Abstract
Although there is strong evidence that short-term exposure to particulate matter is associated with health risks, less is known about whether some subpopulations face higher risks. We identified 108 papers published after 1995 and summarized the scientific evidence regarding effect modification of associations between short-term exposure to particulate matter and the risk of death or hospitalization. We performed a meta-analysis of estimated mortality associations by age and sex. We found strong, consistent evidence that the elderly experience higher risk of particular matter–associated hospitalization and death, weak evidence that women have higher risks of hospitalization and death, and suggestive evidence that those with lower education, income, or employment status have higher risk of death. Meta-analysis showed a statistically higher risk of death of 0.64% (95% confidence interval (CI): 0.50, 0.78) for older populations compared with 0.34% (95% CI: 0.25, 0.42) for younger populations per 10 μg/m3 increase of particulate matter with aerodynamic diameter ≤10 μm. Women had a slightly higher risk of death of 0.55% (95% CI: 0.41, 0.70) compared with 0.50% (95% CI: 0.34, 0.54) for men, but these 2 risks were not statistically different. Our synthesis on modifiers for risks associated with particulate matter can aid the design of air quality policies and suggest directions for future research. Studies of biological mechanisms could be informed by evidence of differential risks by population, such as by sex and preexisting conditions.
Keywords: age, effect modifiers, hospital admissions, mortality, particulate matter, PM10, PM2.5, socioeconomic status
Particulate matter is estimated to cause more than 3.7 million deaths per year worldwide (1). The Environmental Protection Agency (Washington, DC) estimated that the benefits of the Clean Air Act were more than 30 times higher than the costs, with many of those benefits from averted deaths from decreased particulate matter (2). Still, more than 74 million people in the United States live in areas with levels of particulate matter that exceed regulations (3). Although the evidence that particulate matter affects health is strong and consistent (4–8), the evidence regarding susceptibility, vulnerability, and modifying factors is inconclusive (9). The Environmental Protection Agency is mandated to set health-based regulations with adequate margins of safety for sensitive individuals, and physicians need information on which populations are most affected. Furthermore, understanding vulnerable populations may provide scientific evidence related to credible pathological mechanisms.
The terms “susceptibility” and “vulnerability” are often used interchangeably for populations with disproportionate health burdens; however, “susceptibility” often refers to factors inherent to physical predisposition (e.g., genetics), and “vulnerability” often refers to external factors (e.g., occupational exposure) (10). Here, we refer to “effect modifiers” as individual-level or area-level factors related to susceptibility or vulnerability.
A challenge in understanding effect modification is the tremendous heterogeneity among study designs and populations, with a variety of health outcomes, pollutants, confounders, regions, and effect modifiers. Studies draw conclusions from data aggregated at different temporal and spatial resolution. Despite mounting evidence, there is no consensus on which effect modifiers are most important. The assessment of susceptibility to air pollutants is a priority research area for the Environmental Protection Agency and a key focus of the agency's Clean Air Research Centers and Particulate Matter Centers (11, 12).
We reviewed scientific evidence and identified consistencies across published epidemiologic studies regarding effect modification of associations between short-term exposure to particulate matter and death and hospitalization, and we performed meta-analyses for select modifiers. Systematic reviews with meta-analyses are useful for decision makers, physicians, and researchers to synthesize large quantities of information and to convey consistent findings (13).
MATERIALS AND METHODS
We searched the National Library of Medicine's MEDLINE database through PubMed (14) for population-based studies of short-term exposure to particulate matter with aerodynamic diameter ≤10 μm (PM10) or ≤2.5 μm (PM2.5) and mortality, hospital admissions, or emergency room visits. We conducted 3 searches. Search A included the terms “effect modification,” “effect modifier,” “effect modifiers,” “effect” and “modifiers,” or “effect” and “modifying”; and “time series,” “case crossover,” “air pollution,” “air pollutant,” “air pollutants,” “PM,” “PM10,” “PM2.5,” “particles,” or “particulate matter.” Search B included the terms “modified,” “modification,” “modify,” or “modifying”; and “effect” or “effects”; and “PM10” or “PM2.5.” Search C included the terms “emergency department,” “emergency visits,” “emergency room,” “hospital,” “hospitals,” “hospitalizations,” or “mortality”; and “time series” or “case crossover”; and “short term”; and “PM,” PM10,” “PM2.5,” “particulate matter,” or “particles.”
To meet the inclusion criteria, studies had to be population-based; explore PM2.5 or PM10; consider short-term exposure (i.e., same day or few days); explore deaths, hospital admissions, and/or emergency department visits; examine data on adults; report results on effect modification of risk estimates; be written in English; be peer-reviewed; and be indexed by July 26, 2012. We included both single-city and multicity studies. In addition, 1 relevant article known to us but not returned through searches was added.
We obtained each article's study location, time frame, form of particulate matter, lag structure, health outcome, study population, effect modifiers considered, results for effect modification, and statistical methods to assess particulate matter health associations (e.g., time-series, case-crossover) and effect modification (e.g., stratification, interaction). Modifiers were categorized as individual level (e.g., a person's age), daily (e.g., daily temperature), or community level (e.g., county's unemployment rate). Results on emergency room visits were reported with hospitalizations. For each study included in the meta-analysis, we also extracted the estimated relative risk of death (e.g., percent increase in risk), a measure of the uncertainty associated with the estimated risk (e.g., confidence interval, standard error of the estimated regression coefficient), and the increment of pollution (e.g., 10 μg/m3) used in effect estimates.
We performed meta-analysis by random effects modeling (15) for a subset of modifiers (sex and age) for which studies used similar methods of assigning levels of the modifiers. In cases in which modifiers were defined differently by study (e.g., employment categorized as percent unemployed vs. occupational categories), we could not meaningfully combine the estimated effects quantitatively. Meta-analyses were conducted for total mortality or total nonaccidental mortality and not cause-specific mortality. We did not perform meta-analyses for hospital admissions, because most such studies considered specific hospitalization causes. Meta-analyses were considered if estimates were available from at least 5 studies that used individual-level data.
Results reported in various forms (e.g., percent increase in risk, relative risk) were converted to equivalent regression coefficients and their standard errors for pooling. If studies presented risk estimates for multiple lags, meta-analysis incorporated results from the key lag presented by study authors or the single-day lag closest to the day of death (i.e., lag 0, if available). For studies with city-specific estimates, those estimates were included separately. Overall meta-analysis estimates were calculated for PM10. Studies’ PM2.5 estimates were converted to PM10 by using a PM2.5/PM10 ratio calculated from information in the original article when available or 0.6 otherwise; the true PM2.5/PM10 ratio varies by location and meteorological conditions (16–18).
We calculated the uncertainty parameter (I2) representing the percent of total variance in the observed results explained by heterogeneity (19). Publication bias was assessed with Egger's test for asymmetry (20), funnel plots (21), and the “trim and fill” method, which estimates overall risk adjusted for potential publication bias (22).
The meta-analysis combined effect estimates from time-series or case-crossover studies. Case-crossover analysis that uses conditional logistic regression has been shown to be equivalent to time-series analysis (23), and comparison of estimates for air pollution's association with hospitalizations and death showed comparable results when using the 2 approaches (24, 25).
The systematic search and meta-analysis were conducted with consideration of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26, 27).
A priori, we identified the following key potential modifiers: sex, age, race, and the socioeconomic status (SES) indicators of education, income, employment, and poverty. For these modifiers, we synthesized the overall evidence by using categories loosely based on those established by Institute of Medicine committees (28) and applied by the US Congress, other US government entities, and researchers. The categories are, in increasing order of certainty, no, weak, limited/suggestive, and strong evidence of effect modification. The overall state of scientific evidence for each effect modifier was assigned to a category on the basis of our assessment of the quality and quantity of studies providing consistent and significant evidence compared with those of conflicting findings. These categories are intended to provide qualitative information based on the consistency of scientific evidence, not definitive assessments, and provide a way to summarize evidence for effect modifiers for which meta-analysis was unfeasible.
RESULTS
Search results
The searches identified 772 papers published from 1996 to 2012, including 716 unique papers, of which 109 met our inclusion criteria. We omitted 1 peer-reviewed agency report (29) for which relevant results were duplicated in an identified peer-reviewed paper, bringing the total number of studies to 108. We found 63 studies for death and 48 for hospitalization, including 3 studies examining death and hospitalization. Web Tables 1 and 2, available at http://aje.oxfordjournals.org/, provide information on each study's location and time period, the exposure considered (e.g., PM10), lag structure, health outcome, potential effect modifiers considered, and statistical methods used to assess particulate matter health risks and effect modification.
Most studies focused on North America and Europe. The United States was the most represented country (33 of 108 studies). Thirty-one studies were based in Europe (including 12 in Italy), 24 in Asia, 8 in Canada, 7 in Latin America, and 1 each in Russia and Australia. One study examined London and Hong Kong. Two additional studies were meta-analyses combining results from multiple regions (mostly North America and Europe). Although the range of confounding variables differed by study, common confounders were weather (e.g., temperature, dew point), temporal trend and seasonality (e.g., nonlinear functions for variable representing time), and day of the week. Common approaches used to assess individual-level effect modifiers were interaction terms in regression modeling and stratified analysis. For community-level effect modifiers, second-stage analysis (e.g., Bayesian hierarchical modeling) and meta-regression were common approaches in the identified papers.
Appendix Table 1 summarizes evidence for selected effect modifiers with a conclusion on the strength of evidence for each effect modifier based on our assessment. It notes particulate matter studies that found statistically significant evidence of effect modification and in what direction the modification was detected, as well as studies that did not find statistically significant evidence of effect modification. Studies are categorized on the basis of whether they examined potential effect modifiers at the individual level (e.g., a person's age) or the community level (e.g., percent of a city's population above a certain age) and by health outcome (hospital admission or death).
Below, we summarize the state of evidence for each potential effect modifier. Meta-analyses were conducted for risk of death for studies that used individual-level data for sex (men, women) and age (younger populations, older populations). Evidence for the other modifiers was not summarized by using meta-analytical methods because of the substantial heterogeneity in how these effect modifiers were defined (see also our inclusion criteria in the Methods).
Effect modification by sex
In general, estimated associations between short-term exposures to particulate matter and death and hospitalization risks were higher for women than for men, but many of the 36 studies examining this issue did not find evidence of effect modification by sex. Two of the 22 mortality studies showed significantly higher particulate matter exposure risks in women than in men (30, 31). Thirteen of the 14 hospitalization studies did not find statistically significant evidence of effect modification by sex. For the remaining study, estimates of the association between PM10 and hospital admission were higher for arrhythmias in men and for heart failure in women (32).
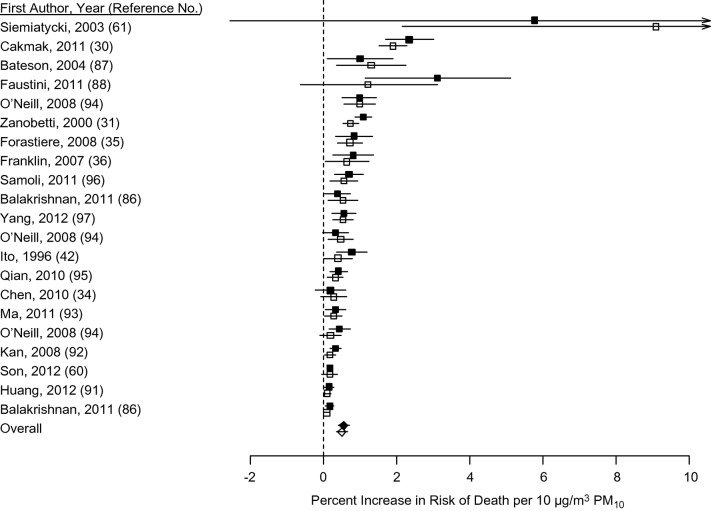
Separate meta-analyses for men and women based on 21 pairs of risk estimates from 18 studies found slightly higher but not statistically different estimated effects of particulate matter exposure on total mortality for men and women (Figure 1). The uncertainty parameter I2 was 87.0% (95% CI: 81.5, 90.9) for women and 88.1% (95% CI: 83.2, 91.6) for men, indicating substantial heterogeneity among the reported estimates. Based on meta-analysis, a 10 μg/m3 increase in exposure to PM10 was associated with a 0.55% (95% CI: 0.41, 0.70) increase in death for women and a 0.50% (95% CI: 0.34, 0.65) increase in death for men. These risk estimates are not statistically different. Egger's test for heterogeneity indicated a potential publication bias (P < 0.05 for men and women). The overall estimates adjusted for publication bias were increases in death of 0.34% (95% CI: 0.19, 0.49) and 0.28% (95% CI: 0.11, 0.44) per 10 μg/m3 PM10 for women and men, respectively (Web Figure 1). We concluded that there is weak evidence that particulate matter exposure risks are higher for women than for men.
Figure 1.
Meta-analysis of the association of sex with increased risk of death by exposure to particulate matter with aerodynamic diameter ≤10 µm (PM10). Solid points represent results for women; open points represent results for men. Points reflect central estimates; horizontal lines represent 95% confidence intervals. Boxes represent individual study results; diamonds represent results from the meta-analysis.
Effect modification by age
We examined studies that compared particulate matter exposure estimates across age groups (e.g., <64 years vs. ≥65 years) or on the basis of a subpopulation of a specific age (e.g., the percent elderly). We clustered results on the basis of whether higher (or lower) risks were observed for older populations, although studies specified age differently (e.g., older persons defined as ≥65 years vs. ≥75 years). Thirty-eight studies examined whether age modifies associations between particulate matter exposure and death. For studies that used individual-level data, 9 found statistically higher associations between particulate matter exposure and death for older persons (30, 33–40), whereas 22 did not find such evidence. Among mortality studies based on community-level age distribution, 1 study found that communities with a higher fraction of elderly persons had statistically higher particulate matter–associated risks (5), 1 study found the opposite result (statistically lower risk estimates with higher age) (41), and 5 studies found no evidence of effect modification.
Some studies found effect modification for some causes of death but not others. In 1 study, older populations had statistically higher particulate matter–associated risk estimates than younger populations for total and stroke deaths but not for respiratory or cardiovascular deaths (36). Another study found that older populations had statistically higher particulate matter–associated death risk estimates than did younger populations, but did not observe effect modification by age for cardiovascular, respiratory, or stroke deaths (39). Of the 26 studies that investigated whether age modifies particulate matter–associated hospitalizations, risk estimates were statistically higher for older populations than for younger populations in 4 studies and lower in 1 study, with no statistically significant evidence of effect modification in the remaining 21 studies.
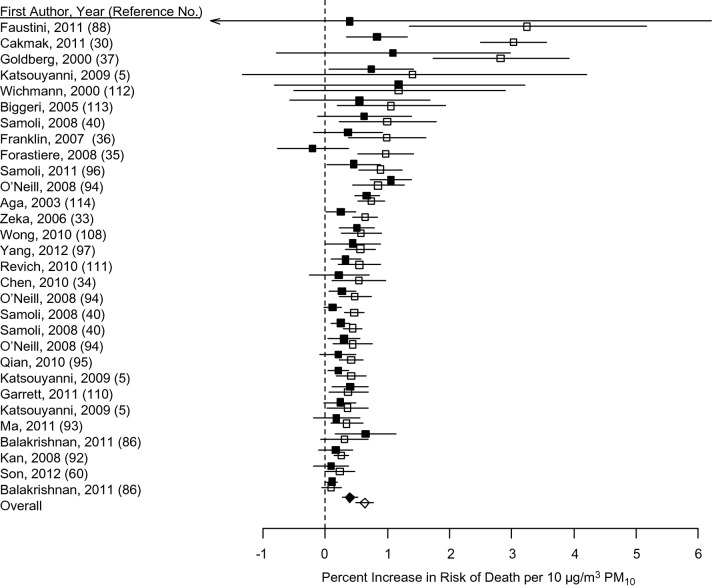
Figure 2 provides meta-analysis results for age from 30 pairs of estimates from 23 studies that used individual-level data. Other studies that used individual-level data were excluded from meta-analysis because of differences in study designs (e.g., results for respiratory deaths only). Because authors used different age categorizations, we considered “older” populations as the oldest age group (e.g., ≥65, ≥66, ≥75, ≥76, ≥80, or ≥85 years). For “younger” populations, we considered the age strata that most closely matched adult populations where available (e.g., 20–64, 35–64, or 45–64 years) or nonelderly populations including children (e.g., 5–64, <65, <70, or <75 years). Some studies presented estimates for an older population versus all ages, in which case we included “all ages” estimates with those of “younger” populations.
Figure 2.
Meta-analysis of the association of age with increased risk of death by exposure to particulate matter with aerodynamic diameter ≤10 µm (PM10). Solid points represent results for younger populations; open points represent results for older populations. Points reflect central estimates; horizontal lines represent 95% confidence intervals. Boxes represent individual study results; diamonds represent results from the meta-analysis.
The uncertainty parameter I2 was 62.1% (95% CI: 43.8, 74.5) for younger populations and 84.6% (95% CI: 79.1, 88.7) for older populations, indicating heterogeneity. Meta-analysis results showed that a 10 μg/m3 increase in PM10 exposure was associated with 0.34% (95% CI: 0.25, 0.42) and 0.64% (95% CI: 0.50, 0.78) increases in risk of death for younger and older populations, respectively. Risks for older populations were 0.30% (95% CI: 0.14, 0.47) higher than for younger persons. Results remained essentially unchanged under sensitivity analysis that removed studies that compared “all ages” with “older” populations. The remaining 26 pairs of estimates from 19 studies resulted in 0.29% (95% CI: 0.20, 0.38) and 0.66% (95% CI: 0.50, 0.82) increases in risk of death per 10 μg/m3 of PM10 exposure for younger and older populations, respectively.
Egger's test for heterogeneity indicated potential publication bias (P < 0.05 for both younger and older populations). Overall estimates adjusted for publication bias were increases of 0.20% (95% CI: 0.10, 0.30) and 0.50% (95% CI: 0.34, 0.66) in risk of death for younger and older populations, respectively (Web Figure 2). By using these results, we found risk estimates to be 0.30% (95% CI: 0.11, 0.49) higher for older populations than for younger populations. We concluded that there is strong evidence that the risk of death associated with short-term particulate matter exposure is higher in older populations than in younger populations.
Effect modification by race/ethnicity
No statistically significant associations were reported in the 9 studies that examined effect modification by race (31, 33, 42–48). Thus, we concluded that these studies present no evidence of effect modification by race; however, the investigation of race was limited. All studies were conducted in the United States. In all cases, race was categorized simplistically, such as percent African American (45) or nonwhite (44). Individual data were assessed with dichotomous categories of black and white (31, 33, 42) or white and “other” (46, 47). Only 1 study considered more than 2 racial/ethnic categories, which were the percentages of a community that were Hispanic, African American, or white (43).
Effect modification by SES indicators
The most commonly studied SES indicator was education, which generally was based on educational attainment. Two of the 10 mortality studies (30, 49) with individual-level data on education and 1 of the 6 mortality studies (50) with community-level data on education found higher particulate matter–associated risks with lower educational level; the remaining studies found no statistically significant evidence of effect modification. One study examined whether the risk of hospitalization was affected by educational level and found no such evidence when using community-level data (51). Overall, we found limited/suggestive evidence of higher risk with lower educational level.
Income level was examined for particulate matter–associated death risk estimates in 8 studies with community-level data (e.g., median household income); 4 studies found higher risk with lower income (30, 50, 52, 53). For the 3 studies examining community-level income data and hospitalization risk, 1 found higher risk in lower income communities (54), whereas the remaining studies did not find evidence of effect modification (51, 55). There exists limited/suggestive evidence of higher risk with lower income, although no studies examined individual-level income data.
Poverty was examined only as a community-level variable in 3 mortality studies (44, 56, 57) and 4 hospitalization studies (48, 55, 58, 59). One study found lower SES to be associated with lower particulate matter–associated hospitalization risk; during the warm season, risk estimates were lower in communities in 36 US cities with higher proportions of persons over 65 years of age living in poverty (59). Overall, we found no evidence of effect modification by poverty, although no studies examined individual-level poverty data.
Effect modification for particulate matter–associated death by employment status was analyzed in 7 mortality studies and no hospitalization studies. Based on individual-level data, risk estimates were higher for those with lower employment status, for unemployed persons compared with white-collar employees (30), and for blue-collar workers or never employed persons compared with white-collar workers (49). Effect modification was not identified in 2 other studies that used occupational categories (60) and an occupational “dirtiness score” (61). Risk estimates were higher for communities with higher unemployment rates in 2 studies (5, 40) but not in a third community-level study (44). Evidence of higher particulate matter–associated risks with lower employment status was limited/suggestive.
DISCUSSION
We found that age is the most consistent effect modifier of the association between short-term exposure to particulate matter and death and hospitalization, with older persons experiencing higher risks. In addition to physiological changes that accompany age, older persons likely have different indoor/outdoor activity patterns, occupational exposures, and social networks. Our analysis of age compared risks for older and younger populations; however, the very young may also be susceptible. Children could face higher risks because their biological systems are under development, they breathe more air per body weight than do adults, and they typically spend more time outdoors. Exposures to PM2.5 and PM10 are associated with the risk of death for infants and children in the United States (62, 63). Future work could investigate whether particulate matter risks are modified for infants and children.
We found weak evidence of higher particulate matter–associated risks for women than for men, which may result from differences in physiology, exposure patterns, and/or activity patterns. A recent review discussed potential reasons for effect modification by sex on respiratory outcomes associated with exposure to PM2.5 and nitrogen dioxide. Exposures related to occupation, cooking, physical activity, smoking status, and personal care products vary by sex. Men and women differ in dermal absorption, lung function, and absorption of gases through the respiratory system. Hormonal changes can affect relationships between dose and effective dose. A recent review found that most studies of adults observed stronger air pollution risks in women than in men and recommended more research to identify the relevant pathways, noting that differences between sexes differ by society (64).
Health status differs by race/ethnicity, such as in higher death rates in the US for black and American Indian infants than for white infants (65). Exposures also differ by race/ethnicity; non-Hispanic blacks had higher levels than whites for 13 of 14 PM2.5 components (66). Although our analysis did not provide evidence that race modifies particulate matter–associated risks, the identified studies are limited. All studies used simplistic race categorizations (e.g., white and “other”). Actual race/ethnicity is more complex, involving community patterns, national origin, and mixed ancestries (67). Great Britain, Canada, and the United States have revised their census surveys to include multirace choices (68). Researchers have noted that hypotheses on health disparities by race are largely characterized by 3 mechanisms (69), which could be extrapolated to differences in particulate matter–associated health risks by race. The first is a biological mechanism of genetic susceptibility to disease by race. Because racial groups are based not only on genetics but also on social and community relationships, this explanation is unlikely to fully explain differences by race. The second mechanism is race as an indicator of SES. Race and SES can be correlated, challenging efforts to disentangle their effects; however, this pathway also is unlikely to fully explain health differences because race is not a fully adequate SES surrogate. For example, in the United States during 2007–2011, more than 9 million blacks or African Americans (25.8%) were in poverty, as were more than 25.5 million whites (11.6%) (70). Some have proposed a more multifaceted third mechanism of race and class as separate influences, with potential interactions (e.g., race affecting class) (69).
Overall, the identified studies suggest that those with lower SES face higher particulate matter–associated risks, although we found only limited/suggestive evidence for modification by educational level, income, and employment status. SES could modify particulate matter–associated health risks through differences in access to health care, baseline health status, occupational exposures, and nutrition. Studies investigating multiple SES indicators generally had consistent within-study results. For example, evidence of effect modification was identified for all of the SES indicators considered in several mortality studies (e.g., employment, education, and income (30)) and hospital admission studies (e.g., education and income (51)). No associations were observed for any of the multiple indicators considered in other studies (e.g., occupation and education (60, 61)). However, this was not true in all cases (e.g., unemployment but not education was identified as an effect modifier in a multicity mortality study (5)). Furthermore, although evidence for effect modification by lower SES was generally consistent within a given study, some studies found such evidence, some did not, and 1 study found the opposite result (i.e., lower risk with higher poverty) (59). Evidence on effect modification by SES has been limited by the use of community-level data. Health is associated with individual characteristics, as well as the community in which a person lives (71), although few studies have evaluated SES by using individual-level data.
The indicators discussed here do not fully represent true SES. Limitations stem from the reliability of each indicator's measurement, the inability to capture lifetime history of SES, unmeasured assets (e.g., home ownership), and misclassification of SES (e.g., retired persons or women who do not work outside the home categorized as unemployed (72)). The use of occupational data to gauge SES can affect estimates differently by sex because women have less heterogeneity in occupations than men (73). Although SES indicators are generally correlated, this correlation can vary by population, including among races within an area (74). Relationships between SES and access to medical care differ by region (e.g., because of the presence or lack of universal healthcare). Some SES indicators are more associated with overall health status than others. There is some evidence that economic indicators such as income have stronger associations with health than do indicators based on occupation or education (75, 76), and that SES is more related to health in some areas than in others (75).
The potential effect modifiers examined here are not independent of each other or of other potential modifiers. In addition to correlations among SES indicators, sex is related to SES, such as in higher income for men. Levels of physical activity change with age and differ by sex and age (77, 78). Smoking rates are often higher for men (e.g., 57% for Japanese men compared with 17% for Japanese women (79)) and can differ by income. Studies are needed on effect modification within the complex system of multiple social, economic, and environmental factors, which may vary across regions in terms of the direction and level of effect modification and their relationships with each other (e.g., different degrees of income inequality by sex).
Regarding our categories of degree of evidence, results such as weak or no evidence of effect modification reflect the current scientific evidence, although modification may indeed exist. Limitations of this study include problems inherent in the designation of results as statistically significant (80–82) and in publication bias (83, 84), under which results (e.g., for lag with the highest association) may be selectively reported and published. Thus, results from studies that did not find evidence of effect modification may be underrepresented in the literature. In fact, the results of our meta-analyses indicate publication bias. Further, many studies that did not find statistically significant evidence did find higher risks for some groups than for others. Our methodology was designed to allow the manageable review and presentation of papers; however, studies without statistically significant results should not be interpreted as definitive evidence of the absence of effect modification. Most studies were designed to investigate hypotheses other than effect modification, so a study designed to address effect modification specifically may differ from those used (e.g., in sample size).
Although we focused on selected effect modifiers, the identified studies considered many other effect modifiers, primarily addressing season, weather, location, pollution, and health status. Effect modification was examined with respect to season and weather (e.g., temperature, synoptic classification) on the day of death as well as communities' long-term weather (e.g., temperature, humidity). Pollutants as effect modifiers were studied by using long-term levels of copollutants (e.g., PM2.5 chemical components), pollutant emissions (e.g., population-weighted traffic emissions), information on particulate matter sources (e.g., industry, traffic), and the presence of gas stoves in the home. Health status was evaluated with individual-level data for comorbidities, such as causes of previous hospitalizations or concurrent conditions, smoking status, dietary intakes, and community-level, age-standardized death rates. Other potential effect modifiers considered include individual-level data on housing type (e.g., government housing for low-income families), exposure to known lung carcinogens, and location of death (in the hospital vs. out of the hospital), and community-level information on percent of adults with non-English language, degree of urbanization (e.g., population density), prevalence of air conditioning, and the number and density of air pollution monitors. Although we summarized evidence for several key modifiers, a multitude of other individual and environmental factors may modify particulate matter–associated health risks.
A better understanding of vulnerability and susceptibility and, more generally, of effect modification, can provide evidence on which to base the targeting of local air quality efforts to specific populations. It can also inform our understanding of biological mechanisms (e.g., differences by sex) and can help design regulations that protect sensitive populations with an adequate margin of safety. Future efforts are needed to further investigate effect modification and the suggestive evidence summarized here. To the degree feasible, researchers should address factors that may modify air pollution estimates and incorporate them into analyses.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: School of Forestry and Environmental Studies, Yale University, New Haven, Connecticut (Michelle L. Bell); Department of Environmental Health, School of Public Health, Harvard University, Boston, Massachusetts (Antonella Zanobetti); and Department of Biostatistics, School of Public Health, Harvard University, Boston, Massachusetts (Francesca Dominici).
Funding for this work was provided by the National Institutes of Health (grants R01ES019560, R01ES019587, R01ES019955, R01ES016317, R21ES020152, and R21ES021427), the US Environmental Protection Agency (grants RD 83479801, RD 83490001, and R834894), and the Health Effects Institute (grant HEI 4909).
Conflict of interest: none declared.
APPENDIX
Appendix Table 1.
Summary of Scientific Evidence for Effect Modifiers of Particulate Matter–Associated Death and Hospitalization
| Effect Modifier | Statistically Significant Evidence | Lack of Statistically Significant Evidence | Summary of Evidence |
|---|---|---|---|
| Sexa | Weak evidence of higher risk for women than for men | ||
| Mortality | Higher risk in women: 2 studies (30, 31) | 20 Studies (34–36, 42, 53, 60, 61, 85–97) | |
| Hospitalization | Higher risk in women: 1 study (32); higher risk in men: 1 study (32) | 13 studies (46, 47, 54, 98–107) | |
| Age | Strong evidence of higher risk for older persons | ||
| Mortality | |||
| Individual | Higher risk with higher age: 9 studies (30, 33–40) | 22 Studies (5, 42, 53, 60, 85–89, 91–97, 108–113) | |
| Community | Higher risk with higher age: 1 study (5); lower risk with higher age: 1 study (41) | 5 Studies (50, 56, 114–116) | |
| Hospitalization | |||
| Individual | Higher risk with higher age: 4 studies (32, 58, 117, 118); lower risk with higher age: 1 study (119) | 21 Studies (46, 47, 54, 98–103, 105–107, 113, 120–127) | |
| Race | No evidence of effect modification by race | ||
| Mortality | |||
| Individual | No studies | 4 Studies (31, 33, 42, 43) | |
| Community | No studies | 2 Studies (44, 45) | |
| Hospitalization | |||
| Individual | No studies | 2 Studies (46, 47) | |
| Community | No studies | 2 Studies (45, 48) | |
| Education | Limited/suggestive evidence of higher risk with lower education | ||
| Mortality | |||
| Individual | Lower risk with higher education: 1 study (30); lower risk for those with primary education compared with those with no education: 1 study (49) | 8 Studies (31, 33, 43, 60, 61, 92, 94, 97) | |
| Community | Lower risk with higher education: 1 study (50) | 5 Studies (5, 44, 45, 57, 87) | |
| Hospitalization | |||
| Individual | No studies | No studies | |
| Community | No studies | 1 Study (51) | |
| Income | Limited/suggestive evidence of higher risk with lower income | ||
| Mortality | |||
| Individual | No studies | No studies | |
| Community | Higher risk with lower income: 4 studies (30, 50, 52, 53) | 4 Studies (45, 86, 128, 129) | |
| Hospitalization | |||
| Individual | No studies | No studies | |
| Community | Higher risk with lower income: 1 study (54) | 2 Studies (51, 55) | |
| Povertyb | No evidence of effect modification by poverty status | ||
| Mortality | No studies | 3 Studies (44, 56, 57) | |
| Hospitalization | Lower risk with higher poverty: 1 study (59) | 4 Studies (48, 55, 58, 59) | |
| Employment | Limited/suggestive evidence of higher risk at lower employment status | ||
| Mortality | |||
| Individual | Higher risk with lower employment: 2 studies (30, 49) | 2 Studies (60, 61) | |
| Community | Higher risk with lower employment: 2 studies (5, 40) | 1 Study (44) |
a Individual-level effect modifier.
b Community-level effect modifier.
REFERENCES
- 1.Anenberg SC, Horowitz LW, Tong DQ, et al. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect. 2010;118(9):1189–1195. doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Environmental Protection Agency, Office of Air and Radiation. The Benefits and Costs of the Clean Air Act from 1990 to 2020. Washington, DC: US Environmental Protection Agency; 2011. [Google Scholar]
- 3.US Environmental Protection Agency. Washington, DC: US Environmental Protection Agency; 2012. The Green Book nonattainment areas for criteria pollutants http://www.epa.gov/oar/oaqps/greenbk/index.html. (Accessed August 21, 2012) [Google Scholar]
- 4.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsouyanni K, Samet JM, Anderson HR, et al. Air pollution and health: a European and North American approach (APHENA) Res Rep Health Eff Inst. 2009;)(142):5–90. [PubMed] [Google Scholar]
- 6.Katsouyanni K, Schwartz J, Spix C, et al. Short term effects of air pollution on health: a European approach using epidemiologic time series data: the APHEA protocol. J Epidemiol Community Health. 1996;50(suppl):12S–18S. doi: 10.1136/jech.50.suppl_1.s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samet JM, Zeger SL, Dominici F, et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst. 2000;94(Pt 2):5–70. discussion 71–79. [PubMed] [Google Scholar]
- 8.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makri A, Stilianakis NI. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211(3-4):326–336. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.The Interagency Working Group on Climate Change. A Human Health Perspective on Climate Change: A Report Outlining the Research Needs of the Human Health Effects of Climate Change. Research Triangle Park, NC: National Institutes of Environmental Health Sciences; 2010. [Google Scholar]
- 11.US Environmental Protection Agency. Notification of upcoming meeting of the Science Advisory Board Particulate Matter Research Centers Program Advisory Panel. Fed Regist. 2008;73(136):40576–40577. [Google Scholar]
- 12.US Environmental Protection Agency. Washington, DC: US Environmental Protection Agency; 2009. Funding opportunity: clean air research centers http://www.epa.gov/ncer/rfa/2009/2009_star_clean_air.html. (Accessed July 26, 2012) [Google Scholar]
- 13.Mulrow CD. Rationale for systematic reviews. BMJ. 1994;309(6954):597–599. doi: 10.1136/bmj.309.6954.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Bethesda, MD: National Institutes of Health; 2012. PubMed http://www.ncbi.nlm.nih.gov/pubmed/ (Accessed July 26, 2012) [Google Scholar]
- 15.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. ( doi:10.1186/1756-0500-5-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C-H, Lin Y-C, Wang C-S. Relationships among particle fractions of urban and non-urban aerosols. Aerosol Air Qual Res. 2003;3(1):7–15. [Google Scholar]
- 17.Vanderstraeten P, Forton M, Lenelle Y, et al. Elevated PM10 concentrations and high PM2.5/PM10 ratio in the Brussels urban area during the 2006 Car-Free Sunday. Int J Environ Waste Manag. 2010;6(3-4):264–279. [Google Scholar]
- 18.Vega E, Reyes E, Ruiz H, et al. Analysis of PM2.5 and PM10 in the atmosphere of Mexico City during 2000–2002. J Air Waste Manag Assoc. 2004;54(7):786–798. doi: 10.1080/10473289.2004.10470952. [DOI] [PubMed] [Google Scholar]
- 19.Thorlund K, Imberger G, Johnston BC, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One. 2012;7(7):e39471. doi: 10.1371/journal.pone.0039471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics. 2007;8(2):337–344. doi: 10.1093/biostatistics/kxl013. [DOI] [PubMed] [Google Scholar]
- 24.Fung KY, Krewski D, Chen Y, et al. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003;32(6):1064–1070. doi: 10.1093/ije/dyg246. [DOI] [PubMed] [Google Scholar]
- 25.Kan H, Chen B. A case-crossover and time-series study of ambient air pollution and daily mortality in Shanghai, China. Epidemiology. 2004;15(4):S55. [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulco C. Gulf War and Health. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 29.Kan H, Chen B, Zhao N, et al. Public Health and Air Pollution in Asia (PAPA: coordinated studies of short-term exposure to air pollution and daily mortality in four cities: Part 1. A time-series study of ambient air pollution and daily mortality in Shanghai, China. Res Rep Health Eff Inst. 2010;(154):17–78. [PubMed] [Google Scholar]
- 30.Cakmak S, Dales RE, Rubio MA, et al. The risk of dying on days of higher air pollution among the socially disadvantaged elderly. Environ Res. 2011;111(3):388–393. doi: 10.1016/j.envres.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Zanobetti A, Schwartz J. Race, gender, and social status as modifiers of the effects of PM10 on mortality. J Occup Environ Med. 2000;42(5):469–474. doi: 10.1097/00043764-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Colais P, Faustini A, Stafoggia M, et al. Particulate air pollution and hospital admissions for cardiac diseases in potentially sensitive subgroups. Epidemiology. 2012;23(3):473–481. doi: 10.1097/EDE.0b013e31824d5a85. [DOI] [PubMed] [Google Scholar]
- 33.Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163(9):849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, Pan G, Kan H, et al. Ambient air pollution and daily mortality in Anshan, China: a time-stratified case-crossover analysis. Sci Total Environ. 2010;408(24):6086–6091. doi: 10.1016/j.scitotenv.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Forastiere F, Stafoggia M, Berti G, et al. Particulate matter and daily mortality: a case-crossover analysis of individual effect modifiers. Epidemiology. 2008;19(4):571–580. doi: 10.1097/EDE.0b013e3181761f8a. [DOI] [PubMed] [Google Scholar]
- 36.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg MS, Bailar JC, 3rd, Burnett RT, et al. Identifying subgroups of the general population that may be susceptible to short-term increases in particulate air pollution: a time-series study in Montreal, Quebec. Res Rep Health Eff Inst. 2000;(97):7–113. discussion 115–120. [PubMed] [Google Scholar]
- 38.Gouveia N, Fletcher T. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Community Health. 2000;54(10):750–755. doi: 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G, Zhou M, Cai Y, et al. Does temperature enhance acute mortality effects of ambient particle pollution in Tianjin City, China. Sci Total Environ. 2011;409(10):1811–1817. doi: 10.1016/j.scitotenv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Samoli E, Peng R, Ramsay T, et al. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA Study. Environ Health Perspect. 2008;116(11):1480–1486. doi: 10.1289/ehp.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsouyanni K, Touloumi G, Samoli E, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 Project. Epidemiology. 2001;12(5):521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Ito K, Thurston GD. Daily PM10/mortality associations: an investigations of at-risk subpopulations. J Expo Anal Environ Epidemiol. 1996;6(1):79–95. [PubMed] [Google Scholar]
- 43.Ostro BD, Feng WY, Broadwin R, et al. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup Environ Med. 2008;65(11):750–756. doi: 10.1136/oem.2007.036673. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz J. Harvesting and long term exposure effects in the relation between air pollution and mortality. Am J Epidemiol. 2000;151(5):440–448. doi: 10.1093/oxfordjournals.aje.a010228. [DOI] [PubMed] [Google Scholar]
- 45.Bell ML, Ebisu K, Peng RD, et al. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179(12):1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am J Cardiol. 2006;97(3):404–408. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 47.Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect. 2000;108(9):841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanobetti A, Schwartz J, Dockery DW. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ Health Perspect. 2000;108(11):1071–1077. doi: 10.1289/ehp.001081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ou CQ, Hedley AJ, Chung RY, et al. Socioeconomic disparities in air pollution-associated mortality. Environ Res. 2008;107(2):237–244. doi: 10.1016/j.envres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Martins MC, Fatigati FL, Vespoli TC, et al. Influence of socioeconomic conditions on air pollution adverse health effects in elderly people: an analysis of six regions in Sao Paulo, Brazil. J Epidemiol Community Health. 2004;58(1):41–46. doi: 10.1136/jech.58.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell ML, Ebisu K, Peng RD, et al. Adverse health effects of particulate air pollution: modification by air conditioning. Epidemiology. 2009;20(5):682–686. doi: 10.1097/EDE.0b013e3181aba749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forastiere F, Stafoggia M, Tasco C, et al. Socioeconomic status, particulate air pollution, and daily mortality: differential exposure or differential susceptibility. Am J Ind Med. 2007;50(3):208–216. doi: 10.1002/ajim.20368. [DOI] [PubMed] [Google Scholar]
- 53.Serinelli M, Vigotti MA, Stafoggia M, et al. Particulate matter and out-of-hospital coronary deaths in eight Italian cities. Occup Environ Med. 2010;67(5):301–306. doi: 10.1136/oem.2009.046359. [DOI] [PubMed] [Google Scholar]
- 54.Burra TA, Moineddin R, Agha MM, et al. Social disadvantage, air pollution, and asthma physician visits in Toronto, Canada. Environ Res. 2009;109(5):567–574. doi: 10.1016/j.envres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Zanobetti A, Franklin M, Koutrakis P, et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58. doi: 10.1186/1476-069X-8-58. ( doi:10.1186/1476-069X-8-58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy JI, Hammitt JK, Spengler JD. Estimating the mortality impacts of particulate matter: What can be learned from between-study variability? Environ Health Perspect. 2000;108(2):109–117. doi: 10.1289/ehp.00108109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson WE, Mar TF, Koenig JQ. Influence of exposure error and effect modification by socioeconomic status on the association of acute cardiovascular mortality with particulate matter in Phoenix. J Expo Sci Environ Epidemiol. 2007;17(2 suppl):11S–19S. doi: 10.1038/sj.jes.7500620. [DOI] [PubMed] [Google Scholar]
- 58.Haley VB, Talbot TO, Felton HD. Surveillance of the short-term impact of fine particle air pollution on cardiovascular disease hospitalizations in New York State. Environ Health. 2009;8:42. doi: 10.1186/1476-069X-8-42. ( doi:10.1186/1476-069X-8-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medina-Ramon M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006;163(6):579–588. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- 60.Son JY, Lee JT, Kim H, et al. Susceptibility to air pollution effects on mortality in Seoul, Korea: a case-crossover analysis of individual-level effect modifiers. J Expo Sci Environ Epidemiol. 2012;22(3):227–234. doi: 10.1038/jes.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siemiatycki J, Krewski D, Shi Y, et al. Controlling for potential confounding by occupational exposures. J Toxicol Environ Health A. 2003;66(16-19):1591–1603. doi: 10.1080/15287390306428. [DOI] [PubMed] [Google Scholar]
- 62.Woodruff TJ, Parker JD, Schoendorf KC. Fine particulate matter (PM2.5) air pollution and selected causes of postneonatal infant mortality in California. Environ Health Perspect. 2006;114(5):786–790. doi: 10.1289/ehp.8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodruff TJ, Darrow LA, Parker JD. Air pollution and postneonatal infant mortality in the United States, 1999–2002. Environ Health Perspect. 2008;116(1):110–115. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clougherty JE, Eisen EA, Slade MD, et al. Gender and sex differences in job status and hypertension. Occup Environ Med. 2011;68(1):16–23. doi: 10.1136/oem.2009.049908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams DR, Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annu Rev Sociol. 1995;21:349–386. [Google Scholar]
- 66.Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699–1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez AD, Hirschman C. The changing racial and ethnic composition of the US population: emerging American identities. Popul Dev Rev. 2009;35(1):1–51. doi: 10.1111/j.1728-4457.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson D. Making (mixed-)race: census politics and the emergence of multiracial multiculturalism in the United States, Great Britain, and Canada. Ethn Racial Stud. 2012;35(8):1409–1426. [Google Scholar]
- 69.Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood) 2005;24(2):343–352. doi: 10.1377/hlthaff.24.2.343. [DOI] [PubMed] [Google Scholar]
- 70.US Census Bureau. 2007–2011 American Community Survey. Washington, DC: US Census Bureau; 2012. [Google Scholar]
- 71.Undurraga EA, Nyberg C, Eisenberg DT, et al. Individual wealth rank, community wealth inequality, and self-reported adult poor health: a test of hypotheses with panel data (2002–2006) from native Amazonians, Bolivia. Med Anthropol Q. 2010;24(4):522–548. doi: 10.1111/j.1548-1387.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- 72.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 73.Ljung R, Hallqvist J. Socioeconomic position, clustering of risk factors, and the risk of myocardial infarction. Am J Public Health. 2007;97(11):1927–1928. doi: 10.2105/AJPH.2007.119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braveman P, Cubbin C, Marchi K, et al. Measuring socioeconomic status/position in studies of racial/ethnic disparities: maternal and infant health. Public Health Rep. 2001;116(5):449–463. doi: 10.1093/phr/116.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von dem Knesebeck O, Lüschen G, Cockerham WC, et al. Socioeconomic status and health among the aged in the United States and Germany: a comparative cross-sectional study. Soc Sci Med. 2003;57(9):1643–1652. doi: 10.1016/s0277-9536(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 76.Daly MC, Duncan GJ, McDonough P, et al. Optimal indicators of socioeconomic status for health research. Am J Public Health. 2002;92(7):1151–1157. doi: 10.2105/ajph.92.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamauchi T, Kim SN, Lu Z, et al. Age and gender differences in the physical activity patterns of urban schoolchildren in Korea and China. J Physiol Anthropol. 2007;26(2):101–107. doi: 10.2114/jpa2.26.101. [DOI] [PubMed] [Google Scholar]
- 78.Spittaels H, Van Cauwenberghe E, Verbestel V, et al. Objectively measured sedentary time and physical activity time across the lifespan: a cross-sectional study in four age groups. Int J Behav Nutr Phys Act. 2012;9:149. doi: 10.1186/1479-5868-9-149. ( doi:10.1186/1479-5868-9-149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda Y, Nakamura K, Takano T. Socioeconomic pattern of smoking in Japan: income inequality and gender and age differences. Ann Epidemiol. 2005;15(5):365–372. doi: 10.1016/j.annepidem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Ioannidis JP. Effect of formal statistical significance on the credibility of observational associations. Am J Epidemiol. 2008;168(4):374–383. doi: 10.1093/aje/kwn156. discussion 384–390. [DOI] [PubMed] [Google Scholar]
- 81.Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25(4):225–230. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 82.Gelman A, Stern H. The difference between “significant” and “not significant” is not itself statistically significant. Am Stat. 2006;60(4):328–331. [Google Scholar]
- 83.Anderson HR, Atkinson RW, Peacock JL, et al. Ambient particulate matter and health effects: publication bias in studies of short-term associations. Epidemiology. 2005;16(2):155–163. doi: 10.1097/01.ede.0000152528.22746.0f. [DOI] [PubMed] [Google Scholar]
- 84.Ji M, Cohan DS, Bell ML. Meta-analysis of the association between short-term exposure to ambient ozone and respiratory hospital admissions. Environ Res Lett. 2011;6(2) doi: 10.1088/1748-9326/6/2/024006. ( doi:10.1088/1748-9326/6/2/024006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliveira MS, Leon AP, Mattos IE, et al. Differential susceptibility according to gender in the association between air pollution and mortality from respiratory diseases. Cad Saude Publica. 2011;27(9):1827–1836. doi: 10.1590/s0102-311x2011000900016. [DOI] [PubMed] [Google Scholar]
- 86.Balakrishnan K, Ganguli B, Ghosh S, et al. Part 1. Short-term effects of air pollution on mortality: results from a time-series analysis in Chennai, India. Res Rep Health Eff Inst. 2011;(157):7–44. [PubMed] [Google Scholar]
- 87.Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15(2):143–149. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- 88.Faustini A, Stafoggia M, Berti G, et al. The relationship between ambient particulate matter and respiratory mortality: a multi-city study in Italy. Eur Respir J. 2011;38(3):538–547. doi: 10.1183/09031936.00093710. [DOI] [PubMed] [Google Scholar]
- 89.Forastiere F, Stafoggia M, Picciotto S, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. Am J Respir Crit Care Med. 2005;172(12):1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- 90.Goldberg MS, Burnett RT, Yale JF, et al. Associations between ambient air pollution and daily mortality among persons with diabetes and cardiovascular disease. Environ Res. 2006;100(2):255–267. doi: 10.1016/j.envres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 91.Huang W, Cao J, Tao Y, et al. Seasonal variation of chemical species associated with short-term mortality effects of PM2.5 in Xi'an, a central city in China. Am J Epidemiol. 2012;175(6):556–566. doi: 10.1093/aje/kwr342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kan H, London SJ, Chen G, et al. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the Public Health and Air Pollution in Asia (PAPA) Study. Environ Health Perspect. 2008;116(9):1183–1188. doi: 10.1289/ehp.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma Y, Chen R, Pan G, et al. Fine particulate air pollution and daily mortality in Shenyang, China. Sci Total Environ. 2011;409(13):2473–2477. doi: 10.1016/j.scitotenv.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 94.O'Neill MS, Bell ML, Ranjit N, et al. Air pollution and mortality in Latin America: the role of education. Epidemiology. 2008;19(6):810–819. doi: 10.1097/EDE.0b013e3181816528. [DOI] [PubMed] [Google Scholar]
- 95.Qian Z, He Q, Lin HM, et al. Public Health and Air Pollution in Asia (PAPA): coordinated studies of short-term exposure to air pollution and daily mortality in four cities: Part 2. Association of daily mortality with ambient air pollution, and effect modification by extremely high temperature in Wuhan, China. Res Rep Health Eff Inst. 2010;(154):91–217. [PubMed] [Google Scholar]
- 96.Samoli E, Kougea E, Kassomenos P, et al. Does the presence of desert dust modify the effect of PM10 on mortality in Athens, Greece? Sci Total Environ. 2011;409(11):2049–2054. doi: 10.1016/j.scitotenv.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 97.Yang C, Peng X, Huang W, et al. A time-stratified case-crossover study of fine particulate matter air pollution and mortality in Guangzhou, China. Int Arch Occup Environ Health. 2012;85(5):579–585. doi: 10.1007/s00420-011-0707-7. [DOI] [PubMed] [Google Scholar]
- 98.Bunch TJ, Horne BD, Asirvatham SJ, et al. Atrial fibrillation hospitalization is not increased with short-term elevations in exposure to fine particulate air pollution. Pacing Clin Electrophysiol. 2011;34(11):1475–1479. doi: 10.1111/j.1540-8159.2011.03200.x. [DOI] [PubMed] [Google Scholar]
- 99.Dales RE, Cakmak S, Vidal CB. Air pollution and hospitalization for headache in Chile. Am J Epidemiol. 2009;170(8):1057–1066. doi: 10.1093/aje/kwp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nuvolone D, Balzi D, Chini M, et al. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: results of the Cardiovascular Risk and Air Pollution in Tuscany (RISCAT) Study. Am J Epidemiol. 2011;174(1):63–71. doi: 10.1093/aje/kwr046. [DOI] [PubMed] [Google Scholar]
- 101.Oudin A, Stromberg U, Jakobsson K, et al. Estimation of short-term effects of air pollution on stroke hospital admissions in southern Sweden. Neuroepidemiology. 2010;34(3):131–142. doi: 10.1159/000274807. [DOI] [PubMed] [Google Scholar]
- 102.Tramuto F, Cusimano R, Cerame G, et al. Urban air pollution and emergency room admissions for respiratory symptoms: a case-crossover study in Palermo, Italy. Environ Health. 2011;10:31. doi: 10.1186/1476-069X-10-31. ( doi:10.1186/1476-069X-10-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wellenius GA, Bateson TF, Mittleman MA, et al. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol. 2005;161(11):1030–1036. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong CM, Yang L, Thach TQ, et al. Modification by influenza on health effects of air pollution in Hong Kong. Environ Health Perspect. 2009;117(2):248–253. doi: 10.1289/ehp.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113(8):978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qorbani M, Yunesian M, Fotouhi A, et al. Effect of air pollution on onset of acute coronary syndrome in susceptible subgroups. East Mediterr Health J. 2012;18(6):550–555. doi: 10.26719/2012.18.6.550. [DOI] [PubMed] [Google Scholar]
- 107.Middleton N, Yiallouros P, Kleanthous S, et al. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: the effect of short-term changes in air pollution and dust storms. Environ Health. 2008;7:39. doi: 10.1186/1476-069X-7-39. ( doi:10.1186/1476-069X-7-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong C-M, Thach TQ, Chau PYK, et al. Public Health and Air Pollution in Asia (PAPA): coordinated studies of short-term exposure to air pollution and daily mortality in four cities: Part 4. Interaction between air pollution and respiratory viruses: time-series study of daily mortality and hospital admissions in Hong Kong. Res Rep Health Eff Inst. 2010;(154):283–362. [PubMed] [Google Scholar]
- 109.De Leon SF, Thurston GD, Ito K. Contribution of respiratory disease to nonrespiratory mortality associations with air pollution. Am J Respir Crit Care Med. 2003;167(8):1117–1123. doi: 10.1164/rccm.200205-409OC. [DOI] [PubMed] [Google Scholar]
- 110.Garrett P, Casimiro E. Short-term effect of fine particulate matter (PM2.5) and ozone on daily mortality in Lisbon, Portugal. Environ Sci Pollut Res Int. 2011;18(9):1585–1592. doi: 10.1007/s11356-011-0519-z. [DOI] [PubMed] [Google Scholar]
- 111.Revich B, Shaposhnikov D. The effects of particulate and ozone pollution on mortality in Moscow, Russia. Air Qual Atmos Health. 2010;3(2):117–123. doi: 10.1007/s11869-009-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wichmann HE, Spix C, Tuch T, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany: Part I. Role of particle number and particle mass. Res Rep Health Eff Inst. 2000;(98):5–86. discussion 87–94. [PubMed] [Google Scholar]
- 113.Biggeri A, Baccini M, Bellini P, et al. Meta-analysis of the Italian Studies of Short-Term Effects of Air Pollution (MISA), 1990–1999. Int J Occup Environ Health. 2005;11(1):107–122. doi: 10.1179/oeh.2005.11.1.107. [DOI] [PubMed] [Google Scholar]
- 114.Aga E, Samoli E, Touloumi G, et al. Short-term effects of ambient particles on mortality in the elderly: results from 28 cities in the APHEA2 project. Eur Respir J Suppl. 2003;21(40 suppl):28S–33S. doi: 10.1183/09031936.03.00402803. [DOI] [PubMed] [Google Scholar]
- 115.Zeka A, Zanobetti A, Schwartz J. Short term effects of particulate matter on cause specific mortality: effects of lags and modification by city characteristics. Occup Environ Med. 2005;62(10):718–725. doi: 10.1136/oem.2004.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Analitis A, Katsouyanni K, Dimakopoulou K, et al. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17(2):230–233. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- 117.Belleudi V, Faustini A, Stafoggia M, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. 2010;21(3):414–423. doi: 10.1097/EDE.0b013e3181d5c021. [DOI] [PubMed] [Google Scholar]
- 118.Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: Are diabetics more susceptible? Epidemiology. 2002;13(5):588–592. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 119.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125(2):367–373. doi: 10.1016/j.jaci.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 120.Namdeo A, Tiwary A, Farrow E. Estimation of age-related vulnerability to air pollution: assessment of respiratory health at local scale. Environ Int. 2011;37(5):829–837. doi: 10.1016/j.envint.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Bhaskaran K, Hajat S, Armstrong B, et al. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. ( doi:10.1136/bmj.d5531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buadong D, Jinsart W, Funatagawa I, et al. Association between PM10 and O3 levels and hospital visits for cardiovascular diseases in Bangkok, Thailand. J Epidemiol. 2009;19(4):182–188. doi: 10.2188/jea.JE20080047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Canova C, Dunster C, Kelly FJ, et al. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effect of individual characteristics. Epidemiology. 2012;23(4):607–615. doi: 10.1097/EDE.0b013e3182572563. [DOI] [PubMed] [Google Scholar]
- 124.Fung KY, Luginaah I, Gorey KM, et al. Air pollution and daily hospital admissions for cardiovascular diseases in Windsor, Ontario. Can J Public Health. 2005;96(1):29–33. doi: 10.1007/BF03404010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lanki T, Pekkanen J, Aalto P, et al. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: the HEAPSS Study. Occup Environ Med. 2006;63(12):844–851. doi: 10.1136/oem.2005.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sousa SI, Pires JC, Martins EM, et al. Short-term effects of air pollution on respiratory morbidity at Rio de Janeiro—Part II: health assessment. Environ Int. 2012;43:1–5. doi: 10.1016/j.envint.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 127.Zanobetti A, Schwartz J. Are diabetics more susceptible to the health effects of airborne particles? Am J Respir Crit Care Med. 2001;164(5):831–833. doi: 10.1164/ajrccm.164.5.2012039. [DOI] [PubMed] [Google Scholar]
- 128.Villeneuve PJ, Burnett RT, Shi Y, et al. A time-series study of air pollution, socioeconomic status, and mortality in Vancouver, Canada. J Expo Anal Environ Epidemiol. 2003;13(6):427–435. doi: 10.1038/sj.jea.7500292. [DOI] [PubMed] [Google Scholar]
- 129.Wong CM, Ou CQ, Chan KP, et al. The effects of air pollution on mortality in socially deprived urban areas in Hong Kong, China. Environ Health Perspect. 2008;116(9):1189–1194. doi: 10.1289/ehp.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.