Abstract
Rearing young rodents in socially isolated or environmentally enriched conditions has been shown to affect numerous components of the dopamine system as well as behavior. Methylphenidate (MPH), a commonly used dopaminergic agent, may affect animals differently based on rearing environment. Here we examined the interaction between environment and chronic MPH treatment at clinically relevant doses, administered via osmotic minipump. Young Sprague Dawley rats (PND 21) were assigned to environmentally enriched, pair-housed, or socially isolated rearing conditions, and treated with either 0, 2, 4, or 8 mg/kg/day MPH for three weeks. At the end of the treatment period, animals were tested for locomotor activity and anxiety-like behavior. The densities of D1-like and D2-like receptors were measured in the striatum using in vitro receptor autoradiography. Locomotor activity and anxiety-like behavior were increased in isolated animals compared to pair-housed and enriched animals. The density of D1-like receptors was greater in isolated animals, but there were no differences between groups in D2-like receptor density. Finally, there were no effects of MPH administration on any reported measure. This study provides evidence for an effect of early rearing environment on the dopamine system and behavior, and also suggests that MPH administration may not have long-term consequences.
Keywords: Environment, Enrichment, Isolation, Dopamine, Methylphenidate
1. Introduction
Early life experiences can have a significant impact on behavioral and brain development. It has been suggested that children raised in impoverished living conditions are at a greater risk for the development of psychiatric disorders such as anxiety, addiction, and attention deficit hyperactivity disorder (ADHD) than children raised in more positive environments (De Bellis, 2002; Jaffee et al., 2012; Latimer et al., 2012; Solinas et al., 2010). Similarly, rodents raised in isolated/impoverished conditions display greater levels of anxiety-like behavior (Bickerdike et al., 1993; Chappell et al., 2013; Hellemans et al., 2004; Lodge and Lawrence, 2003; Lukkes et al., 2009; McCool and Chappell, 2009; Wright et al., 1991; Yorgason et al., 2013), inattention (Ouchi et al., 2013; Schrijver and Wurbel, 2001), and impulsivity (Baarendse et al., 2008; Lovic et al., 2011; Perry et al., 2008). In contrast, rats reared in more enriched housing and social conditions exhibit, for example, improved performance on learning and memory tasks (Fares et al., 2013; Galani et al., 2007; Pamplona et al., 2009; Pappas et al., 1992), decreased levels of anxiety-like behavior (Fares et al., 2013; Pritchard et al., 2013; Urakawa et al., 2013), and decreased levels of depressive-like behaviors (Brenes Saenz et al., 2006), that have been accompanied by increases in neurogenesis (Fares et al., 2013; Ueda et al., 2005) and dendritic complexity (Wang et al., 2012). Enriched rodents also show reduced effects of repeated stimulant administration (Bardo et al., 1995; Gipson et al., 2011; Puhl et al., 2012) and decreased rates of drug self-administration (Alvers et al., 2012; Bardo et al., 2001; Deehan et al., 2011; Stairs and Bardo, 2009). Contrarily, those raised in isolation exhibit higher rates of stimulant drug and alcohol self-administration (Bardo et al., 2001; Chappell et al., 2013; Deehan et al., 2007; McCool and Chappell, 2009; Schenk et al., 1990; Wolffgramm, 1990), increased drug seeking behavior (Lynch et al., 2005), and more rapid acquisition of cocaine self-administration (Kosten et al., 2000).
These behavioral distinctions are associated with significant differences in brain neurochemistry, particularly in monoamine systems. For example, environmental enrichment has been shown to result in higher levels of 5-HT concentrations in the prefrontal cortex that are associated with lower levels of depressive-like behavior in rodents (Brenes et al., 2008a), as well as decreases in tryptophan-hydroxylase positive cells in the dorsal raphe nucleus, an effect similar to that seen following anti-depressant treatment (MacGillivray et al., 2012). Norepinephrine has been shown to be decreased in the ventral striatum by social isolation (Brenes et al., 2008b), and increased in the parieto-temporo-occipital cortex by environmental enrichment (Naka et al., 2002). The dopamine system, which is thought to play a fundamental role in psychiatric disorders such as addiction and ADHD, is known to be particularly sensitive to environmental manipulations. For example, long-term isolation has also been shown to reduce dendritic spine density and complexity of dopamine neurons (Wang et al., 2012), and increase basal dopamine concentration in the nucleus accumbens (Miura et al., 2002) and prefrontal cortex (Han et al., 2011). Conversely, environmental enrichment has been shown to decrease basal dopamine concentration in the striatum (Bowling et al., 1993), increase dopamine clearance from the medial prefrontal cortex (Neugebauer et al., 2004), enhance dopaminergic neuron migration from the midbrain to the striatum (Urakawa et al., 2013) and increase glucose utilization in the nucleus accumbens (Lack et al., 2010). Thus, the basal tone of dopaminergic systems can be significantly influenced by environmental variables.
Stimulant drugs that act on the dopamine system such as methylphenidate and amphetamine are the most frequently used psychotropic medications in childhood and adolescence. Currently, an estimated 6–8% of school-aged children are prescribed methylphenidate (MPH; Trade names Ritalin®, Concerta®, Metadate CD™) to treat ADHD (Biederman, 2005). Prescriptions for MPH and similar stimulants such as amphetamine have increased at a rapid rate, from 2.5 million in 1991 to almost 10 million in 1999 (Safer et al., 1996; Spencer et al., 2000; Zito et al., 2000). MPH exerts its effects in the brain by blocking the dopamine transporter (DAT) (Madras et al., 2005; Volkow et al., 1998), a key regulator of dopaminergic transmission. Rodent studies have documented numerous MPH-induced alterations of the dopamine system including changes in dopamine reuptake rates (Harvey et al., 2011), long-term loss of dopamine neurons (Sadasivan et al., 2012), decreases in DAT density (Simchon et al., 2010), and increased basal dopamine levels in the prefrontal cortex (Koda et al., 2010). Behaviorally, acute MPH treatment at low doses has been shown to be anxiolytic on the elevated plus maze (Gray et al., 2007; Koike et al., 2009; Zhu et al., 2010), improve attention (Zhu et al., 2010), and decrease impulsivity (Perry et al., 2008). Taken together, the literature clearly shows that MPH treatment affects many of the same systems and behaviors as social isolation and environmental enrichment, however few studies have examined the interaction of these two variables. Although there are several reports investigating the effects of the acute administration of MPH on animals reared under differing conditions (Perry et al., 2008; Wooters et al., 2011), the effects of chronic treatment remain largely unexplored. The purpose of this study, therefore, was to determine whether the effects of chronic MPH treatment on striatal dopamine systems would differ depending on environmental rearing conditions. As other psychostimulants, such as cocaine, have been shown to have effects on the concentrations of DA D1-like and D2-like receptors in rats (Kleven et al., 1990; Unterwald et al., 1996), and monkeys (Beveridge et al., 2009; Nader et al., 2002), these two targets were chosen for examination. Additionally, because early rearing conditions profoundly alter the expression of locomotor (Bardo et al., 1995; Bowling et al., 1993; Fabricius et al., 2011; Hoffmann et al., 2009; Shao et al., 2009; Smith et al., 1997; Varty et al., 2000) and anxiety-like behaviors (Bickerdike et al., 1993; Chappell et al., 2013; Lodge and Lawrence, 2003; Lukkes et al., 2009; McCool and Chappell, 2009; Wright et al., 1991; Yorgason et al., 2013), we hypothesized that chronic MPH treatment would produce differential effects on the expression of these behaviors.
In recent years, it has become common clinical practice to prescribe long-acting formulations of MPH such as Concerta® and Metadate CD™, as opposed to immediate-release formulations such as Ritalin®. These drugs are designed to provide an immediate bolus of drug followed by a steady release phase which maintains drug blood levels around the therapeutic range of 10–15 ng/mL (Volkow and Swanson, 2003). Long-acting formulations are reported to be clinically effective for as long as 12 hours after dosing (Pelham et al., 2001; Swanson et al., 2004). Most rodent studies of chronic treatment use twice daily intraperitoneal injection or oral administration, which more closely models the immediate release formulations of MPH. Therefore, questions remain surrounding the effects of chronic dosing with the extended release formulations of MPH that are most commonly prescribed. Here, we use Osmotic MiniPumps (Alzet©; Durect Corporation, Cupertino, CA) to administer MPH continuously during a chronic treatment period, to model some aspects of long-acting formulations of the drug.
To this end, young rats (postnatal day 21) were housed for four weeks in three distinct environmental conditions: enriched environment, standard pair-housed environment, and isolated environment. They were treated for three weeks with saline or 2, 4, or 8 mg/kg/day MPH delivered subcutaneously via osmotic minipump. At the end of the treatment period, animals were tested for locomotor behavior and anxiety-like behavior. Finally, the concentrations of dopamine D1-like and D2-like receptors were measured using in vitro receptor autoradiography. We hypothesized chronic MPH treatment would differentially affect behaviors and dopaminergic measurements depending on the early rearing environment.
2. Results
2.1 Blood Drug Levels
Three animals were removed from this analysis due to insufficient quantities of blood drawn during the procedure. Thus, 24 animals in the 2 mg/kg/day group, 22 animals in the 4 mg/kg/day group, and 23 animals in the 8 mg/kg/day group were included. Osmotic minipump administration of MPH achieved final blood levels of (mean ± SD) 4.4 ± 2.14 ng/mL in the 2 mg/kg/day group, 8.4 ± 3.32 ng/mL in the 4 mg/kg/day group, and 15.4 ± 5.27 ng/mL in the 8 mg/kg/day group. Blood levels of drug did not correlate with any behavioral or dopaminergic measures (data not shown).
2.2 Locomotor Activity
There was a main effect of housing condition on spontaneous locomotor activity in a novel environment on measures of total distance travelled, horizontal beam breaks and stereotypy (tot dist: F2,84 = 83.297; p < 0.001; horiz act: F2,84 = 41.153; p < 0.001; stereo: F2,84 = 51.124; p < 0.001) (Figure 1 A–C). In contrast, there was no effect of MPH treatment (tot dist: F3,84 = 0.399; horiz act: F3,84 = 1.781; stereo: F3,84 = 1.732) and no interaction (tot dist: F6,84 = 0.104; horiz dist: F6,84 = 0.463; stereo: F6,84 = 0.317). Post-hoc analysis (Bonferroni) confirmed that isolated animals exhibited significantly higher levels of locomotor activity (distance traveled) than either paired (p < 0.001) or enriched animals (p < 0.001) over the one hour test. Higher levels of locomotor activity were also measured in pair-housed animals when compared to environmentally enriched animals (p < 0.001). On the measure of horizontal activity, isolated animals had significantly higher beam breaks than both paired (p < 0.001) and enriched animals (p < 0.001), and enriched animals were also significantly different from paired animals (p < 0.02). Finally, enriched animals exhibited significantly reduced levels of stereotypy as compared with either paired (p < 0.001) or isolated animals (p < 0.001). MPH treatment did not significantly alter measures of spontaneous locomotor behavior in a novel environment.
Figure 1. Behavior in the locomotor chamber.
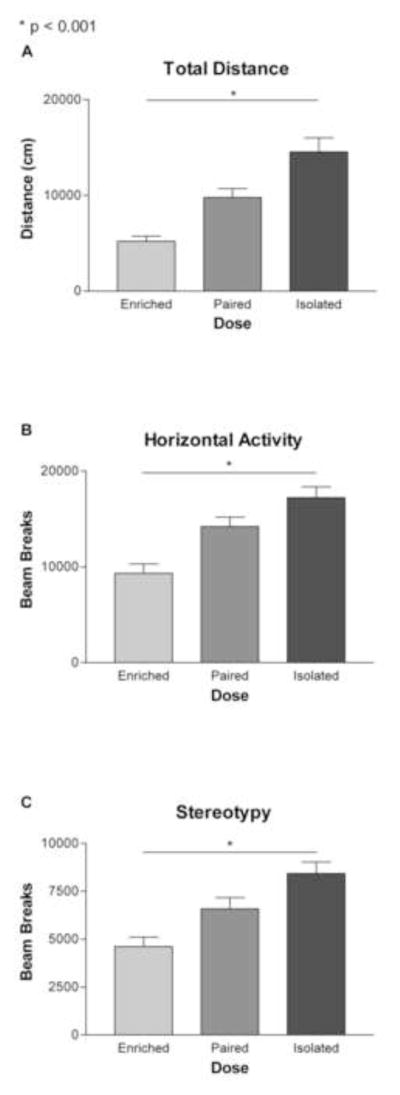
A) There was a main effect of housing condition on total distance travelled (p < 0.001). Isolated animals travelled the greatest distance in one hour, followed by paired and then enriched animals. B) There was a main effect of housing condition on the measure of horizontal activity (p < 0.001). Isolated animals had the highest number of broken beams in the horizontal plane, followed by paired, and then enriched animals. C) Enriched animals had significantly lower expression of stereotypical behavior than paired or isolated animals (p < 0.001). Paired and isolated animals were not significantly different from each other.
2.3 Elevated Plus Maze
On the elevated plus maze, greater amounts of time spent on the open arms indicates lower levels of anxiety-like behavior. Over the course of a 5 minute test, there was a main effect of housing condition (F2,84 = 5.502; p < 0.01), no effect of MPH treatment (F3,84 = 1.646) and no interaction (F6,84 = 0.521) on time spent in the open arms (Figure 2). Isolated animals spent significantly less time in the open arms than pair-housed (p < 0.01) or enriched animals (p < 0.002). Paired and enriched animals did not differ from each other in time spent in the open arms.
Figure 2. Time spent in the open arms of the elevated plus maze.
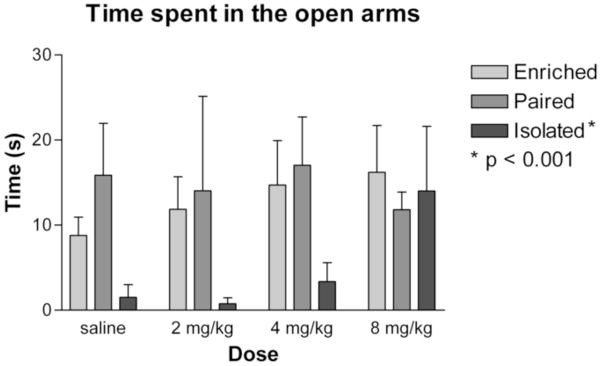
There was a main effect of housing condition on anxiety-like behavior as measured by time spent in the open arms of the elevated plus maze (p < 0.01). Post hoc tests confirmed that isolated animals spent significantly less time in the open arms than pair-housed (p < 0.01) or enriched animals (p < 0.002). There was no difference between enriched and paired animals, there was no effect of MPH, and there was no significant interaction.
2.4 Dopamine D1-like Receptor Density
Three animals were removed from analysis due to problems arising from the tissue processing. Thus, all groups were n = 8 except PH 2 mg/kg, PH 4 mg/kg, and SI 4 mg/kg, which each had 7 animals. In all three brain regions there was a significant main effect of housing condition (CPu: F2,81 = 12.050, p < 0.001; Core: F2,81 = 10.538, p < 0.001; Shell: F2,81 = 10.966, p < 0.001) on D1-like receptor density. However, there was no effect of MPH (CPu: F3,81 = 0.488; Core: F3,81 = 0.463; Shell: F3,81 = 0.209) and there were no significant interactions (CPu: F6,81 = 0.493; Core: F3,81 = 0.207; Shell: F3,81 = 0.358) (Table 1). Planned comparisons showed that there was significantly greater D1-like receptor density in the all three brain regions among isolated animals when compared to both paired (CPu: p < 0.005; Core: p < 0.05; Shell: p < 0.02) and enriched animals (CPu: p < 0.001; Core: p < 0.001; Shell: p < 0.001). There were, however, no differences in D1-like receptor density between paired and enriched animals (Table 1).
Table 1.
The density of D1-like receptors in the striatum, fmol/mg wet weight tissue (mean ± SEM)
| Housing Condition | Dose (mg/kg/day) | Enriched | Paired | Isolated* |
|---|---|---|---|---|
| CPu | 0 | 127.0 ± 5.3 | 134.1 ± 9.5 | 162.2 ± 14.4 |
| 2 | 120.6 ± 3.7 | 129.6 ± 11.3 | 147.9 ± 16.7 | |
| 4 | 126.3 ± 7.0 | 128.1 ± 11.9 | 176.0 ± 14.9 | |
| 8 | 113.7 ± 3.8 | 140.2 ± 13.4 | 156.6 ± 15.9 | |
| Core | 0 | 120.4 ± 3.2 | 134.7 ± 11.6 | 162.8 ± 14.1 |
| 2 | 118.3 ± 4.3 | 129.0 ± 10.9 | 145.3 ± 17.3 | |
| 4 | 122.4 ± 8.7 | 136.2 ± 13.6 | 165.5 ± 14.9 | |
| 8 | 111.5 ± 2.8 | 138.8 ± 12.8 | 152.5 ± 17.1 | |
| Shell | 0 | 107.4 ± 2.3 | 119.8 ± 9.7 | 149.4 ± 12.7 |
| 2 | 110.8 ± 4.1 | 116.3 ± 10.6 | 133.2 ± 16.1 | |
| 4 | 110.3 ± 9.6 | 120.2 ± 11.5 | 150.6 ± 14.4 | |
| 8 | 99.8 ± 3.5 | 128.4 ± 12.2 | 141.6 ± 16.9 |
p <0.05, There was a main effect of housing on D1-like receptors. Isolated animals had significantly greater concentrations of D1-like receptors than pair housed or enriched animals.
2.5 Dopamine D2-like Receptor Density
As above, two-way ANOVAs were employed to compare the density of D2-like receptors in each region of interest. Again, one animal was removed from analysis due to errors in tissue processing and so the PH 4 mg/kg group had only 7 animals. There were no significant effects of housing condition (CPu: F2,83 = 0.375; Core: F2,83 = 0.483; Shell: F2,83 = 0.209) or drug (CPu: F3,83 = 0.220; Core: F3,83 = 0.320; Shell: F3,83 = 0.572), and no significant interactions (CPu: F6,83 = 0.228; Core: F6,83 = 0.275; Shell: F6,83 = 0.458) on D2-like receptor density in any region of the striatum (Table 2).
Table 2.
The density of D2-like receptors in the striatum, fmol/mg wet weight tissue (mean±SEM)
| Housing Condition | Dose (mg/kg/day) | Enriched | Paired | Isolated |
|---|---|---|---|---|
| CPu | 0 | 104.6 ± 3.9 | 103.9 ± 3.8 | 109.6 ± 4.8 |
| 2 | 111.4 ± 5.3 | 103.3 ± 2.5 | 119.0 ± 6.9 | |
| 4 | 104.8 ± 5.7 | 116.2 ± 6.3 | 113.5 ± 9.0 | |
| 8 | 108.2 ± 4.4 | 106.3 ± 3.0 | 100.4 ± 3.4 | |
| Core | 0 | 62.4 ± 3.8 | 62.8 ± 2.9 | 66.6 ± 5.0 |
| 2 | 69.5 ± 4.2 | 59.9 ± 3.8 | 77.4 ± 8.2 | |
| 4 | 64.1 ± 6.7 | 78.7 ± 7.5 | 71.3 ± 8.3 | |
| 8 | 65.7 ± 4.1 | 64.8 ± 2.9 | 64.6 ± 4.4 | |
| Shell | 0 | 50.9 ± 4.1 | 50.5 ± 2.4 | 59.5 ± 6.6 |
| 2 | 59.7 ± 3.8 | 45.9 ± 4.0 | 66.7 ± 9.3 | |
| 4 | 54.1 ± 6.9 | 64.4 ± 8.7 | 64.6 ± 9.2 | |
| 8 | 55.8 ± 4.6 | 56.7 ± 4.4 | 56.7 ± 4.6 |
3. Discussion
The results of these studies demonstrate that rearing environment during early life has significant effects on both spontaneous behaviors and the dopamine system when measured late in adolescence. Socially isolated animals displayed higher levels of locomotor activity and greater levels of anxiety-like behavior on the elevated plus maze. Additionally, these animals had significantly higher D1-like receptor density throughout the entire striatum. In contrast, chronic MPH, administered via osmotic minipump at clinically relevant doses, had no effect on the behaviors or the dopamine systems of these animals. Perhaps more important, there were no interactions of chronic methylphenidate treatment with housing conditions on any of the measures of behavior or dopamine system regulation. These data suggest that chronic MPH treatment, particularly with extended-release formulations, may not have consequences for the dopamine system or effects on behavioral outcomes, a finding that is supported by recent studies in nonhuman primates (Gill et al., 2012; Soto et al., 2012).
Despite the absence of significant effects of MPH treatment observed here, there were substantial effects of rearing condition on both locomotor activity and anxiety-like behaviors. Rats reared in isolation had higher rates of spontaneous locomotor activity in the open field as compared to the pair-housed animals and those raised in enriched environments when tested after 4 weeks of exposure to the rearing conditions. These data are in concert with numerous previous studies that have clearly shown greater baseline activity levels in rodents following early life isolation (Bardo et al., 1995; Bowling et al., 1993; Fabricius et al., 2011; Hoffmann et al., 2009; Shao et al., 2009; Smith et al., 1997; Varty et al., 2000). Additionally, enriched animals had significantly lower measures of total distance travelled, horizontal activity, and stereotypy than paired animals and isolated animals. This most likely indicates a reduced response to novelty in this group, again consistent with a number of previous studies (Cain et al., 2006; Elliott and Grunberg, 2005). As opposed to our hypothesis, however, MPH treatment had no effect on levels of locomotor activity in any of the rearing conditions. This finding is similar to a recent study in which chronic oral MPH did not have any effect on activity levels in adolescent rats (Yates et al., 2012). However, these data are contradictory to other studies that have shown decreases in locomotor activity after chronic MPH treatment by intraperitoneal injection (Bolanos et al., 2003) or by oral administration (Bethancourt et al., 2011). In addition, repeated stimulant treatment has been shown to have a greater locomotor sensitizing effect in animals that have been reared in isolated conditions as compared to enriched animals (Bardo et al., 1995). There are considerable differences between the current study and others that have shown significant effects of stimulant treatment in adolescent animals, including the use of doses within the range of those considered clinically relevant in children, rat strain, duration of rearing conditions, and age at testing, which all may have contributed to the discrepant results. Additionally, the continuous infusion of drug via osmotic minipump may have led to behavioral tolerance to the effects of MPH that is not present when using non-continuous routes of administration such as injection and oral dosing. Previous studies comparing continuous and non-continuous administration of other psychostimulants, cocaine and d-amphetamine, have documented differences in locomotor behavior and stereotypy after drug injections that are not present after continuous administration (Nelson and Ellison, 1978; Zeigler et al., 1991).
In addition to greater locomotor activity in the open field, isolated rats displayed higher levels of anxiety-like behavior as measured on the elevated plus maze. This is consistent with previous studies that have demonstrated similar findings in a number of paradigms (Bickerdike et al., 1993; Chappell et al., 2013; Lodge and Lawrence, 2003; Lukkes et al., 2009; McCool and Chappell, 2009; Wright et al., 1991; Yorgason et al., 2013). However, in the present study on the elevated plus maze, the enriched animals were not different from pair-housed animals, most likely because of the high degree of variability in time spent on the open arms in these two groups. Again, there was an absence of any significant effects of MPH treatment on anxiety-like behaviors. Despite the absence of significant drug effects, previous reports have shown anxiolytic effects of MPH on the elevated plus maze (Koike et al., 2009). In our data, there is some indication that isolated animals that were treated with the highest dose of MPH (8 mg/kg/day) spent more time in the open arms than saline- or low-dose-treated isolated animals. This interaction did not reach statistical significance, but may have with higher doses of MPH.
Isolation rearing was also associated with higher levels of D1-like receptor density throughout the striatum, regardless of MPH dose. D1-like receptor activation in the striatum is known to influence spontaneous locomotor activity in juvenile as well as adult rats, with agonists generally increasing (Charntikov et al., 2011; Desai et al., 2005), and antagonists decreasing (Peters et al., 2007; Schindler and Carmona, 2002) levels of locomotor activity. In addition, D1-like receptor knock-out mice show alterations in locomotor activity compared to controls (Tran et al., 2005) similar to those seen in other studies of isolated animals. It is possible that the altered levels of D1-like receptor density in isolated animals influenced their locomotor activity in the open field in this study.
There are only a few previous reports of alterations to D1-like receptors that have been associated with rearing condition. In mice, D1-like receptors have been shown to be elevated after social isolation (Gariepy et al., 1995), while in rats, another study documented decreases in D1-like receptor density associated with housing in an enriched environment (Del Arco et al., 2007). Yet, another study reported no changes to D1-like or D2-like receptors associated with rearing condition (Bardo and Hammer, 1991). As a model of early life stress, however, social isolation has been associated with numerous changes to other aspects of the dopamine system, such as reduced dendritic spine density and complexity of dopamine neurons (Wang et al., 2012), increased basal dopamine concentration in the nucleus accumbens (Miura et al., 2002) and prefrontal cortex (Han et al., 2011), increased dopamine turnover (Hall et al., 1998; Heidbreder et al., 2000), and increase evoked dopamine overflow and reuptake rates (Yorgason et al., 2013). Social isolation has also been shown to alter corticosterone levels (Miachon et al., 1993; Rivier and Vale, 1987; Sandstrom and Hart, 2005), indicating that it does, indeed, modulate neural stress systems such as the hypothalamic-pituitary-adrenal axis. Other methods of early life stress, such as chronic exposure to restraint stress, have also been shown to result in increased D1-like receptor density in the prefrontal cortex (Mizoguchi et al., 2000). Additionally, D1-like receptors were increased in the monkey striatum following long-term cocaine self administration (Nader et al., 2002), a pharmacological stressor, and were even further increased after a 30 day period of abstinence (Beveridge et al., 2009). Thus, there is evidence that stressful experiences in general may increase D1-like receptor density, whether in the form of early social isolation, chronic restraint stress, or pharmacological stress.
While D1-like receptor densities were elevated in socially isolated animals, D2-like receptors were unaffected by housing condition. Previous reports have documented differences in the concentration of D2-like receptors associated with rearing condition. However, the direction of the changes is unclear, with reports of both increases (Djouma et al., 2006; King et al., 2009) and decreases (Hall et al., 1998) after social isolation. There are also several other studies that have failed to see effects of environment on D2-like receptors, similar to the present data (Bardo and Hammer, 1991; Del Arco et al., 2004; Jones, 1992; Malone et al., 2008; Rilke et al., 1998). Additionally, there were no differences in D2 autoreceptor activity in the nucleus accumbens between isolated and group housed animals, despite increases in dopamine release and reuptake in this region (Yorgason et al., 2013). Behaviorally, isolated animals have been shown to exhibit profiles consistent with lower levels of D2-like receptors, most notably in their vulnerability to drugs and alcohol (McCool and Chappell, 2009; Schenk et al., 1986; Schenk et al., 1987; Stairs and Bardo, 2009). Vulnerability to substance abuse is clearly associated with low levels of D2-like receptor density, as has been shown in nonhuman primates (Morgan et al., 2002) and humans (Dalley et al., 2007; Fehr et al., 2008; Volkow et al., 1996; Volkow et al., 2001; Wang et al., 1997). Conversely, enrichment has been shown to have a protective effect against substance abuse (for review see Stairs and Bardo, 2009), which is associated with higher D2-like receptor density (Morgan et al., 2002; Thanos et al., 2001; Thanos et al., 2004; Volkow et al., 2006). Despite the behavioral profiles that would suggest otherwise, it remains that D2-like receptors have been unaffected by housing condition in numerous studies, including the present one.
In contrast to the effects of rearing conditions on the dopamine system, chronic MPH treatment did not significantly alter the concentrations of either dopamine D1-like or D2-like receptors in the striatum. The present data draw a distinction from work by Thanos and colleagues who showed that chronic, oral MPH treatment was associated with a decrease in D2-like receptor availability after two months, and an increase in availability after eight months of exposure (Thanos et al., 2007). MPH treatment has also been shown to have a lasting effect on presynaptic striatal dopamine function (Sproson et al., 2001), and to increase dendritic spine density on both D1- and D2-expressing medium spiny neurons in the shell of the nucleus accumbens (Kim et al., 2009). The methodologies of these studies differ from the current investigation in both duration of treatment (2 or 8 months versus 3 weeks), dose of MPH (15 mg/kg/day vs 2–8 mg/kg/day), and route of administration (oral or intraperitoneal injection versus osmotic minipump), among other factors. As mentioned above, it is additionally possible that the constant infusion of MPH via osmotic minipump over the course of the study resulted in the development of tolerance to the drug that would not occur with non-continuous administration.
One major goal of this study was to explore the effects of chronic MPH treatment at clinically equivalent doses to those used in children in adolescent rodents. To determine whether this dosing regimen was accurately modeling therapeutic dose ranges as found in children of 10–15 ng/ml (Swanson et al., 2004), we tested blood levels of MPH at the conclusion of the study. Blood levels ranged from an average of 4.4 ± 2.14 ng/mL in the 2 mg/kg/day group, to an average of 15.4 ± 5.27 ng/mL in the 8 mg/kg/day group. There were no correlations between blood levels of drug and any behavioral or dopaminergic measures. Importantly, by administering the drug in an osmotic minipump, drug was infused constantly throughout the study. This type of administration differs from the clinical scenario where extended-release formulations are taken once daily and last for 12 hours. Thus, while blood levels reflected therapeutic dose ranges in children, there are differences in the pattern of dosing between this rodent model and MPH treatment in children.
This dosing regimen is also different from the majority of other rodent studies of MPH treatment and it is likely the primary factor underlying the discrepancies between the results here and the results of previous studies. Indeed, one previous study that directly compared intraperitoneal injection to minipump administration found that acutely injected animals were hyperlocomotive and had enhanced reactions to cocaine, while animals receiving a constant infusion of MPH via minipump exhibited less locomotor activity and were less responsive to cocaine (Griggs et al., 2010). Similarily, behavioral profiles have been shown to vary after the chronic administration of cocaine and d-amphetamine depending on whether the drug was administered continuously or intermittently (Nelson and Ellison, 1978, Zeigler et al., 1991). In humans, pharmacokinetic studies have shown much slower absorption and much longer duration of action with extended-release formulations of MPH when compared to immediate-release forms, though peak concentrations are equivalent (Spencer et al., 2006). Though animal studies of the pharmacokinetics of extended-release MPH treatment or continuous administration are lacking, it is likely that extended absorption and longer duration of action account for differences between continuous and intermittent treatment regimens.
The findings of the present study agree with a recently published study in nonhuman primates that utilized an extended release formulation of MPH which was carefully controlled to maintain doses in the clinically-relevant range as used in children (Gill et al., 2012). In that study, and another study in rhesus monkeys that also titrated doses to the clinically-relevant range (Soto et al., 2012), there were no effects of MPH on growth, availability of dopamine D2-like receptors or DATs, or future vulnerability to cocaine self-administration. All of these results support the notion that chronic MPH treatment in extended release formulations may not have long-term consequences. However, despite the fact that these doses were chosen to replicate clinically-relevant doses in children based upon blood drug levels, it is important to note that it is impossible to determine whether the doses utilized here are behaviorally effective in a rodent. We did not investigate changes in measures of attention or impulsivity that are the primary targets of MPH treatment. Therefore we cannot state for certain that these doses would be behaviorally effective in a rat as they are in children at these blood levels. It remains possible that higher doses must be used in rodent models to achieve clinical relevance.
In conclusion, there were significant effects of early rearing environment on the dopamine system, specifically on the concentrations of D1-like receptors in striatal brain regions, which were greater in socially isolated animals when compared to paired or enriched animals. Similar to other stressors that are environmental or pharmacological, this finding highlights the impact of early life isolation as a form of stress that can significantly affect the dopamine system and associated behaviors. As disturbances to the dopamine system have been associated with disorders such as ADHD and substance abuse, knowledge of how early life influences can alter this system is critical for understanding the development of these pathologies.
Contrary to our hypothesis, MPH treatment did not differentially affect the behavioral outcomes or dopamine system changes that were associated with rearing environment. This study focused on clinically-relevant doses that were administered via osmotic minipump to model the extended release formulations of MPH that are most commonly prescribed. In agreement with our recent findings in nonhuman primates (Gill et al., 2012), this study provides additional support for the lack of long-term dopaminergic effects associated with chronic MPH treatment.
4. Experimental Procedures
4.1 Subjects and Housing Conditions
Ninety-six male Sprague Dawley rats were acquired at postnatal day (PND) 21 from Harlan Industries. Upon arrival, they were immediately placed into one of three housing conditions (32 rats per housing condition): environmentally enriched, pair-housed, or socially/environmentally isolated. Enriched animals were housed 4 animals per cage in large (280 square inches floor, 8 inches high) clear plastic cages. They were furnished with multiple toys including rodent houses, climbing structures, wooden block chew toys, and kong toys, for example. Toys were rotated twice weekly and all animals were handled daily by the experimenter. Pair-housed animals were housed 2 animals per cage in standard size (142 square inches floor, 8 inches high) clear plastic cages. They did not have any toys and were handled on a limited basis, approximately twice per week. Isolated animals were housed singly in standard size opaque cages. They did not receive toys and were only handled once per week for weighing immediately prior to surgeries. Enriched and paired animals were housed in one satellite housing unit, while isolated animals were housed in a separate unit. Thus, isolated animals were not exposed to any form of experimenter interaction except during daily food and water maintenance, 3 minor surgeries, and final behavioral testing. All animals had 24 hour access to food and water and lights were maintained on a 12 hour on/off schedule with lights coming on at 8:00 am. All studies were carried out in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
4.2 Drug Dosing
Methylphenidate hydrochloride was obtained from Mallinckrodt (Covidien Pharmaceuticals; Hazelwood, MO). After one week of habituation to the housing conditions, animals were assigned to one of four conditions: 0, 2, 4, or 8 mg/kg/day MPH (8 animals per housing condition/drug dose). Animals that were housed together in the same cage were assigned to the same drug condition. On the morning of the scheduled surgery, each animal was weighed and its dose for the week was calculated. As young rats grow rapidly in this stage, end of the week body weights were estimated using normative growth charts for Sprague Dawley rats published by Harlan Industries (2008). The estimated end of the week weight was, on average, within 4% of the actual measured weight at the end of the week. The “dose weight” was the average of the current body weight and the estimated end of the week weight. After dose weights were calculated, the correct amount of MPH for each animal was mixed into a sterile saline solution (0.9% NaCl) and loaded into osmotic minipumps.
4.3 Surgical Procedures
Osmotic minipumps were implanted subcutaneously to deliver a steady dose of MPH over the drug administration period. Animals were anesthetized with 3–4% isoflurane and given a dose of ketoprofen (5 mg/kg; s.c.) for pain relief. The skin on the animal’s back was shaved and prepared with a three stage wash of betadine surgical scrub, 70% isopropyl alcohol, and betadine surgical solution. A small incision was made in the skin between the scapulae. Using a hemostat, a small pocket was formed by spreading the subcutaneous connective tissue apart. The pump was inserted into the pocket and the skin was closed with Gluture® (Abbott Animal Health; Abbott Park, IL) tissue adhesive. Each minipump lasted for 7 days, at which point, old pumps were removed and replaced with new pumps in a separate surgery. Drug treatment lasted for 21 days so each animal had a total of three surgeries, with each surgery lasting under 5 minutes.
4.4 Locomotor behavior
Near the end of the drug treatment period, on PND 48 or 49, spontaneous locomotor activity was measured in all animals. Animals were removed from their home cages and placed into novel locomotor chambers (Med Associates; St. Albans, Vermont) constructed of acrylic glass measuring 43×43×30 cm and containing two infrared beam arrays. Beam breaks were recorded by a computer for one hour. The following measures were calculated: total distance (cm) travelled, horizontal activity (the number of beam breaks in the horizontal plane), and stereotypy. Following the session, rats were immediately returned to their home cages and housing room.
4.5 Elevated Plus Maze
Also on PND 48 or 49 (order of tests counterbalanced among drug and housing conditions), rats were tested for anxiety-like behavior on the elevated plus maze (Med Associates; St. Albans, VT). The elevated plus mazes consisted of two opposite open arms and two opposite closed arms that were elevated 40 cm above the floor. Photosensors on each arm recorded the entries and time spent in each individual arm. At the beginning of the assay, animals were placed in the center of the plus maze facing an open arm. The session lasted for 5 minutes during which entries into each arm and the total time spent in each arm were recorded. Similar parameters on this test have been effective in revealing differences in anxiety-like behavior between rats reared in different environments in previous studies (Chappell et al., 2013; McCool and Chappell, 2009). At the end of 5 minutes, animals were immediately returned to their home cages.
4.6 Blood Sampling
On PND 50, animals were sacrificed by an overdose of sodium pentobarbital (100 mg/kg) by intraperitoneal injection. Approximately 3 mLs of blood was drawn from the cardiac chambers and placed into EDTA treated tubes and frozen at −20°C. Analysis for blood MPH levels was performed using gas chromatography (Medtox Laboratories, St. Paul, MN).
4.7 In vitro receptor autoradiography
Immediately after bloods were taken, brains were harvested, flash frozen in isopentane, and stored at −80°C until sectioning. Coronal sections (20μm) were cut in a cryostat maintained at −22°C. Sections were picked up on charged slides, and then stored at −80°C. Assays included striatal regions that have been shown to be sensitive to environmental manipulation and MPH including the nucleus accumbens (core and shell regions) and caudate putamen (CPu). In vitro receptor autoradiography methods were adapted from Lidow et al. (1991) and Bardo and Hammer (1991).
Dopamine D1-like receptor binding site densities were determined with [3H]SCH 23390 (specific activity 85 Ci/mmol; PerkinElmer, Boston, MA). Sections were preincubated for 20 min in buffer (50 mM Tris, 120 mMNaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.4, 25°C) to remove endogenous dopamine. Sections were then incubated for 30 min in the same buffer, pH 7.4, 25°C, containing 1 mM ascorbic acid, 40 nM ketanserin, and 1 nM [3H]SCH 23390. After incubation, sections were rinsed twice for 20 s in buffer containing 1 mM ascorbic acid at pH 7.4, 4°C, then dipped in distilled water at 4°C, and dried under a stream of cool air. Nonspecific binding was defined by incubation of adjacent sections in the incubation solution in the presence of 5 mM ( + )-butaclamol.
The density and distribution of dopamine D2-like receptor binding sites was determined with [3H]raclopride (specific activity, 74.4 Ci/mmol; PerkinElmer, Boston, MA). Sections were preincubated for 20 min in buffer (50mM Tris, 120mM NaCl, 5mM KCl, pH 7.4, 25°C) to remove endogenous dopamine. Slides were then incubated for 30 min in the same buffer, containing 5mM ascorbic acid and 2 nM [3H]raclopride. Sections were rinsed 3×2 min in buffer at pH 7.4, 4°C, then dipped in distilled water at 4°C, and dried under a stream of cool air. Nonspecific binding was defined by incubation of adjacent sections in the incubation solution in the presence of 1 mM (+)–butaclamol.
For all experiments, sections, along with calibrated [3H] autoradiographic standards, were exposed to Kodak Biomax MR film for 6 weeks. Films were developed with Kodak GBX developer, stopbath and Rapid Fixer (VWR; West Chester, PA), and then rinsed. Analysis of autoradiograms was conducted by quantitative densitometry with a computerized image processing system (MCID, Imaging Research; InterFocus Imaging Ltd, Cambridge, UK). Optical density values were converted to fmol/mg (of wet-weight tissue) by reference to calibrated [3H] standards. Specific binding was determined by digitally subtracting images of nonspecific binding from superimposed adjacent images of total binding.
4.8 Statistical Analysis
Pearson Product-Moment correlations were used to assess the relationship between drug blood levels and the following measures: time spent in the open arms on the elevated plus maze, total distance travelled, horizontal activity, and stereotypy in the locomotor chamber, D1-like receptor density in the CPu and nucleus accumbens core and shell, and D2-like receptor density in the CPu and nucleus accumbens core and shell. For correlations, blood levels of drug for rats in all housing groups and dosing conditions were combined to evaluate the relationship between blood levels and other end points. To examine the effects of, and interactions between, MPH and rearing condition on dopamine receptors and behavior, two-way analyses of variance (ANOVAs) (drug × housing condition) were performed on the following measures: time spent in the open arms on the elevated plus maze, total distance travelled, horizontal distance, and stereotypy in the locomotor chamber, and D1-like and D2-like receptor density in the CPu and nucleus accumbens core and shell. For all tests, SPSS software (IBM; Armonk, NY) was used and effects were considered significant if p < 0.05. Post-hoc tests with a Bonferroni correction were used for planned comparisons between housing conditions where there were significant effects as revealed by ANOVAs. Again, p < 0.05 was considered statistically significant.
Highlights.
Isolated rats had greater locomotor behavior than paired or enriched rats
Isolated rats had greater anxiety-like behavior than paired or enriched rats
Isolated rats had greater D1-like receptor density than paired or enriched rats
Rearing conditions did not affect the density of D2-like receptors in the striatum
Chronic methylphenidate treatment did not affect any reported measure
Acknowledgments
We would like to thank Mack Miller and Ann Chappell for their technical assistance with these studies. These studies were funded by grants from NIDA and NIAAA, DA 06634 (LJP, TJRB), DA 20648 (LJP), and T32-AA00756.
Footnotes
Author Roles and Conflict of Interest
Kathryn Gill contributed to study design, performed data collection and data analysis, interpreted results, and prepared the manuscript. Thomas Beveridge contributed to study design and manuscript preparation. Hilary Smith contributed to data collection and manuscript preparation. Linda Porrino contributed to study design, interpretation of results, and manuscript preparation, and had oversight of the research implementation. None of the authors declare any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–7. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJ, van Grootheest G, Jansen RF, Pieneman AW, Ogren SO, Verhage M, Stiedl O. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus. 2008;18:11–9. doi: 10.1002/hipo.20356. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Hammer RP., Jr Autoradiographic localization of dopamine D1 and D2 receptors in rat nucleus accumbens: resistance to differential rearing conditions. Neuroscience. 1991;45:281–90. doi: 10.1016/0306-4522(91)90226-e. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bethancourt JA, Vasquez CE, Britton GB. Sex-dependent effects of long-term oral methylphenidate treatment on spontaneous and learned fear behaviors. Neurosci Lett. 2011;496:30–4. doi: 10.1016/j.neulet.2011.03.080. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–71. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickerdike MJ, Wright IK, Marsden CA. Social isolation attenuates rat forebrain 5-HT release induced by KCI stimulation and exposure to a novel environment. Behav Pharmacol. 1993;4:231–236. [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–20. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–29. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2008a doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Rodriguez O, Fornaguera J. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav. 2008b;89:85–93. doi: 10.1016/j.pbb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Brenes Saenz JC, Villagra OR, Fornaguera Trias J. Factor analysis of Forced Swimming test, Sucrose Preference test and Open Field test on enriched, social and isolated reared rats. Behav Brain Res. 2006;169:57–65. doi: 10.1016/j.bbr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Processes. 2006;73:360–6. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Der-Ghazarian T, Herbert MS, Horn LR, Widarma CB, Gutierrez A, Varela FA, McDougall SA. Importance of D1 and D2 receptors in the dorsal caudate-putamen for the locomotor activity and stereotyped behaviors of preweanling rats. Neuroscience. 2011;183:121–33. doi: 10.1016/j.neuroscience.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–70. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exp Res. 2007;31:1692–8. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Palmatier MI, Cain ME, Kiefer SW. Differential rearing conditions and alcohol-preferring rats: consumption of and operant responding for ethanol. Behav Neurosci. 2011;125:184–93. doi: 10.1037/a0022627. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Zhu S, Terasmaa A, Mohammed AH, Fuxe K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology (Berl) 2004;171:148–55. doi: 10.1007/s00213-003-1578-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114:43–8. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Desai RI, Terry P, Katz JL. A comparison of the locomotor stimulant effects of D1-like receptor agonists in mice. Pharmacol Biochem Behav. 2005;81:843–8. doi: 10.1016/j.pbb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur J Neurosci. 2006;23:3319–27. doi: 10.1111/j.1460-9568.2006.04864.x. [DOI] [PubMed] [Google Scholar]
- Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–96. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Helboe L, Fink-Jensen A, Wortwein G, Steiniger-Brach B. Pharmacological characterization of social isolation-induced hyperactivity. Psychopharmacology (Berl) 2011;215:257–66. doi: 10.1007/s00213-010-2128-9. [DOI] [PubMed] [Google Scholar]
- Fares RP, Belmeguenai A, Sanchez PE, Kouchi HY, Bodennec J, Morales A, Georges B, Bonnet C, Bouvard S, Sloviter RS, Bezin L. Standardized environmental enrichment supports enhanced brain plasticity in healthy rats and prevents cognitive impairment in epileptic rats. PLoS One. 2013;8:e53888. doi: 10.1371/journal.pone.0053888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165:507–14. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Galani R, Berthel MC, Lazarus C, Majchrzak M, Barbelivien A, Kelche C, Cassel JC. The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol Learn Mem. 2007;88:1–10. doi: 10.1016/j.nlm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Gariepy JL, Gendreau PL, Mailman RB, Tancer M, Lewis MH. Rearing conditions alter social reactivity and D1 dopamine receptors in high- and low-aggressive mice. Pharmacol Biochem Behav. 1995;51:767–73. doi: 10.1016/0091-3057(95)00028-u. [DOI] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–65. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011;214:557–66. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, Zupan B, Menzer D, Rice J, Drake CT, Romeo RD, Brake WG, Torres-Reveron A, Milner TA. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J Neurosci. 2007;27:7196–207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs R, Weir C, Wayman W, Koeltzow TE. Intermittent methylphenidate during adolescent development produces locomotor hyperactivity and an enhanced response to cocaine compared to continuous treatment in rats. Pharmacol Biochem Behav. 2010;96:166–74. doi: 10.1016/j.pbb.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–72. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Han X, Wang W, Shao F, Li N. Isolation rearing alters social behaviors and monoamine neurotransmission in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res. 2011;1385:175–81. doi: 10.1016/j.brainres.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Harlan Industries. Sprague Dawley Growth Curve. 2008 http://www.harlan.com/products_and_services/research_models_and_services/research_models/sprague_dawley_outbred_rat.hl.
- Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–47. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–15. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hoffmann LC, Schutte SR, Koch M, Schwabe K. Effect of “enriched environment” during development on adult rat behavior and response to the dopamine receptor agonist apomorphine. Neuroscience. 2009;158:1589–98. doi: 10.1016/j.neuroscience.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Hanscombe KB, Haworth CM, Davis OS, Plomin R. Chaotic homes and children’s disruptive behavior: a longitudinal cross-lagged twin study. Psychol Sci. 2012;23:643–50. doi: 10.1177/0956797611431693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH. Social isolation and individual differences: behavioural and dopaminergic responses to psychomotor stimulants. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):253A–254A. doi: 10.1097/00002826-199201001-00132. [DOI] [PubMed] [Google Scholar]
- Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci U S A. 2009;106:2915–20. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KC. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63:476–83. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532:265–70. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–70. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, Nabeshima T, Yoneda Y, Yamada K. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202:114–21. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Lack AK, Gill KE, Porrino LJ. Local cerebral glucose utilization in rats exposed to an enriched environment: A comparison to impoverishment. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer K, Wilson P, Kemp J, Thompson L, Sim F, Gillberg C, Puckering C, Minnis H. Disruptive behaviour disorders: a systematic review of environmental antenatal and early years risk factors. Child Care Health Dev. 2012;38:611–28. doi: 10.1111/j.1365-2214.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–71. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The effect of isolation rearing on volitional ethanol consumption and central CCK/dopamine systems in Fawn-Hooded rats. Behav Brain Res. 2003;141:113–22. doi: 10.1016/s0166-4328(02)00328-5. [DOI] [PubMed] [Google Scholar]
- Lovic V, Keen D, Fletcher PJ, Fleming AS. Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav Neurosci. 2011;125:481–91. doi: 10.1037/a0024367. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–56. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Mangini LD, Taylor JR. Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology. 2005;30:322–9. doi: 10.1038/sj.npp.1300594. [DOI] [PubMed] [Google Scholar]
- MacGillivray L, Reynolds KB, Rosebush PI, Mazurek MF. The comparative effects of environmental enrichment with exercise and serotonin transporter blockade on serotonergic neurons in the dorsal raphe nucleus. Synapse. 2012;66:465–70. doi: 10.1002/syn.21511. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Malone DT, Kearn CS, Chongue L, Mackie K, Taylor DA. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152:265–72. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–82. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miachon S, Rochet T, Mathian B, Barbagli B, Claustrat B. Long-term isolation of Wistar rats alters brain monoamine turnover, blood corticosterone, and ACTH. Brain Res Bull. 1993;32:611–4. doi: 10.1016/0361-9230(93)90162-5. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 2002;926:10–7. doi: 10.1016/s0006-8993(01)03201-2. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–74. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Naka F, Shiga T, Yaguchi M, Okado N. An enriched environment increases noradrenaline concentration in the mouse brain. Brain Res. 2002;924:124–6. doi: 10.1016/s0006-8993(01)03257-7. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Ellison G. Enhanced stereotypies after repeated injections but not continuous amphetamines. Neuropharmacology. 1978;17:1081–4. doi: 10.1016/0028-3908(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153:213–23. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ouchi H, Ono K, Murakami Y, Matsumoto K. Social isolation induces deficit of latent learning performance in mice: a putative animal model of attention deficit/hyperactivity disorder. Behav Brain Res. 2013;238:146–53. doi: 10.1016/j.bbr.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Pandolfo P, Savoldi R, Prediger RD, Takahashi RN. Environmental enrichment improves cognitive deficits in Spontaneously Hypertensive Rats (SHR): relevance for Attention Deficit/Hyperactivity Disorder (ADHD) Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1153–60. doi: 10.1016/j.pnpbp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Murtha SJ, Park GA, Condon KT, Szirtes RM, Laventure SI, Ally A. Neonatal brain dopamine depletion and the cortical and behavioral consequences of enriched postweaning environment. Pharmacol Biochem Behav. 1992;42:741–8. doi: 10.1016/0091-3057(92)90023-9. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:E105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JR, Vallie B, Difronzo M, Donaldson ST. Role of dopamine D1 receptors in novelty seeking in adult female Long-Evans rats. Brain Res Bull. 2007;74:232–6. doi: 10.1016/j.brainresbull.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Van Kempen TA, Zimmerberg B. Behavioral effects of repeated handling differ in rats reared in social isolation and environmental enrichment. Neurosci Lett. 2013;536:47–51. doi: 10.1016/j.neulet.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav Pharmacol. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilke O, Jahkel M, Oehler J. Dopaminergic parameters during social isolation in low- and high-active mice. Pharmacol Biochem Behav. 1998;60:499–505. doi: 10.1016/s0091-3057(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Diminished responsiveness of the hypothalamic-pituitary-adrenal axis of the rat during exposure to prolonged stress: a pituitary-mediated mechanism. Endocrinology. 1987;121:1320–8. doi: 10.1210/endo-121-4-1320. [DOI] [PubMed] [Google Scholar]
- Sadasivan S, Pond BB, Pani AK, Qu C, Jiao Y, Smeyne RJ. Methylphenidate exposure induces dopamine neuron loss and activation of microglia in the basal ganglia of mice. PLoS One. 2012;7:e33693. doi: 10.1371/journal.pone.0033693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98:1084–8. [PubMed] [Google Scholar]
- Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behav Brain Res. 2005;156:289–96. doi: 10.1016/j.bbr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav. 1986;24:1793–6. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–31. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–6. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72:857–63. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Wurbel H. Early social deprivation disrupts attentional, but not affective, shifts in rats. Behav Neurosci. 2001;115:437–42. [PubMed] [Google Scholar]
- Shao F, Jin J, Meng Q, Liu M, Xie X, Lin W, Wang W. Pubertal isolation alters latent inhibition and DA in nucleus accumbens of adult rats. Physiol Behav. 2009;98:251–7. doi: 10.1016/j.physbeh.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Simchon Y, Weizman A, Rehavi M. The effect of chronic methylphenidate administration on presynaptic dopaminergic parameters in a rat model for ADHD. Eur Neuropsychopharmacol. 2010 doi: 10.1016/j.euroneuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–92. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Ator NA, Riddle MA, Wong DF, Weed MR. Long-Term Exposure to Oral Methylphenidate or dl-Amphetamine Mixture in Peri-Adolescent Rhesus Monkeys: Effects on Physiology, Behavior, and Dopamine System Development. Neuropsychopharmacology. 2012;37:2566–79. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T. Pharmacotherapy of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2000;9:77–97. [PubMed] [Google Scholar]
- Sproson EJ, Chantrey J, Hollis C, Marsden CA, Fonel KC. Effect of repeated methylphenidate administration on presynaptic dopamine and behaviour in young adult rats. J Psychopharmacol. 2001;15:67–75. doi: 10.1177/026988110101500202. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–82. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, Kollins S, Nguyen AS, DeCory HH, Hirshe Dirksen SJ, Hatch SJ. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study) Pediatrics. 2004;113:e206–16. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–8. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–33. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci U S A. 2005;102:2117–22. doi: 10.1073/pnas.0409726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Sakakibara S, Yoshimoto K. Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning. Neuroscience. 2005;135:395–402. doi: 10.1016/j.neuroscience.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur J Pharmacol. 1996;318:31–5. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- Urakawa S, Takamoto K, Hori E, Sakai N, Ono T, Nishijo H. Rearing in enriched environment increases parvalbumin-positive small neurons in the amygdala and decreases anxiety-like behavior of male rats. BMC Neurosci. 2013;14:13. doi: 10.1186/1471-2202-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–73. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–8. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–31. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–82. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012;217:337–51. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–9. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, Dwoskin LP, Midde NM, Gomez AM, Mactutus CF, Booze RM, Zhu J. Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res. 2011;219:98–107. doi: 10.1016/j.bbr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–32. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Yates JR, Darna M, Gipson CD, Dwoskin LP, Bardo MT. Isolation rearing as a preclinical model of attention/deficit-hyperactivity disorder. Behav Brain Res. 2012;234:292–8. doi: 10.1016/j.bbr.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013 doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler S, Lipton J, Toga A, Ellison G. Continuous cocaine administration produces persisting changes in brain neurochemistry and behavior. Brain Res. 1991;552:27–35. doi: 10.1016/0006-8993(91)90655-f. [DOI] [PubMed] [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. The multifaceted effects of oral administration of methylphenidate in juvenile rats: anxiety, activity, and attention. Eur Neuropsychopharmacol. 2010;20:236–44. doi: 10.1016/j.euroneuro.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dos Reis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. Jama. 2000;283:1025–30. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]


