Abstract
Morbidity and mortality attributable to hypertension are higher in African American (AAEH) compared to Caucasian essential hypertensive (EH) patients, possibly related to differential effects on vascular injury and repair. While circulating endothelial progenitor cells (EPC) preserve endothelial integrity, inflammatory endothelial cells (IEC) detach from sites of injury and represent markers of vascular damage. We hypothesized that blood levels of IEC and inflammatory markers would be higher in AAEH compared to EH patients. Inferior-vena-cava and renal-vein levels of CD34+/KDR+ (EPC) and VAP-1+ (IEC) cells were measured by fluorescence-activated cell sorting in EH and AAEH under fixed sodium intake and blockade of the renin-angiotensin-system, and compared to systemic levels in normotensive control subjects (n=19 each). renal-vein and Inferior-vena-cava levels of inflammatory cytokines and EPC homing factors were measured by Luminex. Blood pressure, serum creatinine, lipids, and antihypertensive medications did not differ between EH and AAEH patients, and EPC levels were decreased in both. Circulating IEC levels were elevated in AAEH, and inversely correlated with EPC levels (R2=0.58, p=0.0001). Systemic levels of inflammatory cytokines and EPC homing factors were higher in AAEH compared to EH patients, and correlated directly with IEC. renal-vein inflammatory cytokines, EPC and IEC did not differ from their circulating levels. Most IEC expressed endothelial markers, fewer expressed progenitor cell markers, but none showed lymphocyte or phagocytic cell markers. Thus, increased release of cytokines and IEC in AAEH may impair EPC reparative capacity and aggravate vascular damage, and accelerate hypertension-related complications.
Keywords: hypertension, endothelial progenitor cells, inflammation, African Americans
Introduction
African American individuals have a higher incidence and prevalence of essential hypertension (EH) compared to Caucasians1. Furthermore, morbidity and mortality rates are higher in African American (AAEH) compared to Caucasian EH patients2, 3. Over the past decade, factors suggested to explain this ethnic disparity included dietary habits, obesity, and insulin resistance4. Augmented activity of the Na-K-2Cl co-transport in the thick ascending limb of Henle’s loop5, a strong correlation between uric acid levels and total peripheral resistance6, and increased activity of the amiloride-sensitive epithelial sodium channel7 have been also reported. Furthermore, studies using mapping by admixture disequilibrium have identified a strong association between gene variants in a region on chromosome 22 and the development of hypertensive renal disease in African American individuals8, 9. Although the mechanisms responsible for development of hypertension in AAEH carrying these gene variants remains unknown10, mutations in the non-muscle myosin heavy chain gene (MYH)9 has been associated with disease syndromes that include leucocyte inclusion bodies, suggesting higher propensity for inflammation11.
In turn, inflammation plays a central role in mediating vascular dysfunction in hypertensive patients12. For example, elevated circulating levels of transforming growth factor (TGF)-β13, interleukin (IL)-6, tumor necrosis factor (TNF)-α, and matrix metalloproteinase (MMP)-2 directly correlate with the extent of pulse pressure in AAEH14. Furthermore, endothelial activation is higher in ethnic minority participants (comprised of African-, Hispanic- and Asian-Americans) than among European-American populations, suggesting an important role of inflammation, endothelial activation, and matrix remodeling in the pathogenesis of hypertension and its complications in ethnic minorities15.
Circulating inflammatory endothelial cells (IEC), which detach from the vessel wall in sites of vascular injury, hold potential to become unique markers of endothelial inflammation. These cells are characterized by the expression of vascular adhesion protein (VAP)-1, a cell adhesion molecule that contributes to leucocyte extravasation and promotes formation of hydrogen peroxide, increasing oxidative stress and magnifying the inflammatory response16. Importantly, an increased inflammatory status may impair repair mechanisms such as recruitment of endothelial progenitor cells (EPC) to the site of injury. These bone-marrow derived cells have the ability to proliferate, migrate, and differentiate into mature endothelial cells, contributing to vascular repair17, 18. However, whether the number of circulating EPC and IEC is altered in AAEH remains unknown. Therefore, we hypothesized that greater levels of inflammation in AAEH patients with relatively preserved renal function would be associated with elevated circulating IEC and lower EPC levels compared to healthy individuals. Moreover, considering their increased propensity for hypertensive renal injury, we also tested whether renal-vein levels of inflammatory markers, EPC, and IEC were particularly pronounced in AAEH patients.
Methods
This study was approved by the Institutional Review Board at the Mayo Clinic. African American (n=19) and Caucasian (n=19) patients with diagnosis of essential hypertension were enrolled in the study from January 2008 to January 2012. The control group consisted of 19 age- and sex-matched normotensive healthy volunteers, prospectively recruited through the Mayo Clinic Biobank.
In all patients, clinical and laboratory parameters were collected via the electronic medical records. Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) formula. Urine protein levels (mg/24hr) were measured by standard procedures in samples collected from EH and AAEH patients, and 16 consenting healthy age-matched potential kidney donors.
Inflammatory biomarkers and EPC homing factors
Peripheral blood (in HV), renal-vein and inferior-vena-cava, in EH and AAEH) samples were collected. Renal-vein and circulating levels of soluble E-selectin (sE Selectin), soluble vascular cell adhesion molecule (sVCAM-1), intercellular adhesion molecule (ICAM)-1, myeloperoxidase (MPO), plasminogen activator inhibitor (PAI)-1, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein-(MIP)-1δ, TNF-α, interleukin (IL)-6, adiponectin, matrix metalloproteinase (MMP)-9, stromal cell-derived factor (SDF)-1 and stromal cell factor (SCF) were measured by luminex18, 19.
EPC and IEC
Mononuclear cells were isolated from fresh blood samples by the density-gradient method and subsequently characterized for antigen expression of EPC and IEC markers. Systemic and renal-vein levels of CD34+/KDR+ (EPC) and VAP-1+ (IEC) cells were determined by fluorescence-activated cell sorting (FACS), as previously described18. Results were expressed as EPC or IEC % (per 100,000 cell counts). To further characterize IEC, VAP-1+ cells were also stained with endothelial (CD31), lymphocyte (CD3 and CD45), monocyte/macrophage (CD16 and CD14) and progenitor cell (CD34 and CD133) markers and analyzed using FACS.
For detailed Methods and statistical analysis see the online-only Data Supplement.
Results
Table 1 shows clinical, laboratory, and demographic characteristics of the patients included in the study. BMI was higher in AAEH compared to HV and EH patients (both p<0.05). Systolic (SBP), diastolic (DBP), and mean (MAP) blood pressure were similarly higher in both hypertensive groups compared to HV, while cholesterol fractions and triglyceride levels did not differ among the groups. Antihypertensive medications were similar between EH and AAEH patients. CRP levels were similarly higher in both hypertensive groups compared to normal. Serum creatinine levels were similar among the groups, but eGFR was higher in AAEH compared to HV and EH patients. Urinary protein levels did not differ among the groups.
Table 1.
Clinical, laboratory, and demographic data of healthy volunteers (HV), Caucasian essential hypertensive (EH) and African American essential hypertensive (AAEH) patients.
| Parameters | HV | EH | AAEH |
|---|---|---|---|
| Demographics: | |||
| Number of patients | 19 | 19 | 19 |
| Age (years) | 57.5±12.5 | 56.6±17.6 | 52.1±5.1 |
| Gender (Male/Female) | 6/13 | 6/13 | 6/13 |
| Clinical: | |||
| Weight (Kg) | 80.4±17.7 | 75.7±16.4 | 92.5±14.2*† |
| Height (cm) | 173.1±10.9 | 167.8±10.9 | 168.3±8.6 |
| Body Mass Index | 26.6±4.3 | 26.8±4.8 | 32.9±6.2*† |
| Related laboratory measures: | |||
| Systolic blood pressure (mmHg) | 120.1±11.6 | 130.3±17.6* | 130.9±17.1* |
| Diastolic blood pressure (mmHg) | 69.3±7.0 | 75.5±6.8* | 78.3±11.8* |
| Mean blood pressure (mm Hg) | 86.2±7.3 | 93.8±9.3* | 95.9±12.1* |
| C-reactive protein (μg/ml)) | 2.11±1.70 | 3.63±2.93* | 5.77±8.13* |
| Total cholesterol (mg/dL) | 172.2±33.7 | 188.2±28.8 | 190.1±36.7 |
| High-density lipoprotein (mg/dL) | 59.2±15.1 | 53.1±9.8 | 53.3±12.7 |
| Low-density lipoprotein (mg/dL) | 89.5±30.1 | 107.4±23.7 | 113.7±34.3 |
| Triglycerides (mg/dL) | 117.4±42.7 | 138.8±65.0 | 101.3±36.7 |
| Use of concomitant medication (n/%): | |||
| Antihypertensive drugs (range) | 0 | 3 (0–5)* | 2 (1–5)* |
| Diuretic | 0 | 14 (73.7)* | 16 (84.2)* |
| Calcium-channel blocker | 0 | 6 (31.6)* | 6 (31.6)* |
| Beta-blocker | 0 | 9 (47.4)* | 6 (31.6)* |
| Angiotensin converting enzyme inhibitor | 0 | 11 (58.0)* | 10 (52.6)* |
| Angiotensin receptor blocker | 0 | 9 (47.4)* | 8 (42.1)* |
| Alpha-blocker | 0 | 1(0.05)* | 3 (15.8)* |
| Statins | 0 | 8 (42.1)* | 4 (21.0)* |
| Renal function: | |||
| Serum creatinine (mg/dl) | 0.92±0.14 | 0.88±0.22 | 0.91±0.28 |
| eGFR (ml/min/1.73/m2) | 78.2±14.9 | 82.9±20.6 | 96.0±22.7*† |
| Urinary protein (mg/24hr)† | 42.4±12.9 | 70.3±56.1 | 65.4±49.1 |
eGFR (estimated glomerular filtration rate),
p≤0.05 vs. HV,
p≤0.05 vs.EH.
Urine protein levels (mg/24hr) in HV were measured in samples collected from 16 consenting healthy age-matched potential kidney donors.
Inflammatory biomarkers and EPC homing factors
Systemic and renal-vein levels of sE-Selectin, sVCAM-1, and SDF-1 were elevated in both hypertensive groups compared to HV, but higher in AAEH compared to EH (Table 2). In contrast, systemic and renal-vein levels of MPO, PAI-1, MCP-1, MIP-1δ, and SCF were only elevated in AAEH (table 2, p<0.05 vs. HV, p<0.05 vs. EH). Contrarily, systemic and renal-vein levels of sICAM-1, TNF-α, IL-6, and adiponectin were similar among the groups.
Table 2.
Systemic and renal-vein (mean±SD) cytokine levels in healthy volunteers (HV), Caucasian essential hypertensive (EH) and African American essential hypertensive (AAEH) patients.
| Parameters | HV | EH | AAEH | ||
|---|---|---|---|---|---|
|
| |||||
| Systemic | Systemic | Renal-vein | Systemic | Renal-vein | |
| Inflammatory markers | |||||
| sE-Selectin (ng/ml) | 15.2±8.3 | 27.8±14.7* | 25.5±15.8* | 48.9±37.5*† | 49.7±36.9*† |
| sVCAM-1(pg/ml) | 683.8±299.7 | 1087.5±295.9* | 1034.3±293.7* | 1842.6±1773.4*† | 1873.4±1841.8*† |
| sICAM-1 (pg/ml) | 173.2±57.0 | 182.4±117.5 | 176.2±118.4 | 128.6±167.7 | 124.2±173.2 |
| MPO (pg/ml) | 16.1±6.9 | 22.7±22.3 | 15.3±9.2 | 54.5±64.8*† | 38.0±34.8*† |
| PAI-1(pg/ml) | 22.9±20.8 | 33.2±29.8 | 31.9±28.9 | 309.1±466.2*† | 310.8±474.4*† |
| MCP-1 (pg/ml) | 118.7±29.8 | 143.9±55.9 | 129.3±50.1 | 185.9±75.0*† | 174.8±78.9*† |
| MIP-1δ (pg/ml) | 4109.9±3328.1 | 4777.2±2291.7 | 4304.3±2490.4 | 10591.6±8972.5*† | 9876.5±8949.0*† |
| TNF-α (pg/ml) | 3.7±1.4 | 4.9±2.6 | 4.8±2.8 | 4.2±2.4 | 3.9±1.9 |
| IL-6 (pg/ml) | 13.2±12.7 | 14.4±29.5 | 11.2±21.8 | 14.4±25.6 | 15.5±30.7 |
| Adiponectin (pg/ml) | 7012.7±854.0 | 7618.0±14868.6 | 7723.8±5470.3 | 8420.5±7162.7 | 7836.8±6287.1 |
| MMP-9 (pg/ml) | 80.12±45.2 | 76.2±40.4 | 74.6±38.9 | 82.2±41.4 | 74.2±37.5 |
| EPC homing factors | |||||
| SDF-1 (pg/ml) | 1380.8±426.3 | 2108.8±1567.2 | 1767.2±1458.6 | 3241.7±2406.5*† | 2818.1±2168.2*† |
| SCF (pg/ml) | 9.5±10.6 | 18.7±17.7* | 19.1±19.5* | 136.0±57.1*† | 149.0±73.9*† |
p≤0.05 vs. HV,
p≤0.05 vs.EH.
EPC and IEC
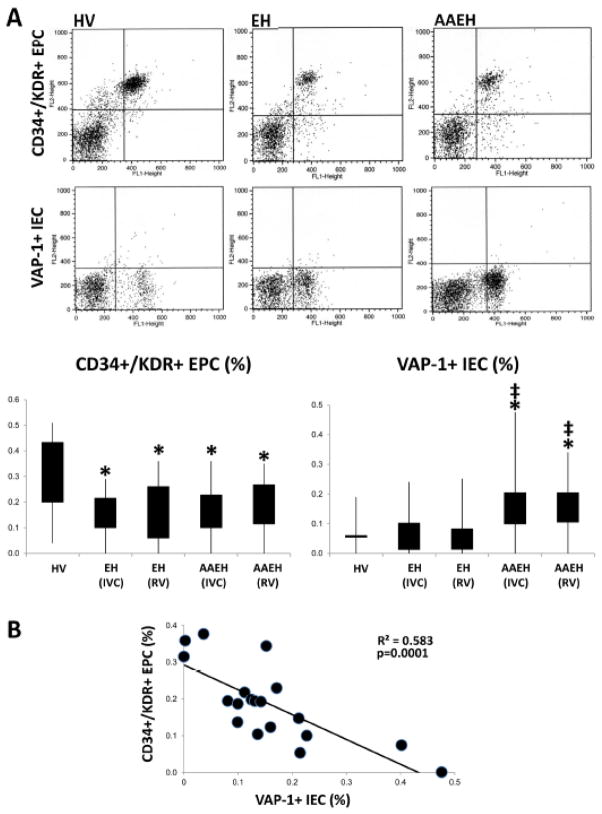
Systemic and renal-vein EPC levels were both similarly lower in both hypertensive groups compared to HV, whereas IEC levels were higher in AAEH compared to HV and EH patients (Figure 1A).
Figure 1.
A: Representative flow cytometric dot plots for CD34+/KDR+ endothelial progenitor cells (EPC) and VAP-1+ inflammatory endothelial cells (IEC) and quantification of their inferior-vena-cava (IVC) and renal-vein (RV) levels in healthy volunteers (HV), Caucasian essential hypertensive (EH) and African American essential hypertensive (AAEH) patients. B: Systemic levels of IEC inversely correlated with circulating EPC levels in AAEH patients (E). *p≤0.05 vs. HV, ‡p≤0.05 vs.EH.
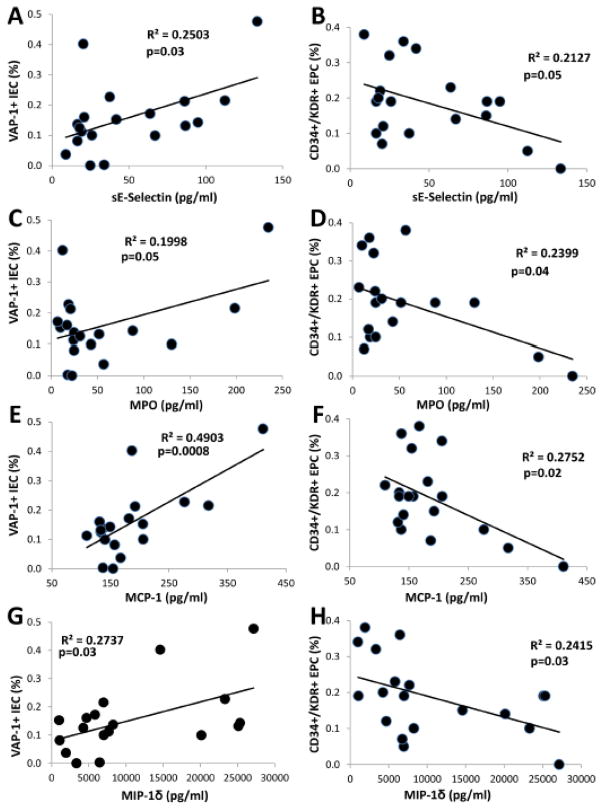
Statistical differences in the levels of EPC and IEC between the groups persisted after adjustment for BMI and eGFR (Figure 1sA–D). Notably, an inverse correlation was found between the number of circulating EPC and IEC in AAEH patients (Figure 1B), but not in EH (R2=0.03, p=0.47). Furthermore, systemic levels of the inflammatory cytokines E-Selectin, MPO, MCP-1, and MIP-1δ in AAEH patients correlated directly with IEC levels (Figure 2A, C, E, G), but inversely with EPC levels (Figure 2B, D, F, H). renal-vein levels of IEC and EPC showed similar relationships (data not shown).
Figure 2.
Systemic levels of soluble E selectin (sE Selectin), myeloperoxidase (MPO), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1 delta (MIP-1δ) correlated directly with the number of circulating inflammatory endothelial cells (IEC, A, C, E, G), but inversely with the number of circulating endothelial progenitor cells (EPC, B, D, F, H).
IEC characterization
A large fraction of VAP-1+ cells co-stained with CD31, but not with CD3, CD45, CD16, or CD14 (Figure 2sA–E). Finally, an important proportion of VAP-1+ cells expressed CD34 and CD133 (Figure 2sF, G).
Discussion
The current study shows elevated circulating and renal-vein levels of IEC and inflammatory cytokines in AAEH compared to Caucasian EH patients, despite controlled blood pressure and preserved renal function. Furthermore, we found reduced levels of renal-vein and circulating EPC in both hypertensive groups, which may suggest impaired reparative mechanisms. These observations may imply higher propensity for vascular injury in AAEH compared to Caucasian EH patients, and introduce IEC as novel markers of inflammatory endothelial injury in hypertensive patients. The current study may also suggest a differential mechanism of end-organ damage in AAEH compared to Caucasian EH, which may have therapeutic implications.
Hypertension remains one of the major risk factors for cardiovascular and cerebrovascular diseases that affects one in three US adults, with estimated annual costs of $50.6 billion1. Importantly, recent projections show that an additional 27 million people could have hypertension by 2030, a 9.9% increase in prevalence from 201020. There is now compelling evidence that hypertension affects African American individuals disproportionally, as their prevalence of hypertension remains one of the highest in the world21. Furthermore, AAEH develop hypertension at an earlier age and cardiac (left ventricular mass and posterior wall thickness) and renal hemodynamic involvement is more severe in these patients compared to Caucasian EH22, suggesting greater end-organ damage.
Polymorphisms in several genes involved in blood pressure regulation have been associated with development of hypertension in AAEH patients7, 23, 24. For example, studies identified variants within a 60kb region of chromosome 22 containing part of the apolipoprotein (APO)L19 and MYH98 genes (associated with increased risk of hypertensive renal disease) in African-Americans, underscoring important contributions of genetic factors to development of hypertension in African-American individuals. Furthermore, heterozygous mutations in the MYH9 have been associated with four autosomal dominant clinical syndromes characterized by neutrophil inclusion bodies, implying increased susceptibility for systemic inflammation in these individuals11.
Indeed, inflammation and endothelial activation play a critical role in impairing vascular function in hypertensive patients. De la Sierra et al reported increased levels of E-selectin, P-selectin, MCP-1, and tissue inhibitor of metalloproteinases-1 in patients with impaired acetylcholine-dependent vasodilation12. In turn, endothelial dysfunction is associated with a higher incidence of myocardial infarction, angina, coronary revascularization procedures, cerebrovascular events, and aortoiliac occlusive disease in hypertensive patients, underscoring the prognostic role of endothelial dysfunction in development of cardiovascular complications25. However, inflammatory markers alone may lack specificity in predicting disease progression in hypertensive patients26, emphasizing the need for alternative markers of endothelial injury.
Circulating IEC, released from sites of vascular injury, might reflect the extent of endothelial injury in kidney and vascular diseases16. These cells are characterized by expression of VAP-1, a cell adhesion molecule that mediates binding, rolling, and transmigration of lymphocytes to the endothelium27. Moreover, VAP-1 is a semicarbazide-sensitive amine oxidase that catalyzes a reaction that promotes the formation of hydrogen peroxide and ammonium28, increasing oxidative stress and enhancing the inflammatory response. Recent data suggest that VAP-1 may predict cardiovascular mortality in type II diabetic subjects29. Likewise, VAP-1 levels are elevated in chronic kidney disease30 and fall with time after kidney transplantation31. However, whether circulating and renal-vein levels of VAP-1 expressing cells are elevated in hypertensive individuals remained unknown.
This study shows higher renal-vein and circulating IEC levels in AAEH compared to Caucasian EH and HV, which may implicate these cells in the pathogenesis or sequelae of hypertension in African American individuals. Importantly, neither BMI nor eGFR alone seem to have accounted for the increase in IEC levels in our study, because the differences persisted after adjustment for both measurements.
We also found that systemic and renal-vein levels of multiple inflammatory biomarkers were elevated in AAEH compared to HV and Caucasian EH, which may indicate increased systemic inflammation in this ethnic group. In particular, systemic levels of sE-Selectin and sVCAM-1 were higher in AAEH compared to EH and HV, suggesting higher endothelial activation in this ethnic group, as previously documented12, 15. Conversely, circulating and renal-vein levels of several inflammatory markers remained within normal range in Caucasian EH patients, in agreement with previous observations from our group that showed similar levels of interferon (IF)-γ, TNF-α, TNF receptor (TNFR)-1, and MIP-1δ between HV and Caucasian EH treated with ACEi and ARB19. Notably, the lack of gradient between circulating and renal-vein cytokine or IEC levels might argue against the kidney as the main source of systemic inflammation in AAEH, suggesting increased systemic vascular injury. This in turn may induce end-organ damage in AAEH compared to Caucasian EH patients. In agreement, increased inflammation32 and oxidative stress33, 34 in AAEH patients is associated with diminished endothelial function, which may imply a critical role for these pathways in aggravating cardiovascular disease.
Importantly, systemic inflammation can trigger mobilization, homing, and transdifferentiation of EPC, which play a major role in regulation and protection of the endothelium after vascular injury35–37. Previous studies have shown that endothelial repair capacity of EPC is reduced in pre-hypertensive patients, which might represent an early event in development of hypertension38. Similarly, we have previously shown that circulating EPC levels are lower in EH patients compared to HV, suggesting inadequate reparative capacity18. In our study, renal-vein and circulating EPC levels were similarly reduced in both hypertensive groups, which may argue against ethnic disparities in their mobilization. However, the inverse correlation between circulating IEC and EPC levels in AAEH may suggest that IEC might interfere with the recruitment or functional capacity for endothelial repair by recruited EPC.
In agreement with a previous study16, IEC expressed endothelial markers, but not lymphocyte, monocyte, or macrophage markers. Interestingly, we found that an important fraction of these cells expressed the progenitor cell markers CD133 and CD34, suggesting that some of these cells might represent inflammatory progenitor cells. Whether these VAP-1+ progenitor cells have impaired reparative capacity or mediate vascular injury warrants further investigation.
Our study is limited by its cross-sectional nature and relatively small study population. In addition, treatment with calcium channel blockers, ACEi, ARB, or statins might have masked elevation in some circulating cytokines or IEC in both AAEH and EH39–41. Further studies are also needed to explore in more detail the precise cause and effect relationships between IEC levels and reparative EPC capacity in AAEH patients.
Perspectives
Our results show that despite preserved kidney function and controlled blood pressure, renal-vein and circulating inflammatory markers were elevated in AAEH and correlated with increased IEC and decreased EPC levels. Increased release of cytokines and IEC in AAEH may impair EPC reparative capacity and predispose to hypertensive vascular injury. This process may aggravate vascular damage and accelerate hypertension-related morbidity/mortality rates in AAEH. Therefore, future management strategies may need to consider the propensity for increased inflammation in AAEH patients, which might be amenable to anti-inflammatory interventions.
Supplementary Material
What Is New?
Our study implies higher propensity to vascular injury in African American essential hypertensive compared to Caucasian essential hypertensive patients, associated with higher circulating levels of inflammatory endothelial cells.
What Is Relevant?
African American individuals have a higher incidence and prevalence of essential hypertension compared to Caucasians, possibly related to a differential effect on vascular injury and repair.
Summary
Inflammatory endothelial cells are novel markers of inflammatory endothelial injury in hypertensive patients, which may aggravate vascular damage and accelerate hypertension-related morbidity/mortality rates in African American essential hypertensive patients.
Acknowledgments
Sources of Funding
This study was partly supported by NIH grant numbers DK73608, HL77131, HL085307, UL1-RR024150, the American Heart Association, and the Mayo Clinic Center for Individualized Medicine.
Footnotes
Disclosures: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (esrd) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis. 1988;12:285–290. doi: 10.1016/s0272-6386(88)80221-x. [DOI] [PubMed] [Google Scholar]
- 3.Mayet J, Shahi M, Foale RA, Poulter NR, Sever PS, Mc GTSA. Racial differences in cardiac structure and function in essential hypertension. BMJ. 1994;308:1011–1014. doi: 10.1136/bmj.308.6935.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folsom AR, Burke GL, Byers CL, Hutchinson RG, Heiss G, Flack JM, Jacobs DR, Jr, Caan B. Implications of obesity for cardiovascular disease in blacks: The cardia and aric studies. Am J Clin Nutr. 1991;53:1604S–1611S. doi: 10.1093/ajcn/53.6.1604S. [DOI] [PubMed] [Google Scholar]
- 5.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713. doi: 10.1161/01.HYP.0000120155.48024.6f. [DOI] [PubMed] [Google Scholar]
- 6.Palmer IM, Schutte AE, Huisman HW. Uric acid and the cardiovascular profile of african and caucasian men. J Hum Hypertens. 2010;24:639–645. doi: 10.1038/jhh.2010.1. [DOI] [PubMed] [Google Scholar]
- 7.Pratt JH, Ambrosius WT, Agarwal R, Eckert GJ, Newman S. Racial difference in the activity of the amiloride-sensitive epithelial sodium channel. Hypertension. 2002;40:903–908. doi: 10.1161/01.hyp.0000039749.75068.f4. [DOI] [PubMed] [Google Scholar]
- 8.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. Myh9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic apol1 variants with kidney disease in african americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB. Apolipoprotein l1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in african americans. Kidney Int. 2013;83:114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murea M, Freedman BI. Essential hypertension and risk of nephropathy: A reappraisal. Curr Opin Nephrol Hypertens. 2010;19:235–241. doi: 10.1097/MNH.0b013e3283366344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Sierra A, Larrousse M. Endothelial dysfunction is associated with increased levels of biomarkers in essential hypertension. J Hum Hypertens. 2010;24:373–379. doi: 10.1038/jhh.2009.91. [DOI] [PubMed] [Google Scholar]
- 13.Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, Schwartz JE, August P. Transforming growth factor-beta 1 hyperexpression in african-american hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A. 2000;97:3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutinho T, Turner ST, Mosley TH, Kullo IJ. Biomarkers associated with pulse pressure in african-americans and non-hispanic whites. Am J Hypertens. 2012;25:145–151. doi: 10.1038/ajh.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DS, Larson MG, Lunetta KL, Dupuis J, Rong J, Keaney JF, Jr, Lipinska I, Baldwin CT, Vasan RS, Benjamin EJ. Clinical and genetic correlates of soluble p-selectin in the community. J Thromb Haemost. 2008;6:20–31. doi: 10.1111/j.1538-7836.2007.02805.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmen C, Elsheikh E, Stenvinkel P, Qureshi AR, Pettersson E, Jalkanen S, Sumitran-Holgersson S. Circulating inflammatory endothelial cells contribute to endothelial progenitor cell dysfunction in patients with vasculitis and kidney involvement. J Am Soc Nephrol. 2005;16:3110–3120. doi: 10.1681/ASN.2005040347. [DOI] [PubMed] [Google Scholar]
- 17.Gulati R, Jevremovic D, Witt TA, Kleppe LS, Vile RG, Lerman A, Simari RD. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H512–517. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 18.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34:540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant. 2012;27:4153–4161. doi: 10.1093/ndt/gfs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 21.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 22.Frohlich ED. Hemodynamic differences between black patients and white patients with essential hypertension. State of the art lecture. Hypertension. 1990;15:675–680. doi: 10.1161/01.hyp.15.6.675. [DOI] [PubMed] [Google Scholar]
- 23.Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, Eckhart AD. G protein-coupled receptor kinase 2 expression and activity are associated with blood pressure in black americans. Hypertension. 2009;54:71–76. doi: 10.1161/HYPERTENSIONAHA.108.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y, Zhu H, Sagnella GA, Carter ND, Cook DG, Cappuccio FP. Association between the c825t polymorphism of the g protein beta3-subunit gene and hypertension in blacks. Hypertension. 1999;34:1193–1196. doi: 10.1161/01.hyp.34.6.1193. [DOI] [PubMed] [Google Scholar]
- 25.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 26.Torun D, Ozelsancak R, Yigit F, Micozkadioglu H. Increased inflammatory markers are associated with obesity and not with target organ damage in newly diagnosed untreated essential hypertensive patients. Clin Exp Hypertens. 2012;34:171–175. doi: 10.3109/10641963.2011.577489. [DOI] [PubMed] [Google Scholar]
- 27.Salmi M, Tohka S, Berg EL, Butcher EC, Jalkanen S. Vascular adhesion protein 1 (vap-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes. J Exp Med. 1997;186:589–600. doi: 10.1084/jem.186.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalkanen S, Salmi M. Cell surface monoamine oxidases: Enzymes in search of a function. EMBO J. 2001;20:3893–3901. doi: 10.1093/emboj/20.15.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li HY, Jiang YD, Chang TJ, Wei JN, Lin MS, Lin CH, Chiang FT, Shih SR, Hung CS, Hua CH, Smith DJ, Vanio J, Chuang LM. Serum vascular adhesion protein-1 predicts 10-year cardiovascular and cancer mortality in individuals with type 2 diabetes. Diabetes. 2011;60:993–999. doi: 10.2337/db10-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin MS, Li HY, Wei JN, Lin CH, Smith DJ, Vainio J, Shih SR, Chen YH, Lin LC, Kao HL, Chuang LM, Chen MF. Serum vascular adhesion protein-1 is higher in subjects with early stages of chronic kidney disease. Clin Biochem. 2008;41:1362–1367. doi: 10.1016/j.clinbiochem.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Koc-Zorawska E, Malyszko J, Malyszko JS, Mysliwiec M. Vap-1, a novel molecule linked to endothelial damage and kidney function in kidney allograft recipients. Kidney Blood Press Res. 2012;36:242–247. doi: 10.1159/000343413. [DOI] [PubMed] [Google Scholar]
- 32.Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Kashem AM, Brown MD. Endothelial-dependent flow-mediated dilation in african americans with masked-hypertension. Am J Hypertens. 2011;24:1102–1107. doi: 10.1038/ajh.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: Predisposition of african americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 34.Textor SC, Gloviczki ML, Flessner MF, Calhoun DA, Glockner J, Grande JP, McKusick MA, Cha SS, Lerman LO. Association of filtered sodium load with medullary volumes and medullary hypoxia in hypertensive african americans as compared with whites. Am J Kidney Dis. 2012;59:229–237. doi: 10.1053/j.ajkd.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 36.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 38.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 39.Farah R, Khamisy-Farah R, Shurtz-Swirski R. Calcium channel blocker effect on insulin resistance and inflammatory markers in essential hypertension patients. Int Angiol. 2013;32:85–93. [PubMed] [Google Scholar]
- 40.Ascer E, Bertolami MC, Venturinelli ML, Buccheri V, Souza J, Nicolau JC, Ramires JA, Serrano CV., Jr Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis. 2004;177:161–166. doi: 10.1016/j.atherosclerosis.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Di Raimondo D, Tuttolomondo A, Butta C, Miceli S, Licata G, Pinto A. Effects of ace-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des. 2012;18:4385–4413. doi: 10.2174/138161212802481282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.