Abstract
The anti-diabetic effects of Brazilian propolis were examined using ob/ob mice. Although repeated injection of an ethanol extract of Brazilian propolis (100 mg/kg, ip, twice a week for 12 weeks) did not affect body weight gain and food intake of ob/ob mice, blood glucose and plasma cholesterol levels were significantly attenuated. Moreover, the propolis extract partially restored glucose tolerance and insulin resistance, indicating anti-diabetic properties of the extract. The propolis-treated mice exhibited lower weight gain in mesenteric adipose tissue, while weight gains in inguinal and epididymal adipose tissues were not modulated. Flow cytometric and microscopic analyses suggested that the extract promoted accumulation of eosinophils into mesenteric and epididymal adipose tissues. Alternatively, the ratio of M1-like macrophages to M2-like macrophages in mesenteric adipose tissue was reduced by the propolis injection, coincident with the decrement of the number of interleukin-12A+ cells. Levels of M1 macrophage markers, such as Itgax and Il12b transcripts, were decreased in the vascular stromal fraction of mesenteric adipose tissue, whereas those of pan-macrophage markers Emr1 and Cd68 were not influenced. Microarray and subsequent gene ontology term analyses suggested that propolis attenuated immune activation in mesenteric adipose tissues. Taken together, this indicates that Brazilian propolis improves diabetes in ob/ob mice, presumably through modification of immune cells in mesenteric adipose tissues.
Keywords: propolis, type 2 diabetes, metaflammation, chronic inflammation, eosinophils, adipose tissue macrophage, ob/ob mouse
Introduction
Obesity and its associated type 2 diabetes have reached epidemic proportions, becoming major health issues in modern society. A major cause of type 2 diabetes is prolonged nutrient overload, which leads to insulin resistance. Increasing evidence indicates that the root cause of insulin resistance is chronic inflammation, namely, metaflammation, in adipose tissues.1,2 Adipose tissue inflammation is caused by dominance of proinflammatory cells, such as M1-like macrophages, neutrophils, and CD4− CD8+ T lymphocytes.2-5 Alternatively, M2-like macrophages, eosinophils, and regulatory T cells sequester inflammation.2,3,6,7 Thus, control of the immune cell populations in adipose tissues is believed to prevent type 2 diabetes.
Propolis is a hive product of the honeybee (Apis mellifera) that consists of resinous materials and secretion. The chemical composition of propolis is dependent on the location of origin, owing to variations in the species of bees and plants. Brazilian propolis is recognized as a highly-effective medicinal substance.8 It contains high levels of Baccharis dracunculifolia extract, which has several pharmacological properties.9 Indeed, several studies have demonstrated that Brazilian propolis has anti-inflammatory,10,11, anti-tumor,12 microbicidal,13,14 and anti-oxidative15 effects in animal models, which indicates the potential for medical usage of this substance. In addition, previous papers have indicated that Brazilian propolis also modulates energy metabolism. Ichi et al. verified that supplementation of propolis (5 g/kg diet) in food decreased fat pad as well as blood triglyceride and cholesterol in mice fed with a high fat diet (HFD).16 Moreover, daily administration of Brazilian propolis restored insulin resistance in obese rat models.17,18 However, despite these pioneer studies, the mechanisms that underlie the anti-diabetic effects of propolis in vivo are not fully understood. In particular, no studies have investigated whether immune cells are involved in the effects of propolis. In this study, we demonstrate the anti-diabetic effects of a Brazilian propolis ethanol extract in genetically obese ob/ob mice. We also identify the changes in the immune cell population in adipose tissues that accompany changes in gene expression in this mouse model.
Results
Intraperitoneal injection of a Brazilian propolis ethanol extract attenuated progression of diabetes, regardless of changes in body weight and food intake
Previous studies have demonstrated that propolis improves energy metabolism in several rat models16-18 and a lean mouse model.19 Thus, we first examined whether propolis also restores metabolic defects in obese mouse models. In the preliminary study, we intraperitoneally administrated a Brazilian propolis ethanol extract (100 mg/kg) to HFD-induced obese mice twice a week from 5 weeks of age. The mice showed a significant decrease in food intake and body weight gain compared with the vehicle-treated mice (data not shown). By contrast, obese C57BL/6JHamSlc-ob/ob mice (ob/ob mice) did not exhibit significant changes in food intake after the propolis treatment (Fig. 1A). In agreement, the body weight gain of ob/ob mice was only marginally affected by the propolis treatment. To focus on the peripheral effects of Brazilian propolis, independently of body weight change, we selected ob/ob mice for further analyses.
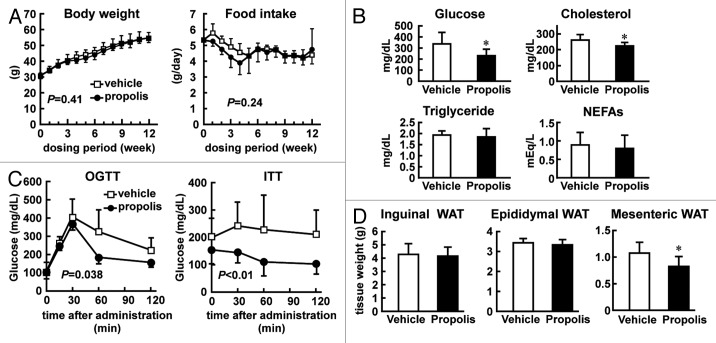
Figure 1. Effects of intraperitoneal injection of Brazilian propolis on metabolic states of ob/ob mice. (A) Effects of a Brazilian propolis ethanol extract on body weight (left) and food intake (right) in ob/ob mice. Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle was injected into ob/ob mice for 12 weeks. Values are the mean ± SD of 9 mice. The P values calculated by two-way repeated measures ANOVA are also shown. (B) Casual plasma glucose and lipid levels. Following 12 weeks of propolis injection, plasma was collected and subjected to analyses. Values are the mean ± SD of 9 mice. *P < 0.05 vs vehicle treated mice. (C) Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). Following 12–13 weeks of propolis injection, mice were subjected to OGTT and ITT. Values are the mean ± SD of 6 (OGTT) and 11 (ITT) mice. The P values calculated by two-way repeated-measures ANOVA are also shown. (D) Wet tissue weight of white adipose tissues (WAT). Following 12 weeks of propolis injection, inguinal, epididymal, and mesenteric adipose tissues were collected. Values are the mean ± SD of 9–10 mice. *P < 0.05 vs the vehicle-treated mice.
We next monitored the plasma glucose levels of ob/ob mice treated with the propolis extract or vehicle for 12 weeks. As shown in Figure 1B, the propolis extract decreased plasma glucose levels by approximately two-thirds compared with the vehicle in ob/ob mice. Total cholesterol level was also slightly attenuated in the propolis-treated mice, while triglyceride and non-esterified free fatty acids (NEFAs) were not affected by the treatment. Thus, the propolis extract improved glucose metabolism in ob/ob mice.
Given that propolis extract inhibits the progression of diabetes, glucose tolerance might be restored by the propolis treatment. To examine this, we performed an oral glucose tolerance test (OGTT) on the propolis- and vehicle-treated ob/ob mice. As shown in Figure 1C, by 30 min after the glucose administration, both the propolis- and vehicle-treated mice displayed increased blood glucose levels. However, the propolis-treated mice displayed a more rapid decrease in blood glucose than the vehicle-treated mice, indicating that the feedback response worked more effectively in the propolis-treated mice. An insulin tolerance test (ITT) was also conducted on the propolis- and vehicle-treated ob/ob mice, with the results indicating a clear decrease in glucose in the propolis-treated mice after intraperitoneal injection of insulin. By contrast, the vehicle-treated mice failed to modulate their glucose levels. Taken together, these findings suggest that the propolis treatment improved insulin sensitivity in ob/ob mice.
It is well known that visceral and subcutaneous adipose tissues play distinct roles in the pathogenesis of type 2 diabetes.20 Accordingly, we examined the effects of propolis on the wet weight of each tissue type in ob/ob mice. The wet weight of inguinal adipose tissues was not influenced by the 12-week treatment with the propolis ethanol extract, as compared with the vehicle treatment (Fig. 1D). Similarly, the weight of epididymal adipose tissue was undistinguishable between the propolis- and vehicle-treated mice. By contrast, the weight of mesenteric adipose tissue was decreased by ~80% in the propolis-treated mice, as compared with the vehicle-treated mice, indicating the site selective effects of propolis in ob/ob mice.
Intraperitoneal injection of a Brazilian propolis ethanol extract modulated immune cell populations in the visceral adipose tissues of ob/ob mice
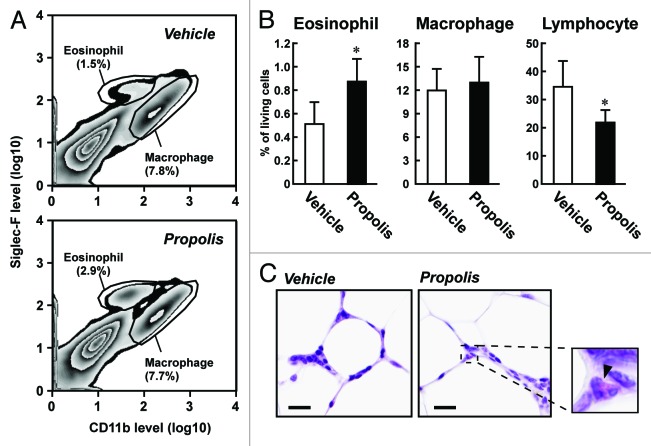
It is reasonable to suggest that the anti-diabetic effects of propolis may be linked to the regulation of immune cell populations due to the roles that adipose tissue immune cells play in the progression of type 2 diabetes.1,2 To elucidate this, we isolated the vascular stromal fraction (VSF) of the adipose tissues of the propolis- and vehicle-treated ob/ob mice, and profiled the populations of immune cells by fluorescence-activated cell sorter (FACS) analysis. As shown in Figures 2 and 3 and Table 1, the most apparent change in the propolis-treated mice was the accumulation of sialic acid-binding immunoglobulin-like lectin-F (Siglec-F)+ CD11bmid eosinophils in the epididymal and mesenteric adipose tissues. A histological approach also detected a higher frequency of eosin-stained eosinophils in the visceral adipose tissues of the propolis-treated mice (Fig. 3C). A slight decrease in the number of Siglec-F− CD11bhigh macrophages was observed in the epididymal adipose tissue, but not in the mesenteric adipose tissue of the propolis-treated mice (Figs. 2B and 3B; Table 1). On the other hand, total lymphocytes, defined by forward scatter (FSC) and side scatter (SSC), were decreased in the mesenteric adipose tissues, whereas the abundance of these cells was not changed in the epididymal adipose tissue (Fig. 3B; Table 1). The numbers of CD11b+ Gr-1+ neutrophils in the visceral adipose tissues that we examined were negligibly affected by the propolis treatment (Table 1). In inguinal adipose tissue, propolis did not affect the abundance of Siglec-F+ CD11bmid eosinophils, Siglec-F− CD11bhigh macrophages, CD11b+ Gr-1+ neutrophils, and total lymphocytes (Table 1).
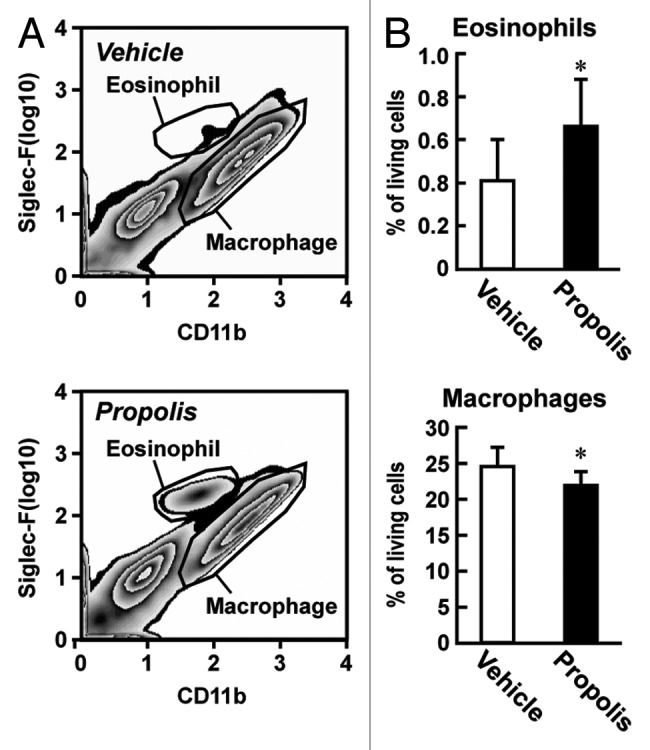
Figure 2. Effects of intraperitoneal injection of a Brazilian propolis ethanol extract on the proportion of eosinophils and macrophages in epididymal adipose tissue of ob/ob mice. A Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle was injected into ob/ob mice for 12 weeks. The vascular stromal fraction (VSF) of epididymal adipose tissue was used for FACS analysis. (A) Representative images of FACS analysis of eosinophils and macrophages. After removal of dead cells, eosinophils and macrophages were defined by the staining pattern of Siglec-F and CD11b. (B) The proportion of eosinophils and macrophages in living cells in the VSF of epididymal adipose tissue. Values are the mean ± SD of 7 mice. *P < 0.05 vs the vehicle-treated mice.
Figure 3. Effects of intraperitoneal injection of a Brazilian propolis ethanol extract on the proportion of eosinophils, macrophages, and total lymphocytes in the mesenteric adipose tissue of ob/ob mice. A Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle was injected into ob/ob mice for 12 weeks. (A) Representative images of FACS analysis of eosinophils and macrophages in the VSF of mesenteric adipose tissue. After removal of dead cells, eosinophils and macrophages were defined by the staining pattern of Siglec-F and CD11b. (B) The proportion of immune cells in living cells in the VSF of mesenteric adipose tissue. Total lymphocytes were defined by forward scatter (FSC) and side scatter (SSC). Values are the mean ± SD of 7 mice. *P < 0.05 vs the vehicle-treated mice. (C) Hematoxylin and eosin staining of mesenteric adipose tissue. Representative images of three experimental series are shown. An enlarged view of the propolis-treated sample is also shown. An arrowhead indicates an eosinophil. Bars represent 20 μm.
Table 1. Summary of the effects of a Brazilian propolis ethanol extract on immune cells in adipose tissues.
| Macrophage | Eosinophil | Neutrophil | Lymphocytes | |
|---|---|---|---|---|
|
Marker |
Siglec-F-, CD11bhigh |
Siglec-F+, CD11bmid |
CD11b+, Gr1+ |
FSClow, SSClow |
|
Mesenteric WAT |
- |
increase |
- |
decrease |
|
Epididymal WAT |
decrease |
increase |
- |
- |
| Mesenteric WAT | - | - | - | - |
A Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle was injected into ob/ob mice for 12 weeks. FACS analysis was performed using the VSF from epididymal, mesenteric, and inguinal adipose tissues. The analysis was performed using 7 replicates. When the P value calculated by the Student t test was less than 0.05 vs the vehicle treated group, we judged as increase or decrease. WAT, white adipose tissue.
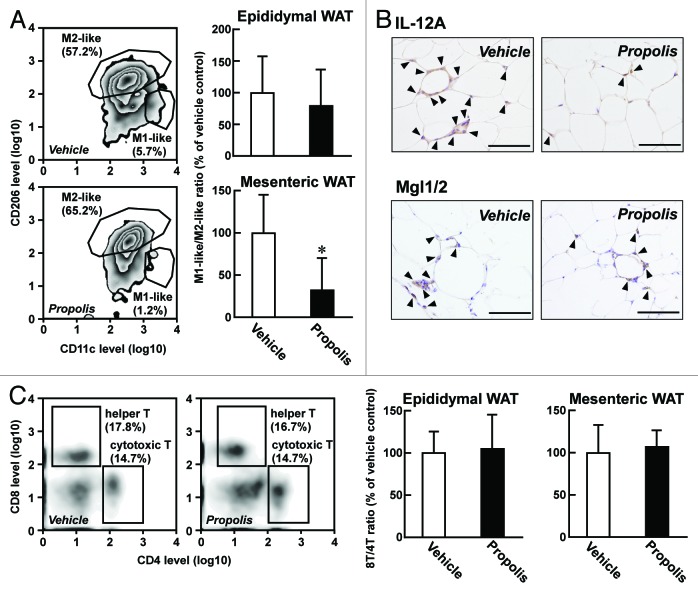
Macrophages can be classified into two categories: F4/80+ CD11c+ CD206- M1-like macrophages and F4/80+ CD11c− CD206+ M2-like macrophages, which promote and attenuate type 2 diabetes, respectively.1,2 To evaluate whether the propolis extract tested was able to influence macrophage subpopulations, we elucidated the ratio of M1-like macrophages to M2-like macrophages in visceral adipose tissues. In epididymal adipose tissue, the ratio was only marginally affected by the propolis treatment (Fig. 4A). In contrast, the ratio in mesenteric adipose tissue was clearly decreased by ~30%. In agreement with these, the immunohistochemical study indicated that interleukin (IL)-12A+ M1-like macrophages were decreased in mesenteric adipose tissue after injection of the propolis extract, while the number of macrophage galactose-type calcium-type lectin1/2 (Mgl1/2)+ M2-like macrophages in the tissue were not modulated (Fig. 4B). Hence, the propolis extract seemed to promote anti-diabetic changes in macrophage subpopulations in mesenteric adipose tissue, although the total number of macrophages in the tissue did not change (Fig. 3B).
Figure 4. Effects of a Brazilian propolis ethanol extract on subpopulations of macrophages and T lymphocytes. A Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle were injected into ob/ob mice for 12 weeks. (A) Effects of propolis on the proportion of macrophage subpopulations. (Left) Representative FACS images of CD11c+ M1-like macrophages and CD206+ M2-like macrophages in mesenteric adipose tissue. The percentage proportions of each macrophage subpopulation in F4/80+ cells are also shown. (Right) The ratio of M1-like macrophages to M2-like macrophages. Values are the mean ± SD of 7 mice. *P < 0.05 vs the vehicle-treated mice. (B) Imunohistochemical detection of M1-like and M2-like macrophages in mesenteric adipose tissue. M1-like and M2-like macrophages were detected using antibodies for IL-12A and Mgl1/2, respectively. Arrowheads indicate immunosignals. Scale bars represent 100 μm. (C) Effects of proportion of T-lymphocyte subpopulations. (Left) Representative FACS images of CD4+ CD8− helper T lymphocytes and CD4− CD8+ cytotoxic T lymphocytes in mesenteric adipose tissue. The percentage proportions of each T-lymphocyte populations are also shown. (Right) The ratio of cytotoxic T lymphocytes to helper T lymphocytes (8T/4T ratio). Values represent the mean ± SD of 7 mice. WAT, white adipose tissue.
In HFD-induced obese mice, it has been reported that the number of CD4− CD8+ cytotoxic T lymphocytes (8T) was elevated, whereas the number of CD4+ CD8− helper T lymphocytes (4T) was diminished.4 Thus, the 8T/4T ratio is another index of progression of type 2 diabetes. As shown in Figure 4C, the repeated injection of the propolis extract did not modify the 8T/4T ratio, both in epididymal and mesenteric adipose tissues of ob/ob mice, suggesting that cytotoxic T lymphocytes are not involved in the anti-diabetic effects of propolis.
Transcriptome analysis of the VSF of mesenteric adipose tissue of ob/ob mice following the propolis treatment
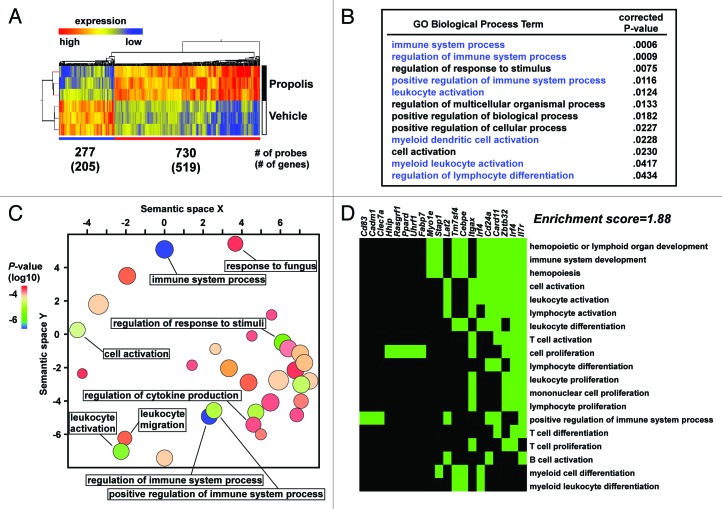
Utilizing microarrays, we performed transcriptome analysis of the VSF of mesenteric adipose tissue of ob/ob mice following the 12-week propolis or vehicle-treatment. Analysis of the microarray data identified 519 genes that were >2-fold significantly (P < 0.05) upregulated and 205 genes that were >2-fold significantly (P < 0.05) downregulated in the VSF following the propolis treatment (Fig. 5A). We then performed gene ontology (GO) term analyses using the GOrilla and REViGO programs in order to elucidate overrepresented functions in the propolis-affected genes.21,22 We found that although the propolis-potentiated genes displayed a wide variety of functional properties (Fig. S1; Table S2), the propolis-attenuated genes selectively exhibited immune associated properties, such as positive immune system regulation and leukocyte activation (Fig. 5B and C). Moreover, the DAVID program23 indicated that the set of propolis-attenuated genes included a cluster consisting of lymphocyte/myeloid cell-associated genes, such as Itgax (also known as Cd11c), Stap1, and Tm7sf4 (also known as Dc-stamp) (Fig. 5D). This suggests that the propolis extract modulates immune functions at the gene expression level in mesenteric adipose tissue.
Figure 5. Effects of a Brazilian propolis ethanol extract on the transcriptome of the VSF of mesenteric adipose tissue. A Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle were injected into ob/ob mice for 12 weeks. Triplicate total RNA samples were prepared from the VSF of mesenteric adipose tissue, and subjected to a microarray analysis. (A) Heatmap representation of expression of the propolis-affected genes. Numbers of increased and decreased genes were 519 and 205 genes, which corresponded to 730 and 277 probes, respectively. Clustering trees are also depicted. (B) Gene Ontology (GO) term analysis of the propolis-attenuated genes using the GOrilla program. We selected Biological Process ontology terms for this analysis. Terms and the P value are shown. Terms associated with immune responses are noted in blue. (C) A scatter plot representation of GO analysis data by the REViGO program. The scatter plot shows a two dimensional cluster representative of the Biological Process ontology terms’ semantic similarities. The color of the circles represents the P value; size indicates the frequency of the GO term in the Gene Ontology Annotation data set. (D) An annotation cluster of the propolis-attenuated genes. Functional clustering of genes and annotation terms were performed by the DAVID program. A cluster showing high enrichment score includes immune activation genes. Green areas indicate the term–gene pairs.
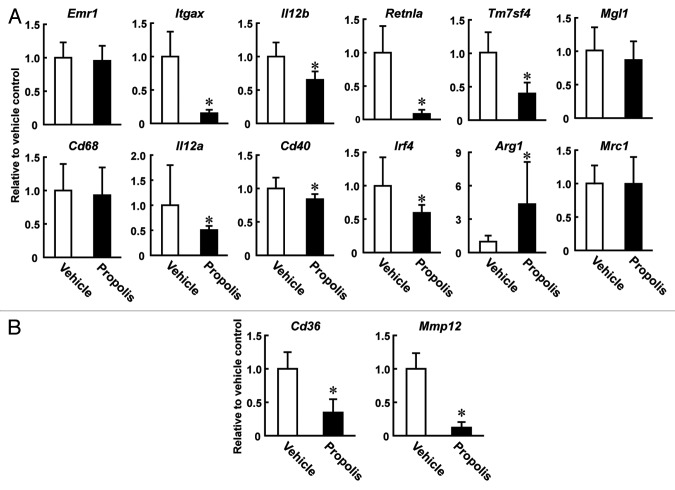
The FACS and immunohistochemical analyses indicated that the propolis injection caused a decrease in the ratio of M1-like to M2-like macrophages in the VSF of mesenteric adipose tissue (Fig. 4A). To confirm this, we monitored the mRNA levels of macrophage markers in the VSF of mesenteric adipose tissue. Quantitative reverse transcription-PCR (qRT-PCR) analyses indicated that transcripts for the pan-macrophage markers Emr-1 (F4/80) and Cd68 were not influenced by injection of the propolis extract (Fig. 6A). On the other hand, levels of transcripts for the M1 macrophage markers Itgax, Il12a, Il12b, and Cd40 were decreased. M2 macrophage markers showed distinct patterns following the treatment. Levels of Retnla (also known as Fizz1), Irf4, and Tm7sf4 transcripts were significantly decreased by the treatment, while those of Mgl1 and Mrc1 (also known as CD206) were marginally affected. Levels of Arg1 transcripts were significantly increased after the treatment. Of note, the propolis extract also modulated Cd36 and Mmp12 expression, both of which are known to be critical modulators of atherosclerosis (Fig. 6B).24,25 Collectively, these results indicate that the propolis extract modulates macrophage subpopulations in the mesenteric adipose tissues of ob/ob mice at the gene expression level, presumably by attenuating “metaflammatory” responses in the tissues.
Figure 6. qRT-PCR analysis of macrophage molecules using the mesenteric adipose tissues of the propolis-treated ob/ob mice. A Brazilian propolis ethanol extract (100 mg/kg, ip, twice a week) or vehicle was injected into ob/ob mice for 12 weeks. Total RNA isolated from the VSF of mesenteric adipose tissue was subjected to qRT-PCR analysis. Expression of (A) Pan, M1, and M2 marker genes, and (B) genes participating in pathogenesis of atherosclerosis were measured. Values are normalized to Hprt1 mRNA levels and represented as the mean ± SD of 4–5 mice. Log10-transformed values were subjected to the Student t test. *P < 0.05 vs vehicle treated mice. All primers used are listed in Table S1.
Discussion
Previous reports have demonstrated that propolis improves insulin sensitivity during the prediabetic and diabetic stages in obese rats.15-18 Moreover, propolis was proven to promote glucose uptake in mouse myocyte cell lines, as well as in the ICR mouse strain.19 The results of our study are in agreement with these previous findings, i.e., that a propolis ethanol extract prevented progression of diabetes in obese mice. Thus, propolis and its derived polyphenols seem to have the potential for use as therapeutic agents against type 2 diabetes. When we treated HFD-induced obese mice with the Brazilian propolis extract (100 mg/kg), we observed remarkable repression of food intake followed by prevention of body weight gain. On the other hand, the treatment did not result in significant changes in food intake or body weight gain in ob/ob mice. Considering that ob/ob mice have a missense mutaion in leptin gene,26 propolis might activate the leptin signaling in the HFD-induced obese mice.
Ichi et al. previously reported that oral administration of propolis clearly decreased blood cholesterol and triglyceride levels in rats. Total fat pad weight was also decreased; however, the effects on body weight were limited.16 Of interest, the authors showed a remarkable reduction in mesenteric adipose tissue weight, while epididymal adipose tissue weight was not significantly altered. In agreement, we observed a significant reduction of mesenteric adipose tissue weight, but not inguinal and epididymal adipose tissues weights, in ob/ob mice after the propolis treatment. This suggests that mesenteric adipose tissue is a dominant target of the propolis-derived metabolites. Considering that mesenteric adipose tissue plays crucial roles in the development of type 2 diabetes,27,28 the anti-diabetic effects of the propolis extract might be attributed to prevention of tissue responses in mesenteric adipose tissue.
One of the most striking findings of the current study is the increase of eosinophils in the visceral adipose tissues of ob/ob mice. Eosinophils are known to be a key cellular population involved in parasite immunity. In addition, a recent study revealed a novel function of this cell population.6,29 Eosinophils located in healthy adipose tissues are proposed to maintain the M2 macrophage-dominant state through IL-4 and IL-13 signaling. Indeed, eosinophil-induced models, such as Il5 transgenic mice and Nippostrongylus brasiliensis-infected mice exhibit improved insulin sensitivity and blood glucose levels.6 Conversely, eosinophil-deficient mice developed increased body weight, and impaired glucose tolerance and insulin resistance.6 Thus, accumulation of eosinophils in visceral adipose tissues seems to contribute to the anti-diabetic effects of the propolis extract.
Macrophages are one of the most prominent effecter cells regulating diabetic and anti-diabetic trends. M1-like macrophages confer insulin resistance through metaflammation, whereas M2-like macrophages sequester this response.1,2,30 In our experiments, the M1-like/M2-like macrophage ratio, defined by CD11c and CD206 expression, was dramatically decreased in mesenteric adipose tissue of the propolis-treated mice. Moreover, treatment with the propolis extract decreased number of IL-12A+ M1-like macrophages in coincidence with downregulated Itgax and Il12b, which are M1 macrophage marker molecules, in the VSF of the mesenteric adipose tissue. These results suggest that propolis repressed infiltration of M1-like macrophages and/or transformation of resident M2-like macrophages to M1-like macrophages in mesenteric adipose tissue.
Previous reports demonstrated that M2 macrophage markers are differentially expressed in several situations, including following prolonged periods on a HFD.31-33 These results indicate that the expression of each M2 macrophage marker is controlled by distinct mechanisms. Alternatively, a subpopulation of M2 macrophages might differentially emerge in lesions. In this study, the propolis extract had different effects on the expression of M2 macrophage markers: Retnla and Irf4 were remarkably decreased, whereas other markers, such as Arg1 and Mrc1, were increased or unchanged. Of interest, Retnla, Irf4, and Tm7sf4 are downstream targets of Jmjd3, and participate in differentiation of M2 macrophages for parasite immunity.34 Therefore, the propolis extract seems to attenuate the function and/or differentiation of Jmjd3-dependent M2 macrophages. Further studies are needed to clarify the roles of the Jmjd3-independent M2 macrophages in the anti-diabetic effects of Brazilian propolis.
Although we demonstrated the beneficial effects of the propolis extract on eosinophils and macrophages in visceral adipose tissues, we did not verify direct targets of cell types in the present study. Our preliminary data indicates significant increment of eosinophils in visceral adipose tissues at 4 weeks after beginning of the propolis injection, and negligible effect on 8T/4T and M1/M2 ratios at that time. Thus, eosinophils might be an initial target of the propolis extract. Alternatively, innate lymphoid type 2 cells (ILC2s) might be involved in the response. ILC2s activated by IL-33 sustained the number of M2 macrophages and eosinophils in visceral adipose tissues even after a high-fat diet.35 Interestingly, our microarray data indicated that expression of Il33 in the VSF of mesenteric adipose tissue was higher (>5-fold) in the propolis-treated mice than in the vehicle-treated mice (data not shown). Therefore, propolis might promote secretion of IL-33 from certain stromal cells in the visceral adipose tissue, resulting in activation of the ILC2-eosinophil-M2 macrophage cascade.
The pathogeneses of several metabolic diseases, such as type 2 diabetes, atherosclerosis, and nonalcoholic fatty liver disease share similar cellular and molecular events. In particular, local inflammation evoked by macrophages is commonly observed in the diseases.36 In this study, transcriptome analyses indicated that the propolis extract clearly downregulated Cd36 and Mmp12, both of which are known to form atherosclerotic plaques.24,25 Therefore, Brazilian propolis seems to be applicable to therapies against a wide variety of metabolic diseases.
In conclusion, Brazilian propolis modulates gene expression and constituent immune cells in the stromal region of mesenteric adipose tissue. These cellular changes may repress hyperplasia of the tissue, resulting in the attenuation of type 2 diabetes in ob/ob mice.
Materials and Methods
Mice and treatments
Male C57BL/6JHamSlc-ob/ob mice and C57BL/6 mice were purchased from Japan SLC C57BL/6 mice were fed with a 60% kcal high fat diet (Research Diet, D12451) from 5 weeks of age. Propolis extracted with 55% ethanol (provided from Yamada Bee Farm) was diluted 5-fold with dimethyl sulfoxide (DMSO), sterilized using a filter membrane (Millipore, SLGP033RS), and stored at −30 °C until usage. Immediately before usage, the propolis solution was diluted with phosphate buffered saline (PBS) to 5%. The diluted propolis (100 mg/kg) or vehicle (PBS containing ethanol and DMSO) was intraperitoneally injected into ob/ob mice twice a week from 6 weeks of age to 18 weeks of age. Body weight was measured once a week and food intake was measured twice a week. Following 12 weeks of the propolis injection, the mice were sacrificed by cervical dislocation, and plasma, visceral (mesenteric and epididymal), and inguinal adipose tissues were collected. All animal experiments were performed in pathogen-free conditions, and approved by the Animal Committee of Nagoya City University.
Plasma biochemical tests
Plasma glucose, cholesterol, triglyceride, and NEFAs were measured using Glucose CII (439-90901), Cholesterol E (439-17501), Triglyceride E (432-40201), and NEFA C (279-75401) Test Wako kits (Wako), respectively.
OGTT and ITT
Mice were fasted for 12 and 6 h before OGTT and ITT, respectively. For OGTT, 1 g/kg of d-glucose (Nacalai, 16805-35) was orally administered to mice. Blood was collected from a tail vein at 0, 15, 30, 60, and 120 min after the glucose administration, and subjected to glucose level measurement using a One Touch Ultra (Johnson and Johnson). For ITT, the mice were intraperitoneally injected with 2.0 U/kg of human insulin (Eli Lily, 2492403A4051), and subjected to measurements of blood glucose at 0, 30, 60, and 120 min after the injection.
Preparation of the VSF and flow cytometric analysis
Adipose tissues were cut into small pieces (<5 mm2) in PBS, and centrifuged at 800 × g for 5 min. Floated pieces were then treated with collagenase (Sigma-Aldrich, C9891) at 37 °C for 20 min with mild agitation. After centrifugation at 800 × g for 5 min, hemolysis was conducted using ammonium-chloride-potassium lysing buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate, 100 μM EDTA, pH 7.2). After filtration, the resultant cells in the VSF were blocked with TruStain FcX (Biolegend, 101320), and labeled with phycoerythrin-conjugated F4/80 (123110), AlexaFluor488-conjugated CD11b (101217), CD11c (117311) or CD8 (100723), or allophycocyanin-conjugated Gr-1 (108412), CD206 (141708), CD4 (100412) purchased from Biolegend, or with phycoerythrin-conjugated Siglec-F (BD Bioscience, 552126) in 3% fetal calf serum-containing PBS. After washing with PBS three times, dead cells were stained with 7-amino-actinomycin D (Biolegend, 420404), and monitored using FACS Canto2 (BD Bioscience). FACS data was analyzed using FlowJo software (Tree Star).
RNA extraction and mRNA analyses
Total cellular RNA was extracted from the VSF of mesenteric adipose tissues using TRIzol reagent (Life Technologies, 15596-018). Microarray experiments were performed using SurePrint G3 Mouse GE arrays (Agilent, G4852A). Microarray data was analyzed using GeneSpring 12.1 GX software package (Agilent). Genes were deemed “significant affected” when the array signals satisfied the following criteria: (1) in all samples the normalized expression value was greater than the background signal for either the propolis or vehicle treated group, (2) the change in the average signal intensity was greater than 2-fold, and (3) a Student t test showed that the change was significant (P < 0.05). Functional annotation analysis was conducted using the GOrilla (http://cbl-gorilla.cs.technion.ac.il/), REViGO (http://revigo.irb.hr/), and DAVID (http://david.abcc.ncifcrf.gov/) programs.21-23 All array data were deposited in Gene Expression Omnibus (Acc. No. GSE46110). Some total RNA samples were subjected to reverse transcription with Molony murine leukemia virus transcriptase (Invitrogen, 28025-021), and then to quantitative PCR with Platinum SYBR Green qPCR SuperMix-UDG reagent (Life Technologies, 11733046) using an ECO real time PCR system (Illumina).
Histological analyses
Adipose tissues were fixed with phosphate-buffered 4% paraformaldehyde solution (Nacalai, 26142-21) at 4 °C, embedded with paraffin, and sectioned at 5 µm. For detection of M1-like and M2-like macrophages, the sections were boiled in a citrate buffer (10 mM citrate buffer with 0.05% Tween20, pH 6.0) for 15 min, and reacted with antibodies for IL-12A (Abcam, ab135455) or Mgl1/2 (R&D System, AF4297) overnight. Subsequently, the diaminobenzidine-positive immunocomplex was detected with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunogulogulin (IgG) or FITC-conjugated anti-goat IgG antibody (Jackson Immunoresearch, 111-095-144 and 711-096-152) and horseradish peroxidase-conjugated anti-FITC (PerkinElmer, NEF710) antibody. Some sections were stained with hematoxylin-eosin (Wako, 032-14635 and 058-00062). The sections were monitored using a BX51 microscope (Olympus) equipped with a computer-controlled digital camera DP70 (Olympus).
Statistical analysis
Statistical analysis was performed by two-way repeated measures ANOVA and the student t test programs using the Kaleida Graph software package (Hulinks).
Supplementary Material
Acknowledgments
The authors are indebted to Yamada Bee Farm for their gift of Brazilian propolis. This work was supported by Yamada Research Grant and JSPS KAKENHI (80312403). The authors acknowledge the editorial assistance of Uni-edit.
Glossary
Abbreviations:
- 4T
CD4+ CD8− helper T lymphocytes
- 8T
CD4− CD8+ cytotoxic T lymphocytes
- DMSO
dimethyl sulfoxide
- FACS
fluorescence-activated cell sorter
- FSC
forward scatter
- GO
gene ontology
- HFD
high fat diet
- IL
interleukin
- ILC2s
innate lymphoid type 2 cells
- ITT
insulin tolerance test
- Mgl1/2
macrophage galactose-type calcium-type lectin 1/2
- NEFAs
non-esterified free fatty acids
- OGTT
oral glucose tolerance test
- PBS
phosphate buffered saline
- qRT-PCR
quantitative reverse transcription-PCR
- Siglec-F
sialic acid-binding immunoglobulin-like lectin-F
- SSC
side scatter
- VSF
vascular stromal fraction
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/25608
References
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 5.Talukdar S, Oh Y, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–62. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–63. doi: 10.1016/S0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 9.Banskota AH, Tezuka Y, Prasain JK, Matsushige K, Saiki I, Kadota S. Chemical constituents of Brazilian propolis and their cytotoxic activities. J Nat Prod. 1998;61:896–900. doi: 10.1021/np980028c. [DOI] [PubMed] [Google Scholar]
- 10.Paulino N, Abreu SR, Uto Y, Koyama D, Nagasawa H, Hori H, et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur J Pharmacol. 2008;587:296–301. doi: 10.1016/j.ejphar.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 11.Machado JL, Assunção AK, da Silva MC, Dos Reis AS, Costa GC, Arruda DdeS, et al. Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evid Based Complement Alternat Med. 2012;2012:157652. doi: 10.1155/2012/157652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu K, Das SK, Hashimoto T, Sowa Y, Yoshida T, Sakai T, et al. Artepillin C in Brazilian propolis induces G(0)/G(1) arrest via stimulation of Cip1/p21 expression in human colon cancer cells. Mol Carcinog. 2005;44:293–9. doi: 10.1002/mc.20148. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci MC, Ferreres F, García-Viguera C, Bankova VS, De Castro SL, Dantas AP, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/S0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 14.Urushisaki T, Takemura T, Tazawa S, Fukuoka M, Hosokawa-Muto J, Araki Y, et al. Caffeoylquinic acids are major constituents with potent anti-influenza effects in brazilian green propolis water extract. Evid Based Complement Alternat Med. 2011;2011:254914. doi: 10.1155/2011/254914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 2005;51:147–52. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Ichi I, Hori H, Takashima Y, Adachi N, Kataoka R, Okihara K, et al. The beneficial effect of propolis on fat accumulation and lipid metabolism in rats fed a high-fat diet. J Food Sci. 2009;74:H127–31. doi: 10.1111/j.1750-3841.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Chen M, Xuan H, Hu F. Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid Based Complement Alternat Med. 2012;2012:981896. doi: 10.1155/2012/981896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamami Y, Takatori S, Koyama T, Goda M, Iwatani Y, Doi S, et al. [Effect of propolis on insulin resistance in fructose-drinking rats] Yakugaku Zasshi. 2007;127:2065–73. doi: 10.1248/yakushi.127.2065. [DOI] [PubMed] [Google Scholar]
- 19.Ueda M, Hayashibara K, Ashida H. Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K- and AMPK-dependent pathways in skeletal muscle. Biofactors. 2013;39:457–66. doi: 10.1002/biof.1085. [DOI] [PubMed] [Google Scholar]
- 20.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/er.21.6.697. [DOI] [PubMed] [Google Scholar]
- 21.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–77. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, et al. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008;172:1419–29. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 27.Yang YK, Chen M, Clements RH, Abrams GA, Aprahamian CJ, Harmon CM. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol Biochem. 2008;22:531–8. doi: 10.1159/000185527. [DOI] [PubMed] [Google Scholar]
- 28.Catalano KJ, Stefanovski D, Bergman RN. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes. 2010;59:1416–23. doi: 10.2337/db08-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–8. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 30.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–82. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 33.Menzies FM, Henriquez FL, Alexander J, Roberts CW. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology. 2011;134:281–91. doi: 10.1111/j.1365-2567.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–44. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 35.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erridge C. Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis. 2011;216:1–6. doi: 10.1016/j.atherosclerosis.2011.02.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.