Abstract
Oudemansiella radicata, one of edible mushrooms belonging to Tricholomataceae of Basidiomycota, has been known to exhibit outstanding therapeutic effects on the hypertension caused by high blood pressure and inhibitory effects on the sarcoma 180 and Erhrlich carcinoma of mice. As one of preliminary experiments for producing fruiting-body of O. radicata, this study was carried out to obtain the basic information for culture conditions of mycelial growth of the fungus. The optimal temperature and pH for the mycelial growth were 25℃ and pH 6, respectively. The medium for favorable mycelial growth of O. radicata was shown in the Lilly medium, whereas compact mycelial density was found in Hamada medium. The carbon and nitrogen sources promoting for mycelial growth of O. radicata were xylose and alanine, respectively. The optimum C/N ratio was about 20 : 1 in case that 3% glucose was supplimented to the basal medium as a carbon source.
Keywords: Cultural conditions, Edible mushroom, Medicinal mushroom, Oudemansiella radicata
Oudemansiella radicata (Relhan ex Fr.), one of edible and medicinal mushrooms belonging to Tricholomataceae of Agaricales has been known to be inhabited on the soil surface or rotted woods located in the broad-leaved forest, mixed forest of broad-leaved and needle-leaved trees for the duration of summer to autumn (Lee, 1988). Many mushroom researchers (Shim et al, 1997; Ha, 2001; Choi et al., 2003; Lee et al., 2004) have been worked to find substances to protect human health from intractable diseases such as a cancer, gastric ulcer and hypertension and so on. Oudenone, one of medicinal substance isolated from fruiting bodies of O. radicata, has been known to exhibit outstanding therapeutic effects on the hypertension caused by high blood pressure and inhibitory effect on Pyricularia oryzae which caused rice blast disease (Park and Lee, 1999; Ying et al., 1987). However, O. radicata has been collected occasionally in the remote regions of Mt. Seorak, Mt. Jiri, Mt. Dukyou and Mt. Sokri, the supply of the fruiting body could not meet the demand. Even though the demand for fruiting body of O. radicata has been increased, no attempt has been made to develop an artificial cultivation method of O. radicata in Korea. To obtain basic information for an artificial cultivation of O. radicata, this study was focused on culture conditions affecting the optimal mycelial growth of O. radicata.
Materials and Methods
The collection and isolation of O. radicata
The fruiting bodies of O. radicata was collected at Donggureung, Guri City, Korea in August, 2003. To obtain the pure culture from fruiting bodies of O. radicata, surface sterilized small pieces of pileus and stipe were transferred to potato dextrose agar (PDA) supplemented with streptomycin (200 µg/l), incubated under the dark condition for 15 days at 25℃ and used for an inoculum in this study. The pure culture of O. radicata was deposited to the "Culture Collection of Wild Mushroom Species" and acquired accession number "IUM00779". Unless otherwise stated, all the tests which the strain was used were performed with 4 replications.
Culture conditions for a mycelial growth of O. radicata
pH
To screen pH value necessary for a favorable growth of O. radicata, a 5 mm diameter plug of an inoculum was removed with cork borer from 10 days old cultures of O. radicata grown on PDA, placed in the center of PDA adjusted to the range of pH 4~9 with 1 N NaOH or HCL and incubated under the dark condition for 10 days at 25℃. The measurement of mycelial growth was performed according to the method described by Shim et al. (1997).
Temperature
To screen pH value necessary for a favorable growth of O. radicata, the fungus was incubated for 10 days at 5 different temperatures. A 5 mm diameter plug of an inoculum was removed with cork borer from 10 days old cultures of O. radicata grown on PDA, placed in the center of PDA adjusted to pH 6, and incubated under the dark condition for 10 days at 15℃, 20℃, 25℃, 30℃ and 35℃, respectively. The measurement of mycelial growth was also performed according to the method described by Shim et al. (1997).
Culture media
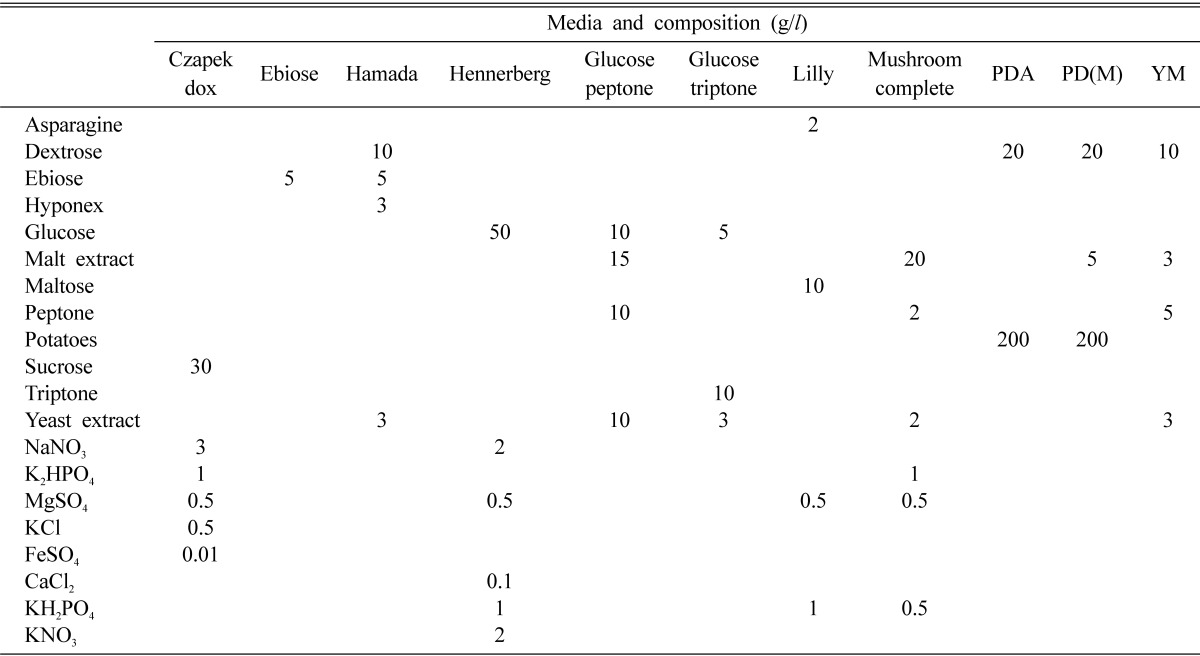
Eleven different culture media were prepared to screen favorable culture media to mycelial growth of O. radicata (Table 1). The culture media were adjusted to pH 6 before sterilization, sterilized for 15 minutes at 121℃ and aseptically poured into a plate. A 5 mm diameter plug of an inoculum was removed from 10 days old cultures of O. radicata grown on PDA, placed in the center of each agar plate of 11 different culture media, and incubated under the dark condition for 10 days at 25℃. After 10 days of incubation, the mycelial growth and density of O. radicata were measured.
Table 1.
Composition of the media for the mycelial growth of Oudemansiella radicata
Effect of favorable nutrient sources
Carbon sources
To screen carbon source favorable to the mycelial growth of O. radicata, the tests were performed on the basal medium supplemented with each of 11 carbon sources. The basal medium was composed of peptone 5 g, MgSO4 0.05 g, KH2PO4 0.46 g, K2HPO4 1.0 g, thiamine-HCl 120 µg, agar 20 g and distilled water 1000 ml. To screen carbon source favorable to the mycelial growth of O. radicata, each carbon source was added to the basal medium at the concentration of 0.1M per 1000 ml and mixed throughly (Shim et al., 1997). The basal medium was adjusted to pH 6 before sterilization, sterilized for 15 minutes at 121℃ and aseptically poured into a plate. A 5 mm diameter plug of an inoculum was removed from 10 days old cultures of O. radicata grown on PDA, placed in the center of a basal medium containing each of 11 carbon sources and incubated under the dark condition for 10 days at 25℃. After 10 days of incubation, the mycelial growth and density of O. radicata were measured.
Nitrogen sources
To screen nitrogen source favorable to the mycelial growth of O. radicata, the basal medium was composed of MgSO4 0.05 g, KH2PO4 0.46 g, K2HPO4 1.0 g, thiamine-HCl 120 µg, agar 20 g and distilled water 1000 ml (Sung et al., 1993) and then supplemented with each of 16 nitrogen sources. Also, D-glucose was supplemented to the basal medium at the concentration of 2% (w/v) and used as carbon source for expediting the mycelial growth of O. radicata. Each nitrogen source was added to the basal medium at the concentration of 0.02M (Shim et al., 1997). The basal medium containing each nitrogen source was adjusted to pH 6 before sterilization, sterilized for 15 minutes at 121℃ and aseptically poured into a plate. A 5 mm diameter plug of an inoculum was placed in the center of a basal medium containing each nitrogen source and incubated under the dark condition for 10 days at 25℃. After 10 days of incubation, the mycelial growth and density of O. radicata were measured.
C/N ratio
On the basal media which were mixed with 1, 2, 3 and 4% glucose (w/v) as carbon source and then mixed continually with NaNO3 as nitrogen source, the mycelial growth of O. radicata was measured. The C/N ratio (NaNO3 versus D-glucose) was adjusted to 10 : 1, 20 : 1 , 30 : 1 and 40 : 1 in each medium (Shim et al., 1997). The basal medium which was adjusted to each C/N ratio (such as 10 : 1, 20 : 1, 30 : 1 and 40 : 1) was adjusted to pH 6 before sterilization, sterilized for 15 minutes at 121℃, aseptically poured into a plate and inoculated with an inoculum (such as a 5 cm diameter plug). After Inocula of O. radicata were incubated under the dark condition for 10 days at 25℃, its colony diameter was measured.
Results and Discussion
Culture conditions for O. radicata
Effect of pH
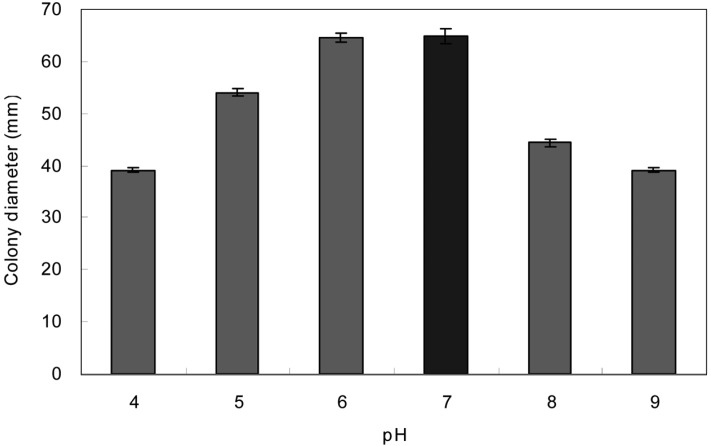
Of 6 pH values, the mycelial growth of O. radicata was exceedingly favorable at pH 6 (Fig. 1). Shim et al. (1997) reported that the mycelial growth of G. umbellata was exceedingly favorable at pH 4 and suppressed in proportion to the rise of pH value. Shim et al. (2003) clarified that the pH value suitable for a favorable growth of P. fumosoroseus was obtained in the range of pH 6~9. Though the mycelial growth of O. radicata was exceedingly favorable at pH 6, the favorable growth of O. radicata was generally obtained in the range of pH 5~9. Since the result was similar to that of P. fumosoroseus, it is reasonable to clarify that there was no a wide difference of mycelial growth referring to O. radicata between pH 5 and 9. Presumably, O. radicata seems to show a favorable growth in the range of wide pH values.
Fig. 1.
Mycelial growth of Oudemansiella radicata on the PDA at different pHs for 10 days at 25℃.
Effect of the temperature
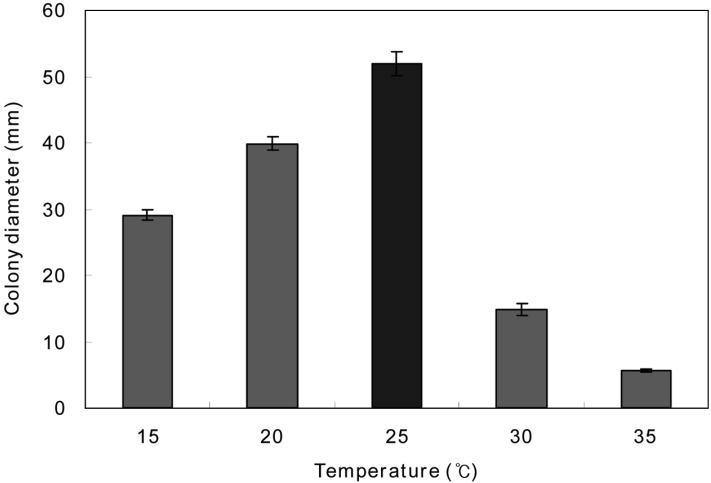
The temperature suitable for the mycelial growth of O. radicata was obtained at 25℃ (Fig. 2). After the favorable growth of O. radicata peaked at 25℃, its mycelial growth was suppressed rapidly at the temperature higher than 25℃. Also, this case was similar to that of P. fumosoroseus (Shim et al., 2003).
Fig. 2.
Mycelial growth of Oudemansiella radicata on the PDA for 10 days at different temperatures.
Screening of the favorable culture media
Eleven different culture media were prepared to screen suitable culture media to mycelial growth of O. radicata (Table 1). The mycelial growth of O. radicata was most outstanding in the Lilly medium, whereas mycelial density Hamada medium (Table 2). Although the size of colony diameter was more large in the Lilly medium than Hamada medium, the difference of their sizes seemed to be scanty between Lilly and Hamada medium. The mycelial density of O. radicata was more compact in the Hamada medium than Lilly medium. Generally, it is reasonable to mention the fact that the mycelial density of filamentous fungi has been determined as the volumes of their accumulated mycelia on the culture media. In case of O. radicata, the criterion can be too obscure to decide if the meaning of suitable culture media should be focused on either the size of colony diameter or volume of accumulated mycelia. Though the application of criterion is somewhat obscure in case of O. radicata, it may be unnecessary to worry the obscurity of criteria. It would be resonable to imply that the application of criteria can be flexible to coincide with various purposes of related researches in the future. Since the spectrum of criteria can be flexible for any purpose of related researches, it is desirable to emphasize that flexible criteria should be applied to induce various researches of O. radicata in the future.
Table 2.
Mycelial growth of Oudemansiella radicata on the various culture media
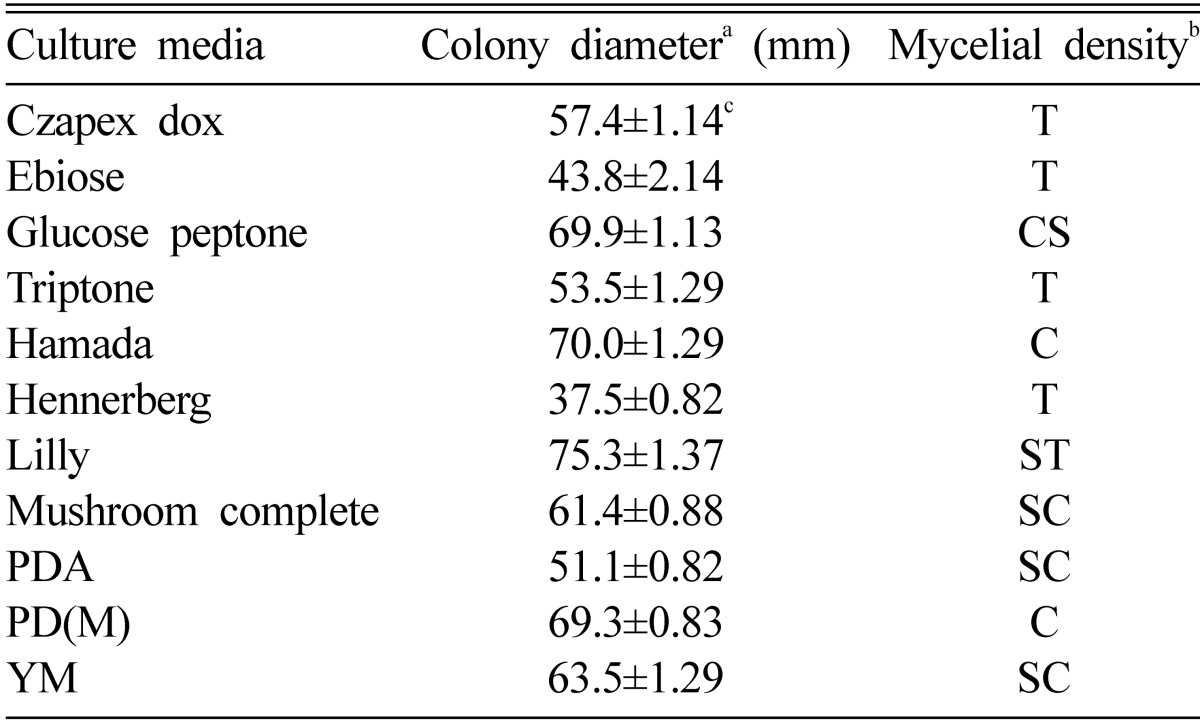
aThe colony diameter was measured after 10 days of incubation.
bMycelial density: C, Compact; SC, Somewhat compact; ST, Somewhat thin; T, Thin.
cValues are average of 4 replicates and standard error.
Effect of favorable nutrient sources
Carbon and nitrogen sources
The carbon and nitrogen source suitable for promoting a mycelial growth of O. radicata were xylose and alanine, respectively (Table 3 and 4). Of 11 carbon sources, xylose showed colony diameter of 65.6 mm. The mycelial density of O. radicata was somewhat compact in xylose. Of 16 nitrogen sources, Alanine showed colony diameter of 66.8 mm. The mycelial density of O. radicata was compact in alanine. According to the above results, xylose and alanine seems to be effective to promoting a mycelial growth of O. radicata.
Table 3.
Effect of carbon sources for the mycelial growth of Oudemansiella radicata in the basal mediuma
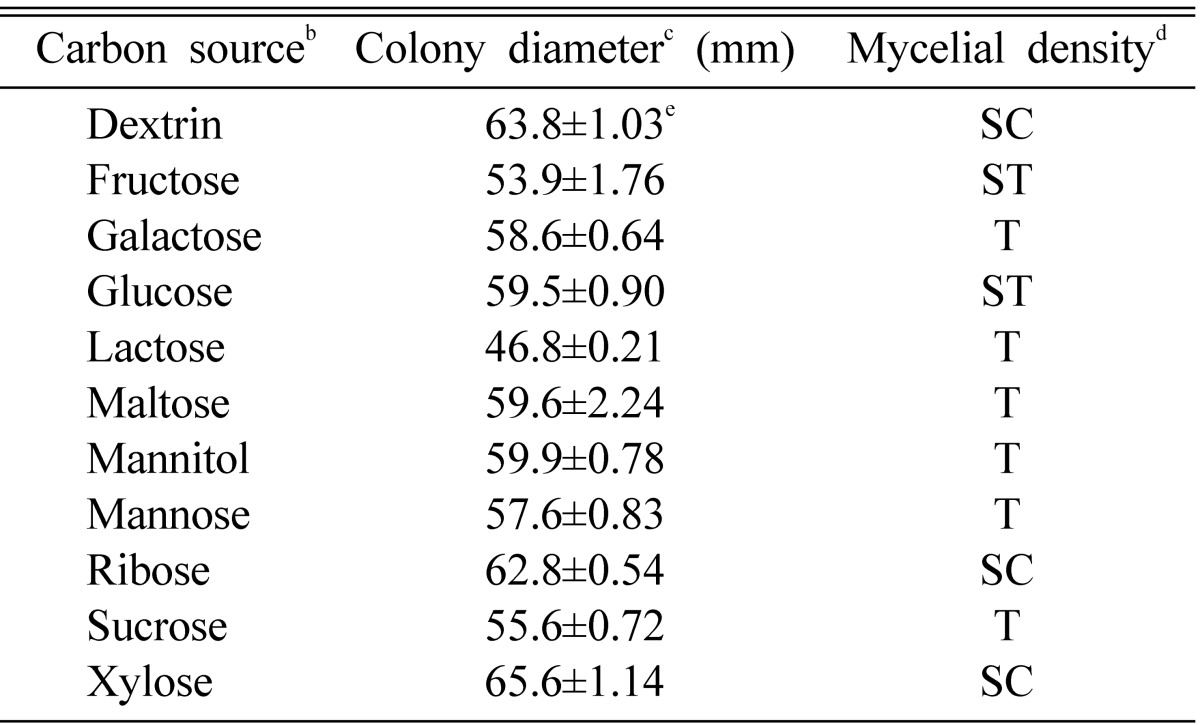
aThe basal medium was composed of peptone 5 g, MgSO4 0.05 g, KH2PO4 0.46 g, K2HPO4 1.0 g, Thiamine-HCl 120 µg, agar 20 g and D.W. 1000 ml.
bEach carbon source was added to the basal medium at the concentration of 0.1M.
cThe colony diameter was measured after 10 days of incubation.
dMycelial density: C, Compact; SC, Somewhat compact; ST, Somewhat thin; T, Thin.
eValues are average of 4 replicates and standard error.
Table 4.
Effect of nitrogen sources for the mycelial growth of Oudemansiella radicata in the basal mediumb
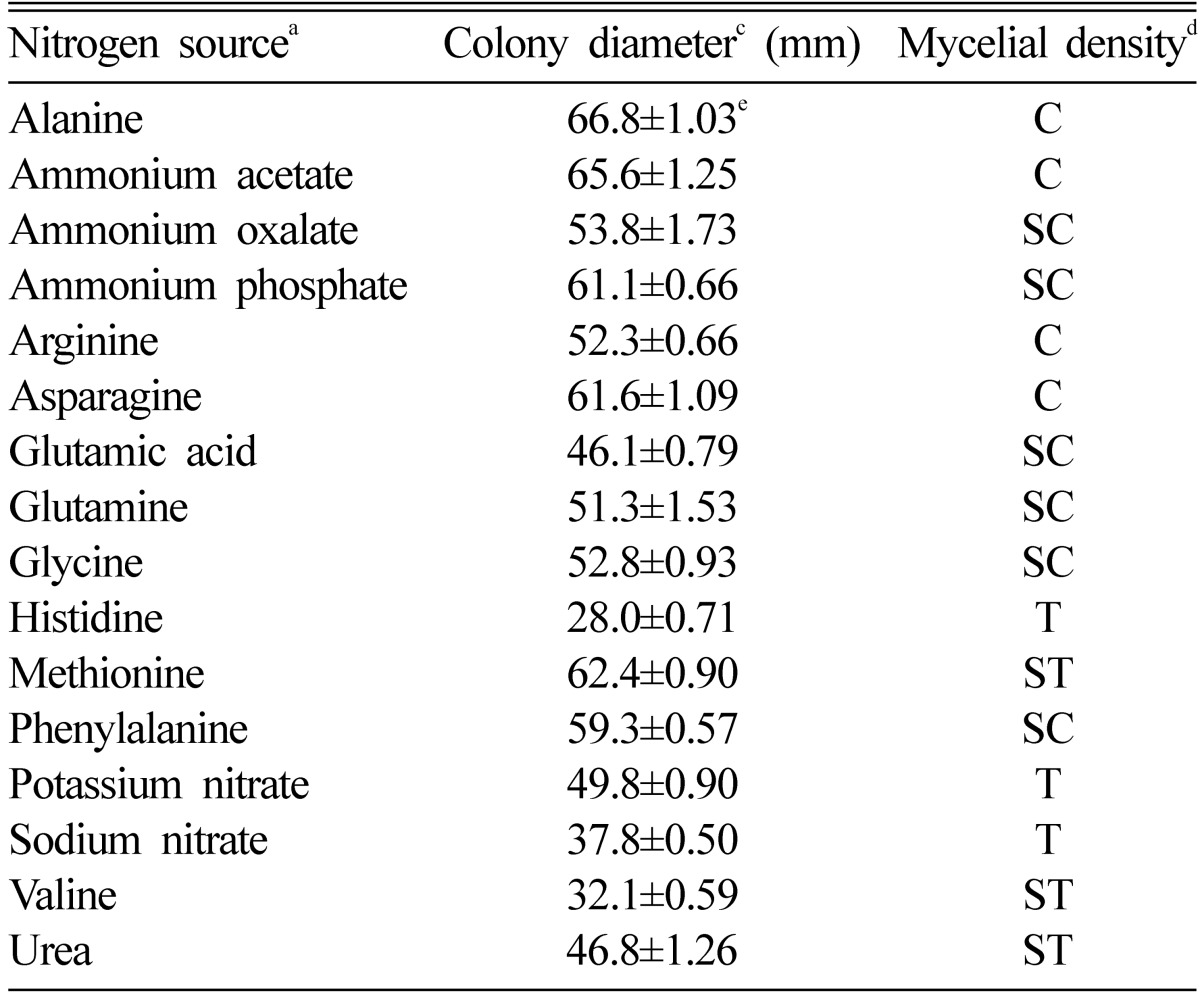
aEach nitrogen source was added to the basal medium at the concentration of 0.02M.
bThe basal medium was composed of glucose 20 g, MgSO4 0.05 g, KH2PO4 0.46 g, K2HPO4 1.0 g, Thiamine-HCl 120 µg, agar 20 g and D.W. 1000 ml.
cThe colony diameter was measured after 10 days of incubation.
dMycelial density: C, Compact; SC, Somewhat compact; ST, Somewhat thin; T, Thin.
eValues are average of 4 replicates and standard error.
C/N ratio
On the culture media which were mixed with 3% glucose as carbon source and then adjusted to the C/N ratio of 20 : 1, O. radicata showed the most favorable mycelial growth (Table 5). Shim et al. (1997) clarified that an excessive concentration of glucose seemed to be attributable to suppress the mycelial growth of Grifola umbellata on the culture media. Also, our results were similar to those of Shim et al. (1997). Generally, the gradual rise of glucose concentration seemed to suppress the mycelial growth of O. radicata.
Table 5.
Mycelial growth of Oudemansiella radicata at various C/N ratio in the basal mediuma
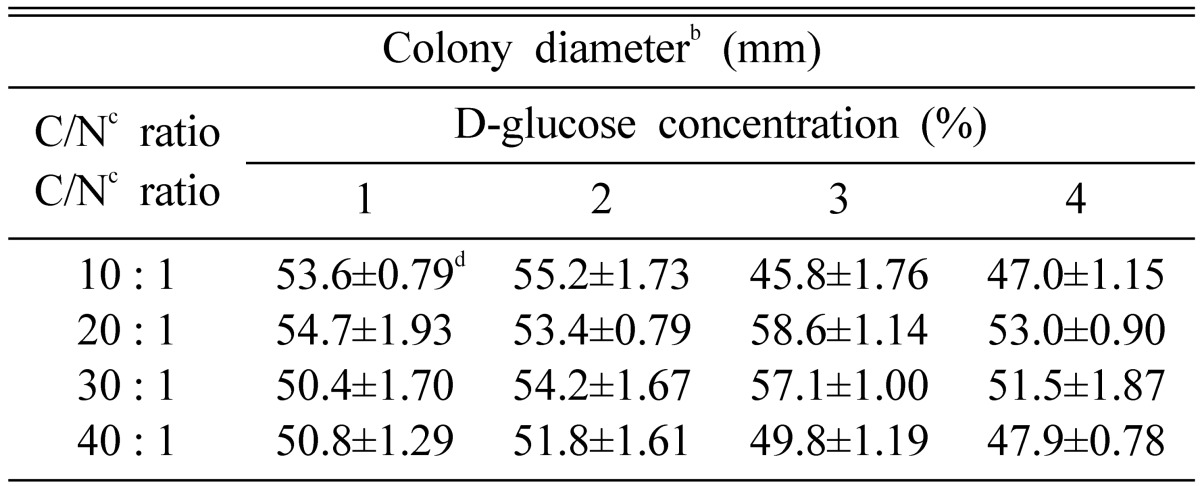
aBasal medium was composed of MgSO4 0.05 g, KH2PO4 0.46 g, K2HPO4 1.0 g, Thiamine-HCl 120 µg, agar 20 g and D.W. 1000 ml.
bThe colony diameter was measured after 10 days of incubation.
cThe ratio of NaNO3 versus D-glucose were adjusted to the rate of 10 : 1, 20 : 1, 30 : 1, 40 : 1, respectively.
dValues are average of 4 replicates and standard error.
Acknowledgement
This work was supported by Agricultural R & D Promotion Center (ARPC), Ministry of Agriculture and Forestry, Korea.
References
- 1.Choi KD, Lee KT, Shim JO, Lee YS, Lee TS, Lee SS, Guo SX, Lee MW. A new method for cultivation of sclerotium of Grifola umbellata. Mycobiology. 2003;31:105–112. [Google Scholar]
- 2.Ha YD. Antitumoral , antioxidant, and antimicrobial activities of solvent fractions from Grifola umbellatus. Korean J Postharvest Sci Technol. 2001;8:481–487. [Google Scholar]
- 3.Lee JY. Coloured Korean mushrooms. Seoul, Korea: Academy Press; 1988. [Google Scholar]
- 4.Lee JM, Kim JY, Choi KD, Han KD, Hur H, Kim SW, Shim JO, Lee TS, Lee MW. Sawdust media affecting the mycelial growth and fruiting body formation of Sparassis crispa. Mycobiology. 2004;32:190–193. [Google Scholar]
- 5.Park WH, Lee HD. Illustrated book of Korean Medicinal mushrooms. Kyo-Hak Publishing C. Ltd; 1999. p. 759. [Google Scholar]
- 6.Shim JO, Son SG, Kim YH, Lee YS, Lee JY, Lee TS, Lee SS, Lee MW. The cultural conditions affecting the mycelial growth of Grifola umbellata. Korean J Mycol. 1997;25:209–213. [Google Scholar]
- 7.Shim SM, Lee KR, Kim SH, Im KH, Kim JW, Lee UY, Shim JO, Lee MW, Lee TS. The optimal culture conditions affecting the mycelial growth and fruiting body formation of Paecilomyces fumosoroseus. Mycobiology. 2003;31:214–220. [Google Scholar]