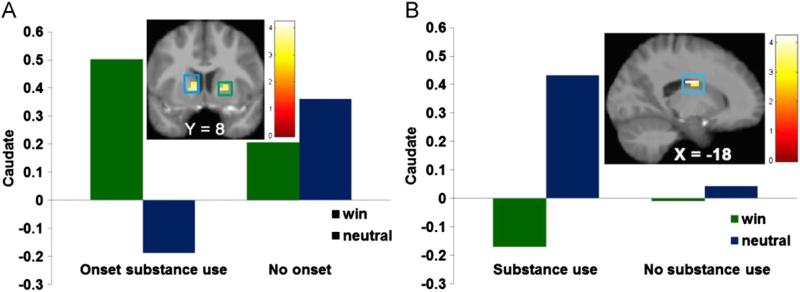
Participants showing future substance-use onset versus those showing continued abstinence exhibited greater left caudate (r = .31) and right putamen (r = .28) response to monetary reward > reward neutral display at baseline (Figure 2A, Table 1). No significant effects were observed to anticipated monetary reward or to receipt or anticipated receipt of food reward.
Figure 2.
Participants showing substance use onset by 1-year follow-up exhibited greater activation in (A) the caudate (Montreal Neurological Institute [MNI] coordinates: –9, 8, 1, Z = 3.93, k = 17) and putamen (MNI coordinates: 21, 11, 1, Z = 3.60, k = 12) in response to receipt of monetary reward compared with those reporting continued abstinence. Participants reporting substance use at baseline showed less activation in (B) the caudate (MNI coordinates: 21, –10, 31, Z = –3.98, k = 35) in response to receipt of monetary reward compared with participants reporting abstinence at baseline.
Relation of Neural Responsivity Baseline Substance Use
Participants reporting substance use at baseline showed less left caudate (r = –.31) response to monetary reward receipt relative to those reporting abstinence at baseline (Figure 2B1, Table 2; Figure S1 in Supplement 1). No significant effects were observed to anticipated monetary reward or to receipt or anticipated receipt of food reward.
Discussion
Greater activation in the dorsal striatum (caudate, putamen) in response to monetary reward receipt at baseline predicted future substance use onset over 1-year follow-up. Caudate activation has been associated with expectation of a positive reward (54), exposure to cues with increased incentive value (55), and exposure to drug stimuli for substance-dependent participants (56). Putamen activation has been associated with dopaminergic signaling and the reception of input from premotor and sensory regions (57). It was noteworthy that despite the use of whole brain analyses, the only peaks that significantly predicted substance use onset were in the dorsal striatum—one of the most characterized reward regions. Also of note was that it was reward region response to monetary reward receipt rather than anticipated receipt that predicted substance use onset. These novel results imply that reward region hyper-responsivity constitutes an initial vulnerability factor for substance use onset, providing support for the reward surfeit theory of substance abuse (30). There was no prospective support for the reward deficit model of substance use (1), which was primarily based on cross-sectional studies that compared substance-dependent individuals with health control subjects (18–20). This pattern of findings suggests that the lower D2 receptor availability and responsivity of reward regions among substance-dependent individuals in prior cross-sectional studies (20–23) is a consequence rather than a cause of substance use, as implied by animal experiments (15–17).
Interestingly, adolescents who had already initiated substance use showed less dorsal striatum response to monetary reward. Results accord with studies that have found that substance-dependent versus nondependent adults show less ventral striatal response to monetary reward (16,23). The current finding provides the first evidence that even a relatively short period of moderate substance use might reduce reward region responsivity to a general conditioned reinforcer. However, because these findings were cross-sectional, it is possible that adolescents who show the weakest reward region responsivity initiate substance use at a younger age and constitute a qualitatively different group at risk for substance use than those with elevated reward region responsivity. Post hoc analyses found no evidence of a quadratic relation between activation in reward regions in response to these paradigms and future substance use onset in the present data. However, future research with larger samples should explore the notion that there are two qualitatively distinct groups at risk for substance use, who are characterized by either hyper- or hypo-responsivity of reward regions.
There was no evidence that elevated responsivity of classic reward regions predicted future overweight/obesity onset in a sample of adolescents with an initially healthy weight. This replicates a lack of main effects between reward region responsivity and future weight gain observed in a previous study (12). It is possible that it is necessary to follow participants for longer than 1 year to be able to identify those who are going to show sustained unhealthy weight gain over the long run, versus transitory weight changes. It is also possible that by recruiting a sample that is largely in the healthy weight range, that we are seeing what might be referred to as dispersion from the mean (the opposite of regression to the mean, which happens when you recruit a sample with elevated pathology). The SD for BMI was 1.9 at pretest and 2.3 at 1-year follow-up. This too would suggest that it will be important to predict longer-term weight gain. We did not replicate the finding that TaqIA A1 allele status interacted with striatal responsivity to predict elevated future weight gain that we observed previously (12), potentially because we predicted initial unhealthy weight gain herein, versus escalation in weight among an overweight sample. It should be noted that the percent change in BMI in the former trial was 3.6 (12), compared with 3.6 in the present sample, suggesting that the differential effects were not rooted in differences in change in weight over follow-up. We should also note that the TaqIA A1 allele status did not moderate the relation of striatal response to receipt and anticipated receipt of food and monetary reward to future substance use onset.
Results indicated that reduced responsivity of the middle temporal gyrus to milkshake receipt and reduced responsivity of the precuneus to anticipated milkshake receipt predicted overweight/obesity onset. Activity in the middle temporal gyrus is typically related to associative and semantic memory (58), but reduced cerebral blood flow in this region has been observed in obese relative to lean individuals during consumption of an energy-dense beverage (59). The precuneus is thought to be prominent in the default network and shown to be functionally connected to multiple cortical and subcortical regions, including projections to reward regions (e.g., striatum and midbrain) (60). Furthermore, it is thought to play a central role in the modulation of higher-order conscious processes (60). Present data hint that the cognitive processes during anticipation of palatable food receipt was less conscious (i.e., more automatic in participants that showed onset of overweight), possibly due to repeated consumption of these types of foods.
Overweight/obesity participants also showed greater activation in the hippocampus and mid cingulate in response to milkshake receipt relative to healthy weight adolescents. The hippocampus has been implicated in memory and learning (61). Del Parigi et al. (62) found that obese and post-obese show greater responsivity in the hippocampus after food intake relative to lean individuals. The mid cingulate has been implicated in memory and emotional valence (63). Thus, results imply that overweight and obese individuals might have stronger memories for the taste of high-fat/high-sugar foods such as milkshake. Overweight/obese versus healthy weight participants also showed greater activation in the fusiform gyrus in response to both receipt and anticipated receipt of monetary reward. This region has been associated with in visual object recognition (64), suggesting that overweight/obese individuals show more recruitment of visual object recognition regions when confronted with monetary reward.
Of note, substance use and future substance use onset were related to receipt of monetary reward but not food reward, whereas current overweight/obesity and overweight/obesity onset was primarily related to receipt/anticipated receipt of milkshake but not monetary reward. This pattern of findings implies that aberrant neural responsivity to food reward is somewhat specific to obesity onset, as one might expect given the role of hyperphagia in obesity. That current substance use and future substance use onset showed stronger relations to aberrant neural responsivity to monetary reward versus food reward might have emerged because money is a universal reward that can be exchanged for any idiosyncratically desired reward, whereas palatable food is a very specific reward. It would be interesting for future studies to test whether current substance use and future substance use onset shows stronger relations to receipt and anticipated receipt of psychoactive substances (e.g., caffeine, methylphenidate) than to monetary reward.
Interestingly, there was little overlap in the participants who showed substance use onset and overweight/obesity onset (r = .14, p = .19), suggesting that—despite the notion that aberrant reward circuitry responsivity is a shared vulnerability factor—adolescents who show onset of one of these appetitive problems are unlikely to show onset of the other. Results imply that individuals might develop habits with specific behaviors that cause DA release rather than multiple behaviors that accomplish this end.
It is important to consider the study limitations. First, there was limited sensitivity for the overweight/obesity onset analyses, because only 17 participants showed this transition over follow-up. Second, we only examined neural response to one palatable food, which might have reduced predictive power. Third, we did not investigate whether individual difference in reward region response to receipt and anticipated receipt of psychoactive substances predicted future substance use onset—an important direction for future research.
Collectively, findings provide novel support for the reward surfeit model of substance use but provide no support for the reward deficit model of substance use. Indeed, findings imply that even a limited substance use history was associated with reduced reward region responsivity, extending results from fMRI studies that compared substance-dependent individuals with healthy control subjects and suggesting that substance use downregulates reward circuitry. These new prospective data seem to resolve seemingly inconsistent findings in the literature with regard to neural vulnerability factors for substance use. The current results provide no evidence that individuals who later show overweight/obesity onset or those that are currently overweight/obese versus in a healthy weight range show aberrant reward circuitry responsivity to food or monetary reward, although there was evidence that overweight/obese adolescents did show greater activation of regions implicated in memory, learning, and emotional valence in response to palatable food receipt. It will be important to test whether aberrant responsivity of reward and related regions to food and monetary reward predict overweight/obesity onset over a longer follow-up. These findings seem to have two primary implications. First, data suggest that young adolescents with elevated reward region responsivity might constitute an important high-risk population to target with evidence-based selective substance abuse prevention programs. Second, results imply that it would be useful to better educate youth about the potential reduction of reward region responsivity that occurs in response to even limited moderate regular substance use, which might make a variety of activities (e.g., sex) less rewarding; this information might deter youth from initiating substance use.
Supplementary Material
Acknowledgments
Support for this work was provided by National Institutes of Health Grant DK-080760. We would like to thank the Lewis Center for Neuroimaging at the University of Oregon for their contribution and assistance in imaging for this investigation.
Footnotes
Because of concern that the peak reflecting reduced caudate activation in participants reporting substance use versus abstinence at baseline might have occurred in white matter, we thought it important to show that this peak did occur in gray matter on the basis of the activation in the gray matter mask created for the sample (Figure S1 in Supplement 1).
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Small D, Jones-Gotman M, Dagher A. Feeding-induced dopa-mine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 4.Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–R910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- 5.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orosco M, Rouch C, Nicolaidis S. Rostromedial hypothalamic monoamine changes in response to intravenous infusions of insulin and glucose in freely feeding obese Zucker rats: A microdialysis study. Appetite. 1996;26:1–20. doi: 10.1006/appe.1996.0001. [DOI] [PubMed] [Google Scholar]
- 7.De Weijer B, van de Giessen E, van Amelsvoort T, Boot E, Braak B, Janssen I, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1:37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 10.Frank G, Reynolds J, Shott M, Jappe L, Yang T, Tregellas J, et al. Anorexia nervosa and obesity are associated with apposite brain reward response. Neuropsychopharmacology. 2012;37:1–16. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res. 2011;1386:109–117. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psych. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed S, Kenny P, Koob G, Markou A. Neurobiological evidence of hedonic allostasis associated with escalating cocaine use. Nat Neuroscience. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 15.Kenny P, Chen S, Kitamura O, Markou A, Koob G. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 17.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang G, Fowler JS, Logan J. Effects of methylphenidate on regional brain glucose metabolism in humans: Relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154:0–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Chang L, Wang G, Fowler JS, Ding Y, Sedler M, et al. Low level of brain dopamine D2 receptors in methampheta-mine abusers: Association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Volkow ND, Fowler JS, Logan J. Dopamine D2 receptor availability in opiate dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- 21.Martinez D, Narendran R, Foltin R, Slifstein M, Hwang D, Broft A, et al. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 22.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral striatum activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Wrase J, Schlagenhauf F, Kienast T, Wustenberg R, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 24.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Val-Laillet D, Layec S, Guérin S, Meurice P, Malbert C. Changes in brain activity after a diet-induced obesity. Obesity. 2011;19:749–756. doi: 10.1038/oby.2010.292. [DOI] [PubMed] [Google Scholar]
- 26.Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 27.Alsio J, Olszewski PK, Norback AH, Gunnarsson ZEA, Levine AS, Pickering C, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171:779–787. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Unterwald EM, Kreek MJ, Cuntapay M. The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res. 2001;900:103–109. doi: 10.1016/s0006-8993(01)02269-7. [DOI] [PubMed] [Google Scholar]
- 29.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 30.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Martin LE, Hosen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2009;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 32.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Ng J, Stice E, Yokum S, Bohon C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite. 2011;57:65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 37.George M, Anton R, Bloomer C, Teneback C, Drobes D, Lorberbaum J, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 38.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 39.Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg A, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- 40.Kiyatkin EA, Gratton A. Electrochemical monitoring of extra-cellular dopamine in nucleus-accumbens of rats lever-pressing for food. Brain Res. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 41.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am J Med Genet. 1997;74:162–166. [PubMed] [Google Scholar]
- 43.Tupala E, Hall H, Bergstrom K, Mantere T, Rasanen P, Sarkioja T, et al. Dopamine D2 receptors and transporters in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum Brain Mapp. 2003;20:91–102. doi: 10.1002/hbm.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chouinard-Decorte F, Felsted J, Small D. Increased amygdala response and decreased influence of internal state on amygdala response to food in overweight compared to healthy weight individuals. Appetite. 2010;54:639. [Google Scholar]
- 45.Demos K, Heatherton T, Kelley W. Individual differences in nucleus accumbens activity to food and sexual images predicts weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. 2011;19:1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohman TG. Assessment of body composition in children. Pediatr Exerc Sci. 1989;1:19–30. doi: 10.1123/pes.1.1.19. [DOI] [PubMed] [Google Scholar]
- 48.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am J. Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obesity. 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 50.Stice E, Barrera M, Chassin L. Prospective differential prediction of adolescent alcohol use and problem use: Examining mechanisms of effect. J Abnorm Psychol. 1998;107:616–628. doi: 10.1037//0021-843x.107.4.616. [DOI] [PubMed] [Google Scholar]
- 51.Saelens BE, Epstein LH. The reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 52.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance Neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 53.Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 54.Kawagoe R, Takikawa Y, Hikosaka O. Reward-predicting activity of dopamine and caudate neurons: A possible mechanism of motivational control of saccadic eye movement. J Neurophysiol. 2004;91:1013–1024. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- 55.Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- 56.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 57.Marchand WR, Lee JN, Thatcher JW, Hsu EW, Rashkin E, Suchy Y, et al. Putamen coactivation during motor task execution. Neurore-port. 2008;19:957–960. doi: 10.1097/WNR.0b013e328302c873. [DOI] [PubMed] [Google Scholar]
- 58.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 59.Del Parigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA. Sensory experience of food and obesity: A positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24:436–443. doi: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 60.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 61.Morris RGM. Theories of hippocampal function. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; New York: 2006. pp. 581–713. [Google Scholar]
- 62.Del Parigi A, Chen K, Hill DO, Wing RR, Reiman E, Tataranni PA. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obesity. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 63.Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 64.Karnath HO, Ruter J, Mandler A, Himmelbach M. The anatomy of object recognition—visual form agnosia caused by medial occipitotemporal stroke. J Neurosci. 2009;29:5854–5862. doi: 10.1523/JNEUROSCI.5192-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.