Abstract
Natural killer (NK) cells constitute a minor subset of normal lymphocytes that initiate innate immune responses toward tumor and virus-infected cells. They can mediate spontaneous cytotoxicity toward these abnormal cells and rapidly secrete numerous cytokines and chemokines to promote subsequent adaptive immune responses. Significant progress has been made in the past two decades to improve our understanding of NK cell biology. Here we review recent discoveries, including a better comprehension of the “education” of NK cells to achieve functional competence during their maturation and the discovery of “memory” responses by NK cells suggesting that they may also contribute to adaptive immunity. The improved understanding of NK cell biology has forged greater awareness that these cells play integral early roles in immune responses. In addition, several promising clinical therapies have been used to exploit NK cell functions in treating cancer patients. As our molecular understanding improves, these and future immunotherapies should continue to provide promising strategies to exploit the unique functions of NK cells to treat cancer, infections, and other pathological conditions.
Keywords: Natural killer (NK) cell, Killer cell Ig-like receptors (KIR), Innate immunity, Immune memory, Antibody-dependent cellular cytotoxicity (ADCC), Cancer, Viral infection, Hematopoietic stem cell transplantation, Immunomodulatory drugs, Interferon-γ
What are Natural Killer Cells? More than the name implies
Natural killer (NK) cells constitute about 10% of the lymphocytes in human peripheral blood and provide an important effector arm of the innate immune system. Although named for their capacity to mediate spontaneous “natural” cytotoxicity toward certain tumor and virus-infected cells, NK cells are also a major source of the type 1 cytokine, interferon (IFN)-γ, as well as tumor necrosis factor (TNF)-α, GM-CSF, and other cytokines and chemokines.(1, 2) Production of these soluble factors by NK cells in early innate immune responses significantly influences the recruitment and function of other hematopoietic cells. For example, by contributing both cytokines and cytolysis during viral infections, NK cells enhance antigen-specific T cell responses under conditions of modest viral doses, while limiting excessive T cell responses upon exposure to high viral titers.(3) Collectively, these NK cell activities prevent pathology and mortality. Also, through physical contacts and production of cytokines, NK cells are central players in a regulatory crosstalk network with dendritic cells (DC) and neutrophils to promote or restrain immune responses.(4, 5)
Recently, NK cells were officially classified as the prototypical members of the group 1 innate lymphoid cells (ILC), which are defined by capacity to secrete IFN-γ, but not type 2 cytokines (IL-4, IL-13), IL-17, or IL-22.(6, 7) Group 1 ILC are thus distinct from group 2 ILC that produce IL-13 and group 3 ILC that can produce IL-17 and/or IL-22.(6, 7) Human NK cells are classically defined as CD56+CD3- cells, distinguishing them from CD56+CD3+ cells, which consist of a mixed population of NK-like T (NKT) cells and antigen-experienced T cells that have up-regulated several NK cell markers.(8) The activating receptor NKp46 (NCR1) is also expressed on virtually all human NK cells, but it is important to note that NKp46 and CD56 are also expressed by some group 3 ILCs, although these cells are very rare, especially in peripheral blood.(6, 7) NK cells are primarily found in the blood, spleen, liver, lung, and bone marrow, while limited numbers are localized in lymph nodes.(9) Nonetheless, the few NK cells that reach lymph nodes play key roles there by interacting with DC to promote IFN-γ responses by T cells(10) and preventing the spread of viruses.(11)
Two major subsets of NK cells are found in humans that can be distinguished by their levels of CD56 expression, namely CD56dim and CD56bright.(8) CD56dim NK cells are fully mature, make up about 90% of the NK cells in peripheral blood, and predominantly mediate cytotoxicity responses. In contrast, CD56bright cells are more immature, make up about 5-15% of total NK cells, and have been considered primarily as cytokine producers, while playing a limited role in cytolytic responses. Although CD56bright NK cells are more efficient at producing cytokines overall, the CD56dim NK cells can also contribute significantly to early cytokine production, since they comprise a significantly greater fraction of the total NK cell pool and can more rapidly secrete cytokines.(1, 12, 13) On the other hand, CD56bright cells are better able to leave the vasculature and constitute the majority of NK cells found in either lymph nodes or the decidual tissues of pregnant women. In the first trimester of human pregnancy, CD56bright NK cells can surprisingly make up about 70% of the lymphocytes in the decidual tissue, and recent evidence suggests that they play important roles in promoting angiogenesis during pregnancy.(14)
NK cells can be rapidly activated to spontaneously attack certain abnormal cells in the body, especially tumor or virus-infected cells. Hence, rare individuals who selectively lack NK cells exhibit increased susceptibility to viral infections, especially herpesviruses, and increased tumor incidence.(15) The predominant cytolytic targets of NK cells are uncommon cells that have down-regulated class I MHC (MHC-I), which is expressed on nearly every healthy cell of the body.(16) MHC-I loss is a fairly common mechanism by which tumors and virus-infected cells can evade recognition by the TCR of cytolytic T cells,(17, 18) and NK cells can thereby overcome this potential immunological Achille's heel. NK cytotoxicity is mediated by the directed exocytosis of cytolytic granules to release perforins and granzymes, which perforate the target cell plasma cell membrane and trigger apoptosis, respectively.(19)
How do NK cells work? Inhibition counterbalances activation
The discovery that NK cells selectively attack target cells with diminished MHC-I expression at first puzzled researchers in the early 1990's. The paradigm at that time had established that T and B lymphocytes rearrange genomic elements via RAG-mediated splicing to create clonotypic antigen receptors that can identify foreign pathogens and altered proteins through “non-self” recognition. Therefore, it was unclear how NK cells could detect and respond to the loss of a normal ubiquitous protein.
This puzzle was solved when it was discovered that mature NK cells are tolerized toward normal cells by their expression of germline-encoded inhibitory receptors that recognize MHC-I. When these inhibitory receptors encounter MHC-I on normal cells, they recruit SHP-1 and SHP-2 protein tyrosine phosphatases at immunoreceptor tyrosine-based inhibitory motifs (ITIMs) to dominantly arrest tyrosine kinase-based activation signals.(20) When a mature NK cell encounters a rare abnormal cell lacking MHC-I, however, inhibitory receptors are not engaged, and unsuppressed activating signals trigger targeted attack. The principal inhibitory receptors are killer cell Ig-like receptors (KIR) on human NK cells and Ly49 on murine NK cells. In addition, both mouse and man share the CD94/NKG2A inhibitory receptor, which recognizes a non-classical MHC-I (Qa-1b in mouse and HLA-E in humans). The differences between human and mouse NK cell inhibitory receptors are summarized in Table I.
Table I. Major NK cell receptors with reported ligands in mouse and man.
| Receptor | Species | Reported Ligand(s)* | |
|---|---|---|---|
| Human | Mouse | ||
| Inhibitory Receptors | |||
| KIR2DL1 (CD158a) | × | Group 2 HLA-C | |
| KIR2DL2, KIR2DL3 (CD158b1, -b2) | × | Group 1 HLA-C, some Group 2 HLA-C and some HLA-B | |
| KIR3DL1 (CD158e1) | × | HLA-Bw4 | |
| Ly49A (Klra1) | × | H2-Dd, -Dk, -Ld, -Db, -Kb, -Dp, -M3 | |
| Ly49C (Klra3) | × | H2-Db, -Kb, -Dd, -Kd, -Dk | |
| Ly49E (Klra5) | × | Urokinase plasminogen | |
| Ly49G (Klra7) | × | H2-Dd, -Kd, -Ld, -Db, -Dk, -Dr | |
| Ly49I (Klra9) | × | H2-Kb, -Kd, -Dk, -Kk, m157 (mouse cytomegalovirus) | |
| NKG2A (CD159A)/CD94 | × | × | HLA-E (human), Qa-1b (mouse) |
| LILRB1 (ILT2, LIR1, CD85j) | × | HLA-A, -B, -C, -E, -G, -F, UL18 protein (human cytomegalovirus) | |
| KLRG1 | × | × | E-, N-, and R-cadherin |
| NKR-P1A (CD161) | × | LLT1 | |
| NKR-P1B, NKR-P1D | × | Ocil/Clr-b | |
| Activating Receptors | |||
| NKp46 (NCR1; CD335) | × | × | Human: HSPG, heparin, VM, HA (IV, VV, ECTV), HN (SeV, NDV), PfEMP-1(P. falciparum), F. nucleatum; Mouse: influenza-infected cells, F. nucleatum |
| NKp30 (NCR3; CD337) | × | B7-H6, BAT3, HSPG, HA (VV, ECTV), pp65 (HCMV), PfEMP-1 (P. falciparum) | |
| FcγRIIIA (CD16) | × | × | Fc of human IgG immune complexes |
| NKG2D (CD314) | × | × | Human: MICA/B, ULBP1, -2, -3, -4, -5, -6 Mouse: RAE-1a, -1b, -1d, -1e, -1g, H60a, H60b, H60c, MUTL1 |
| KIR2DS1 (CD158h) | × | HLA-C2 | |
| KIR2DS4 (CD158i) | × | Some HLA-C1 and –C2, HLA-A11 | |
| KIR2DL4 (CD158d) | × | HLA-G | |
| NKp65 (KLRF2) | × | KACL (keratinocyte-associated C-type lectin) | |
| NKp80 (KLRF1) | × | AICL (activation-induced C-type lectin) | |
| DNAM-1 (CD226) | × | × | Nectin-2, PVR |
| NKG2C (CD159C)/CD94, NKG2E/CD94 | × | × | HLA-E (human), Qa-1b (mouse) |
| Ly49D (Klra4) | × | H2-Dd, -Dr, Dsp2 | |
| Ly49H (Klra8) | × | m157 (mouse cytomegalovirus) | |
| Ly49P (Klra16) | × | H2-Dd, -Dk, m04 (mouse cytomegalovirus) | |
| NKR-P1F | × | Clr-c, -d/x, -g | |
| NKR-P1G | × | Clr-dx, -g, -f | |
| Activating/Inhibitory Receptors | |||
| 2B4 (CD244) | × | × | CD48 |
| NKp44 (NCR2; CD336) | × | PCNA, HS, heparin, E-protein (DV, WNV), HA (IV, SeV), HN (NDV), Mycobacterium, N. farcinica, P. aeruginosa | |
Abbreviations used: HSPG: heparan sulfate proteoglycan, VM: vimentin, HA: haemagglutinin, HN: haemagglutinin–neuraminidase, IV: influenza virus, VV: vaccinia virus, ECTV: ectromelia virus, SeV: Sendai virus, NDV: Newcastle disease virus, P. falciparum: Plasmodium falciparum, F. nucleatum: Fusobacterium nucleatum, N. farcinica: Nocardia farcinica, P. aeruginosa: Pseudomonas aeruginosa.
Although the KIR and Ly49 receptor families are strikingly divergent in structure, they each have evolved to perform nearly identical functions and share similar mechanisms of regulated expression.(21-23) KIR are polymorphic type I proteins with Ig-like domains, and each KIR family member recognizes a distinct subset of classical MHC-I (HLA-A, -B, and -C) molecules, while Ly49 are a family of polymorphic type II proteins with C-type lectin domains that similarly bind subsets of H-2 molecules.(24, 25) Distinct NK cells within an individual express different KIR or Ly49 family members, resulting in a mixed repertoire of cells with different MHC-I recognition capacities, and some inhibitory receptors do not recognize the endogenous (self) MHC-I alleles. This diversity is compounded by inheritance of different haplotypes of KIR or Ly49 genes by individuals and minor allelic polymorphisms, which further increase variability of expression and ligand recognition by these inhibitory receptors within human and mouse populations.(26)
Activating receptors expressed on NK cells include FcγRIIIA, activating forms of KIR (KIR2DS, KIR3DS), 2B4, NKG2D, and the Natural Cytotoxicity Receptors (NCR) called NKp30, NKp44, and NKp46 (see Table I).(20, 27) FcγRIIIA (CD16) can trigger antibody-dependent cellular cytotoxicity (ADCC) upon encountering target cells opsinized with IgG, while NKG2D and NCR appear to be the most relevant receptors that stimulate responses to tumor target cells.(28) Ligands for several of these receptors are still undefined, but several known ligands are upregulated on stressed cells, such as MICA and MICB ligands for NKG2D and the B7-H6 ligand for NKp30.(29, 30) Integrins also play important roles in mediating adhesion to target cells, and integrin-mediated signaling is important for stimulating NK cell activation that triggers targeted degranulation.(31-33)
How do NK cells learn to function properly? Inhibitory receptors can also be instructive
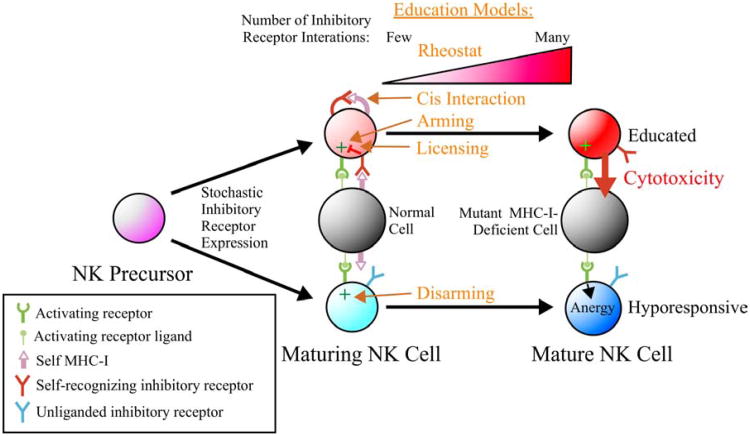
The function of inhibitory receptors is, however, not only to counteract activating receptors as a means to tolerize mature NK cells toward normal cells. Studies in mice support a “regulated sequential” model, in which individual KIR or Ly49 genes are stochastically expressed during NK cell maturation until a receptor recognizes “self” MHC-I, transduces negative signals, and restricts expression of additional inhibitory receptor genes.(34, 35) This creates a repertoire of NK cells, each of which permanently expresses a distinct combination of available inhibitory receptor gene products. Expression of a self-recognizing inhibitory receptor (SRIR) during NK cell development also promotes the maturation of functionally-responsive NK cells in both mice and humans, through a process of “education” (Figure 1).(36-42) It was originally assumed that all mature NK cells express at least one SRIR to assure tolerance,(43, 44) but more recent studies identified a subset of mature cells lacking SRIR. Most of these SRIR- NK cells express inhibitory receptors that cannot recognize endogenous MHC-I and are therefore “unliganded”, and activating receptors in SRIR- NK cells are hyporesponsive to generate cytokine production or cytotoxicity. This hyporesponsive state is characteristic of all NK cells in MHC-I-deficient mice and humans.(45, 46) These results reinforce the need for SRIR interactions to facilitate the development of functional competence of NK cells and the existence of tolerizing mechanisms to prevent autoimmune attack of normal tissues in the absence of SRIR.
Figure 1. Models of NK cell education.
During NK cell development, only some of the stochastically-expressed inhibitory receptors (KIR in humans and Ly49 in mice) recognize self MHC-I ligand (self-recognizing inhibitory receptor; SRIR). SRIR interactions promote NK cell education to mediate cytotoxicity toward an abnormal cell that has lost expression of MHC-I, whereas a mature NK cell expressing only unliganded inhibitory receptors becomes hyporesponsive. Models of NK cell education are indicated: 1) Rheostat: expression of >1 SRIR promotes more robust education, 2) Cis Interaction: interactions between SRIR and MHC-I on the NK cell surface are important, 3) Arming: inhibitory signaling blocks chronic activation signals that would otherwise anergize (“Disarming”); 4) Licensing: an ITIM-mediated signal drives education.
Four models have been proposed to explain the process of NK cell education during development (see Figure 1): 1) Yokoyama introduced the “licensing” model in which signaling through a SRIR directly promotes functional competence mediated by an ITIM-dependent signaling process.(42) 2) Raulet proposed the “disarming” model in which NK cells lacking SRIR are anergic or “disarmed” due to chronic activation signaling, whereas “arming” results when SRIR engage with MHC-I to prevent chronic activation signaling.(41) 3) Höglund first established the “rheostat model” in which stronger inhibitory signaling through more SRIR interactions leads to greater functional responsiveness of NK cells.(37, 47, 48) 4) Held proposed the “cis interaction” model in which cis interactions between Ly49 and MHC-I on the surface of the same NK cell sequesters these SRIR from reaching the immune synapse, thereby making the NK cell more functionally responsive.(49) Although the cis interaction model provides an interesting hypothesis that may be operative for some SRIR, it is unclear whether all Ly49 can interact with MHC-I in cis and whether KIR can interact in cis at all.
Interesting evidence suggests that uneducated NK cells may also play important roles in immune responses toward viral infections in vivo, since SRIR-deficient NK cells responded more strongly than SRIR+ cells toward MCMV.(50) Furthermore, SRIR-deficient NK cells can be “primed” to a functional state upon culture in IL-2.(42) This suggests that these uneducated NK cells may be functionally activated by cytokines at sites of infection and tumors to respond even toward MHC-I-expressing target cells. These results demonstrate the value of a diverse NK cell repertoire and reinforce a need to better understand how the signaling competence of individual NK cells is influenced by SRIR/MHC-I interactions and maturation at the molecular level.
Can NK cells mediate adaptive immunity? Cherishing the memories
Although NK cells have been classified as innate immune cells, exciting recent evidence in mice indicates that NK cells may also mediate long-lived “memory”-like responses, and immune memory is a central attribute of the adaptive immune system. Previous work indicated that mature NK cells transferred into an immune-competent mouse survive with a short half-life of only about one week and undergo minimal proliferation,(51) but they can undergo homeostatic proliferation when transferred to a lymphopenic environment.(52, 53) A more recent study found that murine NK cells undergoing homeostatic expansion in a lymphopenic environment can generate a population that can survive and self-renew for more than 6 months,(54) suggesting that long-lived NK cells can arise under certain circumstances. Related to this finding, three types of long-lived memory responses by NK cells have recently been reported.
First, certain viral infections have been shown to multiply NK cell subpopulations expressing receptors that specifically target the virus. As a classic example, upon infection with mouse cytomegalovirus (MCMV), C57BL/6 mice expand a subpopulation of NK cells expressing the Ly49H activating receptor, which specifically detects the MCMV-derived protein m157. These Ly49H+ cells were found to express markers of terminally mature NK cells,(55) and such cells were previously shown to be long-lived.(56) A subset of these MCMV-experienced NK cells can survive for at least 70 days, and upon secondary MCMV challenge, these cells can proliferate robustly in an IL-12-dependent manner, demonstrate enhanced cytotoxicity and cytokine responses, and provide enhanced protection against secondary viral challenge.(55, 57) A potentially similar phenomenon was observed in human transplant recipients, in which a terminally-mature NKG2C+ memory-like NK cell population expands, is long-lived, and has enhanced responsiveness to secondary CMV infection.(58, 59) Another report found that CMV infected individuals expand a pool of highly differentiated NK cells that express educating SRIR and several activating KIR (KIR2DS4, KIR2DS2, or KIR3DS1) and NKG2C receptors.(60) Also analogous is the expansion and long-term persistence of NKG2C+ NK cells in individuals infected with human hantavirus.(61)
Second, NK cells pre-stimulated in vitro with a cocktail of cytokines (IL-12 + IL-18 + IL-15) were found to survive for at least several weeks when transferred into naïve mice and demonstrated enhanced responses to a variety of stimuli.(62) A subsequent study found that NK cells stimulated with the same cytokine combination significantly reduced tumors in mice, although the effect also required whole body irradiation.(63) The NK cells in this study expanded substantially in vivo in a CD4 T cell-dependent manner and accumulated in tumor sites. Furthermore, human NK cells stimulated in vitro with IL-12 + IL-18 + IL-15 exhibit enhanced IFN-γ responses to restimulation with the same cytokines up to 3 weeks later, indicating the same cytokine memory responses can also occur in humans.(63, 64)
Third, and perhaps most intriguing, is the identification of a liver-derived NK cell population that can generate antigen-specific memory responses. These cells were first identified in Rag2-deficient mice exposed to haptens, which elicited contact hypersensitivity responses upon re-exposure to specific antigen at least four weeks later.(65) Surprisingly, this delayed-type hypersensitivity response was elicited by NK cells derived from the liver, but not from the spleen. Subsequent work from the same group found that vaccination of mice with viral antigens can induce hepatic NK cells that provide antigen-specific protection from challenge with several viruses at least four months later.(66) These hepatic memory NK cells were also found to express and be influenced through the chemokine receptor CXCR6.(66)
While T and B memory cells provide antigen-specific memory responses for many years, these recent findings indicate that NK cells also have the potential to recall previous insults to their environment, leading to improved responsiveness upon secondary challenge for at least a few months. Questions arise regarding the mechanistic foundations for these memory responses, how long they can be maintained, and whether they can be harnessed to combat disease through therapeutic interventions, such as vaccine-based strategies.
Can NK cells be persuaded to improve human health? Killer immunotherapies to the rescue
Due to improved understanding of the important roles played by NK cells in normal immune function, the development of NK cell-targeted therapies has gained traction during the past decade. Not surprisingly, the best examples of treatment strategies to manipulate human NK cell functions have involved immunotherapies to treat cancer.
Some of the clinically approved therapeutic antibodies to treat cancer, such as rituximab (anti-CD20 mAb), trastuzumab (anti-Her2 mAb), cetuximab (anti-EGFR mAb), and mogamulizumab (anti-CCR4 mAb) are considered to function at least partially through triggering NK cell-mediated ADCC activity.(67) Several approaches have been designed to enhance this NK cell-mediated ADCC activity through antibody engineering and use of antibody combinations. For example, mogamulizumab, which was recently approved in Japan, is defucosylated to increase binding by FcγRIIIA and thereby enhance ADCC activity.(67) In addition, since NK cells encountering rituximab-coated target cells upregulate the co-stimulatory molecule CD137, the addition of an agonistic anti-CD137 mAb was shown to potentiate rituximab-mediated ADCC responses.(68) Furthermore, the inhibitory receptor PD-1 was found to be upregulated on NK cells in multiple myelomas that express the cognate ligand, PD-L1, and a PD-1 blocking antibody was shown to potentiate NK cell-mediated cytotoxicity of these tumors.(69) Also, in vitro ADCC responses were potentiated in the presence of antibodies that block NK cell inhibitory receptors from interacting with MHC-I ligands.(70) More recently, an anti-KIR antibody that blocks MHC-I recognition (IPH2101) was shown to boost human NK cell function in vitro, in humanized mice,(71, 72) and most recently in clinical trials in cancer patients.(73-75) In summary, many promising antibody combination strategies designed to boost NK cell ADCC responses to tumors are now in pre-clinical and clinical development.
Since KIR are the predominant receptors regulating NK cell activation in humans, it is not surprising that variations in KIR/HLA interactions can significantly affect human health. Most notably, many population genetic studies have noted differences in disease susceptibility resulting from inheritance of particular combinations of KIR and HLA ligands. For example, individuals homozygous for a low affinity inhibitory KIR/HLA-C combination naturally respond better to hepatitis C virus (weak inhibition is good),(76) whereas pregnancies involving high affinity maternal KIR/fetal HLA-C exhibit greater incidence of pre-eclampsia (strong inhibition is bad).(77) Also, individuals with more gene copies of both activating and inhibitory forms of HLA-B-specific KIR in combination with their high affinity HLA-B ligand(78, 79) are better protected from HIV infection (both strong KIR-mediated activation and inhibition are good).(80-82) Although insightful, the exact mechanisms contributing to many of these genetic correlations are not entirely clear and could involve different thresholds of NK cell activation, differences in NK cell education, or even a role of KIR expressed on T cells.
Inhibitory KIR-HLA ligand mismatch has also been successfully exploited to improve the efficacy of hematopoietic stem cell (HSC) transplantation for treating acute myeloid leukemia (AML), while simultaneously reducing incidence of graft-vs.-host disease.(83-88) Increased survival was also reported for AML patients that received HLA-matched or -mismatched HSC transplants containing KIR “B haplotypes”, which characteristically have more activating KIR.(89) Another study found that patients experienced significantly lower relapse rates if they received T cell-depleted HLA-matched HSC transplants containing three specific KIR genes associated with the B haplotype (KIR2DL5A, KIR2DS1, and KIR3DS1).(90) Furthermore, AML patients receiving HSC allografts from KIR2DS1+ donors were found to experience lower relapse rates compared to those from KIR2DS1- donors.(91) Interestingly, the same study also found that allografts from KIR2DS1+ donors who were homozygous for the KIR2DS1 ligand, HLA-C2, did not provide protection, whereas donors with HLA-C1/C1 or HLA-C1/C2 genotypes provided significantly lower rates of relapse. The results suggest that NK cells expressing the activating KIR2DS1 receptor are tolerized by constitutive stimulatory signals in HLA-C2/C2 donors and this education pattern persists after transplantation. Another study also found that NK-cell education depends on ligands of donor, not recipient, and the education pattern lasted stably for at least three years.(92) These findings demonstrate that KIR/HLA match/mismatch offers a new dimension of variability to consider in HSC transplantation. Although the rules governing the impacts of specific KIR/HLA combinations in donors and recipients continue to be established, evidence is accumulating that allogeneic NK cell reactivity can be beneficial, especially for AML. Also, certain KIR, like the activating KIR2DS1, can play protective roles.
Adoptive transfer of ex vivo-activated mature NK cells is also emerging as a promising immunotherapy for cancer. In several studies, the infusion of enriched populations of haploidentical NK cells, especially those exploiting KIR/HLA allogenicity, has been shown to be safe and effective for treating cancer in adults, children, and elderly patients.(93-95) Although a promising approach, the requirements of specialized processing equipment, good manufacturing practices, and logistical complexities have limited availability of these adoptive NK cell therapies to a few specialized institutions.(96) Nonetheless, centralized processing and safe delivery of viable and active NK cells for infusion at remote clinics has been demonstrated,(97) indicating that the practice may become more widespread as procedures are optimized.
Genetic engineering of NK cells to express chimeric antigen receptors (CAR) also shows promise as an effective anti-tumor therapy. CAR consist of antibody variable domains linked to transmembrane and intracellular activation signaling domains that target engineered effector cells to a target cell-specific marker. The adoptive transfer of patient T cells transduced with lentivirus to express CAR has recently been shown to cause impressive regression of B cell malignancies.(98-100) A disadvantage of this approach is the chronic depletion of normal B cells in these patients, due to the long-term survival of memory-like CAR-expressing T cells. Given the shorter lifespan and potent cytolytic function of NK cells, they are attractive candidate effector cells to express CAR for adoptive immunotherapies. Although not yet tested in clinical trials, several CAR constructs expressed in primary NK cells have been shown to trigger efficient in vitro killing of B cell tumors.(101-103) Limitations of this approach, however, are the difficulty in expressing exogenous genes in primary NK cells and difficulty in expanding the cells in culture to adequate numbers for immunotherapy. Alternatively, some groups have expressed CAR in the human NK-like cell line, NK-92, to engineer a uniformly targeted cytolytic effector cell population.(104, 105) NK-92 cells can be easily expanded in culture and have been well tolerated in phase I clinical trials in humans.(106) Thus, CAR-expressing NK-92 cells may represent a practical NK cell-based immunotherapeutic alternative.
Certain immunomodulatory drugs, such as lenalidomide and bortezomib, can also potentiate NK cell mediated-cytotoxicity or cytokine production responses, which may contribute to their clinical benefits. The thalidomide derivative lenalidomide is approved for the treatment of myelodysplastic syndrome and multiple myeloma (MM) and another derivative pomalidomide was more recently approved for MM. These drugs have has been reported to enhance NK cell-mediated cytotoxicity, possibly through stimulating IL-2 production by T cells(107, 108) and enhancing in vitro ADCC responses to tumor cells.(109) On the other hand, co-treatment with dexamethasone can impair the potentiation of NK cell-mediated cytotoxicity by lenalidomide.(110) The exact mechanism by which lenalidomide stimulates NK cells is still uncertain, however, and it has not been firmly established whether the stimulatory effect of lenalidomide on NK cells directly contributes to treatment outcomes. Bortezomib is a 26S proteasome inhibitor approved for use in treating MM and mantle cell lymphoma.(111) While bortezomib directly induces cell growth inhibition and apoptosis in cancer cells, it also reportedly up-regulates ligands for TRAIL receptors and NKG2D and down-regulates MHC-I on several cancer types, including MM, renal cell carcinoma, leukemia, breast cancer, melanoma, and hepatocellular carcinoma.(112, 113) These effects by bortezomib were found to enhance in vitro NK cell-mediated cytotoxicity responses.(113) Interestingly, in tumor-bearing mouse models, infusion of syngeneic NK cells in combination with bortezomib reduced tumor growth and prolonged survival compared to treatment with bortezomib or NK cells alone.(114) Clinical trials are currently ongoing to evaluate the safety and antitumor effect of adoptively infused ex vivo expanded autologous NK cells following bortezomib treatment in patients with advanced malignancies.(115)
Where do we go from here? Reforming killers for a better future
During the past two decades, significant advances have been made in characterizing the many germline-encoded activating and inhibitory receptors expressed on NK cells, identifying their ligands, and establishing their intricate roles in regulating NK cell activation. Studies in mice and humans have firmly established that this relatively minor subpopulation of lymphocytes provides critical early effector function in a wide variety of immune responses. Further work is needed, however, to fill remaining gaps in knowledge. Surprisingly, the identities of ligands for several NK cell activating receptors still remain elusive, and characterizing these ligands is required to fully define the physiological functions of these receptors. Major efforts are also needed to precisely map the myriad affinities between the many highly polymorphic variants of KIR and HLA ligands found in the human population, as well as the impacts of polymorphism on KIR expression and function. Only once these many diverse variations are taken into account can we fully interpret genetic studies ascribing certain combinations of KIR and HLA alleles to disease risk and accurately predict the impacts of certain donor/recipient combinations on HSC transplantation outcomes. Further work is also required to establish the physiological relevance of and operative mechanisms driving the processes of NK cell “education” and “memory”. Many of the basic mechanisms discovered in NK cells can also be applied to understanding similar mechanisms regulating other immune cells, especially the functionally-related cytolytic T cells.
The NK cell field has reached an exciting stage, in which our improved basic knowledge is rapidly being applied to the development of clinical therapies to modulate NK cell functions in patients. To date, the most promising immunotherapies involving NK cells have been applied to the treatment of cancer. Of particular interest are the evolving improvements in understanding the influences of KIR/HLA matching/mismatching on HSC transplantation and adoptive NK cell transplantation protocols. While these have been most beneficial for treating AML, they need to be tested in other cancers. Further development of immunomodulatory drugs that enhance NK cell functions is also needed, and additional work is required to better characterize their mechanism of action. Effective immunomodulatory drugs can then be incorporated into combination therapies, such as promoting ADCC responses in combination with tumor-targeting antibodies. Future applications are also needed to exploit NK cells in vaccine strategies, overcome mechanisms by which tumors and viruses evade detection by NK cells, and establish therapies that improve the access of NK cells to sites of infection and cancer. While cancer therapy is currently the proving ground, strategies to manipulate NK cell functions can ultimately be applied to treatment of other diseases, such as viral infections, problem pregnancy, autoimmunity, and allergy. Overall, there is a bright future in reforming natural killer cells to improve human health.
Acknowledgments
We thank Drs. Glenn Rall, Adam Cohen, and Amanda Purdy for critical reading of the manuscript.
This work was supported by NIH grants CA083859 (K.S.C) and CA06927 (FCCC) and a Health Research Formula Fund (CURE) grant from the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. J.H. was supported by Daiichi Sankyo Co., Ltd., Tokyo, Japan.
Abbreviations
- NK
Natural killer
- KIR
Killer cell Ig-like receptors
- ADCC
Antibody-dependent cellular cytotoxicity
- IFN
Interferon
- HSC
Hematopoietic stem cell
- TNF
Tumor necrosis factor
- DC
Dendritic cells
- NKT
NK-like T
- MHC-I
Class I MHC
- ITIMs
Immunoreceptor tyrosine-based inhibitory motifs
- NCR
Natural Cytotoxicity Receptors
- MCMV
Mouse cytomegalovirus
- SRIR
self-recognizing inhibitory receptor
- AML
Acute myelocytic leukemia
- MM
Multiple myeloma
- ILC
innate lymphoid cells
- CAR
chimeric antigen receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008;9(5):503–10. doi: 10.1038/ni1582. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
- 3.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481(7381):394–8. doi: 10.1038/nature10624. Epub 2011/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster S, Hurrell B, Tacchini-Cottier F. Crosstalk between neutrophils and dendritic cells: a context-dependent process. Journal of leukocyte biology. 2012 doi: 10.1189/jlb.1012540. Epub 2012/12/20. [DOI] [PubMed] [Google Scholar]
- 5.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. Journal of innate immunity. 2011;3(3):258–63. doi: 10.1159/000323923. Epub 2011/03/18. [DOI] [PubMed] [Google Scholar]
- 6.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews Immunology. 2013;13(2):145–9. doi: 10.1038/nri3365. Epub 2013/01/26. [DOI] [PubMed] [Google Scholar]
- 7.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nature reviews Immunology. 2013;13(2):75–87. doi: 10.1038/nri3349. Epub 2013/01/08. [DOI] [PubMed] [Google Scholar]
- 8.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. doi: 10.1182/blood-2007-09-077438. Epub 2008/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright Natural Killer Cells are Present in Human Lymph Nodes and are Activated by T cell Derived IL-2: a Potential New Link between Adaptive and Innate Immunity. Blood. 2002 doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature immunology. 2004;5(12):1260–5. doi: 10.1038/ni1138. Epub 2004/11/09. [DOI] [PubMed] [Google Scholar]
- 11.Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. The Journal of Experimental Medicine. 2010;207(11):2369–81. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):728–32. doi: 10.1073/pnas.1012356108. Epub 2010/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korbel DS, Norman PJ, Newman KC, Horowitz A, Gendzekhadze K, Parham P, et al. Killer Ig-like receptor (KIR) genotype predicts the capacity of human KIR-positive CD56dim NK cells to respond to pathogen-associated signals. J Immunol. 2009;182(10):6426–34. doi: 10.4049/jimmunol.0804224. Epub 2009/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cellular & molecular immunology. 2011;8(1):1–11. doi: 10.1038/cmi.2010.38. Epub 2010/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orange JS. Human natural killer cell deficiencies. Current opinion in allergy and clinical immunology. 2006;6(6):399–409. doi: 10.1097/ACI.0b013e3280106b65. Epub 2006/11/08. [DOI] [PubMed] [Google Scholar]
- 16.Ljunggren HG, Kärre K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 17.Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol. 2011;23(1):96–103. doi: 10.1016/j.coi.2010.11.005. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 18.Seliger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. Int J Cancer. 2006;118(1):129–38. doi: 10.1002/ijc.21312. Epub 2005/07/09. [DOI] [PubMed] [Google Scholar]
- 19.Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Frontiers in immunology. 2012;3:335. doi: 10.3389/fimmu.2012.00335. Epub 2012/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFarlane AW, 4th, Campbell KS. Signal transduction in natural killer cells. Current topics in microbiology and immunology. 2006;298:23–57. doi: 10.1007/3-540-27743-9_2. [DOI] [PubMed] [Google Scholar]
- 21.Cichocki F, Lenvik T, Sharma N, Yun G, Anderson SK, Miller JS. Cutting Edge: KIR Antisense Transcripts Are Processed into a 28-Base PIWI-Like RNA in Human NK Cells. The Journal of Immunology. 2010;185(4):2009–12. doi: 10.4049/jimmunol.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies GE, Locke SM, Wright PW, Li H, Hanson RJ, Miller JS, et al. Identification of bidirectional promoters in the human KIR genes. Genes and immunity. 2007;8(3):245–53. doi: 10.1038/sj.gene.6364381. Epub 2007/02/23. [DOI] [PubMed] [Google Scholar]
- 23.Saleh A, Davies GE, Pascal V, Wright PW, Hodge DL, Cho EH, et al. Identification of probabilistic transcriptional switches in the Ly49 gene cluster: a eukaryotic mechanism for selective gene activation. Immunity. 2004;21(1):55–66. doi: 10.1016/j.immuni.2004.06.005. Epub 2004/09/04. [DOI] [PubMed] [Google Scholar]
- 24.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5(3):201–14. doi: 10.1038/nri1570. Epub 2005/02/19. [DOI] [PubMed] [Google Scholar]
- 25.Anderson SK, Ortaldo JR, McVicar DW. The ever-expanding Ly49 gene family: repertoire and signaling. Immunol Rev. 2001;181:79–89. doi: 10.1034/j.1600-065x.2001.1810106.x. Epub 2001/08/22. [DOI] [PubMed] [Google Scholar]
- 26.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 27.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. Epub 2008/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Ann Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 29.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–9. doi: 10.1126/science.285.5428.727. Epub 1999/07/31. [DOI] [PubMed] [Google Scholar]
- 30.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–503. doi: 10.1084/jem.20090681. Epub 2009/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AC, Dobbie IM, Alakoskela JM, Davis I, Davis DM. Super-resolution imaging of remodeled synaptic actin reveals different synergies between NK cell receptors and integrins. Blood. 2012;120(18):3729–40. doi: 10.1182/blood-2012-05-429977. Epub 2012/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross CC, Brzostowski JA, Liu D, Long EO. Tethering of Intercellular Adhesion Molecule on Target Cells Is Required for LFA-1–Dependent NK Cell Adhesion and Granule Polarization. The Journal of Immunology. 2010;185(5):2918–26. doi: 10.4049/jimmunol.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1–mediated signaling pathway of actin polarization in natural killer cells. Blood. 2010;116(8):1272–9. doi: 10.1182/blood-2009-12-261487. [DOI] [PubMed] [Google Scholar]
- 34.Hanke T, Takizawa H, Raulet DH. MHC-dependent shaping of the inhibitory Ly49 receptor repertoire on NK cells: evidence for a regulated sequential model. Eur J Immunol. 2001;31(11):3370–9. doi: 10.1002/1521-4141(200111)31:11<3370::aid-immu3370>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Schönberg K, Sribar M, Enczmann J, Fischer JC, Uhrberg M. Analyses of HLA-C– specific KIR repertoires in donors with group A and B haplotypes suggest a ligand-instructed model of NK cell receptor acquisition. Blood. 2011;117(1):98–107. doi: 10.1182/blood-2010-03-273656. [DOI] [PubMed] [Google Scholar]
- 36.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113(11):2434–41. doi: 10.1182/blood-2008-05-156836. Epub 2008/11/01. [DOI] [PubMed] [Google Scholar]
- 38.Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann TL, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110(2):578–86. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105(8):3053–8. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 43.Raulet DH, Held W, Correa I, Dorfman JR, Wu MF, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 44.Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D'Andrea A, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–51. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa H, Yabe T, Watanabe K, Miyamoto R, Miki A, Akaza T, et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I) Human immunology. 1999;60(1):32–40. doi: 10.1016/s0198-8859(98)00097-4. Epub 1999/02/10. [DOI] [PubMed] [Google Scholar]
- 46.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 47.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30(4):143–9. doi: 10.1016/j.it.2009.01.006. Epub 2009/03/14. [DOI] [PubMed] [Google Scholar]
- 48.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182(8):4572–80. doi: 10.4049/jimmunol.0803900. Epub 2009/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalifour A, Scarpellino L, Back J, Brodin P, Devevre E, Gros F, et al. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30(3):337–47. doi: 10.1016/j.immuni.2008.12.019. Epub 2009/03/03. [DOI] [PubMed] [Google Scholar]
- 50.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nature immunology. 2010;11(4):321–7. doi: 10.1038/ni.1849. Epub 2010/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197(8):977–84. doi: 10.1084/jem.20021836. Epub 2003/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172(2):864–70. doi: 10.4049/jimmunol.172.2.864. Epub 2004/01/07. [DOI] [PubMed] [Google Scholar]
- 53.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197(8):967–76. doi: 10.1084/jem.20021847. Epub 2003/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208(2):357–68. doi: 10.1084/jem.20100479. Epub 2011/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–61. doi: 10.1038/nature07665. Epub 2009/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 57.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209(5):947–54. doi: 10.1084/jem.20111760. Epub 2012/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–74. doi: 10.1182/blood-2011-10-386995. Epub 2011/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14725–32. doi: 10.1073/pnas.1110900108. Epub 2011/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–88. doi: 10.1182/blood-2012-10-459545. Epub 2013/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. The Journal of Experimental Medicine. 2011;208(1):13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1915–9. doi: 10.1073/pnas.0813192106. Epub 2009/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–65. doi: 10.1084/jem.20120944. Epub 2012/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–60. doi: 10.1182/blood-2012-04-419283. Epub 2012/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nature immunology. 2006;7(5):507–16. doi: 10.1038/ni1332. Epub 2006/04/18. [DOI] [PubMed] [Google Scholar]
- 66.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature immunology. 2010;11(12):1127–35. doi: 10.1038/ni.1953. Epub 2010/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76. doi: 10.3389/fimmu.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117(8):2423–32. doi: 10.1182/blood-2010-08-301945. Epub 2011/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Benson DM, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood. 2010;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180(9):6392–401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sola C, Andre P, Lemmers C, Fuseri N, Bonnafous C, Blery M, et al. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc Natl Acad Sci U S A. 2009;106(31):12879–84. doi: 10.1073/pnas.0901653106. Epub 2009/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114(13):2667–77. doi: 10.1182/blood-2009-02-206532. Epub 2009/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benson DM, Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(22):4324–33. doi: 10.1182/blood-2012-06-438028. Epub 2012/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–23. doi: 10.1182/blood-2012-06-437558. Epub 2012/09/25. [DOI] [PubMed] [Google Scholar]
- 75.Benson DM, Jr, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118(24):6387–91. doi: 10.1182/blood-2011-06-360255. Epub 2011/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 77.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200(8):957–65. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Connor GM, Yamada E, Rampersaud A, Thomas R, Carrington M, McVicar DW. Analysis of binding of KIR3DS1*014 to HLA suggests distinct evolutionary history of KIR3DS1. J Immunol. 2011;187(5):2162–71. doi: 10.4049/jimmunol.1002906. Epub 2011/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vivian JP, Duncan RC, Berry R, O'Connor GM, Reid HH, Beddoe T, et al. Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature. 2011;479(7373):401–5. doi: 10.1038/nature10517. Epub 2011/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nature genetics. 2002;31(4):429–34. doi: 10.1038/ng934. Epub 2002/07/23. [DOI] [PubMed] [Google Scholar]
- 81.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nature genetics. 2007;39(6):733–40. doi: 10.1038/ng2035. Epub 2007/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pelak K, Need AC, Fellay J, Shianna KV, Feng S, Urban TJ, et al. Copy number variation of KIR genes influences HIV-1 control. PLoS biology. 2011;9(11):e1001208. doi: 10.1371/journal.pbio.1001208. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Velardi A, Ruggeri L, Alessandro, Moretta L. NK cells: a lesson from mismatched hematopoietic transplantation. Trends Immunol. 2002;23(9):438. doi: 10.1016/s1471-4906(02)02284-6. [DOI] [PubMed] [Google Scholar]
- 84.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 85.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–9. [PubMed] [Google Scholar]
- 86.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–61. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hsu KC, Pinto-Agnello C, Gooley T, Malkki M, Dupont B, Petersdorf EW. Hematopoietic stem cell transplantation: killer immunoglobulin-like receptor component. Tissue Antigens. 2007;69(1):42–5. doi: 10.1111/j.1399-0039.2006.759_5.x. [DOI] [PubMed] [Google Scholar]
- 88.McQueen KL, Dorighi KM, Guethlein LA, Wong R, Sanjanwala B, Parham P. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Human immunology. 2007;68(5):309–23. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–9. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(9):1257–64. doi: 10.1016/j.bbmt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. The New England journal of medicine. 2012;367(9):805–16. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haas P, Loiseau P, Tamouza R, Cayuela JM, Moins-Teisserenc H, Busson M, et al. NK-cell education is shaped by donor HLA genotype after unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(3):1021–9. doi: 10.1182/blood-2010-02-269381. [DOI] [PubMed] [Google Scholar]
- 93.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. Epub 2005/01/06. [DOI] [PubMed] [Google Scholar]
- 94.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(6):955–9. doi: 10.1200/JCO.2009.24.4590. Epub 2010/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118(12):3273–9. doi: 10.1182/blood-2011-01-329508. Epub 2011/07/28. [DOI] [PubMed] [Google Scholar]
- 96.Koepsell SA, Miller JS, McKenna DH., Jr Natural killer cells: a review of manufacturing and clinical utility. Transfusion. 2013;53(2):404–10. doi: 10.1111/j.1537-2995.2012.03724.x. Epub 2012/06/08. [DOI] [PubMed] [Google Scholar]
- 97.Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo D, Klein AK, et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013;53(2):412–8. doi: 10.1111/j.1537-2995.2012.03764.x. quiz 1. Epub 2012/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–20. doi: 10.1182/blood-2011-10-384388. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. Epub 2011/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–28. doi: 10.1182/blood-2011-04-348540. Epub 2011/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(15):4857–66. doi: 10.1158/1078-0432.CCR-08-2810. Epub 2009/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–83. doi: 10.1182/blood-2004-12-4797. Epub 2005/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li L, Liu LN, Feller S, Allen C, Shivakumar R, Fratantoni J, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer gene therapy. 2010;17(3):147–54. doi: 10.1038/cgt.2009.61. Epub 2009/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100(4):1265–73. Epub 2002/08/01. [PubMed] [Google Scholar]
- 105.Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer immunology, immunotherapy : CII. 2008;57(3):411–23. doi: 10.1007/s00262-007-0383-3. Epub 2007/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10(6):625–32. doi: 10.1080/14653240802301872. Epub 2008/10/07. [DOI] [PubMed] [Google Scholar]
- 107.McDaniel JM, Pinilla-Ibarz J, Epling-Burnette PK. Molecular action of lenalidomide in lymphocytes and hematologic malignancies. Adv Hematol. 2012;2012:513702. doi: 10.1155/2012/513702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer immunology, immunotherapy : CII. 2008;57(12):1849–59. doi: 10.1007/s00262-008-0512-7. Epub 2008/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 110.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011;117(5):1605–13. doi: 10.1182/blood-2010-04-278432. Epub 2010/10/28. [DOI] [PubMed] [Google Scholar]
- 111.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–53. doi: 10.2174/156800911794519752. Epub 2011/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, et al. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(11):3520–8. doi: 10.1158/1078-0432.CCR-07-4744. [DOI] [PubMed] [Google Scholar]
- 113.Krieg S, Ullrich E. Novel immune modulators used in hematology: impact on NK cells. Front Immunol. 2012;3:388. doi: 10.3389/fimmu.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113(24):6120–7. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lundqvist A, Berg M, Smith A, Childs RW. Bortezomib Treatment to Potentiate the Anti-tumor Immunity of Ex-vivo Expanded Adoptively Infused Autologous Natural Killer Cells. J Cancer. 2011;2:383–5. doi: 10.7150/jca.2.383. Epub 2011/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]