Abstract
Chronic obstructive pulmonary disease (COPD) is a major public health problem in India. Although several International guidelines for diagnosis and management of COPD are available, yet there are lot of gaps in recognition and management of COPD in India due to vast differences in availability and affordability of healthcare facilities across the country. The Indian Chest Society (ICS) and the National College of Chest Physicians (NCCP) of India have joined hands to come out with these evidence-based guidelines to help the physicians at all levels of healthcare to diagnose and manage COPD in a scientific manner. Besides the International literature, the Indian studies were specifically analyzed to arrive at simple and practical recommendations. The evidence is presented under these five headings: (a) definitions, epidemiology, and disease burden; (b) disease assessment and diagnosis; (c) pharmacologic management of stable COPD; (d) management of acute exacerbations; and (e) nonpharmacologic and preventive measures. The modified grade system was used for classifying the quality of evidence as 1, 2, 3, or usual practice point (UPP). The strength of recommendation was graded as A or B depending upon the level of evidence.
Keywords: Asthma, chronic obstructive pulmonary disease, chronic bronchitis, emphysema, guidelines
METHODOLOGY
The process of development of guidelines for diagnosis and management of patients of chronic obstructive pulmonary disease (COPD) in India was undertaken as a joint exercise of the two National Pulmonary Associations (Indian Chest Society (ICS) and National College of Chest Physicians (NCCP)), by the Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh. The committee constituted for this purpose included representation of the two associations, and experts from other institutes and medical colleges including those from disciplines of internal medicine, microbiology, pharmacology, radiodiagnosis, and community medicine.
For development of guidelines, an extensive desk-review was followed by a joint workshop. The review of literature was performed by searching electronic sources (PubMed, EmBase). The major international guidelines available from the Global Initiative for Chronic Obstructive Lung Diseases (GOLD), American Thoracic Society (ATS), and National Institute of Clinical Excellence (NICE) were also reviewed.
The search was conducted under five subgroups: (a) definitions, epidemiology, and disease burden; (b) disease assessment and diagnosis; (c) pharmacologic management of stable COPD; (d) management of acute exacerbations; and (e) nonpharmacologic and preventive measures. Important questions were framed on the basis of discussions on issues with reference to the Indian context. Literature review and discussions in each area were coordinated by group chairs and recorded by rapporteurs. The available evidence as well as the questions were circulated to all the group members before the joint workshop. Discussions for grading of evidence and recommendations were held independently in five parallel group sessions, and thereafter together in the joint meeting of all the groups. Final decisions in the joint group were based on a consensus approach on the majority voting.
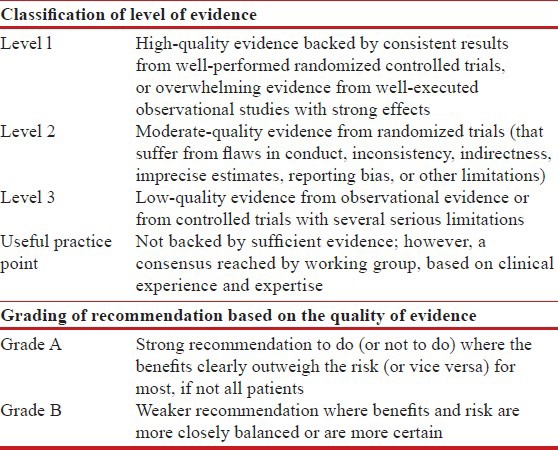
The modified grade system was used for classifying the quality of evidence as 1, 2, 3, or usual practice point (UPP) [Table 1].[1] The strength of recommendation was graded as A or B depending upon the level of evidence [Table 1]. Grade A recommendations in the guidelines should be interpreted as “recommended” and the grade B recommendations as “suggested”. While making a recommendation, the issues of practicality, costs, and feasibility in the country at different levels of healthcare was also taken into consideration.[2]
Table 1.
Classification of level of evidence and grading of recommendation based on the quality of evidence supporting the recommendation
The final document was reviewed by all the committee members, as well as by other external experts.
Synopsis of recommendations
COPD is a common, preventable lung disorder characterized by progressive, poorly reversible airflow limitation often with systemic manifestations, in response to tobacco smoke and/or other harmful inhalational exposures. As of now valid spirometry-based nationwide prevalence data for COPD in India are not available. In most of the critical analyses, validated questionnaire based data has been accepted as reasonable for assessment of prevalence of COPD despite its limitations. COPD poses enormous burden in terms of morbidity and mortality globally and in India. COPD poses a huge economic burden in terms of direct and indirect costs.
What are the risk factors for COPD?
Tobacco smoking is the most well established risk factor for COPD. (1A)
Both smokeless and smoking forms of tobacco are associated with serious health hazards, although only smoking tobacco is primarily responsible for COPD. (1A)
Bidi and other indigenous forms of tobacco smoking are at least as (or even more) harmful than cigarette smoking. (1A)
Low tar or filtered cigarettes are not “less harmful”. (2B)
There is no minimum number of cigarettes/bidi per day below which the risk for COPD decreases. (1A)
Exposure to environmental tobacco smoke (ETS) is a definitive risk factor for COPD. (1A)
Exposure to biomass fuel smoke is a strong risk factor for COPD. (1A)
There is limited data on the association of ambient air pollution and COPD, and its causative role in COPD needs further evaluation.
There is insufficient evidence to attribute an etiological role of pulmonary tuberculosis in causing COPD.
A subgroup of chronic asthma may clinically behave like COPD; whether it is true COPD remains to be established. (UPP)
When to suspect COPD?
A diagnosis of COPD should be considered in persons having chronic symptoms of cough, sputum production, shortness of breath, and/or wheezing, especially among those with prolonged exposure to risk factors for the disease. (1A)
A diagnosis of COPD should not be excluded in the absence of physical signs. (2A)
Forced expiratory time (FET) of more than six seconds is suggestive of airflow obstruction. (2B)
What is the role of spirometry in the diagnosis of COPD? Whether fixed ratio or lower limit of normal (LLN) should be used for diagnosis?
Spirometry should be performed in all patients suspected of having COPD. (1A)
In the absence of availability of spirometry, patients suspected of having COPD should be referred for spirometric evaluation to a center with the facility. (UPP)
A post-bronchodilator forced expiratory volume in first second (FEV1)/forced vital capacity (FVC) below the LLN (lower fifth percentile of values from a reference population) should be preferably used as the criterion for diagnosis of airflow obstruction. (1A)
However, in the absence of reference equations for LLN, FEV1/FVC < 0.7 may be used as the cutoff for defining airflow obstruction. (1A)
What is the role of reversibility testing in COPD?
Absence of bronchodilator reversibility does not differentiate COPD from asthma, and its presence does not predict the response to treatment (1A). However, all FEV1 values should be reported post-bronchodilator.
What is the role of screening spirometry?
Spirometry should not be used as a screening tool in asymptomatic individuals to detect airflow obstruction. (2A)
What is the role of peak expiratory flow in diagnosis and monitoring of COPD?
PEF should not be routinely used for screening, diagnosis, or monitoring of COPD. (1A)
How should the severity of COPD be classified?
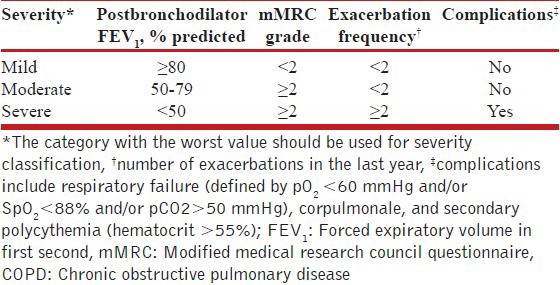
Classification of severity of the disease should be done for all COPD patients based on the FEV1 and exacerbation frequency [Table 2]. (1A)
Level of patient's disability due to symptoms should be assessed using modified Medical Research Council (mMRC) dyspnea questionnaire or the COPD assessment test (CAT) and recorded at each clinical visit. (1A)
Table 2.
Classification of severity of COPD
What is the role of additional investigations in COPD?
All new COPD suspects with cough of more than 2 weeks’ duration should undergo sputum smear examination for acid fast bacilli to rule out pulmonary tuberculosis as per the standard practice of Revised National Tuberculosis Control Program (RNTCP). (UPP)
Pulse oximetry should be used to screen for hypoxemia in stable disease with FEV1 < 50% and in the presence of clinical suspicion of hypoxemia. (3A)
An arterial blood gas analysis should be done if arterial saturation by pulse oximetry is less than 90%. (2A)
Diagnosis of COPD should not be made on the basis of a chest radiograph. (2A)
Chest radiograph may be done during the initial evaluation of COPD to look for comorbidities, complications, and alternative diagnoses. (2B)
Special investigations like high-resolution computed tomography (HRCT) scan, lung volumes, diffusing capacity for carbon monoxide (DLCO), and exercise testing should be done in situations of diagnostic difficulty or whenever clinically indicated. (2A)
6MWT may be used for monitoring of exercise capacity in COPD. (1A)
Testing for alpha-1 antitrypsin deficiency may be done in young patients with lower lobe emphysema. (UPP)
What is the role of multidimensional assessment tools in COPD?
Composite scores including BODE (body mass index (BMI), obstruction, dyspnea, exercise capacity) and DOSE (dyspnea, obstruction, smoking, exacerbation) should not be used to assess severity or prognosis in COPD unless they are validated in Indian patients. (2A)
What are the comorbidities associated with COPD?
COPD patients should be routinely evaluated and appropriately treated for comorbid conditions. (2A)
What is the role of inhaled antimuscarinic agents, inhaled beta-agonists, and inhaled corticosteroids in COPD?
Short-acting antimuscarinic agent (SAMA) can be used as rescue medication to relieve patient symptoms. (1A)
Long term SAMA monotherapy on regular basis is not recommended. (1A)
Long-acting antimuscarinic agents (LAMA) are useful in stable COPD (FEV1 < 80%) to control symptoms and decrease the risk of exacerbations. (1A)
LAMA should be preferred over SAMA. (1A)
We suggest close monitoring of patients with coronary artery disease who are treated with LAMA. (UPP)
Short-acting beta-agonist (SABA) can be used to relieve symptoms of dyspnea as and when needed. (1A)
Long term SABA monotherapy on regular basis is not recommended. (2A)
Long-acting beta-agonist (LABA) monotherapy can relieve symptoms and decrease the exacerbation rate in patients with stable COPD (FEV1 < 80%). (1A)
Patients with symptomatic coronary artery disease receiving inhaled beta-agonists should be closely monitored. (UPP)
ICS have a beneficial effect in subgroup of COPD patients with FEV1 < 50%. (1A)
ICS have a beneficial effect in subgroup of COPD patients with frequent exacerbations (≥ 2 exacerbations/year). (1A)
The risk-benefit profile favors use of ICS in patients with severe COPD. (1A)
LAMA is superior to LABA monotherapy. (1A)
ICS monotherapy should not be used. (1A)
SABA and SAMA are equally effective when used for COPD. (2A)
LAMA plus LABA may be used in patients who continue to have symptoms on monotherapy, except for those with frequent exacerbations. (1A)
LABA plus ICS should be preferred over LABA alone in patients with FEV1 < 50% or those having frequent exacerbations. (1A)
In patients of severe COPD (FEV1 < 50%), triple therapy may be used in those who are symptomatic despite single or dual bronchodilator therapy. (1B)
There is lack of sufficient data to recommend ICS-LABA or ICS-LAMA combination over LAMA monotherapy.
What is the role of oral bronchodilators in management of stable COPD?
Oral methylxanthines are not recommended as first line therapy in patients with COPD. (1A)
-
Oral methylxanthines can be used
- As alternative in patients noncompliant with inhalers for any reason. (1B)
- As add-on therapy in patients continuing to have symptoms despite optimum inhaled therapy. (3A)
Patients on oral methylxanthines need to be monitored for side effects and drug interactions. (UPP)
Roflumilast may be used in frequent exacerbators as an add-on or substitute to ICS. (2B)
What is the role of mucolytic agents?
Routine use of mucolytic agents is not recommended in patients with COPD. (2A)
What should be the Indian strategy for management of COPD?
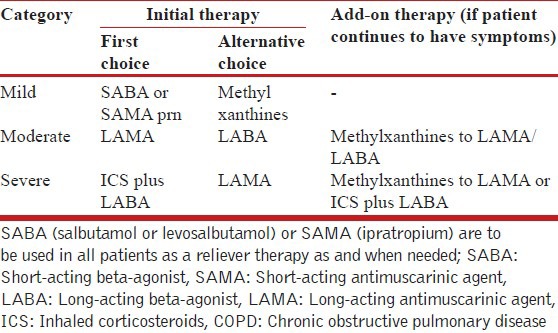
The strategy for management of stable COPD is shown in Table 3 below.
Table 3.
Suggested guidelines for treatment of patients of stable COPD
What is the definition of acute exacerbation of COPD (AECOPD)?
An exacerbation of COPD is an acute event characterized by sustained worsening of any of the patient's respiratory symptoms (cough, sputum quantity and/or character, dyspnea) that is beyond normal day-to-day variation and leads to a change in medication, and where other causes of acute breathlessness have been clinically excluded.
How to investigate an exacerbation of COPD?
No investigations apart from pulse oximetry are routinely required in patients with acute exacerbations managed in an outpatient setting. (IIA)
In those hospitalized with AECOPD, serum electrolytes, liver and renal function tests, complete blood count, chest radiograph, electrocardiogram, and arterial blood gas analysis (if available) should be performed. (IA)
If an infectious exacerbation does not respond to the initial antibiotic treatment, a sputum culture and an antibiotic sensitivity test should be performed. (IIA)
How to decide the site of management of a patient with COPD exacerbation?
The decision to admit the patient can be made on the level of severity as shown in Table 4 below while BAP-65 (elevated blood urean nitrogen (BUN), altered mental status, pulse > 109 beats/min, age > 65 years) score may help in deciding patients who need management in an intensive care unit (ICU) [Figure 1].
Table 4.
Severity assessment (indications for hospitalization) of exacerbation of COPD*

Figure 1.
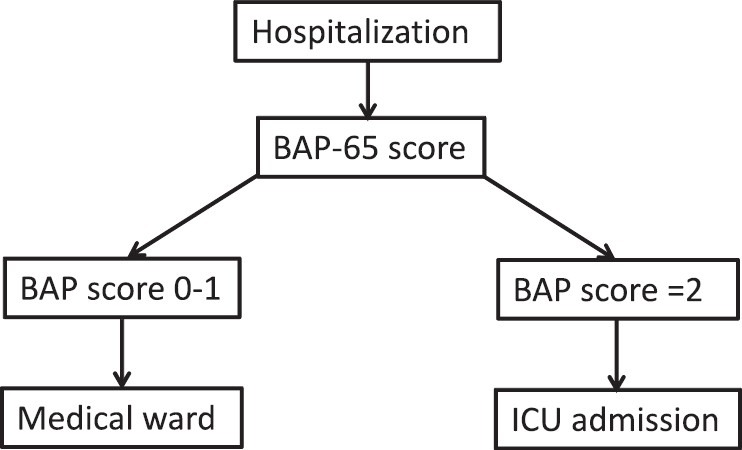
Algorithm for deciding place of management after hospitalization. B: Blood urea nitrogen > 24 mg/dL, A: altered mental status, P: pulse rate > 110/min, 65 = age > 65 years, BAP-65 scores: 0 = age < 65 years, 1 = age > 65 years, 2 = one risk factor, 3 = two risk factors, 4 = three risk factors
What is the role of short-acting bronchodilators in AECOPD?
Inhaled route is the preferred route of administering bronchodilators. (IA)
Inhaled SABAs should be used as the first-line agent because of quicker onset of action (IIIA). SAMAs are however, in no way inferior to SABAs.
Nebulized salbutamol at a dose of 2.5 mg every 20 min (or salbutamol pressurized metered dose inhaler (pMDI) 100 μg 2-4 puffs every 20 min) for 1 h can be given initially. (IIIA) Further dosing would depend on the clinical response, generally every 4-6 h. (IIIA)
If additional bronchodilatation is desired, a combination of ipratropium (500 μg nebulized or 20 μg 2-4 puffs with pMDI) and salbutamol (2.5 mg nebulized or salbutamol pMDI 100 μg 2-4 puffs) every 4-6 h can be used. (IIIA)
Nebulizer or pMDIs with spacer are equally effective. (IIA)
Nebulization should not be driven by oxygen; patients should receive oxygen separately through nasal cannula, with monitoring of oxygen saturation. (IIA)
Intravenous methyl xanthines should not be routinely used. (IA)
The use of intravenous or subcutaneous route of administering bronchodilators should be reserved in the most seriously ill mechanically ventilated patient demonstrating inadequate response to inhaled therapy. (IIIB)
What is the role of glucocorticoids in AECOPD?
Systemic steroids shorten recovery time, improve lung function, oxygenation, reduce length of hospital stay, and are associated with fewer treatment failures. (IA)
A short course of oral prednisolone (or equivalent) at a dose of 30-40 mg/day is recommended for managing acute exacerbations. (IIA)
The duration of systemic steroid therapy should be 5-10 days. (IIA)
Intravenous steroids should be given in patients who are being mechanically ventilated or cannot tolerate oral medication. (UPP)
ICS are not routinely recommended in management of AECOPD. (IA)
Should antibiotics be used in patients with acute exacerbations of COPD?
Antibiotics should be prescribed for all exacerbations of COPD. (IIA)
The choice of antibiotics should be guided by local flora and sensitivity pattern. (IIA)
Fluoroquinolones should not be used routinely in treating AECOPD. (IA)
Patients with AECOPD being managed in the outpatient setting may be treated with first line antibiotics. (IIA)
Hospitalized patients or those requiring mechanical ventilation (noninvasive/invasive) should be treated with second line drugs. (IIA)
The duration of therapy should be 5-7 days. (IIA)
What is the role of procalcitonin in deciding for antibiotic therapy?
Biomarkers do not have any role in management of acute exacerbation of COPD. (IIA)
Procalcitonin should not be used routinely in guiding antibiotic usage in COPD. (IIA)
When should oxygen be prescribed, and at what dose?
Oxygen should be prescribed to hypoxemic patients with a target SpO2 between 88-92%. (IA)
Oxygen should be delivered preferably by a Venturi mask, and by nasal cannula upon recovery. (IIA)
Arterial blood gas monitoring is recommended in patients receiving oxygen therapy, wherever available. (IIA)
What is the indication of noninvasive ventilation during exacerbation of COPD?
NIV should be used early in the management of respiratory failure due to AECOPD. (IA)
NIV can be used even in settings where arterial blood gas monitoring is not routinely available. (UPP)
Should smoking cessation be advised? What are the methods of smoking cessation?
A smoking history, including pack years or smoking index (number of bidis/cigarettes smoked per day multiplied by number of years smoked; mild, moderate, and heavy smokers are defined as having a smoking index of < 100, 100-300, and > 300, respectively) should be documented for all patients with COPD. (UPP)
All COPD patients, regardless of age, should be encouraged to stop smoking, and offered help to do so, at every opportunity. (1A)
Nicotine replacement therapies, varenicline or bupropion, combined with an appropriate support program should be offered to people who are planning to stop smoking. (1A)
What is the role of health education in COPD?
Health education is an integral component of a COPD management program. Special importance should be given to inhaler technique, which should be demonstrated to the patient and accompanying attendants and reinforced at every visit. This is particularly true for elderly patients. (UPP)
For COPD patients who are not active smokers, potential etiological exposures (environmental tobacco smoke, biomass fuel smoke, and others) should be asked for and avoided. (UPP)
What is the role of pulmonary rehabilitation?
Structured pulmonary rehabilitation programs should be set up where feasible. (1A)
In the absence of structured programs, patients should be advised regarding unsupervised daily physical activity. (3A)
All patients should be assessed for nutritional status (at least by BMI) at the initial visit and followed up by serial BMI estimation at every visit. Any patient found to be malnourished should be referred for nutritional advice to a specialist. (UPP)
What are the indications for oxygen therapy in stable COPD?
-
Long term supplemental oxygen therapy is indicated for those with severe daytime resting hypoxemia (1A) defined as:
- PaO2 of < 55 mmHg (or pulse oxygen saturation of < 88%), or
- PaO2 of 56-60 mmHg (or pulse oxygen saturation of 88-92%) with evidence of end-organ dysfunction such as pulmonary hypertension, congestive cardiac failure, and erythrocytosis with hematocrit > 55%
- Determined on two occasions at least 3 weeks apart in the stable patient
The role of oxygen supplementation in other situations is currently not clear and should be decided on a case to case basis. (2B)
Supplemental oxygen should be titrated to achieve a pulse oximetric saturation of 90-92% or a PaO2 of 60-65 mmHg. (3A)
Patients should breathe supplemental oxygen for at least 16 h a day. (1A)
Patients on long-term oxygen therapy should be reviewed at regular intervals with either pulse oximetry or arterial blood gas analysis as indicated. (UPP)
Patients should be warned about the risk of fire if smoking is continued during the period of oxygen supplementation. (UPP)
What is the role of NIV in stable COPD?
NIV may be used in patients with recurrent exacerbations who require frequent use of mechanical and noninvasive ventilation during the acute episodes; the patient should be referred to a specialist center for management. (3A)
The choice of the machine for NIV depends on the presence of coexistent sleep apnea syndromes. (UPP)
What are the bronchoscopic techniques useful in stable COPD?
Bronchoscopic techniques are upcoming modalities of treatment; the data is too sparse to make an evidence-based recommendation.
What are the surgical treatments that can be offered for the treatment of COPD at appropriate centers?
Bullectomy in properly selected patients. (3A)
Lung volume reduction surgery (LVRS) may be offered to properly selected patients. (1A)
Lung transplantation may be offered to properly selected patients. (1A)
Are influenza and pneumococcal vaccinations useful in patients of COPD?
Influenza vaccination is likely to be beneficial in patients with severe COPD and/or frequent exacerbations. (UPP)
Pneumococcal vaccination is likely to be beneficial in patients with severe COPD and/or frequent exacerbations. (UPP)
What is the role of prophylactic antibiotics in COPD?
Antibiotics should not be prescribed as a routine for the prevention of exacerbations of COPD. (2A)
What should be the advice for patients of COPD regarding air travel?
Patients with severe COPD and those on long-term oxygen therapy should be assessed before air travel by a specialist. (UPP)
INTRODUCTION
COPD is a common, preventable lung disorder characterized by progressive, poorly reversible airflow limitation often with systemic manifestations, in response to tobacco smoke and/or other harmful inhalational exposures. As of now valid spirometry-based nationwide prevalence data for COPD in India are not available. In most of the critical analyses, validated questionnaire based data has been accepted as reasonable for assessment of prevalence of COPD despite its limitations. COPD poses enormous burden in terms of morbidity and mortality globally and in India. COPD poses a huge economic burden in terms of direct and indirect costs.
Definition, epidemiology and risk factors of COPD
What is the definition of COPD?
Obstructive airway diseases, emphysema, and chronic bronchitis as separate disease entities were first defined in Ciba Guest symposium in 1958.[3] Later, various organizations came with their own definitions in their respective guidelines. Modifications have been made over the years with subsequent updates of guidelines. Till date there is no single satisfactory definition of this common disease. Different definitions given by various organizations have focused on common features of COPD.[4,5,6,7] The definition endorsed by Global Initiative for Obstructive lung disease (GOLD), in its latest edition is comprehensive and elaborate, but complex.[8] Retaining the key components of various definitions and using simple terms, we recommend the following definition of COPD:
“Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable lung disorder characterized by progressive, poorly reversible airflow limitation often with systemic manifestations, in response to tobacco smoke and/or other harmful inhalational exposures.”
What is the prevalence of COPD?
COPD affects more than 400 million people worldwide. The reported prevalence of COPD is highly variable ranging from 0.2% in Japan to 37% in the United States. According to the 12-site Burden of Obstructive Lung Disease (BOLD) study, the average prevalence of COPD is 10.1%, with wide variations.[9] Prevalence estimates based on spirometry are reported to be higher than those based on questionnaire-based studies.[10,11] Before the turn of 20th century, there were few studies from India, which reported the prevalence of COPD. Most of them were limited by small sample size and were based on unvalidated questionnaire interviews making them unreliable for any national assessment.[12,13,14] Nevertheless, the prevalence of COPD reported in these studies varied from 2-22% in men and from 1.2-19% in women. There were three attempts to systematically review and analyze available data until the results of the ‘Indian Study on Epidemiology of Asthma, Respiratory Symptoms and Chronic Bronchitis in Adults’ (INSEARCH) phase II was published.[13,14,15] All the reviews concluded that data was insufficient to derive national representative figures of prevalence of COPD. After publication of the results of INSEARCH II, some nationwide prevalence data are available. Together, the INSEARCH I and II involved 16 centers across the country, included 121,776 individuals of more than 35 years of age, and was based on a well-validated questionnaire.[16] The study population had rural and urban representation of both genders.[17,18] The prevalence of COPD in India according to these studies was 3.67% (4.46 and 2.86% among males and females, respectively). The estimated burden of COPD in India is about 15 million cases (males and females contributing to 9.02 and 5.75 million, respectively). These figures may however underestimate the true burden since questionnaire based prevalence rates tend to underestimate the true spirometry-based prevalence of COPD.
As of now, valid spirometry based nationwide prevalence data for COPD in India are not available. In most of the critical analyses, validated questionnaire based data has been accepted as reasonable for assessment of prevalence of COPD despite its limitations.
What are the implications of COPD on morbidity and mortality?
Globally, COPD is the ninth leading cause of loss of disability adjusted life years (DALYs) according to the baseline projections made in the Global Burden of Disease Study (GBDS).[19] In India, chronic respiratory diseases (CRDs) account for 3% of DALYs, and COPD is the major cause among CRDs.[20] COPD also accounts for more than 3 million deaths per year globally making it the third leading cause of death worldwide.[21] It accounts for 2.3-8.4% of all deaths. This proportion is more among men than women, and more among the elderly as compared to the young.[11,22] In India, COPD causes about 500,000 deaths per year.[23] A review of data from multiple sources suggested that COPD causes more death than tuberculosis and pneumonia.[24] Recently, the State Health Systems Resource Center reported that COPD is the leading cause of death in Maharashtra state; surpassing coronary artery disease, cerebrovascular accident, and diabetes combined together.[25] According to the preliminary report of the “Million Death Study”, CRDs were the second common cause of death among Indian adults. They are second and the third common causes of death in rural and urban population, respectively.[26]
COPD poses enormous burden in terms of morbidity and mortality globally and in India.
What is the economic impact of COPD?
The estimated economic loss in India due to COPD is about Rs. 35,000 crores for year 2011 and is predicted to exceed Rs. 48,000 crores for year 2016.[20] These economic losses are more than the annual budget of the Ministry of Health and Family Welfare (MOHFW) for year 2010-2011, which was Rs. 25,124 crores.[23] In a study that assessed the costs of treatment amongst 423 COPD patients in India, it was found that patients spent 15% of their annual income on smoking products and 30% on disease management.[27] It has been calculated that proper program-based or guideline-based management of COPD can reduce these costs by approximately 70%.[20]
COPD poses a huge economic burden in terms of direct and indirect costs.
What are the risk factors for COPD?
There are numerous factors that are thought to affect lung function at various stages of development and ageing of lung [Table 5]. Some of them have strong evidence to qualify as being causative for COPD; however there are others for which such causative associations are yet to be established. Exposure to multiple risk factors tends to have an additive effect.
Table 5.
Risk factors for COPD
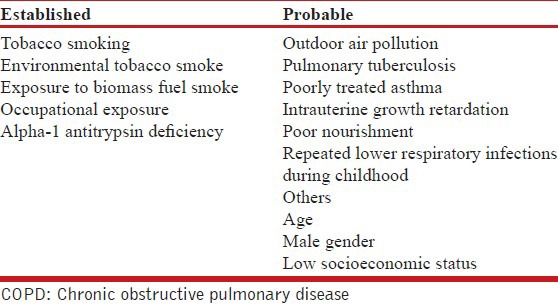
Tobacco smoking
Noncommunicable diseases (NCDs) account for approximately 36 million deaths per year across the globe; among them tobacco alone causes approximately 6 million deaths and is the leading modifiable risk factor for NCDs.[28,29]
Tobacco is abused in two main forms, mainly smoking and smokeless tobacco. Globally, the rate of current smokers is estimated to be about 1 billion.[29] In the INSEARCH study, prevalence of tobacco use was 28.5 and 2.1% among adult men and women, respectively.[18] According to the Global Adult Tobacco Survey (2009-2010) conducted in India, the prevalence of tobacco use in any form was 34.6% (47.9 and 20.3% among men and women, respectively). Of them, 14% (24.3% men and 2.9% women) smoked tobacco. The rise in COPD incidence has paralleled the rise in tobacco smoking throughout the world.[30,31] There is a strong dose-response relationship (for amount and duration) between tobacco smoking and COPD.[16,32] In INSEARCH II, the adjusted odds ratio (OR) for developing COPD among smoker as compared to nonsmoker was 4.08. In addition, smoking tobacco has additive effect with most of the other risk factors for COPD.[29]
Tobacco is consumed in both smoked and smokeless forms. It is well-known that tobacco smoke contains more than 4,000 harmful chemicals, of which over 50 are known carcinogens. Smokeless tobacco (chewing, sniffing, and others) is associated with cancer, hypertension, and heart disease. No association has been reported with COPD. In developing countries including India, indigenous forms of smoking tobacco are more prevalent than cigarette smoking.[32,33] It was thought that, having low tobacco content, bidi smoking would be less harmful than cigarette smoking. However, contrary to this belief, COPD is more common among bidi and hookah smokers as compared to cigarette smokers.[17,18,32,33,34] Chutta, chillum, and hookah are other traditional methods of smoking prevalent in India, and they are associated with even greater risk for developing COPD than cigarette smoking.[32,33,35] Low tar cigarettes are filtered to prevent tar from being inhaled. However, the adverse effects of tobacco smoking are not limited to the amount of tar. Shifting to low tar cigarettes in an attempt to decrease the risk of lung cancer was not effective.[36] Another review suggested that the effect of low tar cigarettes on COPD are inconsistent.[37]
The risk of COPD increases with increase in the number of cigarettes/bidis as well as with the duration of smoking. Any amount of smoking is harmful, although the risks are lower at low dose.[38] In one study the prevalence of CRDs among smokers with 2.5 and > 13.5 pack years was found to be approximately 13 and 60%, respectively.[32] Similarly, another study reported that prevalence of COPD in smokers with less than 20 pack years was 9.6%, which increased to 18% in subjects who smoked more than 20 pack years.[16]
ETS
About 25-45% of patients with COPD are never smokers. Recent evidence suggests that factors other than smoking are strongly associated with COPD.[39,40] In INSEARCH phase II study, approximately 60% of chronic bronchitis patients were nonsmokers.[18] ETS exposure among nonsmokers, especially women and children is common in many Asian countries. The INSEARCH study established the association of ETS exposure with COPD. The adjusted OR for COPD among those with ETS exposure was reported to be 1.99 (95% confidence intervals (CI), 1.69-2.34).[18] The odds for combined childhood and adult exposure was higher than that with ETS exposure during either childhood or adulthood alone, suggesting a cumulative effect.
Burning of biomass fuel
The combustion of biomass fuel such as dried dung, wood, and crop residue is associated with generation of several toxic gases and particles which are responsible for various health hazards, including respiratory problems.[41,42] Globally, about 3 billion people are exposed to biomass fuel smoke, compared with 1.01 billion people who smoke tobacco.[39,43] According to the third National Family Health Survey, about 75% of households in India still continue to use biomass fuel for cooking. Respiratory symptoms were reported in 13% of 3,608 nonsmoking women involved in domestic cooking.[44] One study found the prevalence of airflow limitation to be almost double in residents of households using biomass fuel compared to households using liquefied petroleum gas (LPG) (8.1 vs 3.6%).[45] Contrary to previous studies, this study displayed similar patterns for males as well as females. Two recent meta-analyses suggest that exposure to biomass fuel combustion is an important risk factor for COPD.[46,47] In fact, it has been argued that in India, where more than 70% people use biomass fuel for cooking purposes compared to 25% who smoke, exposure to biomass fuel may be a bigger risk factor for COPD in India.[40]
Occupational exposures
A systematic review of epidemiological data by the ATS suggests that about 15% of COPD cases might be related to exposure at workplace.[48] In subsequent studies, the proportion of patients with COPD attributable to occupation was about 19% overall and 31% in never smokers.[49] The list of occupations associated with increased risk of COPD include; rubber, plastics, and leather manufacturing; textile mill product manufacturing; and food product manufacturing.[50]
Alpha-1 antitrypsin deficiency and other genetic factors
AAT deficiency is the only well-known genetic risk factor for emphysema.[51] While the prevalence of AAT deficiency is significant in Europe and North America, it is not very commonly reported from the Asian continent including India. Two studies from India suggest that few interleukin genotypes, and mutations in glutathione s-transferase 1, have some association with COPD.[52,53]
Outdoor air pollution
Ambient air pollution in metropolitan cities has been frequently implicated as a causative agent for various respiratory diseases including COPD, especially in Asian countries where urban air pollution is high.[54] In one study, respiratory symptoms were found to be more common in the higher pollution zones among 4,171 randomly selected residents. Also, the emergency room visits for COPD increased by 24.9% when the levels of pollutants in ambient air exceeded the acceptable limits.[55]
Pulmonary tuberculosis
The association of pulmonary tuberculosis with COPD has occasionally been described. The prevalence of airflow obstruction varies from 28 to 68% of patients with treated pulmonary tuberculosis.[56] In a nationwide survey of 13,826 adults in South Africa, a history of pulmonary tuberculosis was associated with COPD with odds of 4.9 for men and 6.6 for women.[57] Whether this finding of obstructive functional defect in post tubercular sequel behaves as COPD, or is different, remains to be established in long term-prospective cohort studies.
Asthma
Patients with active asthma were found to have 10-fold increased risk of chronic bronchitis and 17-fold increased risk of emphysema as compared to those without asthma even after adjustment for confounding factors.[58] A subsequent review also suggests that a subset of patients with asthma may have COPD phenotype.[59]
Miscellaneous factors
An increased association of COPD is reported with demographic and socioeconomic factors such as advancing age, low socioeconomic status, and urban residence with lower socioeconomic status. This association may perhaps be attributed to the greater prevalence of smoking and cumulative effects of smoking and other exposures with age. Low socioeconomic status and infections have been listed as additional risks.
Recommendations
Tobacco smoking is the most well-established risk factor for COPD. (1A)
Both smokeless and smoking forms of tobacco are associated with serious health hazards, although only smoking tobacco is primarily responsible for COPD. (1A)
Bidi and other indigenous forms of tobacco smoking are at least as (or even more) harmful than cigarette smoking. (1A)
Low tar or filtered cigarettes are not “less harmful”. (2B)
There is no minimum number of cigarettes/bidi per day below which the risk for COPD decreases. (1A)
Exposure to ETS is a definite risk factor for COPD. (1A)
Exposure to biomass fuel smoke is a strong risk factor for COPD. (1A)
There are limited data on the association of ambient air pollution and COPD, and its causative role in COPD needs further evaluation.
There is insufficient evidence to attribute an etiological role of pulmonary tuberculosis in causing COPD.
A subgroup of chronic asthma may clinically behave like COPD; however whether it is true, COPD remains to be established. (UPP)
Diagnosis and Assessment of COPD
When to suspect COPD?
COPD is one of the important differential diagnosis in patients presenting with symptoms of chronic cough, sputum production, breathlessness, and/or wheezing.[60,61,62] This is especially true when patients have a history of prolonged exposure to risk factors. There is no definite duration of exposure to the risk factors that can result in COPD. However, in general, a prolonged exposure to risk factors is required for the development of disease.
Some patients presenting differently from the aforementioned symptoms (as detailed under the next subheading) might also be detected to have COPD on further evaluation.
Recommendation
A diagnosis of COPD should be considered in persons having chronic symptoms of cough, sputum production, shortness of breath, and/or wheezing; especially among those with prolonged exposure to risk factors for the disease. (1A)
What are the symptoms of COPD?
COPD patients may present to a healthcare facility in four typical ways:
With one or more of the characteristic respiratory symptoms of chronic progressive breathlessness, cough, sputum production, wheezing, and/or chest tightness. Recent studies reveal that presence of one or more of these symptoms increases the odds for the diagnosis of COPD.[60,61,62]
Without respiratory symptoms like breathlessness, because patients might have reduced their physical activity unknowingly to very low levels. They might just complain of fatigue.
With symptoms attributed to complications of the disease like weight loss (COPD related cachexia) or leg swelling (due to cor pulmonale).
With an exacerbation (as discussed in the section on exacerbation).
Breathlessness
Patients may variously describe their breathlessness as: “My breathing requires effort”, “I cannot get enough air in”, “I feel out of breath”, or “I feel hunger for more air”.[63] There may be individual and cultural variations in the description of breathlessness.[64] Breathlessness is usually present on exertion until late in the course of the disease. Orthopnea occurs early and more commonly in heart failure, which is an important differential diagnosis, while it is reported infrequently and late (if ever) in patients with COPD. The severity of breathlessness may be graded on various scales. A simple and widely used scale is the modified Medical Research Council (mMRC) questionnaire,[65] illustrated in Table 6.
Table 6.
Modified medical research council grading of breathlessness

Cough and sputum production
Cough may be the only presenting symptom. On the other hand, a smoker might consider his cough to be a natural consequence of smoking, and might neglect it as a symptom. An increasing intensity, or change in the nature, of cough may be reported. It may be more prominent in the morning. Cough may be accompanied by mucoid or purulent sputum production that may vary greatly in amount.[66] Again, patients may find it a normal phenomenon associated with smoking and might even feel that smoking helps in easing out the passage of sputum. Immediately following smoking cessation, cough and sputum may become more bothersome, but generally improve with continued abstinence.[67] The epidemiological definition of chronic bronchitis (regular production of sputum for 3 or more months for 2 consecutive years) is arbitrary and might not apply to a given patient. However, it might identify an “at risk” individual.
Wheezing
Patients may complain of wheezing, variously described as noisy breathing or a whistling sound. Wheezing may have diagnostic value when seen in the light of other clinical features.[60,61,62]
Chest tightness
Patients may complain of chest tightness, chest congestion, or an obstructed chest.
Chest pain and hemoptysis
These are not the usual symptoms of COPD. Their presence is often a pointer to an alternative diagnosis (e.g., lung malignancy, pulmonary tuberculosis, etc.)
What are the signs of COPD?
COPD patients may demonstrate various physical signs that may either be due to the primary disease or an associated complication [Table 7].[68] Diminished breath sounds and wheezing on auscultation have been shown to increase the odds for a diagnosis of COPD.[60] An important clinical sign is the forced expiratory time (FET).[69] An FET of more than 6 seconds suggests airway obstruction.
Table 7.
Useful physical signs in the diagnosis of COPD

Importantly, COPD patients, especially those presenting early, might lack any of the above mentioned signs. A diagnosis of COPD cannot be rejected due to the mere presence of a normal physical examination.
Recommendations
A diagnosis of COPD should not be excluded in the absence of physical signs. (2A)
Forced expiratory time (FET) of more than 6 seconds is suggestive of airflow obstruction. (2B)
What medical history should be obtained from patients suspected of having COPD?
A patient suspected to have COPD should be asked about the exposure to risk factors, especially tobacco smoking and exposure to smoke from biomass fuel combustion. A history of allergic disorders, asthma and other respiratory diseases, presence of comorbidities, family history of allergic and respiratory disorders, history of previous exacerbations and hospitalizations, and impact of disease on patient's life (including limitations of activities of daily living and the associated psychosocial morbidity) should also be documented.
What is the role of spirometry in the diagnosis of COPD? Whether fixed ratio or LLN should be used for diagnosis?
Demonstration of airflow obstruction is essential to make a definitive clinical diagnosis of COPD. Spirometry is a simple and accurate tool to assess airflow obstruction. Spirometry should be performed as per standard guidelines.[70,71] Table 8 summarizes some of the important points elaborated in these guidelines regarding the equipment and performance of spirometry.[70,71] Both FEV1 and FVC should be assessed, and the FEV1/FVC ratio calculated.
Table 8.
Equipment and performance of spirometry
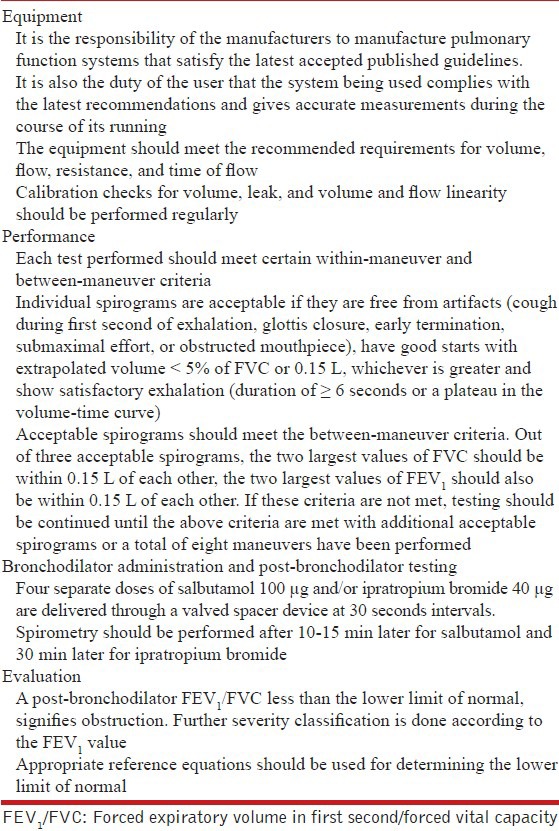
The criterion for defining airflow obstruction has been a subject of much debate in recent years.[72,73] The GOLD committee suggests use of a post-bronchodilator FEV1/FVC less than an arbitrarily fixed value of 0.7 (FR0.7) as the criterion for diagnosis of COPD.[8] However, guidelines on spirometry recommend the use of statistically derived LLN of FEV1/FVC as the cutoff to define airflow obstruction.[70] The LLN is defined as the lower fifth percentile of values in the reference population. The age and gender adjusted reference equations for this purpose are generated from spirometric data from a cohort of normal healthy nonsmoking individuals sampled from the particular population in a geographical area. LLN is then computed as the difference between predicted value and 1.645 times the standard error of estimate of the reference equation.
A systematic review analyzed the findings of 18 studies that compared these two spirometric definitions.[74] Most of these studies reported that use of FR0.7 leads to a much higher proportion of subjects being diagnosed as having airflow obstruction than use of LLN.[75,76,77,78,79,80,81,82,83,84,85,86] FR0.7 misclassifies more than 20% of subjects as having airflow obstruction when compared to LLN.[87] Further, discordance between the two methods in classifying obstruction increases in the elderly as LLN values decrease with advancing age.[88] Thus, elderly individuals having a FEV1/FVC value which might be within a statistically normal range for their age and gender, face the risk of being diagnosed as having COPD by using FR0.7.
Two studies analyzing longitudinal data have concluded that subjects having FEV1/FVC above the LLN, but below FR0.7 (the so called “discordant” group) had an increased risk of mortality than those having FEV1/FVC > 0.7.[80,89] However, the comparison of mortality was between the “discordant” group and a group of healthy individuals without respiratory symptoms, thus it does not hold valid for those who consult the physician for respiratory symptoms.[90] Also, as the relationship between lung function and risk of death is a continuum, it follows that any arbitrary cutoff would potentially have a difference in mortality among subjects falling on either side without representing true disease.[91] A recent study has shown that rate of decline of lung function in the discordant group is half the value observed in those with FEV1/FVC below LLN, while it is similar to those with FEV1/FVC above both LLN and FR0.7 cutoff.[90] Thus, the LLN has better discriminatory value for identifying subjects with a higher rate of lung function decline. An analysis of the aforementioned studies suggests that using FR0.7 criterion leads to over diagnosis of the disease as compared to a more statistically sound LLN criterion. Moreover, studies have also shown that FR0.7 also potentially ‘underdiagnoses’ younger patients with airflow obstruction.[92] Young subjects, especially below the age of 40 years might have a FEV1/FVC below the LLN for their age, but might get misclassified as normal as the ratio may be greater than the fixed cutoff of 0.7.[88]
Going by the current evidence, LLN is a more robust criterion for diagnosis of airflow obstruction than the FR0.7. Several reference equations derived from healthy subjects from various regions of India are available for this purpose.[93,94,95,96,97,98] However, there are still concerns among the experts regarding validity of these equations for populations from all parts of the country. It is imperative that spirometry reference equations should be generated for various population subsets in India. Meanwhile, the criterion of FR0.7 might be used only in the absence of a valid reference equation for FEV1/FVC in a particular region of the country.
Recommendations
Spirometry should be conducted in all patients suspected of having COPD. (1A)
In the absence of availability of spirometry, patients suspected of having COPD should be referred for a spirometric evaluation to a center with the facility. (UPP)
A post-bronchodilator FEV1/FVC below the LLN (lower fifth percentile of values from a reference population) should be used as the criterion for diagnosis of airflow obstruction. (1A)
In the absence of reference equations for LLN, FEV1/FVC < 0.7 may be used as the cutoff for defining airflow obstruction. (2A)
What is the role of reversibility testing in COPD?
It has been traditionally believed that COPD patients do not show reversibility in airflow obstruction after administration of bronchodilators, and this concept was considered useful to differentiate COPD from asthma.[99] Numerous studies have shown that patients with COPD may also show significant spirometric reversibility to bronchodilators.[100,101,102,103] Besides, bronchodilator reversibility is not a variable that essentially signifies the presence of disease; it has also been demonstrated in healthy subjects.[104] Thus, reversibility testing does not help diagnosis of COPD.[105] Also, lack of reversibility in COPD does not preclude a subsequent benefit from long-term maintenance bronchodilator therapy.[103,106] Moreover, the response to ICS is not predicted by bronchodilator reversibility in COPD patients.[107] Finally, bronchodilator reversibility varies temporally and does not correlate with clinically relevant outcomes such as mortality, hospitalization or exacerbation experience, making it an unreliable phenotype.[108]
Recommendation
Absence of bronchodilator reversibility does not differentiate COPD from asthma, and its presence does not predict the response to treatment (1A). However, all FEV1 values should be reported post-bronchodilator.
What is the role of screening spirometry?
Screening spirometry may help in detecting subjects with airflow obstruction before they develop clinical symptoms. This can be potentially beneficial in two ways: (a) diagnosis of COPD might improve smoking cessation rates, and (b) early treatment might alter disease prognosis. However, there is no conclusive evidence for either. Evidence for the notion that a diagnosis of COPD promotes smoking cessation is equivocal.[109,110,111] Rather there is a conceivable risk that tobacco smokers informed to be having a normal lung function might be encouraged to continue smoking.[110]
There are no controlled trials comparing clinical outcomes between screened and non-screened populations. The US Preventive Services Task Force (USPSTF) summarized the evidence on spirometric screening for COPD in 2008,[112] that “screening for COPD using spirometry is likely to identify a predominance of patients with mild to moderate airflow obstruction, who would not experience additional health benefits if labeled as having COPD.”[113] If screening spirometry is limited to smokers of more than 40 years of age, 833 individuals would need to be screened to prevent one exacerbation.[112] In the Indian setting, with a large “at risk” population, screening spirometry thus appears neither feasible nor cost-effective.
Recommendation
Spirometry is not recommended as a screening tool in asymptomatic individuals to detect airflow obstruction. (2A)
What is the role of PEF measurement in diagnosis and monitoring of COPD?
PEF measurement has often been advocated as a surrogate measure for FEV1. The PEF instrument is inexpensive, portable, and easy to operate and maintain.[114] It has been shown that PEF of less than 80% predicted has a sensitivity of 91% and a specificity of 82% in defining airflow obstruction, when using FEV1/FVC < 0.7 and FEV1 < 80% as the gold standard.[115] However, this sensitivity value is rather low for a test to qualify as a good screening test. With a specificity of 82%, the PEF criterion fails to qualify as a good diagnostic test either. Numerous studies have shown absence of parity between PEF% and FEV1 % values, with wide limits of agreement between the two measures.[114,116,117] PEF and change in PEF also cannot be used as a surrogate for standard spirometric criteria for bronchodilator reversibility assessment.[118] Although PEF has been used for the diagnosis, monitoring and prognostication in COPD, the supporting evidence is weak.[115,119,120]
Recommendation
PEF should not be routinely used for screening, diagnosis or monitoring in COPD. (1A)
How should the severity of COPD be classified?
Severity staging of COPD is important for disease prognostication as well as for treatment. GOLD guidelines classify COPD into mild (FEV1 ≥ 80% predicted), moderate (50% ≤ FEV1 < 80%), severe (30% ≤ FEV1 < 50%), and very severe (FEV1 < 30%) disease.[8] Most other guidelines follow the same classification system.[7,121] There is good quality evidence from large studies that worsening airflow limitation is associated with increasing mortality and hospitalization rates, as well as increased risk of exacerbations.[122,123,124] A measure like BODE index might offer additional prognostic information,[125] but there are no data whether treatment can be tailored according to the BODE index.
Most therapeutic considerations (derived from evidence available from large scale trials) differ only between the groups separated by a cutoff of above or below predicted FEV1 of 50%. Evidence regarding treatment of patients having mild airflow obstruction (i.e., predicted FEV1 > 80%) is scarce. Three broad groups based on spirometric severity can be formulated: Patients with FEV1 ≥ 80%, those with FEV1 between 50-79%, and those with FEV1 < 50%. Only the prognostication varies significantly within the last group which, as has been pointed out earlier, has a continuous linear relationship with lung function.[91] An additional group with FEV1 < 30% might be considered redundant.
The course of COPD is punctuated by exacerbations. An increase in frequency of exacerbations is associated with poorer quality of life (QoL), accelerated decline of lung function, and increased mortality.[126,127,128] A history of frequent exacerbations (more than one in a year) implies more severe disease and increased risk of future events.[122] The frequency of exacerbations also needs to be factored in for severity classification of the disease.
The assessment of patient's symptoms is extremely important in understanding the impact of the disease on patient's life. The mMRC questionnaire, as detailed above, helps to assess disability due to breathlessness and correlates well with other measures of health status and mortality risk.[129,130] The CAT is an eight-item questionnaire that comprehensively assesses the patient's symptoms and their impact on patient's life.[131] It is reliable and responsive, and correlates well with the St. George Respiratory Questionnaire (SGRQ) for health status assessment.[132] For simplicity and ease of use, mMRC questionnaire rather than CAT has been incorporated into our proposed classification as a measure of patient symptoms.
Finally, complications like respiratory failure, cor pulmonale, and secondary polycythemia (hematocrit > 55%) signify advanced disease, irrespective of other parameters.[133,134,135] Patients with any of these features should be placed in the category of severe disease. For evidence-based stratification of patients into treatment categories, FEV1, exacerbation frequency, mMRC grade, and presence of complications need to be considered. A proposed classification of severity of COPD is outlined in Table 9.
Table 9.
Classification of severity of COPD
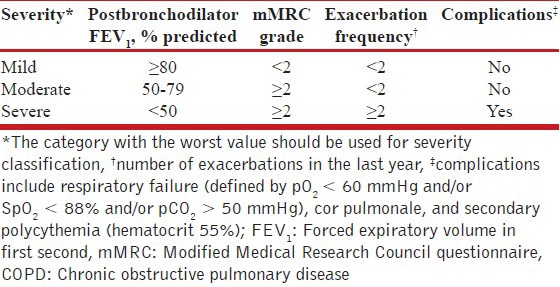
Recommendations
Classification of severity of the disease should be done for all COPD patients based on the FEV1 and exacerbation frequency. (1A)
Level of patient's disability due to symptoms should be assessed using mMRC Council dyspnea questionnaire or the CAT and recorded at each clinical visit. (1A)
What is the role of additional investigations in COPD?
Sputum examination
Smoking increases the risk of both COPD and tuberculosis.[136,137,138] In a country with a high prevalence of tuberculosis, it would be prudent to screen a patient with chronic cough of more than 2 weeks duration for tuberculosis through sputum microscopy.
Pulse oximetry
Pulse oximetry can serve as a screening test for systemic hypoxemia during acute exacerbation of COPD, as well in patients with chronic respiratory failure. Studies have shown that a cutoff of 88-92% has an almost 100% sensitivity to predict hypoxemia during exacerbations.[139,140]
Chest radiography
The radiological abnormalities associated with COPD are nonspecific. Moreover, the sensitivity of chest radiography for the diagnosis of COPD is poor.[141,142,143] It is useful to exclude alternative diagnoses, and to identify other comorbidities and/or complications.[144]
Special investigations: HRCT, lung volumes, and diffusing capacity for carbon monoxide, exercise testing
Although HRCT can be useful in identification and quantification of early emphysematous changes, its clinical utility for this purpose is yet to be determined.[145] HRCT may be useful in identifying other respiratory disorders in patients with symptoms suggestive of COPD. In one such study, among 516 patients with a pulmonary function test (PFT) suggestive of obstruction, HRCT was helpful in establishing an etiology other than COPD in 12.7% patients.[146]
Total lung capacity (TLC) is increased in COPD due to air trapping and emphysema. DLCO is decreased in emphysema due to reduction in the area of alveolar capillary membrane. The reduction in DLCO correlates well with pathological emphysema and emphysematous changes in HRCT.[147,148,149] A decreased DLCO may also help in predicting mortality in patients with COPD. There is no clear threshold value.[150,151] DLCO is typically within normal limits in COPD with a primarily chronic bronchitis phenotype.
Detailed cardiopulmonary exercise testing (CPET) is useful to assess functional status to establish exercise restrictions and to assess the impact of therapeutic interventions.[152,153,154,155] CPET has also been shown to be useful in patients with exertional dyspnea, out of proportion to their lung function abnormality, to assess additional contributing factors like myocardial ischemia.[156,157,158] Six-minute walk test (6MWT), a simpler surrogate for formal CPET, has also been found to be useful in assessment of functional status, effectiveness of therapy, and prognosis in COPD.[159,160,161,162,163]
AAT deficiency
Data on the prevalence of AAT in Indian patients with COPD is sparse.[164] Western data suggest that approximately 3% of patients with COPD might have AAT.[165] In the absence of an effective therapy for AAT, it would be prudent to restrict AAT testing for atypical cases of COPD with a high probability of AAT deficiency (such as a young patient with lower lobe emphysema).
Recommendations
All new COPD suspects with cough of more than 2 weeks’ duration should undergo sputum smear examination for acid fast bacilli to rule out pulmonary tuberculosis as per the standard practice of RNTCP. (UPP)
Pulse oximetry should be used to screen for hypoxemia in stable disease with FEV1 < 50% and in the presence of clinical suspicion of hypoxemia. (3A)
An arterial blood gas analysis should be performed if arterial saturation by pulse oximetry is less than 90%. (2A)
Diagnosis of COPD should not be made on the basis of a chest radiograph. (2A)
Chest radiograph may be done during the initial evaluation of COPD to look for comorbidities, complications, and alternative diagnoses. (2B)
Special investigations like HRCT scan, lung volumes, DLCO, and exercise testing should be done in situations of diagnostic difficulty or whenever clinically indicated. (2A)
6MWT may be used for monitoring of exercise capacity in COPD. (1A)
Testing for alpha-1 antitrypsin deficiency may be done in young patients with lower lobe emphysema. (UPP)
What is the role of multidimensional assessment tools in COPD?
Multidimensional assessment tools in COPD (such as BODE index, DOSE index, etc.) are useful in predicting mortality, exacerbations, and risk of hospitalizations.[166,167] Their predictive ability has been inconsistent when applied to different populations.[168]
Recommendation
Composite scores including BODE and DOSE should not be used to assess severity or prognosis in COPD unless they are validated in Indian patients. (2A)
What are the differential diagnoses of COPD?
The important differential diagnoses of COPD include asthma, congestive heart failure, bronchiectasis, tuberculosis, constrictive bronchiolitis, and diffuse panbronchiolitis [Table 10].
Table 10.
Differential diagnoses of COPD

What are the comorbidities associated with COPD?
COPD is associated with many comorbid diseases, which may be pulmonary or extrapulmonary (coronary vascular disease, congestive heart failure, diabetes mellitus, metabolic syndrome, obstructive sleep apnea, skeletal muscle dysfunction, cachexia, osteoporosis, depression, lung cancer).[169,170,171] Comorbid diseases in COPD are independently associated with a higher risk of hospitalization and mortality.[172]
Recommendation
COPD patients should be routinely evaluated, and appropriately treated, for comorbid conditions. (2A)
Management of stable COPD
What are the goals for the management of patients of stable COPD?
The goals in managing stable COPD include reduction in current symptoms as well as future risk of disease progression, prevention of exacerbations, and reduction in mortality [Table 11].[8] It is also important to avoid treatment associated adverse effects while trying to achieve these goals. The goals should be individualized, and assessed and monitored objectively. For instance, breathlessness can be easily evaluated using the mMRC grading. Similarly, exercise tolerance can be assessed through the 6MWT, and the QoL evaluated by using any one of the several validated questionnaires (such as SGRQ and others). Disease progression is measured by calculating the rate of decline in FEV1.
Table 11.
Treatment goals in a patient of stable COPD

What drugs are available for management of patients of stable COPD, and what are their recommended doses?
The three main groups of drugs available for management of stable COPD include inhaled anticholinergics, inhaled beta2-agonists, and ICS. The currently available drugs and their commonly prescribed doses are summarized in Table 12. Other drugs which can also be used include the oral drugs-beta-agonists, methylxanthines, and selective phosphodiesterase-4 (PDE4) inhibitors.
Table 12.
Commonly used drugs and dosages for pharmacotherapy of stable disease
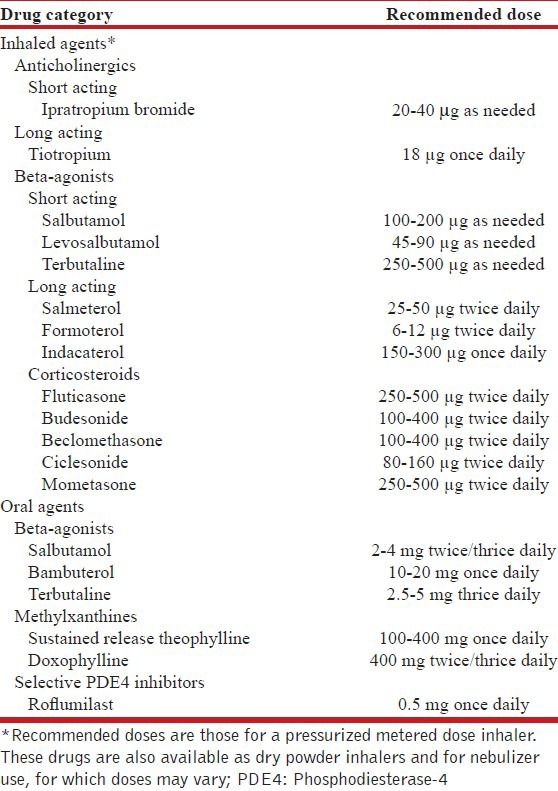
What is the ideal route and method of drug delivery?
Inhaled therapy is now established as the mainstay of treatment for patients with stable COPD. It allows low doses of bronchodilators or corticosteroids to be delivered rapidly and directly into airways, thereby achieving high local concentrations at site of action, while significantly reducing systemic adverse effects as compared to oral or parenteral therapy. Several patients continue to receive oral agents, because of patient preferences, ignorance, financial constraints, and/or lack of availability or acceptance of inhaled drugs.
Three kinds of aerosol devices are available for treatment in COPD namely pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs), and nebulizers. pMDIs are commonly used, but many patients (especially elderly) find it difficult to use them correctly, often because of problems in actuation-inhalation coordination. pMDIs used with a valved spacer further improves drug delivery to airways and reduces oropharyngeal deposition. Breath-actuated pMDIs may also obviate some of these issues. A wide array of DPIs are available that vary significantly in generated particle size and airway delivery. DPIs are breath-actuated, so coordination issues are much less. Even then, many patients are unable to use these devices correctly as relatively higher airflows are required to generate good aerosol. In an observational study of 3811 patients with COPD or asthma, critical errors in technique that potentially affected drug delivery were observed in 28% of pMDI users; and depending on the specific device, 11-32% of DPI users.[173] Another review found that 43, 55, and 59% patients could correctly use pMDI, pMDI with spacer, and DPI, respectively; and teaching substantially improved technique.[174] It is important to educate patients regarding correct inhaler technique, with regular monitoring of technique at follow-up visits. The correct technique of pMDI use is summarized in Table 13. Nebulizers use oxygen, compressed air, or ultrasonic power to generate fine aerosol from drug solutions or suspensions that is passively delivered to airways via mask or mouthpiece. However; they are bulky, much more expensive, require electrical power for operation, and need regular maintenance. Their overall dose delivery is largely comparable to pMDIs with spacer, or good quality DPIs.
Table 13.
Correct technique for using pressurized metered dose inhaler

Two large systematic reviews have concluded that the type of aerosol device has no effect on clinical outcomes in patients with COPD.[174,175] The American College of Chest Physicians and the American College of Asthma, Allergy, and Immunology have also recently concluded that when patients use these inhalation devices as prescribed, all of them work equally well.[175] In real-life clinical practice, selection of a delivery device ideal for a particular patient depends on several factors such as clinician and patient preference, availability, cost, patient age and dexterity, patient motivation and understanding, relative ease of device use, and others.
What is the role of long acting antimuscarinic agents in the management of stable COPD?
The most widely used inhaled LAMA for the management of stable COPD is tiotropium bromide. It has pharmacodynamic specificity for M1 and M3 receptors and has a half-life of more than 24 h, resulting in a convenient once daily dosing. The largest study till date on the use of tiotropium monotherapy in stable COPD is the Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial.[176] There was no difference in the primary end point (rate of decline of lung function) between the two groups, although patients receiving tiotropium had better FEV1 and FVC values at all-time points during the study period. Importantly, there was a significant decrease in the exacerbation rates and mortality with the use of tiotropium. Also, there was improvement in the QoL scores and lesser cardiac and respiratory adverse events in this group. A subgroup analysis of the UPLIFT trial focusing on the patients with moderate COPD (FEV1 50-70%), confirmed the benefits of tiotropium monotherapy in decreasing exacerbations and improving QoL and lung function, although there was no reduction in mortality.[123] Other subgroup analyses have also shown that the beneficial effects of tiotropium are observed in all patients of COPD; irrespective of their gender, ethnicity, or smoking status.[177,178,179] A recent Cochrane review of 22 randomized controlled trials comparing tiotropium monotherapy with placebo also confirmed the beneficial effects of tiotropium.[180] In this meta-analysis, tiotropium use in stable COPD was associated with significant improvement in QoL and reduction in exacerbations (with a number needed to treat of 16 to prevent one exacerbation). A subgroup analysis based on FEV1 (FEV1 > 50% and FEV1 < 50%) showed benefits of tiotropium in both the subgroups.
Aclidinium bromide is another LAMA used in some countries; it is however not available in India at present.[181] As compared to tiotropium, it possesses greater M3 muscarinic receptor selectivity.[182] Two large placebo-controlled randomized trials (AClidinium in COPD 1 (ACCORD COPD 1) trial and Aclidinium To Treat Airway obstruction In COPD patieNts (ATTAIN) study) have shown that aclidinium bromide given twice daily improves dyspnea scores, lung function, and QoL.[183,184] The precise role of this new drug in the management of stable COPD is yet to be established.
What is the role of short acting antimuscarinic agent in the management of stable COPD?
Prior to the introduction of tiotropium, short acting ipratropium was widely used for the management of stable COPD. Only a few short-term randomized controlled trials have compared ipratropium versus placebo.[103,185,186,187] These studies showed a consistent improvement in lung function and dyspnea scores in patients using ipratropium. None of these studies assessed exacerbation and mortality rates. No long-term studies have assessed regular ipratropium use in patients of stable COPD.
Is tiotropium better than ipratropium in pharmacotherapy of stable COPD?
Numerous randomized controlled trials have compared tiotropium with ipratropium using clinical endpoints, and all have consistently shown better outcomes in patients using tiotropium, including prevention of exacerbations.[188,189,190,191,192,193] Tiotropium also results in better patient compliance due to its once daily dosing. In addition, several observational studies have reported increased cardiovascular adverse events with ipratropium use (see next section). Hence, tiotropium is a more effective and a safer alternative to ipratropium in the management of stable COPD. The current position of ipratropium is limited only to its use as a rescue medication for the relief of symptoms.
What are the possible adverse events with the use of inhaled antimuscarinic agents, and does their risk-benefit profile favor their use in COPD?
Pooled analysis of 19 studies, as well as data from UPLIFT trial, shows that dryness of mouth is a significant adverse effect with tiotropium use.[176,194] Systemic side effects of inhaled anticholinergic agents are less common as their systemic absorption from respiratory and gastrointestinal tracts is poor. However, commonly reported minor side effects when using tiotropium include visual blurring, urinary retention, insomnia, and constipation.[176,194]
There has been a considerable debate on cardiovascular safety of inhaled antimuscarinics, especially ipratropium. In the Lung Health Study, mortality and hospitalization due to cardiovascular disease were highest among patients of mild-to-moderate COPD using ipratropium.[195] Two large retrospective observational studies have also shown that recent use of ipratropium is associated with an increased risk of cardiovascular events and mortality.[196,197] Ipratropium use has also been associated with higher risk of arrhythmias and stroke in large retrospective observational studies.[198,199]
In a meta-analysis of 17 studies, Singh et al., concluded that inhaled anticholinergics increase risk of cardiovascular death, myocardial infarction, and stroke.[200] This meta-analysis clubbed trials of tiotropium and ipratropium, and also combined placebo-controlled and active-controlled trials. Further, the healthy survivor effect was not taken into consideration. Three other meta-analyses, and a Cochrane review that included only placebo controlled trials of tiotropium, all concluded that use of tiotropium is not associated with an increased cardiovascular morbidity or mortality.[180,194,201,202] After the results of the UPLIFT study and a pooled analysis of 29 trials, the United States Food and Drug Administration (US-FDA) also concluded that the use of tiotropium was not associated with an increased risk of stroke, cardiovascular events, or death.[203] However, it is also important to note that the two largest trials evaluating tiotropium monotherapy (the UPLIFT trial and the Prevention of Exacerbations with Tiotropium in COPD (POET-COPD) trial) excluded patients with unstable coronary artery or decompensated cardiac diseases.[176,204] Hence, inhaled anticholinergics should be judiciously prescribed to patients with unstable cardiac diseases.
In contrast to tiotropium use by dry powder inhaler, the use of tiotropium by soft mist inhaler (Respimat device, currently not available in India) has been consistently shown to increase the risk of mortality.[205] Though the exact reason is not clear, it is postulated that this increase in adverse events could be due to higher drug deposition seen with soft mist inhalers.
Recommendations
Short-acting antimuscarinic agent (SAMA) can be used as rescue medication to relieve patient symptoms. (1A)
Long-term SAMA monotherapy on regular basis is not recommended. (1A)
Long-acting antimuscarinic agents (LAMA) are useful in stable COPD (FEV1 < 80%) to control symptoms and decrease the risk of exacerbations. (1A)
LAMA should be preferred over SAMA. (1A)
We suggest close monitoring of patients with coronary artery disease who are treated with LAMA. (UPP)
What is the role of long acting beta agonists in the management of stable COPD?
The LABAs available for management of COPD are salmeterol, formoterol, and indacaterol. A Cochrane review of 23 studies concluded that the use of salmeterol 50 μg twice daily was associated with better lung function and QoL scores, and lesser exacerbations (number needed to treat of 24), though there was no effect on mortality.[206] In the towards a revolution in COPD Health (TORCH) study, the use of salmeterol 50 μg twice daily (vs placebo) decreased exacerbation rates and improved SGRQ scores and post bronchodilator FEV1 values.[207] There was however no mortality benefits. A more recent meta-analysis, that also included the results of TORCH trial, concluded that use of either salmeterol or formoterol led to better FEV1 values, better QoL scores, lesser exacerbations (number needed to treat of 30), and lesser use of reliever medication.[208] There is considerable evidence that LABA monotherapy leads to significantly improved lung function, better symptom relief, and lesser exacerbations in patients of stable COPD; but has no significant mortality benefit.
Indacaterol is a novel ultra-long acting selective beta-2 agonist with a half-life of more than 30 h, allowing convenient once daily dosing at doses of 150-300 μg.[209] This agent also has a rapid onset of action within 5 min (similar to salbutamol) because of its high intrinsic efficacy at the receptor level.[210] Although promising, the evidence base for this agent is still limited. Three randomized trials have compared indacaterol with salmeterol (INSIST and INLIGHT study) and formoterol (INVOLVE study) in patients with FEV1 between 30-80%.[211,212,213] Two other randomized trials (INHANCE[214] and INTENSITY[215]) have shown that indacaterol therapy results in better QoL scores and dyspnea relief as compared to tiotropium. A review of all placebo and active controlled trials of indacaterol concluded that indacaterol in daily doses up to 600 μg was safe, with no increase in adverse vascular events or death.[216] Transient cough following inhalation is a common adverse reaction (14-18%) reported in all major trials.
What is the role of short acting beta-agonists in the management of stable COPD?
A recent Cochrane review analyzed 13 short-term studies comparing use of SABA vs placebo in COPD.[217] Use of SABA led to better FEV1 values, better symptom relief, and lesser dropouts from trials. However, none of these studies assessed exacerbation or mortality rates. No long-term studies on SABA use in COPD are available. Similar to that of short acting anticholinergics, the current role of SABA is probably limited to use as rescue medication for symptom relief in patients already using LABA.
Does the risk-benefit ratio of inhaled beta-agonists favor their use in the management of COPD?
Inhaled beta-agonists often lead to some common adverse events like tremors and palpitations, especially in high doses. They have also been shown to cause transient hypoxemia due to their vasodilatory effects on pulmonary vasculature which worsens ventilation-perfusion mismatch in areas of poor ventilation.[218] A single dose of beta-agonist increased the heart rate by 9 beats/min, and decreased serum potassium concentration by 0.36 mmol/L.[219] In the same meta-analysis it was also shown that long-term use of beta-agonists was associated with sinus tachycardia (OR 3.06) and an increase in major cardiovascular events (OR 2.54). A large retrospective cohort study of 76,661 patients has also shown that current use of SABA or LABA increase the risk of arrhythmias.[220] In the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program, a large prospective observational study of patients with heart failure, use of bronchodilators was associated with poorer survival.[221] Another meta-analysis comparing the effects of beta-agonists and anticholinergics to placebo concluded that anticholinergics (but not beta-agonists) decrease the risk of COPD exacerbation, and that beta-agonists actually increase respiratory mortality.[222] The large TORCH study however did not show any increase in risk of cardiovascular adverse events with the use of salmeterol monotherapy.[223] A recent meta-analysis, that included the results of TORCH study, concluded that use of LABA monotherapy decreases exacerbations, and that there was no increase in cardiovascular or respiratory mortality as previously suggested.[208]
Recommendations
SABA can be used to relieve symptoms of dyspnea as and when needed. (1A)
Long-term SABA monotherapy on regular basis is not recommended. (2A)
LABA monotherapy can relieve symptoms and decrease the exacerbation rate in patients with stable COPD (FEV1 < 80%). (1A)
Patients with symptomatic coronary artery disease receiving inhaled beta-agonists should be closely monitored. (UPP)
What is the role of ICS in the management of stable COPD?
There are several long-term studies on the use of ICS in patients with COPD. Four of these studies (including the European Respiratory Society's study on COPD (EUROSCOP) study, the COPD on Primary Care Treatment (CO-OPT) study, and the Lung Health Study) included patients with relatively better lung function (mean FEV1 64-86%).[224,225,226,227] None of these studies showed any significant benefit of ICS use on FEV1 decline or reduction in exacerbations or mortality. Only in the Lung Health Study, the use of triamcinolone led to fewer respiratory symptoms and lesser healthcare visits.[227] Two other studies, the Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) study and the TORCH study, included patients with more severe COPD (mean FEV1 50% and 44%, respectively).[207,228] Both these studies showed that ICS use decreased the risk exacerbation, and led to better QoL scores and better FEV1 values. However, neither study demonstrated any mortality benefit with ICS use. A recent Cochrane review that analyzed 55 studies, concluded that ICS use in COPD decreases exacerbations, results in better QoL scores, and also slows FEV1 decline.[107] However, subgroup analysis based on FEV1 values was not performed in this review. A systematic review and meta-regression on the effect of ICS in preventing exacerbations also concluded that the modest effect of ICS in decreasing exacerbations was apparent only in a subset of patients with FEV1 < 50%.[229] From all these results, it appears that ICS use mainly benefits COPD patients with more severe disease (FEV1 < 50%).
As opposed to bronchial asthma, ICS are believed to have only limited efficacy in COPD. This may partly be due to progressive reduction in histone deacetylase activity (particularly that of histone deacetylase 2), in lungs of patients with increasing severity of COPD.[230] Histone deacetylase 2 is required by corticosteroids to switch off activated inflammatory genes in COPD.
What are the adverse effects of ICS use, and does the risk-benefit ratio favor their use in COPD?
Potential adverse effects of long-term ICS can be either local (oropharyngeal candidiasis, pneumonia, or tuberculosis) or systemic (decreased bone mineral density and fractures, increased cataract and glaucoma, or possibly worsening diabetes control). Of these, the most worrisome are the increased risk of pneumonia and fractures.
Most studies on ICS use in COPD (including the TORCH study) have consistently revealed a higher risk of pneumonia (OR 1.60) as well as serious pneumonia (OR 1.71).[231] There was no increase in pneumonia-specific mortality. Two retrospective observational studies in patients with COPD admitted with pneumonia, have actually shown that ICS users have a lower mortality as compared to non-ICS users.[232,233] One of these studies also showed a decreased need for mechanical ventilation, even after adjustment for potential confounders.[232] Similarly, another prospective observational study of 460 patients of COPD with pneumonia showed that use of ICS did not lead to an increase in mortality.[234] It appears that although ICS use increases risk of developing pneumonia, this does not translate to worse clinical outcomes.
A meta-analysis that exclusively focused on the risk of fractures with ICS use showed a modest but statistically significant increase in the risk of fractures (number-needed-to-harm, 83).[235] This modest risk does not outweigh the beneficial effects of ICS in decreasing COPD exacerbations.
Recommendations
ICS have a beneficial effect in subgroup of COPD patients with FEV1 < 50%. (1A)
ICS have a beneficial effect in subgroup of COPD patients with frequent exacerbations (≥2 exacerbations/year). (1A)
The risk-benefit profile favors use of ICS in patients with severe COPD. (1A)
Which is better for the management of COPD: Tiotropium or LABA?
The largest study comparing tiotropium with LABA (salmeterol) is the POET-COPD trial that randomized 7,376 patients of COPD (FEV1 < 70%) and followed them for 1 year.[204] Tiotropium use was significantly better in decreasing the exacerbation rate in that study. Adverse reaction rates and mortality were similar. Subgroup analysis showed that beneficial effects of tiotropium in decreasing the exacerbations were uniformly seen in all the GOLD severity subgroups, including the GOLD stage 2 (FEV1 50-70%) subgroup.[236]
A recent Cochrane review analyzed six randomized trials comparing tiotropium with LABA monotherapy.[237] The LABA component was salmeterol in three studies, formoterol in one study, and indacaterol in two studies. Tiotropium was better than LABA in decreasing exacerbation rates (OR 0.86). Mortality rates, symptom scores, and QoL scores were similar. An economic evaluation as part of this review also concluded that tiotropium was more cost-effective than LABA monotherapy.
Can ICS monotherapy be an alternative to LABA/LAMA monotherapy?
There is no study which directly compares ICS monotherapy with LAMA monotherapy. A recent review analyzed seven randomized trials that compared ICS/LABA combination with ICS and LABA monotherapy, and extracted data from these studies to compare ICS monotherapy with LABA monotherapy.[238] There was no difference in exacerbation rates, adverse events, or symptom scores between the two groups. Though the QoL score was better with ICS monotherapy, and FEV1 values better with LABA monotherapy; both these differences were below the minimal clinically important threshold. Pneumonia occurred significantly more frequently among patients receiving ICS. The mortality rate was also higher (but not significantly so) with ICS monotherapy. Thus, ICS monotherapy did not confer any added advantage over LABA monotherapy, but increased the risk of pneumonia. Overall evidence therefore does not favor use of ICS monotherapy in management of stable COPD.
Which is better for the management of COPD: SAMA or SABA?
The current role of short acting agents is only as a rescue medication and not as a standalone therapy for the management of stable COPD. A Cochrane review comparing SABA with SAMA concluded that the advantage of regular use of ipratropium over SABA is small if the aim is to improve symptom control, lung function, or exercise capacity.[239]
Recommendations
LAMA is superior to LABA monotherapy. (1A)
ICS monotherapy should not be used. (1A)
SABA and SAMA are equally effective when used for COPD. (2A)
Is a combination of tiotropium plus LABA better than either of these agents used alone?
A Cochrane review analyzed five randomized trials of at least 12 weeks duration, comparing LABA plus tiotropium therapy with LABA or tiotropium monotherapy.[240] All these studies included patients with FEV1 < 70%. The combination therapy resulted in a slightly better QoL, symptom scores, and lung function as compared to monotherapy. There was however no difference in the exacerbation rates or the mortality rates. Another meta-analysis compared tiotropium plus formoterol combination to tiotropium alone, and included eight studies of at least 2 weeks duration.[241] This meta-analysis also concluded that the combination therapy improved lung function and symptom scores, but did not alter the exacerbation or mortality rates. Thus the combination of LABA and LAMA might benefit a subset of patients who continue to be symptomatic despite monotherapy. This combination does not decrease the exacerbation rates and may not be suitable for patients with frequent exacerbations.
What are the benefits of ICS plus LABA combination therapy over LABA monotherapy?
The first landmark trial which assessed the ICS-LABA combination therapy was the TRial of Inhaled STeroids ANd long-acting β2 agonists (TRISTAN) study.[242] This study randomized 1,465 patients and followed them for 1 year. The combination therapy led to better symptom scores, QoL scores, and lung function; as compared to LABA monotherapy. There was however no decrease in the exacerbation rates. The largest study on this issue till date is the TORCH study, which randomized and followed up 6,112 patients for 3 years.[207] The ICS/LABA combination decreased exacerbation rates and improved lung function and QoL when compared to LABA monotherapy. However, there was an increase in pneumonia rates. Also the ICS/LABA combination decreased the risk of cardiovascular adverse events, though the exact mechanism remains unexplained.[223]
A Cochrane review analyzing 14 studies (11,794 patients) compared ICS-LABA combination therapy to LABA monotherapy, and concluded that combination therapy decreased exacerbation rates and improved symptom scores, lung function, and QoL; without affecting mortality.[243] There was an increase in the incidence of pneumonia with the use of combination therapy. Two separate meta-analyses have also shown mortality benefit with use of ICS-LABA combination therapy.[208,244]
What are the benefits of ICS plus LABA combination over LAMA (tiotropium) monotherapy?
The only large study comparing ICS-LABA combination therapy with tiotropium monotherapy is the Investigating New Standards for Prophylaxis in Reduction of Exacerbations (INSPIRE) study that randomized and followed 1,323 patients for 2 years.[245] Though the dropout rates were more in the tiotropium arm (34.5 vs 41.7%), the combination therapy was better than tiotropium in decreasing mortality and improving QoL. There was no difference in the exacerbations in this study. A Cochrane review also addressed this issue, but did not draw any conclusions due to lack of good quality randomized trials and a very high dropout rate in the inspire study.[246]
What are the benefits of triple therapy (ICS plus LABA plus LAMA)?
A total of seven randomized trials and two observational studies (one retrospective and one prospective) have assessed the benefits of triple therapy. All seven randomized trials consistently showed improved lung function and QoL scores with the use of triple therapy.[220,247,248,249,250,251,252] Two of these studies have also shown a beneficial effect of triple therapy in decreasing exacerbations.[249,250] One retrospective observational study of 3,333 patients also showed a 33% decrease in risk of exacerbations.[253] Another small prospective observational study on 46 patients showed that triple therapy improved lung function and QoL scores.[254] A meta-analysis of four randomized trials of triple therapy concluded that such therapy improved the lung function, dyspnea, and QoL scores; however the effect on decreasing exacerbations was not clear.[255]
Recommendations
LAMA plus LABA may be used in patients who continue to have symptoms on monotherapy, except for those with frequent exacerbations. (1A)
LABA plus ICS should be preferred over LABA alone in patients with FEV1 < 50% or those having frequent exacerbations. (1A)
In patients of severe COPD (FEV1 < 50%), triple therapy may be used in those who are symptomatic despite single or dual bronchodilator therapy. (1B)
There is lack of sufficient data to recommend ICS-LABA or ICS-LAMA combination over LAMA monotherapy.
Do oral theophyllines have a role in the management of COPD?
Oral theophyllines have been extensively used in the past. With introduction of inhaled bronchodilators and corticosteroids, which are shown to be more effective, most international guidelines recommend the use of theophylline as a third-line option. Despite this, it is a common practice to prescribe theophyllines in our country due to its low cost.
The bronchodilator effect of theophylline is due to nonselective phosphodiesterase inhibition that occurs at plasma concentrations of 10-20 mg/L. These plasma levels are achieved at doses of 600-800 mg/day (standard dose). Theophyllines also antagonize adenosine receptors, which is responsible for serious adverse events such as seizures and arrhythmias. In view of the narrow therapeutic index, regular monitoring of serum theophylline levels is recommended for patients on long-term theophylline therapy. Such facilities are not routinely available in India. In recent years, theophyllines have also been shown to have a novel anti-inflammatory action related to histone deacetylase (HDAC) activation.[256] Airways of COPD patients have diminished HDAC activity. Corticosteroids act by recruiting HDAC to the transcription complex, leading to suppression of transcription of proinflammatory cytokines. Theophylline directly activates HDAC, independent of its binding to glucocorticoid receptors. Thus the anti-inflammatory action of both agents is synergistic. Theophylline induced HDAC activation occurs at plasma concentration < 10 mg/L. These blood levels can be achieved at much lower doses of 300-400 mg/day (low dose) that cause negligible systemic adverse effects. The standard doses are generally recommended when theophyllines are used as a standalone therapy for their bronchodilating property. Low dose theophyllines can be used to potentiate anti-inflammatory action of steroids.
A systematic review of 22 randomized trials concluded that theophylline use improved lung function, increased PaO2, and decreased PaCO2, and was associated with a higher patient preference as compared to placebo.[257] Low dose theophylline has been shown to improve lung function, QoL, and decrease exacerbations as compared to placebo.[258] A prospective observational study concluded that theophylline is well tolerated when used at doses below 400 mg/day, with nonserious adverse events reported in only 1.38% patients.[259] Adverse events were more common with concomitant use of macrolides.
Theophylline is shown to be inferior to salmeterol as well as formoterol, in improving lung function, QoL scores, or reducing rescue medication use.[260,261,262,263] When added to a combination of formoterol and tiotropium, theophylline failed to show any added advantage.[264] Hence, current evidence does not support the use of theophylline as a primary agent for pharmacotherapy of stable COPD.
Doxophylline, a newer theophylline analogue, may have a better safety profile, with fewer cardiac and neurologic side effects.[265] Doxophylline has not been shown to be superior to theophylline, and long-term studies assessing role of doxophylline in COPD are currently not available.
What is the role of selective phosphodiesterase-4 inhibitors in management of stable COPD?
Roflumilast is an oral selective PDE4 inhibitor approved for the use in COPD at a dose of 500 μg once daily. It is predominantly an anti-inflammatory agent rather than a bronchodilator, and should not be coadministered with theophyllines. The beneficial effects of roflumilast on lung function were initially demonstrated in patients with moderate-to-severe COPD and severe COPD.[266,267] Both studies showed a trend towards lesser exacerbations with the use of roflumilast. Following these early studies, four recent randomized trials confirmed the benefit of roflumilast in decreasing COPD exacerbations.[268,269] A post-hoc analysis of two trials showed that use of roflumilast shifts patients from a frequent to the more stable infrequent exacerbator phenotype.[270] A Cochrane review of 23 randomized trials with selective PDE4 inhibitors concluded that these agents offered benefit over placebo in improving lung function and reducing likelihood of exacerbations in patients of COPD.[271] They had little impact on QoL or symptoms. Gastrointestinal adverse effects and weight loss were also common. A recent study showed that roflumilast is effective and well-tolerated in the Asian population as well.[272] The optimum place of PDE4 inhibitors in COPD management still remains to be defined.
What is the role of oral beta-agonists in management of stable COPD?
Oral beta-agonists are widely used in India as cheaper alternatives to inhaled beta-agonists. But, they need to be administered in much larger doses to achieve comparable bronchodilatation to inhaled agents. Such therapy is commonly associated with systemic adverse events like palpitations, tremors, and arrhythmias. As inhaled bronchodilators have a much better efficacy and safety profile, oral beta-agonists cannot be recommended as a primary treatment for patients with stable COPD. Their use should be considered as a last resort only in situations where a patient does not wish to use inhaled bronchodilators, in the minimum possible doses that balance acceptable bronchodilatation with minimal side effects.
Recommendations
Oral methylxanthines are not recommended as first line therapy in patients with COPD. (1A)
-
Oral methylxanthines can be used as
- an alternative in patients not taking inhalers for any reason. (1B)
- add-on therapy in patients continuing to have symptoms despite optimum inhaled therapy. (3A)
Patients on oral methylxanthines need to be monitored for side effects or drug interactions. (UPP)
Roflumilast may be used in frequent exacerbators as an add-on or substitute to ICS. (2B)
What is the role of mucolytic agents?
Expectoration of sputum is a major bothersome symptom in COPD, and oral mucolytic agents are commonly prescribed for symptom relief. Traditional agents such as bromhexine and guaifenesin showed no significant change in lung function, sputum characteristics, or overall patient well-being.[273] The Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS) study, a 3-year randomized, placebo-controlled trial using N-acetylcysteine in a dose of 600 mg/day in more than 500 patients, failed to show any decrease in rate of decline in FEV1 or reduction in exacerbation rate.[274] A recent meta-analysis of 30 studies on patients with chronic bronchitis or COPD concluded that mucolytic therapy may result in minor reduction in acute exacerbation rates, but has negligible impact on lung function or overall QoL.[275] Quality of several included studies was poor with a high degree of heterogeneity.
Recommendations
Routine use of mucolytic agents is not recommended in patients with COPD. (2A)
What is the Indian strategy for the management of stable COPD?
When a patient initially presents to a physician with a diagnosis of COPD, he should be categorized into one of the three severity categories as per staging criteria described earlier [Table 9]. As initial therapy, physicians can prescribe a preferred treatment schedule, or an alternative schedule if the patient cannot take (or does not want to take) the preferred schedule for some reason [Table 3]. If a patient continues to be symptomatic despite such treatment, other drugs can be given as add-on therapy. During follow-up, if a patient shifts from one severity category to another, therapy should be accordingly modified. For example if a patient of mild COPD develops worsening symptoms, he would be labeled as moderate COPD and the treatment should be changed accordingly. Similarly if a patient with mild or moderate COPD develops frequent exacerbations or FEV1 falls below 50%, he would be categorized as severe COPD and treatment changed accordingly.
Acute Exacerbations of COPD
What is the definition of AECOPD?
An exacerbation of COPD is an acute event characterized by a sustained worsening of any of the patient's respiratory symptoms (cough, sputum quantity and/or character, dyspnea) that is beyond the normal day-to-day variation and leads to a change in medication, and where other causes of acute breathlessness have been clinically excluded.[276,277,278]
What is the impact of exacerbation on patients?
COPD is a progressive disease with a gradual decline in lung function. The course of COPD is however punctuated by exacerbations, some of which may be severe enough to cause hospitalizations; this may not only lead to increase in mortality, but is also associated with increased cost of treatment.[279,280,281,282] Exacerbations are associated with a greater and irreversible decline in lung function.[282,283] The occurrence of a severe exacerbation requiring hospitalization increases the risk of further exacerbation. Patients have 25 times higher risk of readmission to hospital after the tenth hospitalization, as compared to the first hospitalization.[282] In fact, there is five-fold increase in risk of death after tenth hospitalization.[282] Exacerbations are associated with progression of emphysema measured on serial CT scans.[284] Moreover, exacerbations are associated with significant decline in QoL,[285,286] and add to the cumulative economic burden.[287] The mortality is 40% at 1 year in those requiring mechanical ventilation, and all-cause mortality may be as high as 49% at 3 years after hospitalization.[287,288]
What are the precipitating factors for exacerbation?
Several precipitating factors, both infectious and noninfectious, have been implicated in AECOPD [Table 14].[289,290] Infections are the most frequent cause of exacerbations, and both bacterial and viral infections have been detected during exacerbations.[291,292,293,294] Acquisition of a new strain of Hemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, or Pseudomonas aeruginosa is strongly associated with the occurrence of an exacerbation.[295] A pooled analysis of studies utilizing bronchoscopic sampling with the use of a protected specimen brush revealed that bacteria were present in clinically significant concentrations in the airways of 4% of healthy adults, 29% of adults with stable COPD, and 54% of adults with COPD exacerbation.[295,296,297,298]
Table 14.
Causes of exacerbation of COPD#
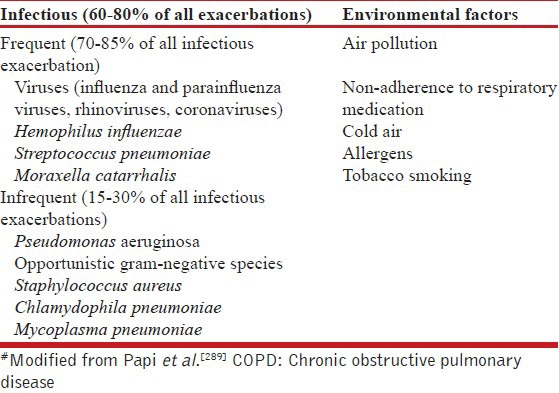
The noninfectious precipitants of acute exacerbations include nonadherence to medication, or inhalation of irritants like tobacco smoke or particles. Air pollution has been implicated in causing AECOPD. The effects of diesel particulates, sulfur dioxide (SO2), and others have been studied and potential mechanisms by which airway inflammation is enhanced (increase in bronchial neutrophils and methyl histamine) have been proposed.[299,300] The role of air pollution in causing exacerbation is primarily based upon epidemiological studies implicating increased air levels of SO2, NO2, and black smoke particulate matter.[301,302]
Conditions like heart failure, pulmonary embolism, cardiac arrhythmias, pneumothorax, pleural effusion, and pneumonia can cause acute worsening of symptoms in patients with COPD and are considered COPD exacerbation mimics.[121,276,290,303,304]
What is the differential diagnosis of AECOPD?
The differential diagnosis of AECOPD includes the 6Ps; pneumonia, pulmonary embolism, pneumothorax, pleural effusion, pulmonary edema (heart failure), and paroxysmal atrial tachycardia (arrhythmias), and these need to be excluded in patients with acute worsening of breathlessness. Pulmonary embolism is especially difficult to differentiate from COPD exacerbation especially when dyspnea is the only symptom. The prevalence of pulmonary embolism in AECOPD was estimated to be about 19.9%.[305] Exacerbation per se may also increase the risk of deep venous thrombosis and pulmonary embolism due to diminution in physical activity.[306,307]
How is an exacerbation of COPD diagnosed?
The diagnosis of an exacerbation is primarily clinical, and is based upon of sudden change of symptoms (baseline dyspnea, cough, and/or sputum production) that is beyond normal day-to-day variation.[297,308] Worsening breathlessness is the cardinal symptom of an exacerbation and is usually accompanied by increased cough, fever, wheezing, chest tightness, and change in the color and/or volume of sputum. There may also be non-specific manifestations such as tachycardia, tachypnea, fever, malaise, insomnia, sleepiness, fatigue, depression, and confusion; these are more common in the elderly.
How is the severity of an exacerbation assessed?
The assessment of severity of an exacerbation is based upon patient's clinical status before exacerbation, symptoms, physical examination, comorbidities, arterial blood gas analysis, and other relevant laboratory tests [Table 15]. These parameters can help in categorizing the severity of an exacerbation and also help in deciding the place of management.
Table 15.
Severity assessment (indications for hospitalization) of exacerbation of COPD*

How to investigate an exacerbation of COPD?
The investigations that should be considered for evaluating an AECOPD are as follows:
Pulse oximetry/Arterial blood gas analysis (wherever available) is helpful to confirm the diagnosis of acute, or acute on chronic, respiratory failure; and also assists in deciding supplemental oxygen therapy. As a general rule, a decline in PaO2 value by 10-15 mmHg suggests an acute deterioration in a patient with chronic respiratory failure.
Chest radiographs are worthwhile in excluding an alternative diagnosis like pneumonia, pneumothorax, pleural effusion, and others.
An electrocardiogram facilitates identification of coexisting cardiac abnormalities.
A complete blood count is useful in identifying anemia, polycythemia (hematocrit > 55%), and/or leukocytosis.
Blood biochemical tests aid in identifying coexisting electrolyte abnormalities or hepatic or renal dysfunction. The use of spirometry during an exacerbation is not recommended, as it can be difficult to perform and the results are inaccurate.[121]
Sputum cultures: Hemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis are the most common bacterial pathogens involved in an exacerbation. In severe exacerbations requiring invasive ventilation, Pseudomonas aeruginosa is an important consideration,[121,309] and sputum cultures may help in identifying the correct pathogen.
Recommendations
No investigations apart from pulse oximetry are routinely required in patients with acute exacerbations managed in an outpatient setting. (IIA)
In those hospitalized with AECOPD, serum electrolytes, liver and renal function tests, complete blood count, chest radiograph, electrocardiogram, and arterial blood gas analysis (if available) should be performed in all patients. (IA)
If an infectious exacerbation does not respond to the initial antibiotic treatment, a sputum culture and an antibiotic sensitivity test should be performed. (IIA)
How to decide the site of management of a patient with COPD exacerbation?
More than 80% of exacerbations can be managed on an outpatient basis.[122,176,310] The decision to hospitalize a patient is based on overall assessment [Table 15]. The presence of any one of the listed features may warrant hospitalization. Old age, poor nutritional status, presence of comorbidities, acidemia, altered mental status, and hypotension are associated with poor outcomes in patients hospitalized with COPD.[311] A prospective study evaluating CURB65 scores in AECOPD reported 30-day mortality rates of 2.0, 6.7, and 21.3% respectively in low risk (scores 0-1), moderate risk (score 2), and high risk (scores 3-5) groups. It was concluded that CURB 65 score could be used for predicting early mortality in patients with AECOPD.[312] In another study; 88,704 patients with AECOPD were assessed for the purpose of stratifying in-hospital mortality. Three variables had high discrimination score of outcomes (blood urea nitrogen > 24 mg/dL, acute mental status change, and heart rate ≥ 110/min). The investigators used these three parameters along with age, and proposed a new score, that is, BAP-65. In those with all three factors, the mortality rates were 13.1 and 14.6% in the derivation and validation cohorts respectively, compared to 0.3% in both cohorts without any of the three factors and age < 65 years.[313] The BAP-65 score was further validated in a study across 177 US hospitals. Mortality increased with increasing BAP-65 class, ranging from 1% in subjects in class I (score of 0) to 25% in those meeting all BAP-65 criteria. The need for mechanical ventilation also increased with escalating score (2% in the lowest risk cohort vs 55% in the highest risk group).[314] In a recent study comparing CURB-65 score with BAP-65 score to evaluate the need for mechanical ventilation, it was concluded that BAP-65 identifies patients with AECOPD at high risk for need of mechanical ventilation more accurately than does CURB-65.[315]
Recommendation
What are the treatment modalities?
The therapeutic components for acute exacerbation include both pharmacological and nonpharmacological modalities. Three classes of medications are generally used in managing an acute exacerbation-bronchodilators, steroids, and antibiotics. The nonpharmacological strategies includes oxygen therapy and use of mechanical ventilation, both noninvasive and invasive.
What is the role of short-acting bronchodilators in AECOPD?
SABA, with or without anticholinergic, are essential for symptomatic relief of airway obstruction, and constitute the first line of treatment.[121,316,317,318,319] Few studies have evaluated the use of bronchodilators in management of AECOPD. There were no significant differences in FEV1 change in patients treated with beta-agonists or ipratropium bromide, and no additive benefit of adding ipratropium to beta-agonist.[320] Also, the optimal dosing and frequency of bronchodilators in AECOPD is not established. There was no difference in clinical outcomes in patients treated every 4 hours with either 2.5 or 5 mg of nebulized salbutamol.[321] There was also no significant difference in improvement in FEV1 between hourly and 20-min dosing of salbutamol.[322] Post-hoc analysis however revealed significantly better improvement with the 20-min protocol in those with FEV1 < 20%.[322] The drugs can be delivered by the inhaled route either using pMDI with spacer or nebulizer. There is no significant difference in outcomes based on either method of administration.[319,323] In severe exacerbations, altered mental status favors the use of nebulized bronchodilators, while in others pMDI with spacer is preferred due to lower costs and lesser chances of infection. In any case, the patient should be switched over to pMDIs with spacer at the earliest. Importantly, the drugs should not be nebulized using oxygen. Rather patients should receive supplemental oxygen separately through nasal prongs while nebulizing drugs using compressed air, with monitoring of oxygen saturation.
Intravenous or subcutaneous routes of administering beta-agonists are associated with significant adverse events without significant additional bronchodilatation. Intravenous methylxanthines (theophylline or aminophylline) are considered second-line therapy, to be used only in select cases when there is insufficient response to short-acting bronchodilators. Current evidence does not favor routine use of methylxanthines in AECOPD. In a recent systematic review, methylxanthines were found to have modest and inconsistent effects and significant adverse effects.[324,325] Two randomized controlled trials have compared intravenous magnesium sulfate (1.5 or 1.2 g) with placebo in patients with AECOPD, and have found modest improvements in lung function.[326,327]
Recommendations
Inhaled route is the preferred route of administering bronchodilators. (IA)
Inhaled SABAs should be used for the first-line because of the quicker onset of action (IIIA), however SAMA are in no way inferior to SABAs.
Nebulized salbutamol at a dose of 2.5 mg every 20 min (or salbutamol pMDI100 μg 2-4 puffs every 20 min) for 1 h can be given initially.(IIIA) Further dosing will depend on the clinical response, generally every 4-6 h. (IIIA)
If additional bronchodilatation is desired, a combination of ipratropium (500 μg nebulized or 20 μg 2-4 puffs with pMDI) and salbutamol (2.5 mg nebulized or salbutamol pMDI100 μg 2-4 puffs) every 4-6 h can be used. (IIIA)
Nebulizer or pMDIs with spacer are equally effective. (IIA)
Patients should not be nebulized with oxygen but should receive oxygen separately through nasal prongs, with monitoring of oxygen saturation. (IIA)
Intravenous methylxanthines should not be routinely used. (IA)
The use of intravenous or subcutaneous route of administering bronchodilators should be reserved in the most seriously ill mechanically ventilated patient demonstrating inadequate response to inhaled therapy. (IIIB)
What is the role of glucocorticoids in AECOPD?
The pathophysiology of AECOPD is due to severe inflammation triggered by infective and/or noninfective causes, and thus systemic corticosteroids have a role in managing this condition. In a systematic review involving ten studies (n = 1051), steroid use resulted in significantly fewer treatment failures (defined as the need to seek additional medical therapy within 30 days), shorter duration of hospitalization, improvement in breathlessness, and FEV1 ; but with no significant effect on mortality. There was an increased likelihood of an adverse event (hyperglycemia, weight gain, or insomnia) associated with corticosteroid treatment with a number needed to harm of any adverse event being 5 (95% CI 4-9).[328] In a study of approximately 80,000 hospitalizations for COPD, there was no advantage of high-dose corticosteroids over low-dose regimens; in fact lower-dose regimens actually improved outcomes.[329] The study of ICS has been restricted largely to its role as chronic therapy for the prevention of exacerbations.[229] Few studies have investigated the role of ICS in management of AECOPD.[330,331,332]
Recommendations
Systemic steroids shorten recovery time, improve lung function, oxygenation, reduce length of hospital stay, and are associated with fewer treatment failures. (IA)
A short course of oral prednisolone (or equivalent) at a dose of 30-40 mg/day is recommended for managing acute exacerbations. (IIA)
The duration of systemic steroid therapy should be 5-10 days. (IIA)
Intravenous steroids should be given in patients who are being mechanically ventilated or cannot tolerate oral medication. (UPP)
ICS are not routinely recommended in management of AECOPD. (IA)
Should antibiotics be used in patients with acute exacerbations of COPD? What should be the dose and duration?
The recommendation for prescribing antibiotics in AECOPD is based on the seminal study where 173 patients of AECOPD were randomly assigned to receive either antibiotics or placebo. Patients receiving antibiotics had a higher success rate in type I exacerbations (defined by increased dyspnea, increased sputum volume, and increased sputum purulence) compared to the placebo group; whereas those with only one or two cardinal symptoms did not benefit from antibiotic therapy.[308] In a systemic review of 16 trials (n = 2068), there was evidence that antibiotics significantly reduce the risk of treatment failure; however hospitalized patients with severe exacerbations had a substantial benefit on treatment failure rates and mortality.[333] Antibiotics can effectively reduce treatment failure and mortality rates in COPD patients with severe exacerbations.[333,334] In a recent multicentric trial, treatment with antibiotics was associated with longer median time to next exacerbation even in those with nonsevere exacerbations.[335] The antibiotics that are used in managing AECOPD can be broadly classified as first-line (amoxicillin 500-1000 mg thrice a day for 5-7 days, doxycycline 100 mg twice a day for 5-7 days, azithromycin 500 mg once a day for 3 days) or second-line (amoxicillin/clavulanic acid 625 mg thrice a day for 5-7 days; second-generation or third-generation cephalosporins, e.g., cefixime 200 mg twice a day for 5-7 days).[336] A meta-analysis of seven randomized controlled trials comparing short (5 days) versus long (7-10 days) treatment with antibiotics (same dosage and same route of administration), revealed no difference in treatment success across the two groups. There were fewer adverse events in the short treatment duration group.[337] Fluoroquinolone use is associated with masking of tubercular infection and increased risk of drug resistance to M. tuberculosis,[338,339,340,341,342] its indiscriminate empirical use in India should be discouraged.[343]
Recommendation
Antibiotics should be prescribed for all exacerbations of COPD. (IIA)
The choice of antibiotics should be guided by local flora and sensitivity pattern. (IIA)
Fluoroquinolones should not be used routinely in treating AECOPD. (IA)
Patients with AECOPD being managed in the outpatient setting may be treated with first line antibiotics. (IIA)
Hospitalized patients or those requiring mechanical ventilation (noninvasive/invasive) should be treated with second line drugs. (IIA)
The duration of therapy should be 5-7 days. (IIA)
What is the role of procalcitonin in deciding for antibiotic therapy?
Several studies have evaluated the role of procalcitonin for initiating and stopping antibiotics in patients with respiratory tract infections. In a recent review evaluating 14 trials (4,221 participants), the authors concluded that procalcitonin can help in guiding the duration of antibiotic treatment in acute respiratory infections. There was significant decrease in total antibiotic exposure without increase in mortality.[344] But, procalcitonin use led to greater duration of antibiotics and mechanical ventilation, and overall increase in cost.[345] Moreover, there is no evidence on the use of procalcitonin to guide antibiotic therapy in AECOPD.
Recommendations
Biomarkers do not have a role in management of acute exacerbation of COPD. (IIA)
Procalcitonin should not be used routinely in guiding antibiotic usage in COPD. (IIA)
When should oxygen be prescribed, and at what dose?
The goal of inpatient oxygen therapy is to maintain PaO2 ≥ 8 kPa (≥60 mmHg) or SpO2 ≥ 90% in order to prevent tissue hypoxia and preserve cellular oxygenation. Because of the characteristics of the oxyhemoglobin dissociation curve, increasing the PaO2 to values greater than 60 mmHg confers little added benefit but significantly increases the risk of CO2 retention, which may lead to respiratory acidosis.[121] The choice of delivery devices depends on the patient's oxygen requirement, efficacy of the device, reliability, ease of therapeutic application, and patient acceptance. A Venturi air-entrainment mask mixes oxygen with room air, and provides an accurate and constant FIO2. The Venturi mask is the oxygen delivery device of choice in AECOPD.[121] The nasal cannula delivers a variable FIO2 depending on the minute ventilation; the lower the minute volume the higher the FIO2.[121] The nasal cannula can be used in AECOPD in those intolerant to Venturi mask, and after the acute phase of the exacerbation. An arterial blood gas analysis or oximetry is advised upon switching delivery devices. Frequent monitoring is advisable in the unstable patient.[346,347]
Recommendation
Oxygen should be prescribed to hypoxemic patients with a target SpO2 between 88-92%. (IA)
Oxygen should be delivered preferably by a Venturi mask, and by nasal cannula upon recovery. (IIA)
Arterial blood gas monitoring is recommended in patients receiving oxygen therapy, wherever available. (IIA)
What is the indication of noninvasive ventilation during exacerbation of COPD?
Noninvasive ventilation has revolutionized the management of AECOPD. Its use is associated with reduced rates of intubation, reduced length of hospital stay, and decline in mortality.[348,349,350] The benefit with NIV is observed even in those with hypercapnic encephalopathy, if it is used judiciously.[351,352] The patient needs to be closely monitored with facilities for intubation being readily available. NIV has also been demonstrated to be useful in weaning from invasive ventilation. In a review of 12 studies involving 530 patients, the use of NIV was associated with significant reduction in mortality, ventilator associated pneumonia, and intensive care unit and hospital lengths of stay.[353,354] The indications and protocol of NIV are summarized in Table 16. Patients should be assessed at 1, 2, 3, 4, and 24 h after initiation of NIV; clinically and by monitoring arterial blood gases (or pulse oximetry).[348,355] The presence of any one factor listed in Table 16 should prompt consideration of invasive mechanical ventilation (Adapted from).[348,355]
Table 16.
Indications, protocol, and parameters of failure of noninvasive ventilation in acute exacerbation of COPD

NIV can be used even in settings where arterial blood gas monitoring is not routinely available in patients with acute onset of breathlessness with tachypnea (RR > 25-30/min) and/or clinical signs of hypercapnia. Facilities for pulse oximetry, positive pressure ventilation, and intubation should be readily available. Patients not improving in 2-4 h should be promptly referred to a center with better facilities for further management.
Recommendations
NIV should be used early in the management of respiratory failure due to AECOPD. (IA)
NIV can also be used in weaning from invasive mechanical ventilation. (IIA)
NIV can be used even in settings where arterial blood gas monitoring is not routinely available. (UPP)
What are the indications of invasive mechanical ventilation?
With the advent of NIV, the need for invasive mechanical ventilation in management of AECOPD has significantly declined. The need for invasive mechanical ventilation is associated with poorer outcomes, the overall mortality among AECOPD patients is lower than mortality among patients ventilated for non-COPD causes.[356] There is evidence to suggest that patients likely to benefit with invasive ventilation are denied admission to intensive care units because of unwarranted prognostic pessimism.[357] The indications and ventilatory protocol of invasive mechanical ventilation are listed in Table 17. Adopted from.[348,355]
Table 17.
Indications and protocol of invasive mechanical ventilation
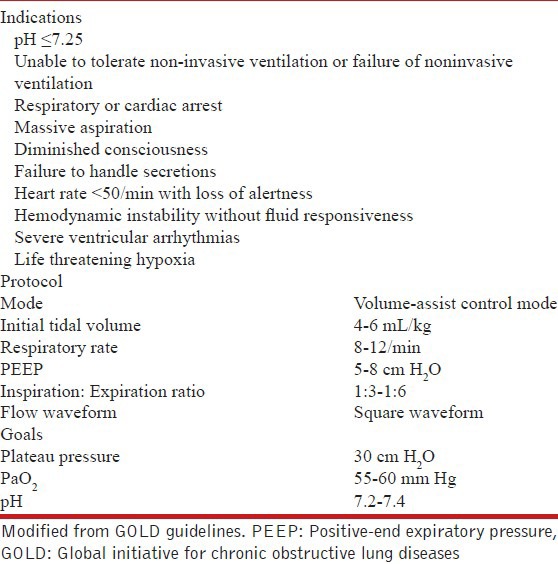
When should the patient be discharged from hospital?
The decision to discharge a patient from the hospital is a clinical art, with scarce data to clearly define the optimal duration of hospitalization in AECOPD.[161,358,359] In general, the patient should be clinically stable for at least 24-48 h, should be able to eat and sleep comfortably, and should be ambulatory for activities of daily living. In addition, there should be minimal requirement of short-acting bronchodilators, and the patient should be able to use long-acting bronchodilators.
When should the patient be followed-up after the discharge from hospital?
Patients should be followed-up 4-6 weeks after discharge from hospital. At every visit, due emphasis should be laid on smoking cessation, the inhaler technique checked, and the effectiveness of each medication monitored.[360] For patients who are hypoxemic during an exacerbation, arterial blood gases, and/or pulse oximetry should be evaluated prior to hospital discharge and in the following 3-6 weeks. If the patient remains hypoxemic, long-term supplemental oxygen therapy may be required.
Non-pharmacological management of COPD
Should smoking cessation be advised? What are the methods of smoking cessation?
Smoking cessation is without doubt the most effective method to prevent COPD.[361] This has been established by a number of studies over the last several decades, and is recommended by all major guidelines.[7,8] In addition to a reduction in the rate of decline of FEV1 in stable COPD, smoking cessation is also associated with reduction in the frequency of exacerbations.[362] Standard guidelines [Table 18] are available to both physicians and patients for quitting smoking; guidelines have also been made available by the National Tobacco Control Program under the MOHFW in India.[361,363,364]
Table 18.
Simple steps in assisting patients on smoking cessation

A variety of pharmacotherapies have also become available. These include:
Nicotine replacement therapies: Act by the supplementation of nicotine during the period of abstinence and help to reduce withdrawal symptoms. Various forms are available including gums, tablets, patches, and inhalers. Use of nicotine replacement therapies has been shown to increase the quit rate by 50-70% compared to placebo.[365]
Bupropion: Acts by inhibiting the reuptake of both dopamine and norepinephrine in the brain; antagonism of the nicotine receptor has also been demonstrated.[366] There are higher odds (OR 2.12) for quitting smoking with the use of bupropion as compared to placebo.[367]
Varenicline: It is a partial agonist of the α4β2 nicotinic receptor. It has a dual mechanism of action. It is both a partial agonist of the nicotinic receptor (wherein it provides some amount of stimulation) as well as simultaneously blocks the effect of nicotine on the receptor in case the patient smokers.[366] A meta-analysis of 69 trials showed that varenicline had the highest odds (2.55; 95% CI = 1.99-3.24) of smoking cessation out of all the available pharmacotherapies.[367]
There is no fixed protocol on the use of these agents. However, pharmacotherapy should be offered to all patients in view of the significantly increased quit rates with these modalities. The quit rate is even better if intensive counseling sessions are combined with pharmacotherapy.[365,368,369,370]
Recommendations
A smoking history, including pack years or smoking index (number of bidis/cigarettes smoked per day multiplied by number of years smoked; mild, moderate, and heavy smokers are defined as having a smoking index of < 100, 100-300, and > 300, respectively[371]) should be documented for all patients with COPD. (UPP)
All COPD patients, regardless of age, should be encouraged to stop smoking, and offered help to do so, at every opportunity. (1A)
Nicotine replacement therapies (varenicline or bupropion), combined with an appropriate support program, should be offered to people who are planning to stop smoking. (1A)
What is the role of health education in COPD?
Intuitively, health education should improve the management of patients with any disease, not only COPD. Health education may include informal out-patient discussions, didactic lectures, group sessions, and others. The role of multimedia, especially the role of the internet and online support groups should also be considered. However, modifying the habits of a lifetime is difficult. Various topics that may be covered in an educational program include:
Risks and benefits of stopping smoking and cessation strategies.
Avoidance of potential risk factors in nonsmokers.
The concept of normal lung function.
Inhaler use: It is important to check that the inhalers being used by the patient are the ones prescribed and that they are being used properly. The inhaler technique should be demonstrated to the patient and accompanying attendants and reinforced at every visit. The demonstration of the inhaler technique to the attendants is especially important in case of the elderly.
Use of other medications, including oxygen.
Nutrition.
Adequate physical exercise, especially if the patient is not enrolled in a pulmonary rehabilitation program.
Breathing strategies.
Advice related to travel and sexuality.
End of life planning.
Recommendations
Health education is an integral component of a COPD management program. Special importance should be given to inhaler technique, which should be demonstrated to the patient and accompanying attendants, and reinforced at every visit. This is particularly true for elderly patients. (UPP)
For COPD patients who are not active smokers, potential etiological exposures (environmental tobacco smoke, biomass fuel smoke, etc.) should be asked for and avoided. (UPP)
What are the components of nonpharmacological management of stable COPD?
Various therapies are available for the management of stable COPD. Some of these modalities may be as good as or better than pharmacotherapy. These methods are meant to be applied to patients with stable COPD, that is, not during a period of acute exacerbations. They include pulmonary rehabilitation, oxygen therapy, noninvasive ventilation, bronchoscopic techniques, and surgery.
What is the role of pulmonary rehabilitation?
The ATS and the European Respiratory Society have defined pulmonary rehabilitation as “an evidence-based, multidisciplinary, and comprehensive intervention for patients with chronic respiratory diseases who are symptomatic and often have decreased daily life activities. Integrated into the individualized treatment of the patient, pulmonary rehabilitation is designed to reduce symptoms, optimize functional status, increase participation, and reduce healthcare costs through stabilizing or reversing systemic manifestations of the disease”.[372]
COPD is a multisystem disorder, affecting the skeletal muscles, bones, and other organs besides the lungs. In general, treatment is targeted to the respiratory system, ignoring the other organ systems involved. However, much of the morbidity of COPD is because of involvement of other organ systems. Pulmonary rehabilitation addresses these issues. Pulmonary rehabilitation has been used as a last resort for those failing medical management. However, this is not an optimal use of this strategy. In fact, patients should be integrated into rehabilitation programs from an early stage in the course of the disease.[372] The various components of pulmonary rehabilitation include: (a) Patient selection and assessment; (b) exercise; (c) nutritional support; (d) psychosocial support; and (e) education.
The major component of pulmonary rehabilitation is exercise training. Exercise training is based on three major principles: Intensity (higher intensity producing greater results), specificity (only those muscles trained showing effect), and reversibility (cessation of regular exercise training resulting in decrease in the effect).[373] Regions to be included in exercise training include the upper limbs, lower limbs, and inspiratory muscles. The type of training also varies; it can either be continuous or interval, and strength or endurance based. The type of exercise sessions carried out is highly variable from center to center. The duration of each session can range from 15-45 min; the sessions can be carried out at intervals ranging from daily to once weekly. The intensity of each workout varies from 50% of the peak VO2 to the maximum tolerated. The duration of the program is also highly variable from 4 to 12 weeks, with greater benefits for longer programs.[8,372,373] A meta-analysis on the duration of pulmonary rehabilitation could not be carried out because of heterogeneity.[374]
Pulmonary rehabilitation has been proven to be effective in almost all aspects of management of COPD. A Cochrane review of 31 trials evaluated the effect of pulmonary rehabilitation on health-related QoL (HRQoL) and exercise capacity. The change in the QoL scores with pulmonary rehabilitation was above the minimal clinically important difference, while the change in exercise capacity was just below it.[375] A Cochrane review, which included nine trials with a total of 432 patients, found a significant improvement in both the QoL and exercise capacity, which was greater than the minimal clinically important difference.[376] The increase in HRQoL was greater with strength training as compared to endurance training; also, interval training was of similar benefit as compared to continuous training. Surprisingly, the evidence favoring high intensity exercise was weak.[377] Another analysis compared interval training to continuous training and found no difference on the QoL and exercise capacity between the two.[378] An advantage of inspiratory muscle training in addition to standard exercise was reported in another meta-analysis.[379] It is also suggested that starting pulmonary rehabilitation soon after an acute exacerbation leads to a reduction in future hospital admissions and mortality.[376]
Although the utility of structured rehabilitation programs is proven without doubt, it is often difficult to set up such programs in resource poor settings. A few small randomized clinical trials available from India have studied home-based rehabilitation and ‘pranayama’ breathing, and found positive results.[380,381,382,383] Exercise training options have also been detailed in resource poor settings.[384] Also, it is intuitive to advise patients to exercise daily as per their capacity because if the associated health benefits.
The use of nutritional therapy as part of structured rehabilitation programs has been the subject of much debate. The presence of malnutrition in patients presenting with COPD has been well-established. Various changes are noted in these patients, the prominent ones being loss of both total body fat mass as well as fat free mass.[372] Some of the methods available to assess the nutritional status of these patients include subjective assessment, assessment of BMI, skinfold anthropometry, bioimpedance analysis, and dual-energy X-ray absorptiometry.
The use of subjective assessment for nutritional status has been assessed in a resource poor setting for the initial evaluation of COPD patients.[385] The debate in this issue stems from the fact that most studies have found inconsistent benefits with nutritional supplementation in COPD[386] However, a recent Cochrane meta-analysis of 17 studies with 632 participants, who received at least 2 weeks of nutritional support, reports a difference in the outcome parameters of respiratory muscle strength and QoL.[387] As of now, the role of nutritional supplementation should be decided on a case to case basis by a specialist.
Recommendations
Structured pulmonary rehabilitation programs should be set up where feasible. (1A)
In the absence of structured programs, patients should be advised regarding unsupervised daily physical activity. (3A)
All patients should be assessed for nutritional status at least by BMI at the initial visit and followed-up by serial BMI estimation at every visit. Any patient found to be malnourished should be referred for nutritional advice to a specialist. (UPP)
What are the indications for oxygen therapy in stable COPD?
The benefits of supplemental oxygen therapy were proven in two landmark trials carried out in the 1980s, that is, the Nocturnal Oxygen Therapy Trial (NOTT) and the MRC trials.[388,389,390,391] At present, supplemental oxygen is recommended in stable COPD for the following group of patients:[392]
Those with severe daytime resting hypoxemia, that is,
PaO2 of < 55 mmHg (or pulse oxygen saturation of < 88%), or
PaO2 56-60 mmHg (or pulse oxygen saturation of 88-92%) with evidence of end-organ dysfunction including pulmonary hypertension, congestive cardiac failure, and erythrocytosis with hematocrit > 55%.
Additionally, hypoxia should be demonstrated on two occasions at least 3 weeks apart in the stable patient.
However, there are other patient groups in whom the evidence is not as clear as to the benefit of oxygen supplementation.[393,394,395] These include patients with moderate daytime hypoxemia, only nocturnal hypoxemia, and only exercise-induced hypoxemia. A recent analysis found no benefit of oxygen therapy in patients with moderate daytime hypoxemia or nocturnal hypoxemia alone.[396] The ongoing Long-Term Oxygen Treatment Trial (LOTT) trial is likely to clarify some of these issues.
There have been numerous studies and meta-analyses evaluating the issue of supplemental oxygen therapy. One analysis included 31 studies with 534 participants and studied the effect of ambulatory oxygen in COPD. The authors concluded that ambulatory oxygen improved all pooled outcomes related to exercise capacity. However, the study was limited by heterogeneity, publication bias, and small sample size.[397] Another meta-analysis, which included only six trials, concluded that oxygen supplementation did not lead to an improvement in the mortality in patients with only nocturnal or mild daytime hypoxemia.[398] The most recent Cochrane review included 18 trials with 431 participants and reports that the use of supplemental oxygen relieved dyspnea in patients with mild or no hypoxemia. Interestingly, these patients were not eligible for oxygen therapy according to either absolute or relative indications.[396,399]
Oxygen therapy may be administered by various delivery devices such as oxygen cylinders, liquid oxygen systems, and oxygen concentrators. Patients should be advised regarding the various options available locally and the expenses involved. Patients should also be given advice regarding the proper use of oxygen, and especially to avoid smoking while receiving oxygen, because of the increased risk of fire and explosions. The prescription for oxygen should be written clearly, detailing all the technicalities involved. Advice should also be given regarding the target oxygen saturation, in order to avoid over-oxygenating the patient and precipitating hypercapnic respiratory failure.[400]
Recommendations
-
Long-term supplemental oxygen therapy is indicated for those with severe daytime resting hypoxemia (1A) defined as:
- PaO2 of < 55 mmHg (or pulse oxygen saturation of < 88%), or
- PaO2 of 56-60 mmHg (or pulse oxygen saturation of 88-92%) with evidence of end-organ dysfunction such as pulmonary hypertension, congestive cardiac failure, and erythrocytosis with hematocrit > 55%.
- Determined on two occasions at least 3 weeks apart in the stable patient.
The role of oxygen supplementation in other situations is currently not clear and should be decided on a case to case basis. (2B)
Supplemental oxygen should be titrated to achieve a pulse oximetric saturation of 90-92% or a PaO2 of 60-65 mmHg. (3A)
Patients should breathe supplemental oxygen for at least 16 h a day. (1A)
Patients on long-term oxygen therapy should be reviewed at regular intervals with either pulse oximetry or arterial blood gas analysis as indicated. (UPP)
Patients should be warned about the risk of fire if smoking is continued during the period of oxygen supplementation. (UPP)
What is the role of noninvasive ventilation in stable COPD?
Although the use of NIV is well-established in the treatment of AECOPD,[401,402] its role in stable COPD is controversial.[7,8] There are many potential benefits, including improvement in respiratory muscle strength, maximum inspiratory pressure, gas exchange, and others.[403,404] A Cochrane review of four studies involving the use of nocturnal NIV for 3 months in hypercapnic patients found no consistent clinically or statistically significant effect on lung function, gas exchange, respiratory muscle strength, sleep efficiency, or exercise tolerance.[405] Another meta-analysis published in 2007 included 15 studies (six randomized control trials and nine non-randomized trials).[406] The randomized trials did not show any benefit in gas exchange or other parameters; while the non-randomized trials demonstrated benefit in QoL, exercise capacity, and lung hyperinflation. However, there was significant heterogeneity in this analysis.[406] A recent meta-analysis and review studied both the short- and long-term effects of NIV.[407] The results showed that there was a short-term benefit of NIV on gas exchange and exercise capacity which was not sustained over longer periods; there was no short- or long-term beneficial effect on FEV1 or mortality. Additionally, there was an overall beneficial effect on breathlessness, but not on the rate of hospitalization.[407] The indications for NIV in stable COPD were laid out at a consensus conference in 1999,[408] and are still relevant and applicable:
Documentation of the diagnosis of COPD by a physician, optimization of other therapies, and exclusion of sleep apnea if required.
-
Presence of both symptoms (such as fatigue, dyspnea, morning headache, etc.) and physiologic criteria (one of the following):
- PaCO2 ≥ 55 mmHg or PaCO2 of 50-54 mmHg and nocturnal desaturation (oxygen saturation by pulse oximeter ≤ 88% for continuous 5 min while receiving oxygen therapy at 2 L/min)
- PaCO2 of 50-54 mmHg and hospitalization related to recurrent (≥ 2 in a 12-month period) episodes of hypercapnic respiratory failure.
Recommendations
Noninvasive ventilation may be used in patients with recurrent exacerbations who require frequent use of mechanical and noninvasive ventilation during the acute episodes; the patient should be referred to a specialist center for management. (3A)
The choice of the machine for NIV depends on the presence of coexistent sleep apnea syndromes. (UPP)
What are the bronchoscopic techniques useful in stable COPD?
Also known as bronchoscopic lung volume reduction, the use of bronchoscopic techniques to achieve atelectasis of emphysematous lung areas has shown an expansion in the past decade.[409] Various devices are available, ranging from spigots to endobronchial valves to extra anatomical bypass tracts.[410,411,412,413,414] At present, the field is still evolving and these treatments are best carried out in specialist centers.
Recommendation
Bronchoscopic techniques are upcoming modalities of treatment, the data are too sparse to make an evidence-based recommendation.
What are the surgical treatments that can be offered for the treatment of COPD?
There are three primary surgical modalities for the treatment of COPD. These are bullectomy, lung volume reduction surgery, and lung transplantation.
Bullectomy
The presence of large bullae is one of the common findings in patients with COPD. However, extensive data on the natural history of bullae is not available. Generally, enlargement of these bullae occurs with complications like dyspnea, hemoptysis, chest pain, and pneumothorax. Some of the accepted indications for bullectomy are the presence of single large bullae compressing the remaining lung, breathlessness due to the bullae, hemoptysis, and reduction in the FEV1 to < 50%.
LVRS
Though the procedure was proposed a long while ago, LVRS came into vogue after results of the National Emphysema Treatment Trial (NETT) were published in 2003.[415] The proposed mechanisms of improvement of lung function include increased elastic recoil pressure, decrease in the degree of hyperinflation, and decreased regional ventilation perfusion mismatch.[416] The NETT trial divided patients of COPD into four groups, namely upper lobe predominant disease with either low or high exercise capacity and non-upper lobe predominant disease with low or high exercise capacity. There was unequivocal benefit for patients with both upper lobe predominant disease and low exercise capacity, and a clear increase in mortality for those with non-upper lobe predominant disease and high exercise capacity; the risk benefit ratio for the other two groups was equivocal.[415] A Cochrane review of eight trials, which was heavily dominated by the NETT trial, demonstrated almost similar results. In addition, there was an increase in 90-day mortality following LVRS; while long-term QoL, lung function, and exercise capacity were better.[416] The standard accepted indications and contraindications for LVRS are predominantly upper lobe emphysema and low post-rehabilitation exercise capacity.[7,8] The contraindications include FEV1 < 20% predicted or either non-upper lobe predominant emphysema or a very low DLCO (< 20% predicted).
Lung transplantation
Lung transplantation has been carried out since the 1960s; however, it came into routine clinical practice only since the mid-1980s.[417] It is an accepted treatment for COPD, which is the most common indication for the procedure. It has been shown to improve the QoL and exercise capacity; however, survival benefit has not been shown consistently.[418] The accepted indications for lung transplantation include a BODE index of 7-10 with an FEV1 < 20% predicted, or a DLCO < 20% and homogenous emphysema or corpulmonale.[7,8]
Recommendation
Bullectomy may be carried out in properly selected patients in appropriate centers. (3A)
LVRS may be offered to properly selected patients at centers capable of performing the procedure. (1A)
Lung transplantation may be offered to properly selected patients at centers capable of doing the procedure. (1A)
Are influenza and pneumococcal vaccinations useful in patients of COPD?
Immunization with influenza and pneumococcal vaccines is generally considered to be useful. The data to support this practice are sparse. Pneumococcal vaccination has been shown to reduce the risk of pneumococcal bacteremia but not that of pneumonia.[419] Although there are data to support the use of influenza vaccination, it is based on the availability of surveillance data on the type of strains circulating in the community. As this data is lacking in this country, giving firm recommendations is difficult. The recent adult immunization guidelines promulgated by the Association of Physicians in India recommended against immunization for this reason.[420]
Two recent meta-analyses showed that influenza vaccination reduced the number of exacerbations in patients of COPD, especially in patients with severe disease; there was no difference in patients with less severe disease.[421,422] Regarding pneumococcal vaccination, there was a discrepancy between two recent meta-analyses. While a Cochrane review did not demonstrate any reduction in the rate of exacerbations, another meta-analysis showed a significant reduction in the rate of exacerbations of patients with severe COPD.[422,423]
Recommendations
Influenza vaccination is likely to be beneficial in patients with severe COPD and/or frequent exacerbations. (UPP)
Pneumococcal vaccination is likely to be beneficial in patients with severe COPD and/or frequent exacerbations. (UPP)
What is the role of prophylactic antibiotics in COPD?
Although prophylactic antibiotics have been used in COPD for at least 40 years, its benefit of use has only recently been demonstrated.[424] The postulated benefit is a decreased risk of exacerbations while the potential risks include adverse effects of the drugs used, development of resistance, and increased cost. The largest trial available compared azithromycin 250 mg daily with placebo for 1 year. A significant decrease in the time to the first exacerbation as well as in the number of total exacerbations was noted.[425] There was increased risk of colonization with macrolide-resistant organisms and impaired hearing in some patients. Other randomized trials have also shown similar results; however, the numbers studied were not as large, except for one trial which used moxifloxacin.[426,427,428]
The data available are limited, though favorable. Further data would be needed, especially on the impact of the use of antibiotics in a high tuberculosis burden setting and also whether this would lead to increased antibiotic resistance in the community.
Recommendation
Antibiotics should not be prescribed as a routine for the prevention of exacerbations of COPD. (2A)
What should be the advice for patients of COPD regarding air travel?
Patients of COPD are liable to develop complications on exposure to high altitudes, including air travel. Though commercial airliners travel at altitudes varying from 25,000-45,000 feet above sea level; the cabin is usually pressurized to an altitude not above 8,000 feet above sea level. This is associated with a decrease in the partial pressure of oxygen in inspired air. COPD patients who require oxygen and those with borderline oxygen saturation at sea level may need additional oxygen during the flight.[429] Other risks include the expansion of air in large bullae because of the low pressure leading on to pneumothorax.
The methods of assessment of fitness for air travel include the hypoxia challenge test and the use of regression equations. Patients of severe COPD, especially those requiring long-term oxygen therapy and those with large bullae should be assessed by a specialist before air travel.[430] These patients may need additional oxygen during air travel; some airlines provide this facility if informed in advance. Otherwise, patients should be given general advice to carry all their medications and spacers with them.
Recommendation
Patients with severe COPD and those on long-term oxygen therapy should be assessed before air travel by a specialist. (UPP)
Vernacular term for COPD
In view of the common terminology, ‘dama’ used for both COPD and asthma in Indian vernacular languages, there has been a long-felt need to coin a separate identifying name for COPD. The point was discussed at length at the workshop. Of various suggestions, that were put forward the working group found the term ‘kaladama’ (black asthma) as an appropriate choice for COPD to distinguish from dama (bronchial asthma). Similar terminology is in common use for some other diseases in India. The distinct terms will be helpful for patient-doctor interactions and public understanding of COPD as a progressive disease. The readers are welcome to offer suggestions and alternate terms with reasonable explanations. Those who agree with the terminology may also send their views (dr.skjindal@gmail.com).
Footnotes
Source of Support: Indian Chest Society and National College of Chest Physicians (India)
Conflict of Interest: None declared.
REFERENCES
- 1.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt GH, Rennie D, Meade MO, Cook DJ. 2nd ed. New York: McGraw Hill; 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-based Clinical Practice. [Google Scholar]
- 3.Ciba Foundation Guest Symposium. Terminology, definitions and classification of chronic pulmonary emphysema and related conditions. Thorax. 1959;14:286–99. [Google Scholar]
- 4.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:S77–121. [PubMed] [Google Scholar]
- 5.Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J. 1995;8:1398–420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- 6.BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax. 1997;52(Suppl 5):S1–28. [PMC free article] [PubMed] [Google Scholar]
- 7.London: National Clinical Guideline Centre; 2010. National Clinical Guideline Centre. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2013. [Last accessed on 2013 Apr 13]. Available from: http://www.goldcopd.org/
- 9.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 10.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: Systematic review and meta-analysis. Eur Respir J. 2006;28:523–32. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 11.Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: A literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–94. doi: 10.2147/COPD.S32330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvi S. COPD: The neglected epidemic. In: Jindal SK, editor. Textbook of Pulmonary and Critical Care Med. 1st ed. New Delhi: Jaypee Publications; 2011. pp. 971–4. [Google Scholar]
- 13.Jindal SK, Aggarwal AN, Gupta D. A review of population studies from India to estimate national burden of chronic obstructive pulmonary disease and its association with smoking. Indian J Chest Dis Allied Sci. 2001;43:139–47. [PubMed] [Google Scholar]
- 14.Jindal SK. Emergence of chronic obstructive pulmonary disease as an epidemic in India. Indian J Med Res. 2006;124:619–30. [PubMed] [Google Scholar]
- 15.McKay AJ, Mahesh PA, Fordham JZ, Majeed A. Prevalence of COPD in India: A systematic review. Prim Care Respir J. 2012;21:313–21. doi: 10.4104/pcrj.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahesh PA, Jayaraj BS, Prahlad ST, Chaya SK, Prabhakar AK, Agarwal AN, et al. Validation of a structured questionnaire for COPD and prevalence of COPD in rural area of Mysore: A pilot study. Lung India. 2009;26:63–9. doi: 10.4103/0970-2113.53226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. A multicentric study on epidemiology of chronic obstructive pulmonary disease and its relationship with tobacco smoking and environmental tobacco smoke exposure. Indian J Chest Dis Allied Sci. 2006;48:23–9. [PubMed] [Google Scholar]
- 18.Jindal SK, Aggarwal AN, Gupta D, Agarwal R, Kumar R, Kaur T, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH) Int J Tuberc Lung Dis. 2012;16:1270–7. doi: 10.5588/ijtld.12.0005. [DOI] [PubMed] [Google Scholar]
- 19.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 20.Murthy KJ, Sastry JG. Economic burden of chronic obstructive pulmonary disease. In: Rao KS, editor. New Delhi: Burden of Disease in India, National Commission on Macroeconomics and Health; 2005. pp. 264–74. [Google Scholar]
- 21.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Global burden of Disease. Projections of mortality and burden of disease 2004-2030. [Last accessed on 2013 Apr 27]. Available from: http://www.who.int/healthinfo/global_burden_disease/projections/en/index.html .
- 23.Salvi S, Agrawal A. India needs a national COPD prevention and control programme. J Assoc Physicians India. 2012;60(Suppl):5–7. [PubMed] [Google Scholar]
- 24.Ramanakumar AV, Aparajita C. Respiratory Disease Burden in India: Review from multiple data sources. Internet J Epidemiol. 2005;2 DOI: 105580/3ed. [Google Scholar]
- 25.Health Status Maharashtra 2009: A report by the State Health Systems resource Centre. 2010:20–1. [Google Scholar]
- 26.Report on causes of death in India (2001-03) 2009. [Last accessed on 2013 Apr 27]. Available from: http://www.censusindia.gov.in/Vital_Statistics/Summary_Report_Death_01_03.pdf .
- 27.Jindal SK. Estimation of costs of management of smoking related COPD and CHD. Project Report, Indian Council of Medical Research. 1998 [Google Scholar]
- 28.Noncommunicable diseases fact sheet 2011. [Last accessed on 2013 Apr 27]. Available from: http://www.who.int/mediacentre/factsheets/fs355/en/index.html .
- 29.Geneva, Switzerland: WHO; 2011. Global status report on noncommunicable diseases 2010. [Google Scholar]
- 30.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–64. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stang P, Lydick E, Silberman C, Kempel A, Keating ET. The prevalence of COPD: Using smoking rates to estimate disease frequency in the general population. Chest. 2000;117:354–9S. doi: 10.1378/chest.117.5_suppl_2.354s. [DOI] [PubMed] [Google Scholar]
- 32.Chhabra SK, Rajpal S, Gupta R. Patterns of smoking in Delhi and comparison of chronic respiratory morbidity among beedi and cigarette smokers. Indian J Chest Dis Allied Sci. 2001;43:19–26. [PubMed] [Google Scholar]
- 33.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. Tobacco smoking in India: Prevalence, quit-rates and respiratory morbidity. Indian J Chest Dis Allied Sci. 2006;48:37–42. [PubMed] [Google Scholar]
- 34.Kumar R, Prakash S, Kushwah AS, Vijayan VK. Breath carbon monoxide concentration in cigarette and bidi smokers in India. Indian J Chest Dis Allied Sci. 2010;52:19–24. [PubMed] [Google Scholar]
- 35.Singh S, Soumya M, Saini A, Mittal V, Singh UV, Singh V. Breath carbon monoxide levels in different forms of smoking. Indian J Chest Dis Allied Sci. 2011;53:25–8. [PubMed] [Google Scholar]
- 36.Westmaas JL, Brandon TH. Reducing risk in smokers. Curr Opin Pulm Med. 2004;10:284–8. doi: 10.1097/01.mcp.0000128431.72268.4d. [DOI] [PubMed] [Google Scholar]
- 37.Kabat GC. Fifty years’ experience of reduced-tar cigarettes: What do we know about their health effects? Inhal Toxicol. 2003;15:1059–102. doi: 10.1080/08958370390228547. [DOI] [PubMed] [Google Scholar]
- 38.Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315–20. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 40.Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138:3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 41.Semple S, Devakumar D, Fullerton DG, Thorne PS, Metwali N, Costello A, et al. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118:j988–91. doi: 10.1289/ehp.0901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qureshi KA. Domestic smoke pollution and prevalence of chronic bronchitis/asthma in a rural area of Kashmir. Indian J Chest Dis Allied Sci. 1994;36:61–72. [PubMed] [Google Scholar]
- 43.Mathers CD, Lopez AD, Murray CJ. The burden of disease and mortality by condition: Data, methods, and results for 2001. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ, editors. Global Burden of Disease and Risk Factors. Washington DC: The International Bank for Reconstruction and Development/The World Bank Group; 2006. [PubMed] [Google Scholar]
- 44.Behera D, Jindal SK. Respiratory symptoms in Indian women using domestic cooking fuels. Chest. 1991;100:385–8. doi: 10.1378/chest.100.2.385. [DOI] [PubMed] [Google Scholar]
- 45.Kurmi OP, Devereux GS, Smith WC, Semple S, Steiner MF, Simkhada P, et al. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J. 2013;41:25–30. doi: 10.1183/09031936.00220511. [DOI] [PubMed] [Google Scholar]
- 46.Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, et al. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 47.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: Systematic review and meta-analysis. Thorax. 2011;66:232–9. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 48.Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, et al. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167:787–97. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- 49.Blanc PD, Toren K. Occupation in chronic obstructive pulmonary disease and chronic bronchitis: An update. Int J Tuberc Lung Dis. 2007;11:251–7. [PubMed] [Google Scholar]
- 50.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: A study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156:738–46. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 51.Foreman MG, Campos M, Celedon JC. Genes and chronic obstructive pulmonary disease. Med Clin North Am. 2012;96:699–711. doi: 10.1016/j.mcna.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shukla RK, Kant S, Bhattacharya S, Mittal B. Association of cytokine gene polymorphisms in patients with chronic obstructive pulmonary disease. Oman Med J. 2012;27:285–90. doi: 10.5001/omj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shukla RK, Kant S, Bhattacharya S, Mittal B. Association of genetic polymorphism of GSTT1, GSTM1 and GSTM3 in COPD patients in a north Indian population. COPD. 2011;8:167–72. doi: 10.3109/15412555.2011.560128. [DOI] [PubMed] [Google Scholar]
- 54.Chhabra SK, Chhabra P, Rajpal S, Gupta RK. Ambient air pollution and chronic respiratory morbidity in Delhi. Arch Environ Health. 2001;56:58–64. doi: 10.1080/00039890109604055. [DOI] [PubMed] [Google Scholar]
- 55.Pande JN, Bhatta N, Biswas D, Pandey RM, Ahluwalia G, Siddaramaiah NH, et al. Outdoor air pollution and emergency room visits at a hospital in Delhi. Indian J Chest Dis Allied Sci. 2002;44:13–9. [PubMed] [Google Scholar]
- 56.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med. 1989;83:195–8. doi: 10.1016/s0954-6111(89)80031-9. [DOI] [PubMed] [Google Scholar]
- 57.Limaye S, Salvi S. Risk factors for COPD. In: Jindal SK, editor. Textbook of pulmonary and critical care med. 1st ed. New Delhi: Jaypee Publications Pvt. Ltd; 2011. pp. 987–92. [Google Scholar]
- 58.Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126:59–65. doi: 10.1378/chest.126.1.59. [DOI] [PubMed] [Google Scholar]
- 59.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 60.Broekhuizen BD, Sachs AP, Verheij TJ, Janssen KJ, Asma G, Lammers JW, et al. Accuracy of symptoms, signs, and C-reactive protein for early chronic obstructive pulmonary disease. Br J Gen Pract. 2012;62:e632–8. doi: 10.3399/bjgp12X654605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui J, Zhou Y, Tian J, Wang X, Zheng J, Zhong N, et al. A discriminant function model as an alternative method to spirometry for COPD screening in primary care settings in China. J Thorac Dis. 2012;4:594–600. doi: 10.3978/j.issn.2072-1439.2012.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137:1345–53. doi: 10.1378/chest.09-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis. 1990;142:1009–14. doi: 10.1164/ajrccm/142.5.1009. [DOI] [PubMed] [Google Scholar]
- 64.Elliott MW, Adams L, Cockcroft A, MacRae KD, Murphy K, Guz A. The language of breathlessness. Use of verbal descriptors by patients with cardiopulmonary disease. Am Rev Respir Dis. 1991;144:826–32. doi: 10.1164/ajrccm/144.4.826. [DOI] [PubMed] [Google Scholar]
- 65.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2:257–66. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bates DV. Chronic bronchitis and emphysema. N Engl J Med. 1968;278:546–51. doi: 10.1056/NEJM196803072781007. [DOI] [PubMed] [Google Scholar]
- 67.Buist AS, Sexton GJ, Nagy JM, Ross BB. The effect of smoking cessation and modification on lung function. Am Rev Respir Dis. 1976;114:115–22. doi: 10.1164/arrd.1976.114.1.115. [DOI] [PubMed] [Google Scholar]
- 68.Tokuda Y, Miyagi S. Physical diagnosis of chronic obstructive pulmonary disease. Intern Med. 2007;46:1885–91. doi: 10.2169/internalmedicine.46.0455. [DOI] [PubMed] [Google Scholar]
- 69.Lal S, Ferguson AD, Campbell EJ. Forced expiratory time: A simple test for airways obstruction. Br Med J. 1964;1:814–7. doi: 10.1136/bmj.1.5386.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 71.Standardization of Spirometry 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 72.Celli BR, Halbert RJ. Point: Should we abandon FEV (1)/FVC < 0.70 to detect airway obstruction? No. Chest. 2010;138:1037–40. doi: 10.1378/chest.10-2049. [DOI] [PubMed] [Google Scholar]
- 73.Enright P, Brusasco V. Counterpoint: Should we abandon FEV (1)/FVC < 0.70 to detect airway obstruction? Yes. Chest. 2010;138:1040–2. doi: 10.1378/chest.10-2052. [DOI] [PubMed] [Google Scholar]
- 74.Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV 1 /FVC < 0.70 in diagnosing COPD: An evidence-based review. Respir Med. 2011;105:907–15. doi: 10.1016/j.rmed.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Schermer TR, Smeele IJ, Thoonen BP, Lucas AE, Grootens JG, van Boxem TJ, et al. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J. 2008;32:945–52. doi: 10.1183/09031936.00170307. [DOI] [PubMed] [Google Scholar]
- 76.Hwang YI, Kim CH, Kang HR, Shin T, Park SM, Jang SH, et al. Comparison of the prevalence of chronic obstructive pulmonary disease diagnosed by lower limit of normal and fixed ratio criteria. J Korean Med Sci. 2009;24:621–6. doi: 10.3346/jkms.2009.24.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko FW, Woo J, Tam W, Lai CK, Ngai J, Kwok T, et al. Prevalence and risk factors of airflow obstruction in an elderly Chinese population. Eur Respir J. 2008;32:1472–8. doi: 10.1183/09031936.00058708. [DOI] [PubMed] [Google Scholar]
- 78.Vollmer WM, Gislason T, Burney P, Enright PL, Gulsvik A, Kocabas A, et al. Comparison of spirometry criteria for the diagnosis of COPD: Results from the BOLD study. Eur Respir J. 2009;34:588–97. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Padilla R, Hallal PC, Vazquez-Garcia JC, Muino A, Maquez M, Lopez MV, et al. Impact of bronchodilator use on the prevalence of COPD in population-based samples. COPD. 2007;4:113–20. doi: 10.1080/15412550701341012. [DOI] [PubMed] [Google Scholar]
- 80.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: What defines abnormal lung function? Thorax. 2007;62:237–41. doi: 10.1136/thx.2006.068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Rochester CL, Yaggi HK, et al. Chronic obstructive pulmonary disease in older persons: A comparison of two spirometric definitions. Respir Med. 2010;104:1189–96. doi: 10.1016/j.rmed.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22:268–73. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 83.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70% Chest. 2007;131:349–55. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 84.Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. COPD. 2006;3:95–100. doi: 10.1080/15412550600651552. [DOI] [PubMed] [Google Scholar]
- 85.Lau AC, Ip MS, Lai CK, Choo KL, Tang KS, Yam LY, et al. Variability of the prevalence of undiagnosed airflow obstruction in smokers using different diagnostic criteria. Chest. 2008;133:42–8. doi: 10.1378/chest.07-1434. [DOI] [PubMed] [Google Scholar]
- 86.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV 1 /FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–51. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 87.Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest. 2011;139:52–9. doi: 10.1378/chest.10-0189. [DOI] [PubMed] [Google Scholar]
- 88.Aggarwal AN, Gupta D, Agarwal R, Jindal SK. Comparison of the lower confidence limit to the fixed-percentage method for assessing airway obstruction in routine clinical practice. Respir Care. 2011;56:1778–84. doi: 10.4187/respcare.01160. [DOI] [PubMed] [Google Scholar]
- 89.Mannino DM, Diaz-Guzman E. Interpreting lung function data using 80% predicted and fixed thresholds identifies patients at increased risk of mortality. Chest. 2012;141:73–80. doi: 10.1378/chest.11-0797. [DOI] [PubMed] [Google Scholar]
- 90.Akkermans RP, Berrevoets MA, Smeele IJ, Lucas AE, Thoonen BP, Grootens-Stekelenburg JG, et al. Lung function decline in relation to diagnostic criteria for airflow obstruction in respiratory symptomatic subjects. BMC Pulm Med. 2012;12:12. doi: 10.1186/1471-2466-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller MR. Lung function data interpretation. Chest. 2012;141:832–3. doi: 10.1378/chest.11-2718. [DOI] [PubMed] [Google Scholar]
- 92.Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, et al. Underestimation of airflow obstruction among young adults using FEV 1 /FVC < 70% as a fixed cut-off: A longitudinal evaluation of clinical and functional outcomes. Thorax. 2008;63:1040–5. doi: 10.1136/thx.2008.095554. [DOI] [PubMed] [Google Scholar]
- 93.Aggarwal AN, Gupta D, Jindal SK. Development of a simple computer program for spirometry interpretation. J Assoc Physicians India. 2002;50:567–70. [PubMed] [Google Scholar]
- 94.Jindal SK, Wahi PL. Pulmonary function laboratory in the tropics: Needs, problems and solutions. In: Sharma OP, editor. Lung Disease in The Tropics. New York: Marcel Dekker; 1991. pp. 523–42. [Google Scholar]
- 95.Vijayan VK, Kuppurao KV, Venkatesan P, Sankaran K, Prabhakar R. Pulmonary function in healthy young adult Indians in Madras. Thorax. 1990;45:611–5. doi: 10.1136/thx.45.8.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Udwadia FE, Sunavala JD, Shetye VM, Jain PK. The maximal expiratory flow-volume curve in normal subjects in India. Chest. 1986;89:852–6. doi: 10.1378/chest.89.6.852. [DOI] [PubMed] [Google Scholar]
- 97.Chatterjee S, Saha D, Chatterjee BP. Pulmonary function studies in healthy non-smoking men of Calcutta. Ann Hum Biol. 1988;15:365–74. doi: 10.1080/03014468800009841. [DOI] [PubMed] [Google Scholar]
- 98.Chatterjee S, Saha D. Pulmonary function studies in healthy non-smoking women of Calcutta. Ann Hum Biol. 1993;20:31–8. doi: 10.1080/03014469300002472. [DOI] [PubMed] [Google Scholar]
- 99.Lynes D, Frankland K. Reversibility testing in patients with asthma and COPD. Nurs Stand. 2008;23:45–9. doi: 10.7748/ns2008.09.23.3.45.c6673. [DOI] [PubMed] [Google Scholar]
- 100.Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742–50. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 101.Bleecker ER, Emmett A, Crater G, Knobil K, Kalberg C. Lung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium bromide/albuterol in COPD: Response by beta-agonist reversibility. Pulm Pharmacol Ther. 2008;21:682–8. doi: 10.1016/j.pupt.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Celli BR, Tashkin DP, Rennard SI, McElhattan J, Martin UJ. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respir Med. 2011;105:1176–88. doi: 10.1016/j.rmed.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 103.Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–65. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 104.Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006;173:1316–25. doi: 10.1164/rccm.200601-023OC. [DOI] [PubMed] [Google Scholar]
- 105.Kesten S, Rebuck AS. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest. 1994;105:1042–5. doi: 10.1378/chest.105.4.1042. [DOI] [PubMed] [Google Scholar]
- 106.Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest. 2003;123:1441–9. doi: 10.1378/chest.123.5.1441. [DOI] [PubMed] [Google Scholar]
- 107.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67:701–8. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 109.Gorecka D, Bednarek M, Nowinski A, Puscinska E, Goljan-Geremek A, Zielinski J. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123:1916–23. doi: 10.1378/chest.123.6.1916. [DOI] [PubMed] [Google Scholar]
- 110.Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: A randomised clinical trial. Respir Med. 2006;100:2012–7. doi: 10.1016/j.rmed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 111.Wilt TJ, Niewoehner D, Kane RL, MacDonald R, Joseph AM. Spirometry as a motivational tool to improve smoking cessation rates: A systematic review of the literature. Nicotine Tob Res. 2007;9:21–32. doi: 10.1080/14622200601078509. [DOI] [PubMed] [Google Scholar]
- 112.Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:529–34. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 113.Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. Screening for chronic obstructive pulmonary disease using spirometry: Summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:535–43. doi: 10.7326/0003-4819-148-7-200804010-00213. [DOI] [PubMed] [Google Scholar]
- 114.Aggarwal AN, Gupta D, Jindal SK. The relationship between FEV 1 and peak expiratory flow in patients with airways obstruction is poor. Chest. 2006;130:1454–61. doi: 10.1378/chest.130.5.1454. [DOI] [PubMed] [Google Scholar]
- 115.Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: Cross sectional survey. BMJ. 2003;327:653–4. doi: 10.1136/bmj.327.7416.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Llewellin P, Sawyer G, Lewis S, Cheng S, Weatherall M, Fitzharris P, et al. The relationship between FEV 1 and PEF in the assessment of the severity of airways obstruction. Respirology. 2002;7:333–7. doi: 10.1046/j.1440-1843.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 117.Choi IS, Koh YI, Lim H. Peak expiratory flow rate underestimates severity of airflow obstruction in acute asthma. Korean J Intern Med. 2002;17:174–9. doi: 10.3904/kjim.2002.17.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aggarwal AN, Agarwal R, Gupta D, Jindal SK. Use of peak expiratory flow for assessing bronchodilator responsiveness. Prim Care Respir J. 2009;18:50–2. doi: 10.3132/pcrj.2008.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murata GH, Kapsner CO, Lium DJ, Busby HK. Patient compliance with peak flow monitoring in chronic obstructive pulmonary disease. Am J Med Sci. 1998;315:296–301. doi: 10.1097/00000441-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 120.de la Iglesia F, Diaz JL, Pita S, Nicolas R, Ramos V, Pellicer C, et al. Peak expiratory flow rate as predictor of inpatient death in patients with chronic obstructive pulmonary disease. South Med J. 2005;98:266–72. doi: 10.1097/01.SMJ.0000152541.89483.AA. [DOI] [PubMed] [Google Scholar]
- 121.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 122.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 123.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): A prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–8. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 124.Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cote CG, Pinto-Plata VM, Marin JM, Nekach H, Dordelly LJ, Celli BR. The modified BODE index: Validation with mortality in COPD. Eur Respir J. 2008;32:1269–74. doi: 10.1183/09031936.00138507. [DOI] [PubMed] [Google Scholar]
- 126.Halpin DM, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–61. doi: 10.2147/COPD.S34186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax. 2006;61:164–8. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–92. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 129.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–40. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 131.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 132.Kwon N, Amin M, Hui DS, Jung KS, Lim SY, Ta HD, et al. Validity of the Chronic Obstructive Pulmonary Disease Assessment Test (CAT) Translated into Local Languages for Asian Patients. Chest. 2012 doi: 10.1378/chest.12-0535. [DOI] [PubMed] [Google Scholar]
- 133.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weitzenblum E, Chaouat A. Cor pulmonale. Chron Respir Dis. 2009;6:177–85. doi: 10.1177/1479972309104664. [DOI] [PubMed] [Google Scholar]
- 135.Chambellan A, Chailleux E, Similowski T. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128:1201–8. doi: 10.1378/chest.128.3.1201. [DOI] [PubMed] [Google Scholar]
- 136.Gajalakshmi V, Peto R, Kanaka TS, Jha P. Smoking and mortality from tuberculosis and other diseases in India: Retrospective study of 43000 adult male deaths and 35000 controls. Lancet. 2003;362:507–15. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
- 137.Dhamgaye TM. Tobacco smoking and pulmonary tuberculosis: A case-control study. J Indian Med Assoc. 2008;106:216–9. [PubMed] [Google Scholar]
- 138.Kolappan C, Gopi PG. Tobacco smoking and pulmonary tuberculosis. Thorax. 2002;57:964–6. doi: 10.1136/thorax.57.11.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guryay MS, Ceylan E, Gunay T, Karaduman S, Bengi F, Parlak I, et al. Can spirometry, pulse oximetry and dyspnea scoring reflect respiratory failure in patients with chronic obstructive pulmonary disease exacerbation? Med Princ Pract. 2007;16:378–83. doi: 10.1159/000104812. [DOI] [PubMed] [Google Scholar]
- 140.Kelly AM, McAlpine R, Kyle E. How accurate are pulse oximeters in patients with acute exacerbations of chronic obstructive airways disease? Respir Med. 2001;95:336–40. doi: 10.1053/rmed.2001.1046. [DOI] [PubMed] [Google Scholar]
- 141.Sanders C. The radiographic diagnosis of emphysema. Radiol Clin North Am. 1991;29:1019–30. [PubMed] [Google Scholar]
- 142.Simon G, Medvei VC. Chronic bronchitis: Radiological aspects of a five-year follow-up. Thorax. 1962;17:5–8. doi: 10.1136/thx.17.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thurlbeck WM, Simon G. Radiographic appearance of the chest in emphysema. AJR Am J Roentgenol. 1978;130:429–40. doi: 10.2214/ajr.130.3.429. [DOI] [PubMed] [Google Scholar]
- 144.Wallace GM, Winter JH, Winter JE, Taylor A, Taylor TW, Cameron RC. Chest X-rays in COPD screening: Are they worthwhile? Respir Med. 2009;103:1862–5. doi: 10.1016/j.rmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 145.Sverzellati N, Molinari F, Pirronti T, Bonomo L, Spagnolo P, Zompatori M. New insights on COPD imaging via CT and MRI. Int J Chron Obstruct Pulmon Dis. 2007;2:301–12. [PMC free article] [PubMed] [Google Scholar]
- 146.Kurashima K, Takayanagi N, Sato N, Kanauchi T, Hoshi T, Tokunaga D, et al. High resolution CT and bronchial reversibility test for diagnosing COPD. Respirology. 2005;10:316–22. doi: 10.1111/j.1440-1843.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 147.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–92. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 148.Gould GA, MacNee W, McLean A, Warren PM, Redpath A, Best JJ, et al. CT measurements of lung density in life can quantitate distal airspace enlargement: An essential defining feature of human emphysema. Am Rev Respir Dis. 1988;137:380–92. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- 149.McLean A, Warren PM, Gillooly M, MacNee W, Lamb D. Microscopic and macroscopic measurements of emphysema: Relation to carbon monoxide gas transfer. Thorax. 1992;47:144–9. doi: 10.1136/thx.47.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boushy SF, Thompson HK, Jr, North LB, Beale AR, Snow TR. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973;108:1373–83. doi: 10.1164/arrd.1973.108.6.1373. [DOI] [PubMed] [Google Scholar]
- 151.Kanner RE, Renzetti AD, Jr, Stanish WM, Barkman HW, Jr, Klauber MR. Predictors of survival in subjects with chronic airflow limitation. Am J Med. 1983;74:249–55. doi: 10.1016/0002-9343(83)90623-x. [DOI] [PubMed] [Google Scholar]
- 152.Gallagher CG. Exercise limitation and clinical exercise testing in chronic obstructive pulmonary disease. Clin Chest Med. 1994;15:305–26. [PubMed] [Google Scholar]
- 153.Gallagher CG. Exercise and chronic obstructive pulmonary disease. Med Clin North Am. 1990;74:619–41. doi: 10.1016/s0025-7125(16)30542-9. [DOI] [PubMed] [Google Scholar]
- 154.Oelberg DA, Kacmarek RM, Pappagianopoulos PP, Ginns LC, Systrom DM. Ventilatory and cardiovascular responses to inspired He-O2 during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1876–82. doi: 10.1164/ajrccm.158.6.9802015. [DOI] [PubMed] [Google Scholar]
- 155.Richardson RS, Sheldon J, Poole DC, Hopkins SR, Ries AL, Wagner PD. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:881–5. doi: 10.1164/ajrccm.159.3.9803049. [DOI] [PubMed] [Google Scholar]
- 156.Killian KJ, Leblanc P, Martin DH, Summers E, Jones NL, Campbell EJ. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis. 1992;146:935–40. doi: 10.1164/ajrccm/146.4.935. [DOI] [PubMed] [Google Scholar]
- 157.Hamilton AL, Killian KJ, Summers E, Jones NL. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med. 1995;152:2021–31. doi: 10.1164/ajrccm.152.6.8520771. [DOI] [PubMed] [Google Scholar]
- 158.O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: Pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–15. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 159.Bernstein ML, Despars JA, Singh NP, Avalos K, Stansbury DW, Light RW. Reanalysis of the 12-minute walk in patients with chronic obstructive pulmonary disease. Chest. 1994;105:163–7. doi: 10.1378/chest.105.1.163. [DOI] [PubMed] [Google Scholar]
- 160.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1185–9. doi: 10.1164/ajrccm.158.4.9802091. [DOI] [PubMed] [Google Scholar]
- 161.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–64. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 162.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet. 1998;351:773–80. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 163.Spence DP, Hay JG, Carter J, Pearson MG, Calverley PM. Oxygen desaturation and breathlessness during corridor walking in chronic obstructive pulmonary disease: Effect of oxitropium bromide. Thorax. 1993;48:1145–50. doi: 10.1136/thx.48.11.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ray D, Kanagasabapathy AS, Jairaj PS. Alpha 1-Antitrypsin deficiency in Indian patients with chronic obstructive airways and other pulmonary diseases. Ceylon Med J. 1994;39:77–81. [PubMed] [Google Scholar]
- 165.Alpha 1-antitrypsin deficiency: Memorandum from a WHO meeting. Bull World Health Organ. 1997;75:397–415. [PMC free article] [PubMed] [Google Scholar]
- 166.Jones RC, Donaldson GC, Chavannes NH, Kida K, Dickson-Spillmann M, Harding S, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease: The DOSE Index. Am J Respir Crit Care Med. 2009;180:1189–95. doi: 10.1164/rccm.200902-0271OC. [DOI] [PubMed] [Google Scholar]
- 167.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 168.Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Anto JM, Agusti AG, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: The updated BODE index and the ADO index. Lancet. 2009;374:704–11. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
- 169.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–85. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 170.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128:2099–107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 171.Murali Mohan BV, Sen T, Ranganath R. Systemic manifestations of COPD. J Assoc Physicians India. 2012;60(Suppl):44–7. [PubMed] [Google Scholar]
- 172.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–9. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 173.Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249–54. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- 174.Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: A systematic review of the literature. Health Technol Assess. 2001;5:1–149. doi: 10.3310/hta5260. [DOI] [PubMed] [Google Scholar]
- 175.Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–71. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 176.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 177.Tashkin D, Celli B, Kesten S, Lystig T, Decramer M. Effect of tiotropium in men and women with COPD: Results of the 4-year UPLIFT trial. Respir Med. 2010;104:1495–504. doi: 10.1016/j.rmed.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 178.Fukuchi Y, Fernandez L, Kuo HP, Mahayiddin A, Celli B, Decramer M, et al. Efficacy of tiotropium in COPD patients from Asia: A subgroup analysis from the UPLIFT trial. Respirology. 2011;16:825–35. doi: 10.1111/j.1440-1843.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- 179.Tashkin DP, Celli B, Kesten S, Lystig T, Mehra S, Decramer M. Long-term efficacy of tiotropium in relation to smoking status in the UPLIFT trial. Eur Respir J. 2010;35:287–94. doi: 10.1183/09031936.00082909. [DOI] [PubMed] [Google Scholar]
- 180.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD009285. doi: 10.1002/14651858.CD009285.pub2. [DOI] [PubMed] [Google Scholar]
- 181.Suppli Ulrik C. Aclidinium Bromide: Clinical benefit in patients with moderate to severe COPD. Open Respir Med J. 2012;6:150–4. doi: 10.2174/1874306401206010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Joos GF. Aclidinium bromide for the treatment of chronic obstructive pulmonary disease. Drugs Today (Barc) 2012;48:757–63. doi: 10.1358/dot.2012.48.12.1885871. [DOI] [PubMed] [Google Scholar]
- 183.Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: The ATTAIN study. Eur Respir J. 2012;40:830–6. doi: 10.1183/09031936.00225511. [DOI] [PubMed] [Google Scholar]
- 184.Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I) COPD. 2012;9:90–101. doi: 10.3109/15412555.2012.661492. [DOI] [PubMed] [Google Scholar]
- 185.Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:778–84. doi: 10.1164/ajrccm.164.5.2007006. [DOI] [PubMed] [Google Scholar]
- 186.Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1087–92. doi: 10.1164/ajrccm.163.5.9903053. [DOI] [PubMed] [Google Scholar]
- 187.Wadbo M, Lofdahl CG, Larsson K, Skoogh BE, Tornling G, Arwestrom E, et al. Effects of formoterol and ipratropium bromide in COPD: A 3-month placebo-controlled study. Eur Respir J. 2002;20:1138–46. doi: 10.1183/09031936.02.00301702. [DOI] [PubMed] [Google Scholar]
- 188.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium nd ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55:289–94. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–16. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 190.Oostenbrink JB, Rutten-van Molken MP, Al MJ, Van Noord JA, Vincken W. One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. Eur Respir J. 2004;23:241–9. doi: 10.1183/09031936.03.00083703. [DOI] [PubMed] [Google Scholar]
- 191.Zheng JP, Kang J, Cai BQ, Zhou X, Cao ZL, Bai CX, et al. Comparison of tiotropium inhalation capsules and ipratropium metered dose inhaler in a randomized, double-blind, double-dummy, efficacy and safety study in patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:363–7. [PubMed] [Google Scholar]
- 192.Hsu JY, Perng RP, Lu JY, Wu CP, Huang MS, Luh KT, et al. Double-blind randomized parallel group study comparing the efficacy and safety of tiotropium and ipratropium in the treatment of COPD patients in Taiwan. J Formos Med Assoc. 2006;105:708–14. doi: 10.1016/S0929-6646(09)60198-4. [DOI] [PubMed] [Google Scholar]
- 193.Voshaar T, Lapidus R, Maleki-Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomized study of tiotropium Respimat Soft Mist inhaler vs. ipratropium pMDI in COPD. Respir Med. 2008;102:32–41. doi: 10.1016/j.rmed.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 194.Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695–703. doi: 10.1378/chest.130.6.1695. [DOI] [PubMed] [Google Scholar]
- 195.Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–9. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- 196.Lee TA, Pickard AS, Au DH, Bartle B, Weiss KB. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149:380–90. doi: 10.7326/0003-4819-149-6-200809160-00004. [DOI] [PubMed] [Google Scholar]
- 197.Ogale SS, Lee TA, Au DH, Boudreau DM, Sullivan SD. Cardiovascular events associated with ipratropium bromide in COPD. Chest. 2010;137:13–9. doi: 10.1378/chest.08-2367. [DOI] [PubMed] [Google Scholar]
- 198.Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: Part 1: Saskatchewan cohort study. Chest. 2012;142:298–304. doi: 10.1378/chest.10-2499. [DOI] [PubMed] [Google Scholar]
- 199.Wang MT, Tsai CL, Lo YW, Liou JT, Lee WJ, Lai IC. Risk of stroke associated with inhaled ipratropium bromide in chronic obstructive pulmonary disease: A population-based nested case-control study. Int J Cardiol. 2012;158:279–84. doi: 10.1016/j.ijcard.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 200.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA. 2008;300:1439–50. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 201.Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest. 2010;137:20–30. doi: 10.1378/chest.09-0011. [DOI] [PubMed] [Google Scholar]
- 202.Rodrigo GJ, Castro-Rodriguez JA, Nannini LJ, Plaza Moral V, Schiavi EA. Tiotropium and risk for fatal and nonfatal cardiovascular events in patients with chronic obstructive pulmonary disease: Systematic review with meta-analysis. Respir Med. 2009;103:1421–9. doi: 10.1016/j.rmed.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 203.Michele TM, Pinheiro S, Iyasu S. The safety of tiotropium: The FDA's conclusions. N Engl J Med. 2010;363:1097–9. doi: 10.1056/NEJMp1008502. [DOI] [PubMed] [Google Scholar]
- 204.Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, et al. POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 205.Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2011;342:d3215. doi: 10.1136/bmj.d3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan MM. Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD001104. doi: 10.1002/14651858.CD001104.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 208.Rodrigo GJ, Nannini LJ, Rodriguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: A systematic review. Chest. 2008;133:1079–87. doi: 10.1378/chest.07-1167. [DOI] [PubMed] [Google Scholar]
- 209.Vogelmeier C, Ramos-Barbon D, Jack D, Piggott S, Owen R, Higgins M, et al. INTIME study investigators (INdacaterol and TIotropium: Measuring Efficacy) Indacaterol provides 24-hour bronchodilation in COPD: A placebo-controlled blinded comparison with tiotropium. Respir Res. 2010;11:135. doi: 10.1186/1465-9921-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Balint B, Watz H, Amos C, Owen R, Higgins M, Kramer B INSURE Study Investigators. Onset of action of indacaterol in patients with COPD: Comparison with salbutamol and salmeterol-fluticasone. Int J Chron Obstruct Pulmon Dis. 2010;5:311–8. doi: 10.2147/copd.s12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C INSIST study group. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: A 12-week study. Respir Med. 2011;105:719–26. doi: 10.1016/j.rmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 212.Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. INLIGHT-2 (Indacaterol Efficacy Evaluation Using 150-μμg Doses with COPD Patients) study investigators. Once-daily indacaterol versus twice-daily salmeterol for COPD: A placebo-controlled comparison. Eur Respir J. 2011;37:273–9. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 213.Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–9. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 214.Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, et al. INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: Indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155–62. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- 215.Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M, et al. INTENSITY study investigators. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38:797–803. doi: 10.1183/09031936.00191810. [DOI] [PubMed] [Google Scholar]
- 216.Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:477–92. doi: 10.2147/COPD.S23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sestini P, Renzoni E, Robinson S, Poole P, Ram FS. Short-acting beta 2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002:CD001495. doi: 10.1002/14651858.CD001495. [DOI] [PubMed] [Google Scholar]
- 218.Santus P, Centanni S, Morelli N, Di Marco F, Verga M, Cazzola M. Tiotropium is less likely to induce oxygen desaturation in stable COPD patients compared to long-acting beta2-agonists. Respir Med. 2007;101:1798–803. doi: 10.1016/j.rmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 219.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: A meta-analysis. Chest. 2004;125:2309–21. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 220.Cazzola M, Ando F, Santus P, Ruggeri P, Di Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther. 2007;20:556–61. doi: 10.1016/j.pupt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 221.Hawkins NM, Wang D, Petrie MC, Pfeffer MA, Swedberg K, Granger CB, et al. CHARM Investigators and Committees. Baseline characteristics and outcomes of patients with heart failure receiving bronchodilators in the CHARM programme. Eur J Heart Fail. 2010;12:557–65. doi: 10.1093/eurjhf/hfq040. [DOI] [PubMed] [Google Scholar]
- 222.Salpeter SR, Buckley NS, Salpeter EE. Meta-analysis: Anticholinergics, but not beta-agonists, reduce severe exacerbations and respiratory mortality in COPD. J Gen Intern Med. 2006;21:1011–9. doi: 10.1111/j.1525-1497.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65:719–25. doi: 10.1136/thx.2010.136077. [DOI] [PubMed] [Google Scholar]
- 224.Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 225.Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med. 2009;103:542–51. doi: 10.1016/j.rmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 226.Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 227.Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 228.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: A systematic review and metaregression of randomized controlled trials. Chest. 2010;137:318–25. doi: 10.1378/chest.09-1305. [DOI] [PubMed] [Google Scholar]
- 230.Barnes PJ. Inhaled corticosteroids in COPD: A controversy. Respiration. 2010;80:89–95. doi: 10.1159/000315416. [DOI] [PubMed] [Google Scholar]
- 231.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: A meta-analysis. Arch Intern Med. 2009;169:219–29. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 232.Chen D, Restrepo MI, Fine MJ, Pugh MJ, Anzueto A, Metersky ML, et al. Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am J Respir Crit Care Med. 2011;184:312–6. doi: 10.1164/rccm.201012-2070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Malo de Molina R, Mortensen EM, Restrepo MI, Copeland LA, Pugh MJ, Anzueto A. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J. 2010;36:751–7. doi: 10.1183/09031936.00077509. [DOI] [PubMed] [Google Scholar]
- 234.Singanayagam A, Chalmers JD, Akram AR, Hill AT. Impact of inhaled corticosteroid use on outcome in COPD patients admitted with pneumonia. Eur Respir J. 2011;38:36–41. doi: 10.1183/09031936.00077010. [DOI] [PubMed] [Google Scholar]
- 235.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: Systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 236.Vogelmeier C, Fabbri LM, Rabe KF, Beeh KM, Schmidt H, Metzdorf N, et al. Effect of tiotropium vs. salmeterol on exacerbations: GOLD II and maintenance therapy naive patients. Respir Med. 2013;107:75–83. doi: 10.1016/j.rmed.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 237.Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD009157. doi: 10.1002/14651858.CD009157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long-acting beta (2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011:CD007033. doi: 10.1002/14651858.CD007033.pub2. [DOI] [PubMed] [Google Scholar]
- 239.Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD001387. doi: 10.1002/14651858.CD001387.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Karner C, Cates CJ. Long-acting beta (2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta (2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;4:CD008989. doi: 10.1002/14651858.CD008989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang J, Jin D, Zuo P, Wang T, Xu Y, Xiong W. Comparison of tiotropium plus formoterol to tiotropium alone in stable chronic obstructive pulmonary disease: A meta-analysis. Respirology. 2011;16:350–8. doi: 10.1111/j.1440-1843.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 242.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 243.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta (2)-agonist in one inhaler versus long-acting beta (2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kliber A, Lynd LD, Sin DD. The effects of long-acting bronchodilators on total mortality in patients with stable chronic obstructive pulmonary disease. Respir Res. 2010;11:56. doi: 10.1186/1465-9921-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA INSPIRE Investigators. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 246.Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long-acting beta2-agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010:CD007891. doi: 10.1002/14651858.CD007891.pub2. [DOI] [PubMed] [Google Scholar]
- 247.Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2007;146:545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 248.Singh D, Brooks J, Hagan G, Cahn A, O’Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63:592–8. doi: 10.1136/thx.2007.087213. [DOI] [PubMed] [Google Scholar]
- 249.Fang LZ, Liang X, Zhang JQ, Liu L, Fu WP, Zhao ZH, et al. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31:811–4. [PubMed] [Google Scholar]
- 250.Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741–50. doi: 10.1164/rccm.200904-0492OC. [DOI] [PubMed] [Google Scholar]
- 251.Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: A randomized controlled study. Respir Med. 2012;106:382–9. doi: 10.1016/j.rmed.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 252.Hanania NA, Crater GD, Morris AN, Emmett AH, O’Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106:91–101. doi: 10.1016/j.rmed.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 253.Chatterjee A, Shah M, D’souza AO, Bechtel B, Crater G, Dalal AA. Observational study on the impact of initiating tiotropium alone versus tiotropium with fluticasone propionate/salmeterol combination therapy on outcomes and costs in chronic obstructive pulmonary disease. Respir Res. 2012;13:15. doi: 10.1186/1465-9921-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Perng DW, Wu CC, Su KC, Lee YC, Perng RP, Tao CW. Additive benefits of tiotropium in COPD patients treated with long-acting beta agonists and corticosteroids. Respirology. 2006;11:598–602. doi: 10.1111/j.1440-1843.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 255.Gaebel K, McIvor RA, Xie F, Blackhouse G, Robertson D, Assasi N, et al. Triple therapy for the management of COPD: A review. COPD. 2011;8:206–43. doi: 10.3109/15412555.2011.560131. [DOI] [PubMed] [Google Scholar]
- 256.Barnes PJ. Theophylline: New perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–8. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- 257.Ram FS. Use of theophylline in chronic obstructive pulmonary disease: Examining the evidence. Curr Opin Pulm Med. 2006;12:132–9. doi: 10.1097/01.mcp.0000208453.95972.e2. [DOI] [PubMed] [Google Scholar]
- 258.Zhou Y, Wang X, Zeng X, Qiu R, Xie J, Liu S, et al. Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year. Respirology. 2006;11:603–10. doi: 10.1111/j.1440-1843.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 259.Makino S, Adachi M, Ohta K, Kihara N, Nakajima S, Nishima S, et al. Safety of Sustained-Release Theophylline and Injectable Methylxanthines Committee; Asthma Prevention and Management Guidelines Committee. A prospective survey on safety of sustained-release theophylline in treatment of asthma and COPD. Allergol Int. 2006;55:395–402. doi: 10.2332/allergolint.55.395. [DOI] [PubMed] [Google Scholar]
- 260.Di Lorenzo G, Morici G, Drago A, Pellitteri ME, Mansueto P, Melluso M, et al. Efficacy, tolerability, and effects on quality of life of inhaled salmeterol and oral theophylline in patients with mild-to-moderate chronic obstructive pulmonary disease. SLMT02 Italian Study Group. Clin Ther. 1998;20:1130–48. doi: 10.1016/s0149-2918(98)80109-4. [DOI] [PubMed] [Google Scholar]
- 261.Broseghini C, Testi R, Polese G, Tosatto R, Rossi A. A comparison between inhaled salmeterol and theophylline in the short-term treatment of stable chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2005;18:103–8. doi: 10.1016/j.pupt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 262.Cazzola M, Noschese P, Centanni S, Santus P, Di Marco F, Spicuzza L, et al. Salmeterol/fluticasone propionate in a Single Inhaler Device versus theophylline + fluticasone propionate in patients with COPD. Pulm Pharmacol Ther. 2004;17:141–5. doi: 10.1016/j.pupt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 263.Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, et al. Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058–69. doi: 10.1378/chest.121.4.1058. [DOI] [PubMed] [Google Scholar]
- 264.Cazzola M, Gabriella Matera M. The additive effect of theophylline on a combination of formoterol and tiotropium in stable COPD: A pilot study. Respir Med. 2007;101:957–62. doi: 10.1016/j.rmed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 265.Shukla D, Chakraborty S, Singh S, Mishra B. Doxofylline: A promising methylxanthine derivative for the treatment of asthma and chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2009;10:2343–56. doi: 10.1517/14656560903200667. [DOI] [PubMed] [Google Scholar]
- 266.Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast: An oral anti-inflammatory treatment for chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2005;366:563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 267.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–61. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 268.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet. 2009;374:685–94. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 269.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: Two randomised clinical trials. Lancet. 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 270.Wedzicha JA, Rabe KF, Martinez FJ, Bredenbroker D, Brose M, Goehring UM, et al. Efficacy of roflumilast in the chronic obstructive pulmonary disease frequent exacerbator phenotype. Chest. 2013;143:1302–11. doi: 10.1378/chest.12-1489. [DOI] [PubMed] [Google Scholar]
- 271.Chong J, Poole P, Leung B, Black PN. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011:CD002309. doi: 10.1002/14651858.CD002309.pub3. [DOI] [PubMed] [Google Scholar]
- 272.Lee SD, Hui DS, Mahayiddin AA, Roa CC, Jr, Kwa KH, Goehring UM, et al. Roflumilast in Asian patients with COPD: A randomized placebo-controlled trial. Respirology. 2011;16:1249–57. doi: 10.1111/j.1440-1843.2011.02038.x. [DOI] [PubMed] [Google Scholar]
- 273.Clarke SW, Thomson ML, Pavia D. Effect of mucolytic and expectorant drugs on tracheobronchial clearance in chronic bronchitis. Eur J Respir Dis Suppl. 1980;110:179–91. [PubMed] [Google Scholar]
- 274.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet. 2005;365:1552–60. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 275.Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;8:CD001287. doi: 10.1002/14651858.CD001287.pub4. [DOI] [PubMed] [Google Scholar]
- 276.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 277.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29:1224–38. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 278.Burge S, Wedzicha JA. COPD exacerbations: Definitions and classifications. Eur Respir J Suppl. 2003;41:46–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 279.Wedzicha JA, Seemungal TA. COPD exacerbations: Defining their cause and prevention. Lancet. 2007;370:786–96. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–31. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Miravitlles M, Murio C, Guerrero T, Gisbert R DAFNE Study Group. Decisiones sobre Antibioticoterapia y Farmacoeconomía en la EPOC. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121:1449–55. doi: 10.1378/chest.121.5.1449. [DOI] [PubMed] [Google Scholar]
- 282.Suissa S, Dell’aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67:957–63. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1653–9. doi: 10.1164/rccm.201009-1535OC. [DOI] [PubMed] [Google Scholar]
- 285.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 286.Kessler R, Stahl E, Vogelmeier C, Haughney J, Trudeau E, Lofdahl CG, et al. Patient understanding, detection, and experience of COPD exacerbations: An observational, interview-based study. Chest. 2006;130:133–42. doi: 10.1378/chest.130.1.133. [DOI] [PubMed] [Google Scholar]
- 287.Wouters EF. The burden of COPD in The Netherlands: Results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S51–9. doi: 10.1016/s0954-6111(03)80025-2. [DOI] [PubMed] [Google Scholar]
- 288.Gunen H, Hacievliyagil SS, Kosar F, Mutlu LC, Gulbas G, Pehlivan E, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J. 2005;26:234–41. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 289.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–21. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 290.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–51. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: A state-of-the-art review. Clin Microbiol Rev. 2001;14:336–63. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease. 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax. 2003;58:73–80. doi: 10.1136/thorax.58.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rohde G, Wiethege A, Borg I, Kauth M, Bauer TT, Gillissen A, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: A case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Seemungal TA, Wedzicha JA. Viral infections in obstructive airway diseases. Curr Opin Pulm Med. 2003;9:111–6. doi: 10.1097/00063198-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 295.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–71. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 296.Rosell A, Monso E, Soler N, Torres F, Angrill J, Riise G, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:891–7. doi: 10.1001/archinte.165.8.891. [DOI] [PubMed] [Google Scholar]
- 297.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. Haemophilus haemolyticus: A human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007;195:81–9. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 298.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–60. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 299.Nordenhall C, Pourazar J, Blomberg A, Levin JO, Sandstrom T, Adelroth E. Airway inflammation following exposure to diesel exhaust: A study of time kinetics using induced sputum. Eur Respir J. 2000;15:1046–51. doi: 10.1034/j.1399-3003.2000.01512.x. [DOI] [PubMed] [Google Scholar]
- 300.Rudell B, Blomberg A, Helleday R, Ledin MC, Lundback B, Stjernberg N, et al. Bronchoalveolar inflammation after exposure to diesel exhaust: Comparison between unfiltered and particle trap filtered exhaust. Occup Environ Med. 1999;56:527–34. doi: 10.1136/oem.56.8.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Garcia-Aymerich J, Tobias A, Anto JM, Sunyer J. Air pollution and mortality in a cohort of patients with chronic obstructive pulmonary disease: A time series analysis. J Epidemiol Community Health. 2000;54:73–4. doi: 10.1136/jech.54.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Anto JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: A case-crossover analysis. Am J Epidemiol. 2000;151:50–6. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- 303.Adams SG, Melo J, Luther M, Anzueto A. Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPD. Chest. 2000;117:1345–52. doi: 10.1378/chest.117.5.1345. [DOI] [PubMed] [Google Scholar]
- 304.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–13. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 305.Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: A systematic review and metaanalysis. Chest. 2009;135:786–93. doi: 10.1378/chest.08-1516. [DOI] [PubMed] [Google Scholar]
- 306.Rodriguez-Roisin R. COPD exacerbations. 5: Management. Thorax. 2006;61:535–44. doi: 10.1136/thx.2005.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Abbatecola AM, Fumagalli A, Bonardi D, Guffanti EE. Practical management problems of chronic obstructive pulmonary disease in the elderly: Acute exacerbations. Curr Opin Pulm Med. 2011;17(Suppl 1):S49–54. doi: 10.1097/01.mcp.0000410748.28582.22. [DOI] [PubMed] [Google Scholar]
- 308.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 309.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–65. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 310.Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: Results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–8. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 311.Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM. 2010;103:817–29. doi: 10.1093/qjmed/hcq126. [DOI] [PubMed] [Google Scholar]
- 312.Chang CL, Sullivan GD, Karalus NC, Mills GD, McLachlan JD, Hancox RJ. Predicting early mortality in acute exacerbation of chronic obstructive pulmonary disease using the CURB65 score. Respirology. 2011;16:146–51. doi: 10.1111/j.1440-1843.2010.01866.x. [DOI] [PubMed] [Google Scholar]
- 313.Tabak YP, Sun X, Johannes RS, Gupta V, Shorr AF. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: Development and validation of a simple risk score. Arch Intern Med. 2009;169:1595–602. doi: 10.1001/archinternmed.2009.270. [DOI] [PubMed] [Google Scholar]
- 314.Shorr AF, Sun X, Johannes RS, Yaitanes A, Tabak YP. Validation of a novel risk score for severity of illness in acute exacerbations of COPD. Chest. 2011;140:1177–83. doi: 10.1378/chest.10-3035. [DOI] [PubMed] [Google Scholar]
- 315.Shorr AF, Sun X, Johannes RS, Derby KG, Tabak YP. Predicting the need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: Comparing the CURB-65 and BAP-65 scores. J Crit Care. 2012;27:564–70. doi: 10.1016/j.jcrc.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 316.National Clinical Guideline Centre (UK). Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. 2010 [Google Scholar]
- 317.Brulotte CA, Lang ES. Acute exacerbations of chronic obstructive pulmonary disease in the emergency department. Emerg Med Clin North Am. 2012;30:223–47. doi: 10.1016/j.emc.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 318.O’Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, et al. Canadian thoracic society recommendations for management of chronic obstructive pulmonary disease-2008 update-highlights for primary care. Can Respir J. 2008;15(Suppl A):1–8A. doi: 10.1155/2008/641965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Turner MO, Patel A, Ginsburg S, FitzGerald JM. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736–44. [PubMed] [Google Scholar]
- 320.McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002:CD003900. doi: 10.1002/14651858.CD003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest. 2005;128:48–54. doi: 10.1378/chest.128.1.48. [DOI] [PubMed] [Google Scholar]
- 322.Emerman CL, Cydulka RK. Effect of different albuterol dosing regimens in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1997;29:474–8. doi: 10.1016/s0196-0644(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 323.Turner MO, Gafni A, Swan D, FitzGerald JM. A review and economic evaluation of bronchodilator delivery methods in hospitalized patients. Arch Intern Med. 1996;156:2113–8. [PubMed] [Google Scholar]
- 324.Barr RG, Rowe BH, Camargo CA. Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003:CD002168. doi: 10.1002/14651858.CD002168. [DOI] [PubMed] [Google Scholar]
- 325.Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA, Jr MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: The Multicenter Airway Research Collaboration. J Am Geriatr Soc. 2003;51:908–16. doi: 10.1046/j.1365-2389.2003.51302.x. [DOI] [PubMed] [Google Scholar]
- 326.Skorodin MS, Tenholder MF, Yetter B, Owen KA, Waller RF, Khandelwahl S, et al. Magnesium sulfate in exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 1995;155:496–500. [PubMed] [Google Scholar]
- 327.Abreu Gonzalez J, Hernandez Garcia C, Abreu Gonzalez P, Martin Garcia C, Jimenez A. Effect of intravenous magnesium sulfate on chronic obstructive pulmonary disease exacerbations requiring hospitalization: A randomized placebo-controlled trial. Arch Bronconeumol. 2006;42:384–7. doi: 10.1016/s1579-2129(06)60551-x. [DOI] [PubMed] [Google Scholar]
- 328.Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009:CD001288. doi: 10.1002/14651858.CD001288.pub2. [DOI] [PubMed] [Google Scholar]
- 329.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–67. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 330.Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, In E. The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J. 2007;29:660–7. doi: 10.1183/09031936.00073506. [DOI] [PubMed] [Google Scholar]
- 331.Maltais F, Ostinelli J, Bourbeau J, Tonnel AB, Jacquemet N, Haddon J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: A randomized controlled trial. Am J Respir Crit Care Med. 2002;165:698–703. doi: 10.1164/ajrccm.165.5.2109093. [DOI] [PubMed] [Google Scholar]
- 332.Stallberg B, Selroos O, Vogelmeier C, Andersson E, Ekstrom T, Larsson K. Budesonide/formoterol as effective as prednisolone plus formoterol in acute exacerbations of COPD. A double-blind, randomised, non-inferiority, parallel-group, multicentre study. Respir Res. 2009;10:11. doi: 10.1186/1465-9921-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257. doi: 10.1002/14651858.CD010257. [DOI] [PubMed] [Google Scholar]
- 334.Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: A randomised placebo-controlled trial. Lancet. 2001;358:2020–5. doi: 10.1016/S0140-6736(01)07097-0. [DOI] [PubMed] [Google Scholar]
- 335.Llor C, Moragas A, Hernandez S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:716–23. doi: 10.1164/rccm.201206-0996OC. [DOI] [PubMed] [Google Scholar]
- 336.Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: A metaanalysis of randomized controlled trials. Chest. 2007;132:447–55. doi: 10.1378/chest.07-0149. [DOI] [PubMed] [Google Scholar]
- 337.Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos Short-versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: A meta-analysis. J Antimicrob Chemother. 2008;62:442–50. doi: 10.1093/jac/dkn201. [DOI] [PubMed] [Google Scholar]
- 338.Chang KC, Leung CC, Yew WW, Lau TY, Leung WM, Tam CM, et al. Newer fluoroquinolones for treating respiratory infection: Do they mask tuberculosis? Eur Respir J. 2010;35:606–13. doi: 10.1183/09031936.00104209. [DOI] [PubMed] [Google Scholar]
- 339.Chen TC, Lu PL, Lin CY, Lin WR, Chen YH. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: A systematic review and meta-analysis. Int J Infect Dis. 2011;15:e211–6. doi: 10.1016/j.ijid.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 340.Devasia RA, Blackman A, Gebretsadik T, Griffin M, Shintani A, May C, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis: The effect of duration and timing of fluoroquinolone exposure. Am J Respir Crit Care Med. 2009;180:365–70. doi: 10.1164/rccm.200901-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Long R, Chong H, Hoeppner V, Shanmuganathan H, Kowalewska-Grochowska K, Shandro C, et al. Empirical treatment of community-acquired pneumonia and the development of fluoroquinolone-resistant tuberculosis. Clin Infect Dis. 2009;48:1354–60. doi: 10.1086/598196. [DOI] [PubMed] [Google Scholar]
- 342.Wang JY, Hsueh PR, Jan IS, Lee LN, Liaw YS, Yang PC, et al. Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas. Thorax. 2006;61:903–8. doi: 10.1136/thx.2005.056887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani GC, et al. Pneumonia Guidelines Working Group. Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: Joint ICS/NCCP (I) recommendations. Lung India. 2012;29:S27–62. doi: 10.4103/0970-2113.99248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Schuetz P, Muller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;9:CD007498. doi: 10.1002/14651858.CD007498.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin And Survival Study (PASS) Group. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: A randomized trial. Crit Care Med. 2011;39:2048–58. doi: 10.1097/CCM.0b013e31821e8791. [DOI] [PubMed] [Google Scholar]
- 346.Kory RC, Bergmann JC, Sweet RD, Smith JR. Comparative evaluation of oxygen therapy techniques. JAMA. 1962;179:767–72. doi: 10.1001/jama.1962.03050100021005. [DOI] [PubMed] [Google Scholar]
- 347.Leigh JM. Variation in performance of oxygen therapy devices. Anaesthesia. 1970;25:210–22. doi: 10.1111/j.1365-2044.1970.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 348.Agarwal R, Gupta R, Aggarwal AN, Gupta D. Noninvasive positive pressure ventilation in acute respiratory failure due to COPD vs other causes: Effectiveness and predictors of failure in a respiratory ICU in North India. Int J Chron Obstruct Pulmon Dis. 2008;3:737–43. doi: 10.2147/copd.s3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Brochard L, Mancebo J, Elliott MW. Noninvasive ventilation for acute respiratory failure. Eur Respir J. 2002;19:712–21. doi: 10.1183/09031936.02.00295502. [DOI] [PubMed] [Google Scholar]
- 350.Carlucci A, Delmastro M, Rubini F, Fracchia C, Nava S. Changes in the practice of non-invasive ventilation in treating COPD patients over 8 years. Intensive Care Med. 2003;29:419–25. doi: 10.1007/s00134-002-1574-1. [DOI] [PubMed] [Google Scholar]
- 351.Khilnani GC, Saikia N, Banga A, Sharma SK. Non-invasive ventilation for acute exacerbation of COPD with very high PaCO (2): A randomized controlled trial. Lung India. 2010;27:125–30. doi: 10.4103/0970-2113.68308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Zhu GF, Zhang W, Zong H, Xu QF, Liang Y. Effectiveness and safety of noninvasive positive-pressure ventilation for severe hypercapnic encephalopathy due to acute exacerbation of chronic obstructive pulmonary disease: A prospective case-control study. Chin Med J (Engl) 2007;120:2204–9. [PubMed] [Google Scholar]
- 353.Burns KE, Adhikari NK, Keenan SP, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127. doi: 10.1002/14651858.CD004127.pub2. [DOI] [PubMed] [Google Scholar]
- 354.Prasad SB, Chaudhry D, Khanna R. Role of noninvasive ventilation in weaning from mechanical ventilation in patients of chronic obstructive pulmonary disease: An Indian experience. Indian J Crit Care Med. 2009;13:207–12. doi: 10.4103/0972-5229.60173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sharma S, Agarwal R, Aggarwal AN, Gupta D, Jindal SK. A survey of noninvasive ventilation practices in a respiratory ICU of North India. Respir Care. 2012;57:1145–53. doi: 10.4187/respcare.01541. [DOI] [PubMed] [Google Scholar]
- 356.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, et al. Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA. 2002;287:345–55. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 357.Wildman MJ, Sanderson C, Groves J, Reeves BC, Ayres J, Harrison D, et al. Implications of prognostic pessimism in patients with chronic obstructive pulmonary disease (COPD) or asthma admitted to intensive care in the UK within the COPD and asthma outcome study (CAOS): Multicentre observational cohort study. BMJ. 2007;335:1132. doi: 10.1136/bmj.39371.524271.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Mushlin AI, Black ER, Connolly CA, Buonaccorso KM, Eberly SW. The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991;266:80–3. [PubMed] [Google Scholar]
- 359.Regueiro CR, Hamel MB, Davis RB, Desbiens N, Connors AF, Jr, Phillips RS. A comparison of generalist and pulmonologist care for patients hospitalized with severe chronic obstructive pulmonary disease: Resource intensity, hospital costs, and survival. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Am J Med. 1998;105:366–72. doi: 10.1016/s0002-9343(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 360.Gravil JH, Al-Rawas OA, Cotton MM, Flanigan U, Irwin A, Stevenson RD. Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment service. Lancet. 1998;351:1853–5. doi: 10.1016/s0140-6736(97)11048-0. [DOI] [PubMed] [Google Scholar]
- 361.Jindal SK, Gupta D, Aggarwal AN. WHO-Government of India Biennium (2002-2003) Programme. Guidelines for management of chronic obstructive pulmonary disease (COPD) in India: A guide for physicians (2003) Indian J Chest Dis Allied Sci. 2004;46:137–53. [PubMed] [Google Scholar]
- 362.Au DH, Bryson CL, Chien JW, Sun H, Udris EM, Evans LE, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457–63. doi: 10.1007/s11606-009-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.National Tobacco Control Programme DGoHS, Ministry of Health and Family Welfare, Government of India; 2011. Tobacco dependence treatment guidelines. [Google Scholar]
- 364.Jindal SK. New Delhi: Vitasta Publishing Pvt Ltd; 2008. Quit Smoking: Why and How. [Google Scholar]
- 365.Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. doi: 10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- 366.Galanti LM. Tobacco smoking cessation management: Integrating varenicline in current practice. Vasc Health Risk Manag. 2008;4:837–45. doi: 10.2147/vhrm.s3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: A meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–44. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser. 2012;12:1–50. [PMC free article] [PubMed] [Google Scholar]
- 369.Singh P, Kumar R. Assessment of the effectiveness of sustained release Bupropion and intensive physician advice in smoking cessation. Lung India. 2010;27:11–8. doi: 10.4103/0970-2113.59262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kumar R, Kushwah AS, Mahakud GC, Prakash S, Vijayan VK. Smoking cessation interventions and continuous abstinence rate at one year. Indian J Chest Dis Allied Sci. 2007;49:201–7. [Google Scholar]
- 371.Jindal SK, Malik SK. Smoking index-a measure to quantify cumulative smoking exposure. Lung India. 1988;6:195–6. [Google Scholar]
- 372.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 373.Nici L, Lareau S, ZuWallack R. Pulmonary rehabilitation in the treatment of chronic obstructive pulmonary disease. Am Fam Physician. 2010;82:655–60. [PubMed] [Google Scholar]
- 374.Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, Brooks D. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease: A systematic review. Chron Respir Dis. 2011;8:129–40. doi: 10.1177/1479972311404256. [DOI] [PubMed] [Google Scholar]
- 375.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 376.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011:CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 377.Puhan MA, Schunemann HJ, Frey M, Scharplatz M, Bachmann LM. How should COPD patients exercise during respiratory rehabilitation? Comparison of exercise modalities and intensities to treat skeletal muscle dysfunction. Thorax. 2005;60:367–75. doi: 10.1136/thx.2004.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Beauchamp MK, Nonoyama M, Goldstein RS, Hill K, Dolmage TE, Mathur S, et al. Interval versus continuous training in individuals with chronic obstructive pulmonary disease: A systematic review. Thorax. 2010;65:157–64. doi: 10.1136/thx.2009.123000. [DOI] [PubMed] [Google Scholar]
- 379.O’Brien K, Geddes EL, Reid WD, Brooks D, Crowe J. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: A systematic review update. J Cardiopulm Rehabil Prev. 2008;28:128–41. doi: 10.1097/01.HCR.0000314208.40170.00. [DOI] [PubMed] [Google Scholar]
- 380.Subin, Rao V, Prem V, Sahoo Effect of upper limb, lower limb and combined training on health-related quality of life in COPD. Lung India. 2010;27:4–7. doi: 10.4103/0970-2113.59260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Singh V, Khandelwal DC, Khandelwal R, Abusaria S. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2003;45:13–7. [PubMed] [Google Scholar]
- 382.Pande A, Singhal P, Kumar R, Gaur SN. Effect of home-based pulmonary rehabilitation programme on disability in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2005;47:217–9. [PubMed] [Google Scholar]
- 383.Katiyar SK, Bihari S. Role of Pranayama in rehabilitation of COPD patients-A randomized controlled study. Indian J Allergy Asthma Immunol. 2006;20:98–104. [Google Scholar]
- 384.Gothi D, Joshi JM. Pulmonary rehabilitation in resource poor settings. Indian J Chest Dis Allied Sci. 2011;53:163–72. [PubMed] [Google Scholar]
- 385.Gupta B, Kant S, Mishra R. Subjective global assessment of nutritional status of chronic obstructive pulmonary disease patients on admission. Int J Tuberc Lung Dis. 2010;14:500–5. [PubMed] [Google Scholar]
- 386.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131:4–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 387.Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi: 10.1002/14651858.CD000998.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Force SD, Choong C, Meyers BF. Lung transplantation for emphysema. Chest Surg Clin N Am. 2003;13:651–67. doi: 10.1016/s1052-3359(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 389.Is 12-hour oxygen as effective as 24-hour oxygen in advanced chronic obstructive pulmonary disease with hypoxemia? (The nocturnal oxygen therapy trial--NOTT) Chest. 1980;78:419–20. doi: 10.1378/chest.78.3.419. [DOI] [PubMed] [Google Scholar]
- 390.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 391.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 392.Jindal SK. Hypoxemia and goals of oxygen therapy. In: Jindal SK, Agarwal R, editors. Oxygen therapy. New Delhi: Jaypee Publications; 2008. pp. 143–51. [Google Scholar]
- 393.Make B, Krachman S, Panos RJ, Doherty DE, Stoller JK. Oxygen therapy in advanced COPD: In whom does it work? Semin Respir Crit Care Med. 2010;31:334–42. doi: 10.1055/s-0030-1254073. [DOI] [PubMed] [Google Scholar]
- 394.Guell Rous R. Long-term oxygen therapy: Are we prescribing appropriately? Int J Chron Obstruct Pulmon Dis. 2008;3:231–7. doi: 10.2147/copd.s1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: Current evidence and the long-term oxygen treatment trial. Chest. 2010;138:179–87. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Jindal SK, Agarwal R. Long-term oxygen therapy. Expert Rev Respir Med. 2012;6:639–49. doi: 10.1586/ers.12.69. [DOI] [PubMed] [Google Scholar]
- 397.Bradley JM, O’Neill B. Short-term ambulatory oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005:CD004356. doi: 10.1002/14651858.CD004356.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005:CD001744. doi: 10.1002/14651858.CD001744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Uronis H, McCrory DC, Samsa G, Currow D, Abernethy A. Symptomatic oxygen for non-hypoxaemic chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011:CD006429. doi: 10.1002/14651858.CD006429.pub2. [DOI] [PubMed] [Google Scholar]
- 400.Jindal SK, Agarwal R. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2008. Oxygen therapy. [Google Scholar]
- 401.Khilnani GC, Bhatta N. Non-invasive ventilation: Current status. Natl Med J India. 2002;15:269–74. [PubMed] [Google Scholar]
- 402.Khilnani GC, Banga A. Noninvasive ventilation in patients with chronic obstructive airway disease. Int J Chron Obstruct Pulmon Dis. 2008;3:351–7. doi: 10.2147/copd.s946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Diaz-Lobato S, Alises SM, Rodriguez EP. Current status of noninvasive ventilation in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2006;1:129–35. doi: 10.2147/copd.2006.1.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Nickol AH, Hart N, Hopkinson NS, Hamnegard CH, Moxham J, Simonds A, et al. Mechanisms of improvement of respiratory failure in patients with COPD treated with NIV. Int J Chron Obstruct Pulmon Dis. 2008;3:453–62. doi: 10.2147/copd.s2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wijkstra PJ, Lacasse Y, Guyatt GH, Goldstein R. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002:CD002878. doi: 10.1002/14651858.CD002878. [DOI] [PubMed] [Google Scholar]
- 406.Kolodziej MA, Jensen L, Rowe B, Sin D. Systematic review of noninvasive positive pressure ventilation in severe stable COPD. Eur Respir J. 2007;30:293–306. doi: 10.1183/09031936.00145106. [DOI] [PubMed] [Google Scholar]
- 407.Group CW. Noninvasive positive pressure ventilation for chronic respiratory failure patients with stable chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser. 2012;12:1–51. [PMC free article] [PubMed] [Google Scholar]
- 408.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation: A consensus conference report. Chest. 1999;116:521–34. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 409.Gasparini S, Zuccatosta L, Bonifazi M, Bolliger CT. Bronchoscopic treatment of emphysema: State of the art. Respiration. 2012;84:250–63. doi: 10.1159/000341171. [DOI] [PubMed] [Google Scholar]
- 410.Leroy S, Marquette CH. VENT: International study of bronchoscopic lung volume reduction as a palliative treatment for emphysema. Rev Mal Respir. 2004;21:1144–52. doi: 10.1016/s0761-8425(04)71590-9. [DOI] [PubMed] [Google Scholar]
- 411.Reilly J, Washko G, Pinto-Plata V, Velez E, Kenney L, Berger R, et al. Biological lung volume reduction: A new bronchoscopic therapy for advanced emphysema. Chest. 2007;131:1108–13. doi: 10.1378/chest.06-1754. [DOI] [PubMed] [Google Scholar]
- 412.Strange C, Herth FJ, Kovitz KL, McLennan G, Ernst A, Goldin J, et al. VENT Study Group. Design of the Endobronchial Valve for Emphysema Palliation Trial (VENT): A non-surgical method of lung volume reduction. BMC Pulm Med. 2007;7:10. doi: 10.1186/1471-2466-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ernst A, Anantham D. Bronchoscopic lung volume reduction. Pulm Med 2011. 2011:610802. doi: 10.1155/2011/610802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Venuta F, Diso D, Anile M, De Giacomo T, Rendina EA, Rolla M, et al. Bronchoscopic lung volume reduction as a bridge to lung transplantation in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2011;39:364–7. doi: 10.1016/j.ejcts.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 415.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 416.Tiong LU, Davies R, Gibson PG, Hensley MJ, Hepworth R, Lasserson TJ, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2006:CD001001. doi: 10.1002/14651858.CD001001.pub2. [DOI] [PubMed] [Google Scholar]
- 417.Patel N, Criner GJ. Transplantation in chronic obstructive pulmonary disease. COPD. 2006;3:149–62. doi: 10.1080/15412550600829364. [DOI] [PubMed] [Google Scholar]
- 418.Mora JI, Hadjiliadis D. Lung volume reduction surgery and lung transplantation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:629–35. doi: 10.2147/copd.s4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Expert Group of the Association of Physicians of India on Adult Immunization in India. The Association of Physicians of India evidence-based clinical practice guidelines on adult immunization. J Assoc Physicians India. 2009;57:345–56. [PubMed] [Google Scholar]
- 421.Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD002733. doi: 10.1002/14651858.CD002733.pub2. [DOI] [PubMed] [Google Scholar]
- 422.Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): An evidence-based review. Ont Health Technol Assess Ser. 2012;12:1–64. [PMC free article] [PubMed] [Google Scholar]
- 423.Walters JA, Smith S, Poole P, Granger RH, Wood-Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010:CD001390. doi: 10.1002/14651858.CD001390.pub3. [DOI] [PubMed] [Google Scholar]
- 424.Staykova T, Black PN, Chacko EE, Poole P. Prophylactic antibiotic therapy for chronic bronchitis. Cochrane Database Syst Rev. 2003:CD004105. doi: 10.1002/14651858.CD004105. [DOI] [PubMed] [Google Scholar]
- 425.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, et al. COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–98. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–47. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 427.He ZY, Ou LM, Zhang JQ, Bai J, Liu GN, Li MH, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80:445–52. doi: 10.1159/000321374. [DOI] [PubMed] [Google Scholar]
- 428.Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA, et al. PULSE Study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: A randomized controlled trial. Respir Res. 2010;11:10. doi: 10.1186/1465-9921-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Mohr LC. Hypoxia during air travel in adults with pulmonary disease. Am J Med Sci. 2008;335:71–9. doi: 10.1097/MAJ.0b013e31815f1e35. [DOI] [PubMed] [Google Scholar]
- 430.Shrikrishna D, Coker RK Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2011;66:831–3. doi: 10.1136/thoraxjnl-2011-200694. [DOI] [PubMed] [Google Scholar]


