Abstract
Background
The metabolic syndrome (MetS) is a constellation of clinical features that include central obesity, hypertension, atherogenic dyslipidemia, and insulin resistance (IR). However, the concept remains controversial; it has been debated whether MetS represents nothing more than simultaneous co-occurrence of individual risk factors, or whether there are common, shared pathophysiologic mechanisms that link the individual components.
Methods and Results
To investigate the emergence of metabolic and cardiovascular components during the development of MetS, we identified MetS-predisposed animals (n=35) in a large population of rhesus macaques (Macaca mulatta, 12.7 ± 2.9 years old, n=408), acclimated them to standardized conditions, and monitored the progression of individual component features over 18 months. In total 18 MetS animals with recently developed fasting hyperinsulinemia, central obesity, hypertension, and atherogenic dyslipidemia, we found that individual metabolic and cardiovascular components track together during the transition from pre-MetS to onset of MetS; MetS was associated with a 60% impairment of flow mediated dilation (FMD), establishing the mechanistic link with vascular dysfunction. Pioglitazone treatment (3 mg/kg body weight/day for 6 weeks), a PPARγ agonist, reversibly improved atherogenic dyslipidemia and IR, and fully restored FMD with persistent benefits.
Conclusions
Co-emergence of metabolic and cardiovascular components during MetS progression and complete normalization of vascular dysfunction with PPARγ agonists suggest shared underlying mechanisms rather than separate processes, arguing for the benefit of early intervention of MetS components. Predictive NHP models of MetS should be highly valuable in mechanistic and translational studies on the pathogenesis of MetS in relation to cardiovascular disease and diabetes.
Keywords: Metabolic Syndrome (MetS), Nonhuman Primates, Cardiovascular Disease, Pathogenesis, PPARγ agonists
The metabolic syndrome (MetS) is a constellation of clinical features that include central obesity, hypertension, atherogenic dyslipidemia, and insulin resistance (IR), as well as systemic inflammation and hypercoagulability 1–4. The prevalence of MetS is increasing dramatically worldwide. By age 60, up to 40% of the US population has MetS, and the prevalence in developing countries like China and India is catching up rapidly 5. MetS increases the risk of developing cardiovascular disease two-fold, and the risk of type 2 diabetes (T2D) five-fold 6. Most definitions of MetS require the presence of three or more of the five component features: central obesity, hypertension, low high density lipoprotein, high triglycerides, and hyperglycemia 7–10. A recent joint statement from the International Diabetes Federation, NHLBI, AHA, WHO, International Atherosclerosis Society, and International Association for the Study of Obesity has harmonized the various definitions, addressing concerns about multiple definitions and taking into account racial and ethnic differences in body habitus 11.
The concept of the MetS has been debated since its inception, and continues to be controversial 6, 12–15. On the one hand, the MetS concept is important because it identifies a subgroup of patients at increased risk of cardiovascular disease who may benefit from intensive medical and lifestyle interventions to lower such risk 1, 5, 14–17. Furthermore, it is generally accepted that each individual component of MetS is mechanistically linked to the development of atherosclerotic cardiovascular disease 18.
On the other hand, there are several arguments that the MetS concept has limited utility from the perspective of a diabetes viewpoint13, 15. First, the risk of cardiovascular disease is no greater with the simultaneous clustering of components of MetS (hypertension, dyslipidemia, and glucose intolerance) than the sum of each risk. Thus, the question arises of what additional information MetS provides for predicting or managing cardiovascular risk over considering each component risk separately. Second, it is not known whether MetS patients all share common pathophysiologic mechanisms. Such unifying underlying mechanisms would have important implications for clinical care. For example, it is not known whether pharmacologic therapy to reduce IR would be beneficial to reduce cardiovascular disease in MetS. Third, a better understanding of the pathogenesis of MetS and its development and progression is needed.
These considerations are more than semantic. They carry important clinical implications for patient care. They are also relevant to the current regulatory landscape for diabetes drugs; while cardiovascular disease is the major cause of death in patients with diabetes, meta-analysis reveals that treatment of diabetes with rosiglitazone is associated with higher cardiovascular mortality 19. Furthermore, several large clinical outcomes studies fail to demonstrate a relationship between glycemic control and cardiovascular disease or death 20, 21. These findings have led to the current FDA position that diabetes drugs must be evaluated for their effects on cardiovascular endpoints, and not simply for efficacy in glycemic control 22, 23.
In this setting, we developed a nonhuman primate (NHP) model of MetS. Phylogenetically, NHPs are more similar to humans in terms of lipoprotein profiles, pathogenesis of atherosclerotic disease, and genetic makeup 24–27. In their pioneering work, Hansen and colleagues demonstrated that, along with aging, some rhesus monkeys spontaneously develop obesity, followed by IR and T2D 28–31. In the present study, we established a rhesus monkey model of spontaneous MetS using population screening approaches. By directly observing the development of MetS in these rhesus monkeys, we were in a position to gain unique insights into the early pathogenesis of MetS and vascular complications. In addition, we investigated the metabolic and vascular responses of this model to an established pharmacologic treatment for diabetes.
Methods
Screening Design
The present study was approved by the Institutional Animal Care and Use Committee of Peking University (PKU) and was in accordance with the principles of laboratory animal care of the National Academy of Sciences/National Research Council. The screening population included 408 male adult rhesus monkeys (Macaca mulatta) from three monkey husbandry sites in China (see Supplement Information for details). The screening parameters include waist circumference (WC), hip circumference, body weight (BW), blood pressure (BP), fasting plasma glucose (FPG), fasting insulin, fasting plasma triglycerides (TGs), fasting plasma high density lipoprotein - cholesterol (HDL-c), fasting low density lipoprotein - cholesterol (LDL-c), total cholesterol (TC). The criteria used for monkey selection were guided by Adult Treatment Panel (ATP) III and the WHO definition for MetS in human11, similar criteria for rhesus monkey31, 32 and the percentiles of our screening parameters (see Results).
Follow-up Tests
Monkey husbandry
Sixty selected monkeys from the screening were brought to the AAALAC International accredited animal facility at PKU, where they were housed individually in cages. The monkeys had free access to water and were fed ad libitum with pellet monkey chow (Beijing HFK Bio-Technology Co. Ltd. China), which contains 7–10% crude fat, 16–20% crude protein, and 55–65% crude carbohydrate.
Blood sampling and biochemical tests
The blood samples were taken from a vein after 14–16 hrs fasting and anaesthesia with ketamine at 10mg/kg body weight. All measurements of plasma lipids and glucose were performed at the Department of Clinical Biochemistry of 301 Hospital, Beijing, China, using kits from Roche. Insulin was measured by Roche Modular Analytics E170 Combinations (Cobas 12017547 122), C-reactive protein (CRP) analyzed by Roche Modular PE (Roche 03002012 122), C-peptide and proinsulin were measured by ELISA (Mercodia 10-1118-01, Mercodia 10-1136-01) and other measurements were performed by a HITACHI 7600-110 auto-analyzer.
Intravenous glucose tolerance test (IVGTT)
IVGTT was performed as described previously 31, 33. IR was calculated by means of the homeostasis model assessment –IR (HOMA-IR): FPG (mmol/L) × fasting insulin (mU/L)/22.5. For glucose disappearance rate, KG = [ln(glucose level at 5 min) − ln(glucose level at 20 min)]/15 min ×100%.
Brachial artery FMD measurement
FMD was performed using a protocol modified from that for humans 34, 35. In brief, monkeys were anesthetized by ketamine at 14mg/kg body weight (a higher dose to reduce motion artifacts during measurement) after overnight fasting. Brachial artery images were acquired at baseline images, 1 min after occlusion and also 1, 2, 3, 4, and 5 min after cuff deflation. Image was analyzed using custom-developed edge detection software. FMD was calculated as [(Dmax-DBL)/DBL] × 100%, where DBL is the baseline brachial artery diameter, and Dmax is the maximum diameter after cuff release.
Validation study with pioglitazone
Twelve obese MetS rhesus monkeys that were cooperative for oral drug administration underwent a baseline phase with vehicle administration for 2 weeks followed by 6 weeks of treatment with pioglitazone at an oral dose of 3mg/kg/day, followed by a 6-week washout period. Metabolic, anthropometric parameters and BP, IVGTT, and FMD were measured at baseline, after 3 and 6 weeks of pioglitazone treatment, and at the end of washout.
Statistical Analysis
All data presented here are expressed as mean ± standard error (SE) unless specified otherwise. Student’s t test and two-way ANOVA with repeated measures followed by Bonferroni’s post hoc correction were used to compare the differences between groups, when appropriate. A p value <0.05 was considered statistically significant.
Results
Overall strategy
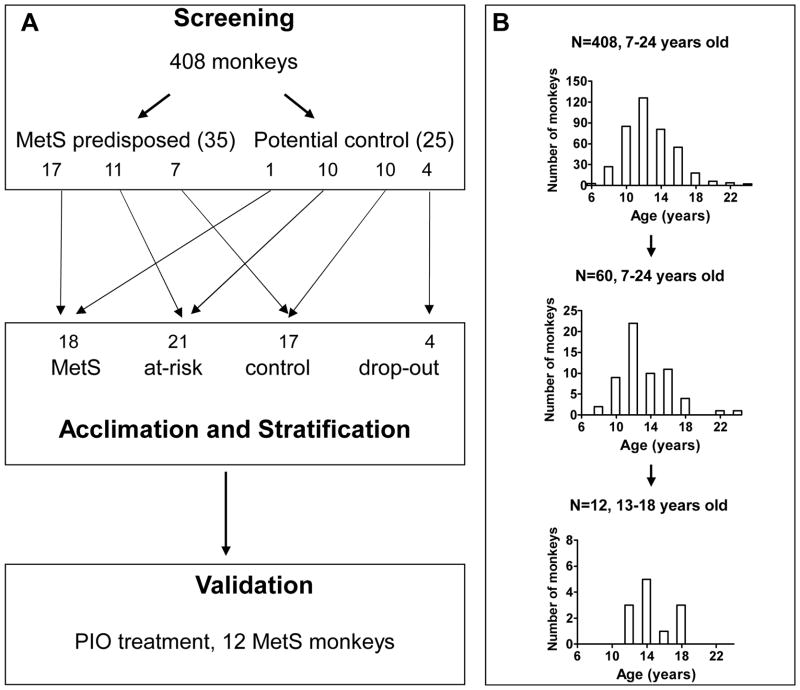
In order to establish a spontaneous NHP model of MetS, we first screened a large population of rhesus macaques using the five defining parameters of MetS (WC, BP, FPG, TG, and HDL-c). We selected animals in the top quintile of these parameters as most predisposed to MetS, and acclimated them under well-controlled laboratory conditions to observe the progression of each parameter. After seven timepoints in-laboratory tests (T1 – T7) over 18 months, we identified MetS animals. We characterized vascular function by measuring FMD of the brachial artery. Finally, the MetS model was subjected to a clinically relevant drug treatment to validate for its predictive power and gain insight into relationship between MetS and cardiovascular dysfunctions. Our overall procedure and the age distributions in different groups are shown in Figure 1.
Figure 1. Strategy for establishing a spontaneous MetS model in rhesus monkey.
(A) Study design. (B) Age distributions for the original in-field group, the in-laboratory tested group and the final drug treatment group. Ages are reported as those at time of experiment.
Screening and acclimation
We performed field screening of 408 male rhesus monkeys of 12.7 ± 2.9 (SD) years age, most metabolic parameters, including TC, TG, HDL-c, LDL-c, and insulin, were lower than previously reported in laboratory rhesus monkeys at comparable ages 31. This pattern closely resembled findings in monkeys on caloric restriction 36–38. Notably, none of the original monkeys screened could be classified as MetS using the criteria in this study later. Table 1 and supplement Figure 1 summarize basic characteristics of all the monkeys screened.
Table 1.
Metabolic and anthropometric parameters in screened rhesus monkeys
| All (n = 408) | MetS-predisposed (n = 35) | Potential control (n = 25) | p | |
|---|---|---|---|---|
| Age (year) | 12.65 ± 0.14 | 13.22 ± 0.50 | 13.75 ± 0.62 | NS |
| SBP (mmHg) | 124.41 ± 1.78 | 143.99 ± 4.75 | 118.92 ± 3.48 | <0.001 |
| DBP (mmHg) | 79.86 ± 1.10 | 91.29 ± 3.24 | 78.94 ± 2.49 | 0.007 |
| WC (cm) | 33.65 ± 0.22 | 38.38 ± 0.79 | 35.49 ± 1.14 | 0.035 |
| BW (kg) | 9.76 ± 0.11 | 11.98 ± 0.37 | 10.26 ± 0.46 | 0.005 |
| TG (mmol/L) | 0.38 ± 0.01 | 0.40 ± 0.03 | 0.34 ± 0.05 | NS |
| HDL-c (mmol/L) | 1.18 ± 0.02 | 1.20 ± 0.05 | 1.31 ± 0.07 | NS |
| LDL-c (mmol/L) | 1.17 ± 0.02 | 1.31 ± 0.10 | 1.20 ± 0.07 | NS |
| TC (mmol/L) | 2.65 ± 0.04 | 2.62 ± 0.13 | 2.62 ± 0.11 | NS |
| FPG (mmol/L) | 3.44 ± 0.04 | 3.68 ± 0.11 | 3.30 ± 0.13 | 0.028 |
| Fasting Insulin (μU/mL) | 15.94 ± 0.90 | 19.00 ± 3.14 | 19.73 ± 3.81 | NS |
| HOMA-IR | 2.54 ± 0.15 | 3.12 ± 0.46 | 3.10 ± 0.66 | NS |
SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; BW, body weight; TG, triglyceride; HDL-c, high density lipoprotein-cholesterol; LDL-c, low density lipoprotein-cholesterol; TC, total cholesterol; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment for insulin resistance. MetS, metabolic syndrome; p, MetS-predisposed vs. potential control; NS, p>0.1.
We sought to identify the animals most predisposed to MetS using the following criteria. Monkeys that met three out of the following five criteria: a) BP ≥ 120/75 mmHg, b) WC ≥ 37 cm, c) FPG ≥ 3.8 mmol/L, or d) TG ≥ 0.45 mmol/L, all above the 80th percentile; or HDL-c ≤ 1.10 mmol/L, below the 20th percentile, were considered MetS-predisposed. Potential control monkeys were chosen among individuals that met three out of the following five criteria: a) BP ≤ 100/64 mmHg, b) WC ≤ 31 cm, c) FPG ≤ 3.27 mmol/L, or d) TG ≤ 0.22 mmol/L, all below the 25th percentile; or e) HDL-c ≥ 1.41 mmol/L, above the 75th percentile.
Using these screening criteria, we identified a total of 35 (8.6%) MetS-predisposed monkeys, along with 25 potential controls. As expected by the way they were selected, MetS-predisposed monkeys showed SBP, diastolic BP (DBP), FPG, WC and BW significantly higher than seen in potential controls. There were no differences between the groups with respect to TG, HDL-c, LDL-c, TC, or insulin (Table 1).
After being brought to the Laboratory Animal Center at PKU, all 60 monkeys were allowed free access to pellet monkey chow after an initial 3 months of acclimation. We then performed seven in-laboratory tests during a one and a half year period. Four potential control monkeys were excluded because of hypersensitivity to ketamine and chronic diarrhea.
Identification of rhesus monkeys with MetS
We set forth the following criteria for MetS and control monkeys: 1) WC ≥ 40 cm and waist/hip ratio ≥ 0.9; 2) FPG ≥ 4.40 mmol/L; 3) TG ≥ 0.90 mmol/L; 4) HDL-c ≤ 1.55 mmol/L; 5) BP ≥ 130/80 mmHg. Individuals displaying three or more components were classified as MetS monkeys, while those displaying none or only one component were classified as control monkeys. The rest, which manifested two MS components, were classified as at-risk monkeys.
By this set of criteria, using the values from T7, we identified 18 MetS, 17 control, and 21 at-risk monkeys. Of these, 17 out of 18 MetS monkeys (94%) were from the original MetS-predisposed group, while 10 out of 17 control monkeys (59%) were from the original potential control group. Monkeys in all groups were of comparable age (Table 2).
Table 2.
Metabolic, anthropometric and inflammatory parameters in MetS and control monkeys
| MetS -T7 (n = 18) | Control -T7 (n = 17) | p | |
|---|---|---|---|
| Age (year) | 14.58 ± 0.47 | 14.89 ± 0.74 | NS |
| SBP (mmHg) | 160.00 ± 7.08 | 141.41 ± 4.97 | 0.036 |
| DBP (mmHg) | 89. 56 ± 4.86 | 75.18 ± 2.98 | 0.015 |
| WC (cm) | 52.14 ± 2.35 | 41.97 ± 2.49 | 0.004 |
| BW (kg) | 16.39 ± 0.98 | 12.27 ± 0.98 | 0.004 |
| TG (mmol/L) | 1.04 ± 0.15 | 0.58 ± 0.05 | 0.005 |
| HDL-c (mmol/L) | 1.56 ± 0.09 | 2.13 ± 0.12 | < 0.001 |
| LDL-c (mmol/L) | 1.33 ± 0.14 | 1.11 ± 0.07 | NS |
| TC (mmol/L) | 3.31 ± 0.19 | 3.50 ± 0.16 | NS |
| FPG (mmol/L) | 4.46 ± 0.21 | 3.90 ± 0.10 | 0.019 |
| Fasting Insulin (μU/mL) | 58.93 ± 15.82 | 18.54 ± 3.57 | 0.018 |
| HOMA-IR | 12.31 ± 3.60 | 3.18 ± 0.58 | 0.020 |
| CRP (mg/dl) | 0.17 ± 0.03 | 0.09 ± 0.02 | 0.023 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; BW, body weight; TG, triglyceride; HDL-c, high density lipoprotein-cholesterol; LDL-c, low density lipoprotein-cholesterol; TC, total cholesterol; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment for insulin resistance; CRP, C-reactive protein. MetS, metabolic syndrome; T7, test 7; p, MetS vs. control in Test 7, respectively; NS, p >0.1.
Metabolic characterization of MetS in rhesus monkeys
Compared to controls, MetS monkeys exhibited clear symptoms of obesity, with greater BW and WC. MetS monkeys showed evidence of IR, with markedly higher insulin levels but comparable glucose levels, with resulting increased levels of the HOMA-IR index. MetS monkeys showed elevated TG and lower HDL-c than control animals, while TC and LDL-c were not significantly different. These changes are summarized in Table 2.
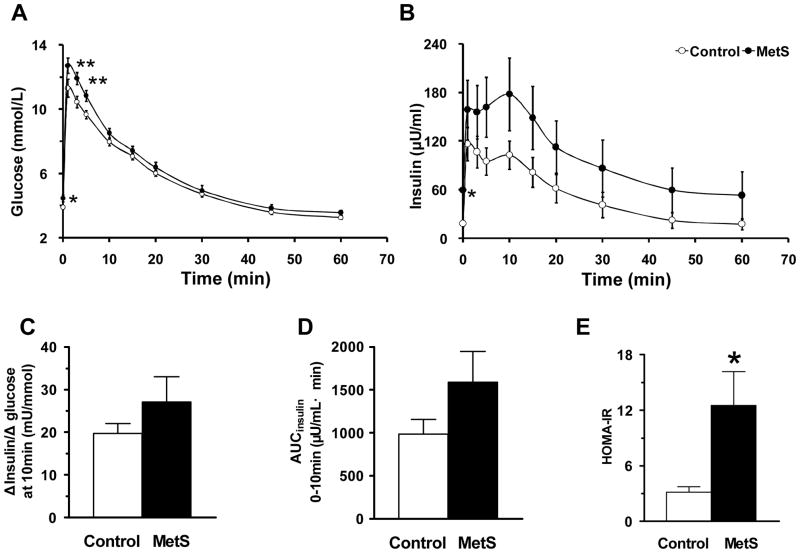
We performed IVGTT to assess the metabolic response to glucose and insulin sensitivity. As seen in Figure 2A, FPG and plasma glucose level 5 min after the glucose challenge were higher in the MetS group (Student’s t test p >0.05). The glucose-induced rise of insulin level showed a trend of increase in the MetS group at multiple time points (Figure 2B). The ratio between Δinsulin and Δglucose at 10 min, an index of insulin sensitivity was higher in the MetS group (Figure 2C), as was the area under the insulin curve (AUCinsulin) (0 – 10 min) (Figure 2D). These measures of insulin sensitivity parallel the HOMA-IR index (Figure 2E).
Figure 2. Plasma glucose and insulin responses during IVGTT.
(A) Glucose excursion. (B) Insulin excursion. (C) Δinsulin/Δglucose (at 10 min). (D) Area under the insulin curve (AUCinsulin) (0 – 10 min). (E) HOMA-IR. Data are expressed as mean ± SEM; n = 18 for MetS and 17 for control. * p <0.05; ** p <0.01.
Taken together, the current NHP model of MetS reproduces virtually all salient features of MetS in humans. These results lend support to the strategy of first identifying NHP most predisposed to MetS, followed by acclimation to standardized conditions with dietary liberalization for cohort stratification. Notably, none of the MetS monkeys have FPG levels above the cutoff value of 6.99 mmol/L for T2D in rhesus monkeys 31, 32, suggesting that this NHP model represents an early stage in the onset of MetS, prior to the development of T2D.
Development and progression of MetS
Because all our MetS monkeys evolved from a non-MetS status at the entry of this study, we were in a unique position to observe the development of MetS. We analyzed data from the T1 to T7, and sought patterns in the emergence of individual components, component pairs, and component triads (i.e., MetS per se).
Of our 18 MetS monkeys, 1, 4, and 13 monkeys displayed 5, 4, and 3 components of MetS, respectively. The two most prevalent components were greater waist size (94%) and high BP (73%). Among ten possible component pairs, the most common combination was waist size + elevated BP (67%), followed by waist size + high FPG (56%), waist size + low HDL (50%), and waist size + high TG (50%). The most common triads were waist size + elevated BP + high FPG (7/18), waist size + elevated BP + low HDL (6/18), and waist size + elevated BP + high TG (5/18). These patterns are similar to those found in humans 39.
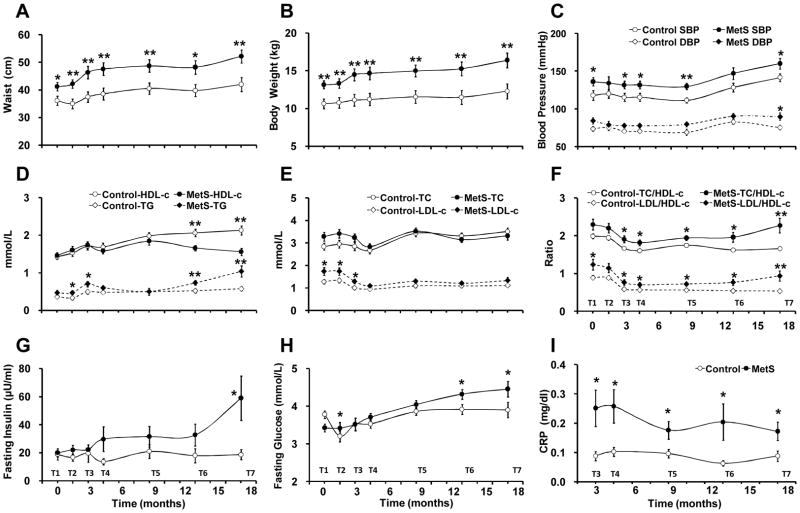
In following the progression of these parameters in the development of MetS, we made several observations. First, BW and WC were greater in MetS than control animals at all seven time points, from the T1 to the T7 (Figure 3A and 3B). SBP was significantly higher in the MetS group than in control animals, while DBP was less well separated (Figure 3C). Second, both TG and HDL-c showed early variability related to acclimation, but were not distinguishable between MetS and control animals until after 9 months. Eventually, MetS monkeys showed progressive increase in TG and decrease in HDL-c. In contrast, control animals showed stable TG and a rise in HDL-c (Figure 3D). TC and LDL-c levels in MetS and control groups converged to similar levels (Figure 3E), but the ratio of TC/HDL-c and LDL-c/HDL-c were higher in MetS than control animals at all seven tests (Figure 3F). Third, there was a clear increase of the insulin level during progression to MetS, but not in control monkeys (Figure 3G). The FPG level increased in the MetS group, while it remained stable in the control group, such that there were significant differences at later timepoints (Figure 3H). Although not a defining parameter, CRP exhibited early and sustained high levels in the MetS group compared to the control group (Figure 3I).
Figure 3. Changes of MetS-related parameters.
(A) Waist circumference. (B) Body weight. (C) Blood pressures; SBP: systolic blood pressure; DBP, diastolic blood pressure. (D) HDL and TG. (E) TC and LDL. (F) Ratios of TC/HDL and LDL/HDL. (G) Fasting insulin. (H) FPG. (I) CRP. T1 – T7: in-laboratory follow-up Test 1 – Test 7 (0 –18 months); data are expressed as mean ± SEM; n = 18 for MetS and 17 for control. * p <0.05; ** p <0.01.
Vascular function in rhesus monkeys with MetS
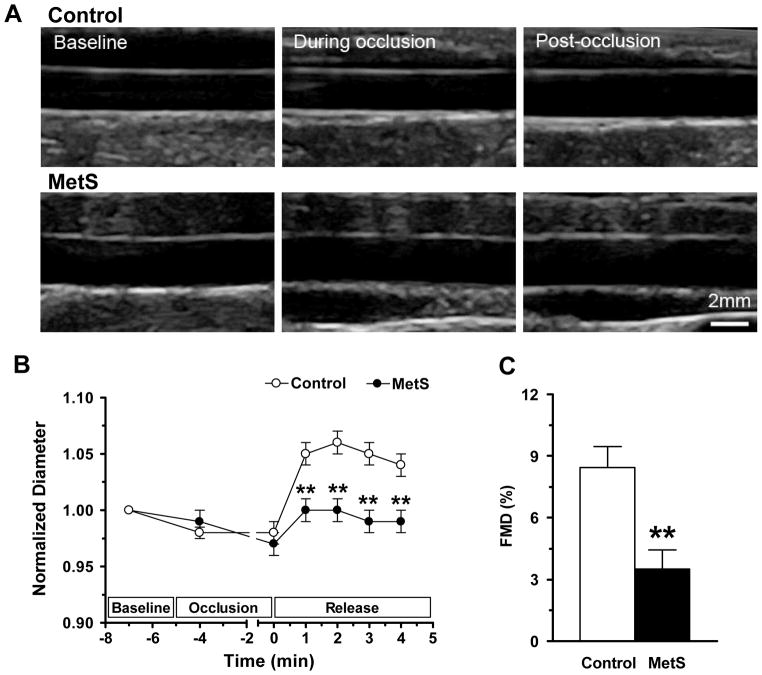
To study how development of MetS affects vascular function, we measured FMD in the brachial artery as a non-invasive assessment of endothelial function. As shown in Figure 4, compared with control monkeys (n = 11), FMD in MetS monkeys (n = 15) showed a markedly abbreviated duration and a 58.6% lower amplitude (Figures 4B and 4C), indicating overtly impaired endothelium-dependent vasodilatation. These results are consistent with previous reports that obesity, IR, and MetS are closely associated with endothelial dysfunction in humans4, 40,41, and support the clinical finding that loss of endothelial reactivity is an early event in progression of MetS.
Figure 4. Flow mediated dilation in MetS monkeys.
(A) Ultrasonic images of the brachial artery during FMD measurement. (B) Average time course of arterial diameter during the FMD measurement. The duration of FMD response was abbreviated in the MetS monkeys. (C) The amplitude of FMD was significantly reduced in MetS monkeys. Data are expressed as mean ± SEM. n = 11 in control and n = 15 in MetS group. *p <0.05, ** p <0.01.
Model validation by pioglitazone treatment
In obese-IR rhesus monkey and in humans with MetS, the PPARγ agonist pioglitazone improves insulin sensitivity and beta-cell function and lowers plasma TG 42, 43. We evaluated the effect of pioglitazone in 12 MetS monkeys. As shown in Table 3 and Figure 5, pioglitazone significantly decreased TG levels and increased HDL-c (Figure 5A). Concurrently, proinsulin and C-peptide were significantly decreased (Figure 5B). By IVGTT, pioglitazone significantly decreased plasma glucose level in the first 5 min after the glucose challenge and glucose-induced insulin levels at multiple time points; the glucose and insulin curves were significantly lowered by pioglitazone (two-way ANOVA with repeated measures, Bonferroni corrected p value was 1.5e-4 or 7.9e-12 for glucose or insulin curve, respectively) (Figures 5C and 5D). The HOMA-IR was significantly decreased after pioglitazone treatment (Figure 5E), and the ratio between Δinsulin and Δglucose (at 10 min) was also significantly lower (Figure 5F). The AUCinsulin (0 – 10 min) was significantly decreased by pioglitazone (Figure 5G), while glucose disappearance rate was unaffected (data not shown). Thus, a 6-week pioglitazone treatment corrected dyslipidemia, normalized glucose tolerance, and restored insulin sensitivity in MetS monkeys. Moreover, data at the end of washout showed that these effects of pioglitazone were largely reversible (Figure 5, Table 3). It is noteworthy that pioglitazone didn’t normalize the blood pressure in the MetS monkeys (Table 3), which is consistent with the findings in MetS patients 43. The present results in onset of MetS are in good agreement with previous observations in rhesus monkeys with overt diabetes or severe hyperglycemia (FPG > 8.3mmol/L, 3 out of 6 monkeys) and severe dyslipidemia (highest TG values > 17mmol/L), except for a lowering of blood pressure by pioglitazone in the diabetes model 42.
Table 3.
Effects of PIO treatment on MetS monkeys.
| Baseline (n = 12) | 6 weeks PIO (n = 12) | 6 weeks washout (n = 12) | p1 | p2 | |
|---|---|---|---|---|---|
| SBP (mmHg) | 140.00 ± 8.33 | 145.33 ± 6.09 | 146.67 ± 5.48 | NS | NS |
| DBP (mmHg) | 88.33 ± 6.61 | 76.00 ± 4.53 | 79.50 ± 4.39 | NS | NS |
| MAP (mmHg) | 105.56 ± 6.09 | 99.11 ± 3.77 | 101.89 ± 3.88 | NS | NS |
| BW (kg) | 16.86 ± 0.98 | 16.94 ± 0.97 | 17.31 ± 0.98 | NS | <0.001 |
| TG (mmol/L) | 0.75 ± 0.11 | 0.54 ± 0.07 | 0.67 ± 0.11 | 0.004 | 0.061 |
| HDL-c (mmol/L) | 1.76 ± 0.07 | 1.92 ± 0.07 | 1.68 ± 0.07 | 0.071 | 0.006 |
| LDL-c (mmol/L) | 1.13 ± 0.05 | 1.18 ± 0.05 | 1.14 ± 0.06 | NS | NS |
| TC (mmol/L) | 3.15 ± 0.11 | 3.25 ± 0.09 | 3.12 ± 0.10 | NS | NS |
| FPG (mmol/L) | 3.63 ± 0.10 | 3.58 ± 0.13 | 3.83 ± 0.12 | NS | 0.051 |
| Fasting Insulin (μU/mL) | 56.57 ± 19.54 | 25.56 ± 4.76 | 46.67 ± 12.95 | 0.069 | 0.042 |
| HOMA-IR | 8.89 ± 2.97 | 3.95 ± 0.70 | 7.13 ± 1.95 | 0.052 | 0.043 |
| HbA1c (%) | 5.49 ± 0.08 | 5.16 ± 0.08 | 5.54 ± 0.09 | 0.001 | 0.007 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; BW, body weight; TG, triglyceride; HDL-c, high density lipoprotein-cholesterol; LDL-c, low density lipoprotein-cholesterol; TC, total cholesterol; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1c, hemoglobin A1c. PIO, pioglitazone; MetS, metabolic syndrome; p1, 6 weeks PIO vs. baseline; p2, 6 weeks PIO vs. washout; NS, p>0.1.
Figure 5. Effects of pioglitazone in MetS monkeys.
(A–G) Effects of Pioglitazone (PIO) treatment on TG and HDL-c (A), C-peptide and proinsulin levels (B), glucose excursion during IVGTT (C), insulin excursion during IVGTT (D), HOMA-IR (E), Δinsulin/Δglucose (at 10 min) during IVGTT (F), area under the insulin curve (AUCinsulin) (0 – 10 min) (G). Note that the effects of PIO were largely reversible. (H) & (I) PIO treatment normalized the FMD duration (H) and amplitude (I) in the MetS monkeys. Note that the effect was persistent after a 6-week washout. Data are expressed as mean ± SEM (n = 12 monkeys). PIO vs baseline (BL): * p<0.05, ** p<0.01, PIO vs washout: # p<0.05, ## p<0.01 (paired T-test).
It was of interest to determine whether and to what extent pioglitazone affects endothelial reactivity in NHPs with MetS. Pioglitazone had a profound effect on both the duration and the amplitude of FMD, such that it completely restored normal endothelial responsiveness at 6 weeks into the treatment (Figures 5H and 5I). When compared to the durability of other pioglitazone effects mentioned above, it was remarkable that the pioglitazone effect on vascular function persisted for 6 weeks after drug washout (Figures 5H and 5I).
Discussion
MetS is important from a clinical and public health standpoint, both because of its sheer prevalence and its effect on risk for cardiovascular disease and T2D 5, 6. The current major controversies over the concept are centered around 1) whether MetS represents a collection of independent cardiovascular risk factors, 2) whether there is shared underlying pathophysiology between the components, and 3) a need for better understanding how MetS predisposes to cardiovascular disease and T2D. The current NHP MetS model has implications for all of these issues.
By identifying 35 MetS-predisposed animals and 25 potential controls among 408 rhesus monkeys of 12.7 years age, we were in a unique position to track and observe how the individual MetS-related parameters evolved during this period under standardized conditions of diet and physical activity. Our data suggests that the MetS NHP share common underlying processes that track together during the transition from pre-MetS to onset of MetS, rather than displaying independent cardiovascular risk factors. During the first three months of acclimation (T1–T3), there appeared to be a resetting of metabolic homeostasis (Figure 3) in both control and MetS groups, likely due to a change in nutritional status and activity. Over time, BW and waist size increased at a faster rate in the eventual MetS monkeys compared with control animals; BP changes similarly in both groups, but the between-group difference remained largely unchanged (Figure 3C). Retrospective analysis of entry characteristics (data from T1) revealed that the group of monkeys that went on to develop overt MetS displayed greater BW and WC, higher BP, but similar insulin, glucose, HDL-c and TG levels and HOMA-IR as compared to control animals (Figure 3). This confirms that visceral obesity and hypertension are central to predisposition to MetS and its pathogenesis, in agreement with the rationale for the definitions of MetS 11.
In contrast to BW, WC, and BP, the fasting insulin were increased in the MetS group from 4 months onward (T4), and rose sharply in the MetS monkeys at 18 months (T7), while it remained steady in the control animals. The fasting glucose levels were also higher in the MetS monkeys than controls, particularly at later timepoints, but were lower than the cutoff for T2D. Thus, the MetS monkeys maintain euglycemia by hyperinsulinemia, which is very similar to the development of T2D in humans.
Previous studies have shown that when rhesus monkeys are placed on caloric restriction with 30% less food, they exhibit significantly reduced BW, body mass index, body fat, and TG than the free-feeding controls. They also show lower levels of insulin and plasma glucose, and increased insulin sensitivity 36–38. Thus the monkeys screened at husbandry sites might have had similar caloric restriction as they were fed with limited rations (see Supplement Information), which prevented full expression of the MetS phenotype. In laboratory conditions, the total amount of food intake was relatively high in MetS (Supplement Figure 2A). However, when the food intake normalized to BW was actually less in the MetS animals than in control monkeys (Supplement Figure 2B). Thus, the divergence between the MetS and control animals was not due merely to a difference in food or caloric intake. Rather, it suggests that inherent genetic and metabolic predispositions interact with environmental factors of food intake and energy expenditure to determine the spontaneous emergence of MetS.
In this study, we applied the FMD technique to assess endothelial reactivity in NHPs for the first time. MetS monkeys showed a 60% decrease of FMD compared to control monkeys, confirming the mechanistic link between MetS with vascular dysfunction 18. Pioglitazone not only normalizes metabolic parameters, but also restores normal FMD after 6 weeks of treatment. These results suggest that early intervention in lipid and glucose metabolism will not only restore insulin sensitivity, but also vascular function.
Despite efficacy in improving IR and glycemic control in man, rosiglitazone appears to increase overall cardiovascular mortality in patients with T2D by meta-analysis of large clinical studies 19, 22, 23. In addition, the two largest randomized clinical trials, the United Kingdom Perspective Diabetes Study and the University Group Diabetes Program, fail to show a convincing link between glycemic control and reduction in cardiovascular events 21. Other large clinical trials, including the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Study, suggest aggressive lowering of glycemia to near normal levels in patients at high risk for cardiovascular mortality 20. These findings suggest that by the time T2D is established, the processes underlying atherogenesis may have proceeded to such an extent that pharmacologic treatment directed to IR or glycemic control may be too late. The improvement of FMD in the MetS rhesus monkey suggests that treatment of MetS, prior to the development of frank T2D, may have profound effects on vascular function and risk for atherogenesis. Of note, the MetS animals are not diabetic based on FPG levels, and by serial monitoring, they only recently developed hyperinsulinemia at T7. The finding of persistent improvement despite washout of drug is an unexpected finding that would be fascinating to replicate in man.
The availability of a primate model for MetS should accelerate both basic and translational research on MetS and cardiovascular disease. Hansen and colleagues demonstrated that with age, some rhesus monkeys spontaneously develop obesity, followed by IR and T2D 28–31. These animals undergo a sequential set of metabolic phases, starting with fasting hyperinsulinemia, impaired glucose tolerance, then fasting hyperglycemia, followed by frank diabetes. The current MetS rhesus monkey model described here would represent an early stage in this sequence, having just recently developed fasting hyperinsulinemia in the setting of central obesity, hypertension, and atherogenic dyslipidemia. As such, it should be a valuable tool for further research into mechanisms of MetS.
Compared to clinical studies in humans, unique features of the MetS NHP model include drug-naive status, ability to control and modulate nutritional composition and physical activity, and freedom from confounding risk factors such as smoking. In addition, a NHP provides better availability of tissue for mechanistic studies, and the ability to perform translational and clinical trials that cannot be performed in humans. Unanswered questions that could be addressed with this NHP MetS model include: comparison of rosiglitazone with pioglitazone; efficacy of metformin, thiazolidinediones, and other T2D agents in reducing cardiovascular disease in MetS prior to the occurrence of T2D; mechanisms by which agents such as rosiglitazone and torcetrapib result unexpectedly in overall increases in cardiovascular mortality; and mechanisms that underlie why glycemic control does not correlate with cardiovascular mortality.
Supplementary Material
Acknowledgments
We express our sincere appreciation to Drs Ken R Chien, Rui-ping Xiao, Gianni Gromo, Karin Conde Knape and Zhiming Zhu for scientific consultancy, Drs. Sabine Sewing, Guido Hartmann as well as Denise Blum, Urs Sprecher and Andrée Roeckel, for analytical support, Yang Nan for technical support, and Zhuan Zhou, Claire Xi Zhang, Jun Li, Profs Desheng Zhu, Jiedong Zhang, Caihong Wu and Xiaocheng Gu for support of the animal care program.
Funding Sources
The present study was supported by F. Hoffmann-La Roche Ltd., the National Basic Research Program of China 2007CB512100, and the National Natural Science Foundation of China (30973584 and 30870996).
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL. A comprehensive definition for metabolic syndrome. Disease models & mechanisms. 2009;2(5–6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM. Metabolic syndrome pandemic. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(4):629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. Journal of the American College of Cardiology. 2006;47(6):1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16(5):442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection E Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 10.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. Journal of atherosclerosis and thrombosis. 2005;12(6):295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM. Does the metabolic syndrome exist? Diabetes care. 2006;29(7):1689–1692. doi: 10.2337/dc05-2307. discussion 1693–1686. [DOI] [PubMed] [Google Scholar]
- 13.Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation. 2007;115(13):1806–1810. doi: 10.1161/CIRCULATIONAHA.106.658336. discussion 1811. [DOI] [PubMed] [Google Scholar]
- 14.Kahn R. Metabolic syndrome--what is the clinical usefulness? Lancet. 2008;371(9628):1892–1893. doi: 10.1016/S0140-6736(08)60731-X. [DOI] [PubMed] [Google Scholar]
- 15.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM. Metabolic syndrome: therapeutic considerations. Handbook of experimental pharmacology. 2005;(170):107–133. doi: 10.1007/3-540-27661-0_3. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(11):2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 18.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends in endocrinology and metabolism: TEM. 2009;20(6):295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 20.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Rosiglitazone for type 2 diabetes mellitus. Cochrane database of systematic reviews (Online) 2007;(3):CD006063. doi: 10.1002/14651858.CD006063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen CJ. The rosiglitazone story--lessons from an FDA Advisory Committee meeting. The New England journal of medicine. 2007;357(9):844–846. doi: 10.1056/NEJMp078167. [DOI] [PubMed] [Google Scholar]
- 23.Rosen CJ. Revisiting the Rosiglitazone Story -- Lessons Learned. The New England Journal of Medicine. 2010 doi: 10.1056/NEJMp1008233. [DOI] [PubMed] [Google Scholar]
- 24.Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. The Journal of nutrition. 2005;135(11):2499–2502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- 25.Dhawan V, Handu SS, Nain CK, Ganguly NK. Chronic L-arginine supplementation improves endothelial cell vasoactive functions in hypercholesterolemic and atherosclerotic monkeys. Molecular and cellular biochemistry. 2005;269(1–2):1–11. doi: 10.1007/s11010-005-1810-4. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs RA, Rogers J, Katze MG, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science (New York, NY. 2007;316(5822):222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 27.Lee AS, Gutierrez-Arcelus M, Perry GH, Vallender EJ, Johnson WE, Miller GM, Korbel JO, Lee C. Analysis of copy number variation in the rhesus macaque genome identifies candidate loci for evolutionary and human disease studies. Human molecular genetics. 2008;17(8):1127–1136. doi: 10.1093/hmg/ddn002. [DOI] [PubMed] [Google Scholar]
- 28.de Koning EJ, Bodkin NL, Hansen BC, Clark A. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta-cell population. Diabetologia. 1993;36(5):378–384. doi: 10.1007/BF00402271. [DOI] [PubMed] [Google Scholar]
- 29.Hansen BC, Jen KL, Schwartz J. Changes in insulin responses and binding in adipocytes from monkeys with obesity progressing to diabetes. International journal of obesity. 1988;12(5):433–443. [PubMed] [Google Scholar]
- 30.Jen KL, Hansen BC, Metzger BL. Adiposity, anthropometric measures, and plasma insulin levels of rhesus monkeys. International journal of obesity. 1985;9(3):213–224. [PubMed] [Google Scholar]
- 31.Tigno XT, Gerzanich G, Hansen BC. Age-related changes in metabolic parameters of nonhuman primates. The journals of gerontology. 2004;59(11):1081–1088. doi: 10.1093/gerona/59.11.1081. [DOI] [PubMed] [Google Scholar]
- 32.Ding SY, Tigno XT, Hansen BC. Nuclear magnetic resonance-determined lipoprotein abnormalities in nonhuman primates with the metabolic syndrome and type 2 diabetes mellitus. Metabolism: clinical and experimental. 2007;56(6):838–846. doi: 10.1016/j.metabol.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Hansen BC, Bodkin NL. Heterogeneity of insulin responses: phases leading to type 2 (non-insulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia. 1986;29(10):713–719. doi: 10.1007/BF00870281. [DOI] [PubMed] [Google Scholar]
- 34.Olthof MR, Bots ML, Katan MB, Verhoef P. Effect of folic acid and betaine supplementation on flow-mediated dilation: a randomized, controlled study in healthy volunteers. PLoS clinical trials. 2006;1(2):e10. doi: 10.1371/journal.pctr.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, Paun M, Gill EA, Kaufman JD. Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC cardiovascular disorders. 2007;7:11. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. The journals of gerontology. 2003;58(3):212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 37.Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. American journal of physiology. 2001;281(4):E757–765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 38.Lane MA, Ingram DK, Roth GS. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci. 1999;52(2 Suppl):41–48. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- 39.Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino RB., Sr Trajectories of entering the metabolic syndrome: the framingham heart study. Circulation. 2009;120(20):1943–1950. doi: 10.1161/CIRCULATIONAHA.109.855817. [DOI] [PubMed] [Google Scholar]
- 40.Mahmud FH, Hill DJ, Cuerden MS, Clarson CL. Impaired vascular function in obese adolescents with insulin resistance. The Journal of pediatrics. 2009;155(5):678–682. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 41.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110(4):380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 42.Kemnitz JW, Elson DF, Roecker EB, Baum ST, Bergman RN, Meglasson MD. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes. 1994;43(2):204–211. doi: 10.2337/diab.43.2.204. [DOI] [PubMed] [Google Scholar]
- 43.Jin J, Yu Y, Yu H, Wang C, Zhang X. Effects of pioglitazone on beta-cell function in metabolic syndrome patients with impaired glucose tolerance. Diabetes Res Clin Pract. 2006;74(3):233–241. doi: 10.1016/j.diabres.2006.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.