Abstract
Background
Studies of prenatal cocaine exposure have primarily examined childhood populations. Studying adolescents is especially important because adolescence is a time of changing motivations and initiation of substance use.
Methods
Using magnetic resonance imaging and whole-brain voxel-based morphometry, we assessed gray matter volume (GMV) differences in 42 prenatally cocaine exposed (PCE) and 21 noncocaine-exposed (NCE) adolescents, aged 14 to 17 years. Associations between GMV differences in significant clusters and the probability of substance use initiation were examined.
Results
PCE relative to NCE adolescents demonstrated three clusters of lower GMV involving a limbic and paralimbic (p < .001, family-wise error [FWE] corrected), superior frontal gyrus (p = .001, FWE corrected), and precuneus (p = .019, FWE corrected) cluster. GMVs in the superior frontal and precuneus clusters were associated with initiation of substance use. Each 1-mL decrease in GMV increased the probability of initiating substance use by 69.6% (p = .01) in the superior frontal cluster and 83.6% (p = .02) in the precuneus cluster.
Conclusions
PCE is associated with structural differences in cortical and limbic regions. Lower GMVs in frontal cortical and posterior regions are associated with substance use initiation and may represent biological risk markers for substance use.
Keywords: Adolescence, gray matter volume, prenatal cocaine exposure, substance use initiation, voxel-based morphometry, whole brain
Longitudinal studies suggest that prenatal cocaine exposure (PCE) affects the developing child’s capacity for sustained attention, working memory, inhibitory control, stress responses and emotion regulation (1–4). Among possible outcomes for PCE children is substance use initiation as adolescents, and PCE has been reported to predict cocaine use at age 14 (5). Although animal models suggest important effects of PCE, neuroimaging data concerning human PCE are limited, as are studies that seek to identify neural markers for effects of PCE on adolescent behaviors including substance use initiation.
Animal models of PCE have found disrupted cortical neurogenesis and migration (6–7); reduced neuron number and density in cortex (8,9) and hippocampus (10); narrowed, more closely spaced cortical mini-columns (11); neurite changes in locus coeruleus, hippocampus, parietal lobes, and anterior cingulate (ACC) (12–14); and reductions in overall brain volume (15). Differences in dopaminergic function in ACC and orbitofrontal cortex (OFC) may predispose to drug abuse in humans (16). Thus, of potential relevance to human PCE and substance use is evidence of altered dopaminergic pathways in PCE rabbits, specifically in the ACC (17) and ventromedial prefrontal cortex (VMPFC) (18), regions involved in attention, cognitive control, and emotional regulation (19,20). Adult PCE rabbits were impaired in a learning discrimination task, suggesting enduring behavioral deficits related to PCE (17). PCE increased reward-potentiation of cocaine in adult mice and was associated with alterations in the sensitivity of dopaminergic systems (21).
In humans, the neurobiological effects of PCE in children and adolescents encompass the direct biological teratogenic effects of cocaine as well as postnatal environmental effects on the brain (2,4). Whereas PCE-related changes in neuropsychological function are reported, structural brain studies in PCE children are limited. Total cortical gray matter volume (GMV) was lower in PCE than non-exposed children (22), and PCE adolescents had lower volumes in the caudate nucleus, a candidate region selected because of its rich dopaminergic innervation and effects of PCE on dopaminergic systems (23). As no whole-brain studies have examined volume differences related to PCE and assessed their relationship to substance use initiation, we conducted whole-brain voxel-based morphometry (VBM) of PCE and non-cocaine-exposed (NCE) adolescents (aged 14–17 years) to investigate between-group GMV differences and the relationship of observed differences to substance-use initiation. We hypothesized lower GMVs in PCE relative to NCE children in brain regions associated with sustained attention, working memory, executive function, and emotional and reward processing including the prefrontal cortex, OFC, and ACC. Because frontal impairments have been associated with substance use propensities (24), we also hypothesized that lower GMV in frontal cortical regions would be associated with substance-use initiation.
Methods and Materials
Adolescent PCE and NCE Participants
Forty-two PCE adolescents (23 boys and 19 girls; mean age [SD] = 14.71 [1.00] years) and 21 NCE adolescents (14 boys and 7 girls; mean age [SD] = 14.57 [.60] years) were studied over three visits, with two for intake assessments and one for the magnetic resonance imaging (MRI) session. Subjects represented a subset of a larger cohort that was recruited at birth and followed longitudinally (2,25). This subset of subjects was eligible for the current study based on their age at the time of the study. All participants were between the ages of 14 and 17 years, inclusive. PCE and NCE subjects did not differ on age (t61 = .60, NS), and there were no significant differences by gender or race/ethnicity (χ26 = 6.97, p = .323) between PCE and NCE subjects and both groups were recruited from the urban inner-city New Haven, Connecticut, area. The groups were not different on levels of parental stress (Table 1) and childhood trauma scores (Table 2). Inclusion criteria were that adolescents be fluent in English, be free of significant medical or mental illness (e.g., psychosis), and have an IQ greater than 80. Exclusion criteria included use of psychotropic medications. There were also no differences between the present group of subjects and the larger cohort on measures of perinatal outcome including birth weight and head circumference, size for gestational age, and obstetric complications (26).
Table 1.
Demographic Characteristics of PCE and NCE Mothers and Their Infants at Time of Birth
| Mother’s Agea Mean (SD) | High School Diplomaa | Parental Stress | BDI | OCS Scalea,b | Infant GA (weeks)a | Birth Weight (g)a | HC (cm)a | |
|---|---|---|---|---|---|---|---|---|
| PCE | 29.46 (4.63) | 17 = No 24 = Yes |
70.53 (12.37) | 5.11 (7.23) | 78.22 (18.82) | 38.00 (2.88) | 2621.05 (628.12) | 32.12 (2.54) |
| NCE | 24.87 (5.20) | 2 = No 19 = Yes |
65.26 (14.80) | 3.75 (3.61) | 93.75 (20.24) | 39.00 (2.26) | 3170.95 (556.47) | 34.25 (2.64) |
BDI, Beck Depression Inventory; GA, gestational age; HC, head circumference; NCE, noncocaine-exposed; OCS, Obstetrical Complications Scale; PCE, prenatally cocaine-exposed.
Significant group differences p < .01.
Higher scores reflect more optimal birth factors (26).
Table 2.
Age, Race/Ethnicity, and Frequency of Substance Use by Prenatally Cocaine-Exposed (PCE) and Noncocaine-Exposed (NCE) Adolescents
| PCE (n = 42) Mean (SD) |
NCE (n = 21) Mean (SD) |
|||
|---|---|---|---|---|
| Boys (n = 23) | Girls (n = 19) | Boys (n = 14) | Girls (n = 7) | |
| Age | 15.00 (1.13) | 14.37 (.68) | 14.64 (.63) | 14.43 (.54) |
| Race/Ethnicity, n (%) | ||||
| African American | 20 (87.0) | 18 (94.7) | 7 (50.0) | 4 (57.2) |
| Biracial | 1 (4.3) | 0 | 4 (8.0) | 1 (14.3) |
| Caucasian | 1 (4.3) | 0 | 2 (4.0) | 0 |
| Hispanic | 0 | 1 (5.3) | 1 (2.0) | 2 (28.6) |
| Native American | 1 (4.3) | 0 | 0 | 0 |
| Substance Use Initiationa | ||||
| Substance use lifetime, n (%) | 14 (60.9) | 9 (47.4) | 4 (28.6) | 2 (28.6) |
| Substance use past 30 days, n (%) | 7 (31.8) | 3 (15.8) | 2 (14.3) | 2 (28.6) |
| Mean Days Used in Past 30 Days (SD)/n | ||||
| Alcohol | 3.00 (2.65) | 2.50 (2.12) | 4.00 (1.41) | 1.00 (.00) |
| n = 3 | n = 2 | n = 2 | n = 2 | |
| Tobacco | 5.00 (0.00) | — | 4.00 (—) | — |
| n = 2 | n = 1 | |||
| Marijuana | 2.33 (1.15) | 1.00 (—) | 4.00 (—) | 2.00 (—) |
| n = 3 | n = 1 | n = 1 | n = 1 | |
| Childhood Trauma Scale Total Score, Mean (SD) | 36.24 (9.42) | 37.54 (10.86) | 35.33 (10.25) | 30.75 (4.65) |
| n = 17 | n = 13 | n = 9 | n = 4 | |
Number of youth using in lifetime and in past 30 days based on combination of urine and self-report on Youth Risk Behavior Survey. Mean days used in past 30 days based on report on Youth Risk Behavior Survey.
One child (a PCE boy) was missing substance use information.
Maternal Recruitment and Characterization of PCE Status
Mothers were recruited over a 5-year time frame at the Women’s Center at a large urban hospital setting either prenatally or immediately following delivery as part of a longitudinal study of PCE (25). The Addiction Severity Index (27) was used via an interview to examine substance use during pregnancy. Urine samples were collected for toxicology screening during pregnancy (for those mothers who received prenatal care) and at delivery for every mother. If either self-reported use of cocaine or urine screens were positive, mothers were considered positive for cocaine use and infants as cocaine exposed. Mothers with cocaine use were not excluded if they used other drugs including tobacco, alcohol, or marijuana, but were excluded for opiate use. From the ongoing longitudinal PCE/NCE study, 63 children who had reached adolescence and the age range eligible for this study, and their parent, assented or consented to the MRI study. Of this subset of cocaine-using mothers who were interviewed prepartum or at delivery, 100% had used cocaine over the previous 30 days and had used an average of .69 g (SD = .62 g) of cocaine per day at the time of perinatal interview. Over the same time period, 59.5% of cocaine-using mothers had used marijuana, 73.2% had used alcohol, and 70.7% had used cigarettes. None of the non-cocaine-using women had used marijuana or other illicit drugs during this 30-day period. However, one NCE mother reported a single use of tobacco (4.8%), and four reported a single use of alcohol (19%; for maternal and infant characteristics at delivery, see Table 1).
PCE and NCE Adolescent Substance Use Initiation
Adolescent substance use initiation was determined by self-reports on the Youth Risk Behavior Survey and urine toxicology results from laboratory-analyzed samples obtained at the first or second study visit. Samples were analyzed for the presence of amphetamines, cocaine, cotinine (for nicotine/tobacco use), ethyl glucuronide (for alcohol use), opiates, phencyclidine, and tetrahydrocannabinol. Substance-use initiation was coded for each adolescent as a dichotomous variable. If an adolescent had indicated ever using any substance via the Youth Risk Behavior Survey or shown positive urine samples for the presence of a substance, they were classified as having initiated substance use. Six NCE adolescents (28.6%) and 23 PCE adolescents (56.1%) reported substance use initiation. The frequency of substance use initiation was higher in PCE than NCE adolescents (χ21 = 4.23, p = .04). Separate analyses revealed that PCE boys reported a significantly higher frequency of substance use initiation that NCE boys (χ21 = 4.21, p = .04), whereas the difference did not reach significance between PCE versus NCE girls (χ21 = .74, p = .39). Notably, whereas 56% of the PCE group and 28.6% of the NCE group had initiated substance use, rates of drug use in the previous 30 days based on self-report and urine toxicology screens were low (Table 2).
Structural Brain Analyses
MRI Data Acquisition
Subjects were scanned on a Siemens 3T (Malvern, Pennsylvania) scanner. Data for each subject consisted of a single sagittally acquired high-resolution T1-weighted magnetization-prepared rapid gradient echo scan: field of view = 256 × 256 mm, data acquisition matrix = 256 × 256 × 176, 1 mm3 isotropic voxels, repetition time = 2530 msec, echo time = 3.66 msec, and flip angle = 7°.
Segmentation and Registration
Image segmentation and registration were performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8) in SPM8. After applying a non-local-means denoising filter to improve signal to noise ratio (28), VBM8 uses an adaptive maximum a posteriori technique (29) and a hidden Markov random field (30) to determine optimal segmentation. The segmentation procedure models partial volume effects to account for image voxels that are mixtures of pure tissue types (31). Because all model parameters are estimated from image data, unlike the SPM segmentation and registration algorithms, VBM8 does not use tissue probability maps for tissue classification. On the basis of our previous experience, we opted to change several default settings in the “VBM8: Estimate and Write” module. Specifically, we used light bias regularization, a 30-mm cutoff for bias full width at half maximum, and no cleanup of gray, white, and cerebrospinal fluid partitions (segmentations). Because manual segmentation is considered the gold standard for evaluating the quality of automated tissue classification (32,33), the resulting segmentations were validated visually (34,35). Particular attention was given to the thickness of the cortical surface, which was compared visually to each subject’s native space image. Gray matter segmentation demonstrated appropriate face validity in all images.
VBM8 segmentation integrates DARTEL (36) normalization into the toolbox. DARTEL is a high-dimensional, diffeomorphic registration algorithm that has performed well in a comparison of registration algorithms (37). VBM8 uses a preexisting DARTEL template (in Montreal Neurological Institute [MNI] space) derived from 550 healthy control subjects of the IXI database (http://www.brain-development.org) to register the images. Outputs were modulated gray matter images that were then smoothed with a 6-mm Gaussian filter. With modulation voxel values reflect relative brain volume differences between images. Gaussian smoothing reduces the effects of residual misregistration on potential group differences and reduces departures from normality that may occur at some voxels (38). Final outputs were smoothed, modulated gray matter segments (1.5 mm3 voxels).
PCE and NCE Group Differences in GMV
Statistical parametric maps were created in SPM8 to perform between-group comparisons using the normalized, modulated, and smoothed gray matter tissue segmentations generated in VBM8. A general linear model was created with exposure group (PCE vs. NCE) and gender as the factors of interest, and GMV as the dependent variable. Estimates of total intracranial volume (TICV) and age were entered into the model as covariates. The analysis therefore compared whole-brain GMV differences adjusted for the effects of age and individual differences in global brain size. Perinatal outcome differences in head circumference and birth weight were not included as covariates because these outcomes are highly associated with PCE (independent variable) and the TICV covariate. However, they were included as covariates in the association with substance use analyses discussed later.
The whole-brain statistical analysis was conducted using random field-based cluster-size testing and family-wise error (FWE) rate correction for multiple comparisons (39). The cluster-size test increases sensitivity, relative to voxel-based tests, for spatially extended signals, and low thresholds increase the power of these tests for signals of large spatial extent (40,41). The analysis of cluster extent also better characterizes the spatially distributed nature of group differences within spatially smoothed data (42). Clusters were formed from contiguous voxels exceeding an uncorrected one-tailed threshold of p < .025. The FWE-corrected threshold for significant cluster size was set at one-tailed p < .025. No minimum cluster extent was used, a priori, to threshold the data, because the random field-based cluster-size testing in SPM produces a multiple-comparisons corrected p value for a cluster of any size in the data.
Anatomic regions within significant clusters were initially identified in SPM outputs from MNI space coordinates using the Automated Anatomy Library within the WFU Pickatlas (43,44). Anatomic regions were confirmed and Brodmann areas determined using the Talairach Atlas (after conversion to Talairach coordinates). MNI to Talairach conversion was performed using the Nonlinear Yale MNI to Talairach Conversion Algorithm (45).
Association of GMV with Substance Use Initiation
To examine whether GMVs in the clusters showing significant differences between PCE and NCE adolescents were associated with substance-use initiation, a region of interest (ROI) analysis was conducted. Each significant cluster was converted to a binary mask using MarsBar (http://marsbar.sourceforge.net). These masks and the ImCalc function in SPM8 were used to extract the modulated gray matter voxels to create the ROI for each subject within each masked region. A MATLAB script (The MathWorks, Natick, Massachusetts; Ged Ridgway, http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m) summed the voxels for each subject within each ROI to calculate total GMVs (in milliliters) for each significant cluster. Binary logistic regression was performed using the extracted ROI GMVs as the independent measure. Because cluster data represented raw GMV within each ROI, each regression model included age, gender, and TICV. In addition, to control for possible effects of individual differences in demographic characteristics of mothers and infants on adolescent brain volume, we calculated semipartial correlations of the maternal and birth characteristics in Table 1 with volumes in each cluster, adjusting for age, gender, and TICV. Head circumference and birth weight were correlated with GMV in all three significant clusters. Mother’s age and the score on the Obstetrical Complications Scale were correlated with the ventral frontal-limbic cluster. These measures were included in the binary logistic regression models for each ROI with which the measure was significantly correlated.
Results
PCE versus NCE GMV Differences
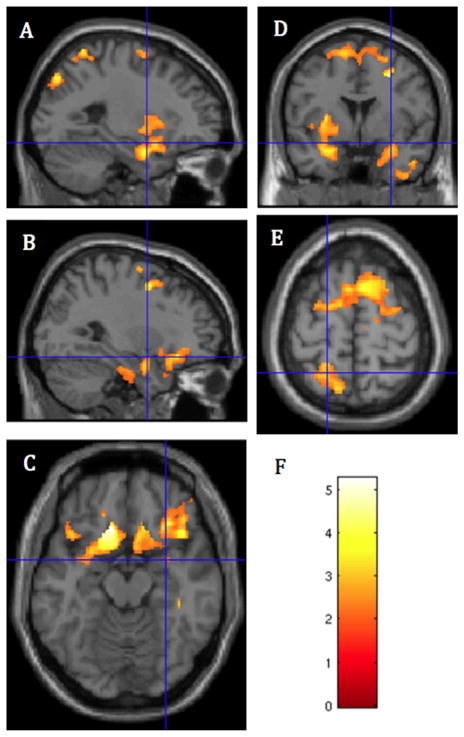
Analysis of the interaction between PCE/NCE status and sex revealed no significant clusters of GMV difference. Therefore, boys and girls were combined in all further analyses. Three clusters were found in which PCE subjects had lower GMV than NCE subjects (Figures 1 and 2, Table 3). The first cluster included regions in the inferior frontal gyrus (IFG), OFC, amygdala, hippocampus, parahippocampal gyrus, insula, and gyrus rectus, bilaterally; left subgenual anterior cingulate, and left ventral striatum (FWE p < .001, cluster = 11,725 voxels). A second cluster in superior frontal cortex included the paracentral lobule, the supplementary motor area (SMA), and presupplementary motor area (pre-SMA), bilaterally; and dorsal ACC (FWE p = .001, cluster = 6087 voxels). A third, posterior cluster included the precuneus, bilaterally; the left superior parietal lobe, right paracentral lobule, and right midcingulate cortex (FWE p = .019, cluster = 3635 voxels).
Figure 1.
Regions of significantly lower gray matter volume in prenatally cocaine-exposed than noncocaine-exposed adolescents: (A) Crosshairs at x = −25, y = 1.5, z = −15; left insula and amygdala. (B–D) Crosshairs at x = 30, y = 1.5, z = −15; (B) right amygdala and inferior frontal gyrus; (C) ventral frontal cortical regions: orbitofrontal cortex and inferior frontal gyrus; (D) superior frontal gyrus, left and right amygdalae, and left insula. (E) Crosshairs at x = −19.5, y = −52.5, z = 63.0; posterior parietal cortex and superior frontal cortex shown. (F) Color bar representing the height of the voxelwise the t statistics in panels A–E.
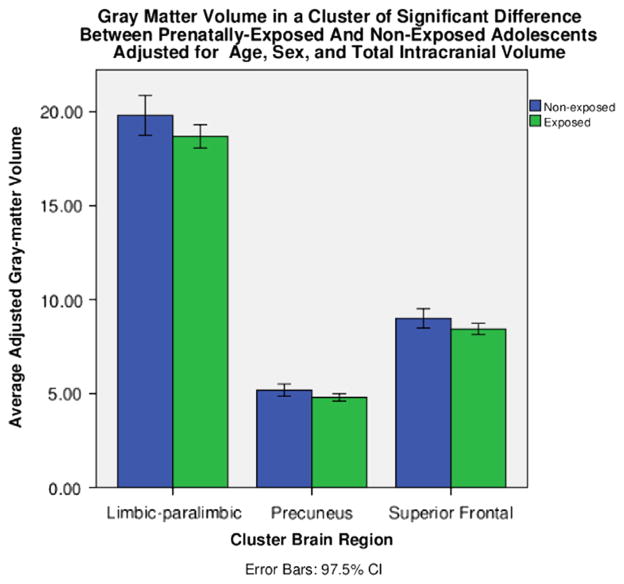
Figure 2.
Average gray matter volume in milliliters by group for each of the three regions of significantly lower gray matter volume in prenatally cocaine-exposed than noncocaine-exposed adolescents (ps < .025 family-wise error corrected). From left to right: limbic and paralimbic cortex; parietal cortex, including the superior parietal lobe and precuneus region; and superior frontal cortex. CI, confidence interval.
Table 3.
Significant Gray Matter Volume Differences in Prenatally Cocaine-Exposed (PCE) and Noncocaine-Exposed (NCE) Adolescents
| Brain Regions | Brodmann Areas | Voxels in Cluster | Family-Wise Error Corrected p Value | Peak Z Value | |
|---|---|---|---|---|---|
| PCE < NCE | Bilateral amygdala, hippocampus, parahippocampal gyrus, insula, inferior frontal gyrus, orbitofrontal cortex, gyrus rectus; left subgenual anterior cingulate, left ventral striatum, putamen, superior temporal pole; right inferior temporal lobe, anterior temporal pole | 11, 38, 45, 47 | 11725 | 3.001 | 4.73 |
| Bilateral paracentral lobule, supplementary and presupplementary motor areas; dorsal anterior cingulated | 3, 6, 8 | 6087 | .001 | 4.44 | |
| Bilateral precuneus; right paracentral lobule, right midcingulate; left superior parietal lobe | 7, 31 | 3635 | .019 | 4.01 | |
| PCE < NCE | None |
Because the frequency of substance use initiation was higher in PCE than NCE subjects but involved low levels of recent past 30-day frequency of substance use (Table 2), secondary voxelwise whole-brain GMV analyses were conducted using the same voxelwise and cluster-extent thresholds, including a dichotomous substance use variable (initiator/noninitiator) to account for the possible effects of substance use on GMV. As in the primary analysis, PCE adolescents were found to have lower GMV in ventromedial frontal cortical areas including the subgenual cingulate, inferior frontal gyrus, OFC, and gyrus rectus, bilaterally, as well as in the ventral striatum, putamen, left posterior insula, and the left amygdala (FWE p = .002, cluster = 4985 voxels). Also in agreement with the earlier analysis, a cluster of lower GMV in the PCE group was found in the right temporal gyrus, including the fusiform gyrus, inferior temporal gyrus, and the temporal pole, and extending into the right inferior frontal gyrus (FWE p = .006, cluster = 4367 voxels). Finally, also similar to the previous analysis was a finding of lower GMV in PCE adolescents in superior frontal cortex, including the paracentral lobule, the SMA, and pre-SMA, bilaterally, extending anteriorly into dorsomedial prefrontal regions (FWE p = .009), cluster = 4123 voxels).
In addition, we also compared substance-initiators versus noninitiators, controlling for PCE status and sex. The significant regions showed no overlap with the primary analysis; specifically, there were no clusters in which the initiators had lower GMV than noninitiators. However, the initiators were found to have significantly greater GMV than noninitiators in a cluster that included the posterior cingulate, inferior temporal lobes, and lingual gyrus of the occipital lobes, and the anterior cerebellar vermis, bilaterally (p = .016, cluster = 3702 voxels).
GMV Associations with Substance-Use Initiation
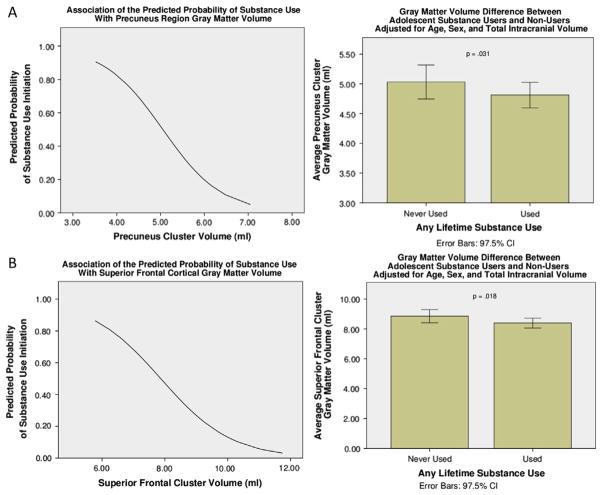
Among the entire sample, extracted GMVs from the posterior cluster were associated with substance-use initiation (β = −1.81, Wald χ2 = 5.38, p = .020; odds ratio = .164). For every 1-mm decrease in GMV, there was an 83.6% increase in the odds of having initiated substance use. In the entire sample, the extracted GMVs from the superior frontal cluster were associated with substance-use initiation (β = −1.19, Wald χ2 = 6.68, p = .010; odds ratio = .304). For every 1-mm decrease in GMV, there was a 69.6% increase in the odds of having initiated substance use (Figure 3). To examine whether there were differences between the PCE and NCE groups in the association of extracted GMVs with the odds of substance use initiation, a similar binary logistic regression model was run separately for each group. The odds of substance use initiation were not significantly associated with the extracted GMVs from any of the three clusters for either group separately.
Figure 3.
Logistic regression curves: region of interest clusters in which lower gray matter volume was associated with higher probability of substance use initiation in the sample. (A) Superior frontal cortex cluster; (B) posterior parietal cortex cluster. CI, confidence interval.
Discussion
This study is the first whole-brain morphometric analysis to report significantly lower regional GMV in PCE relative to NCE adolescents. As predicted, regions of lower GMV in PCE adolescents included the OFC, ACC, and other prefrontal regions. Among the three clusters of lower GMV in PCE adolescents, two clusters, located in the superior frontal cortex and posterior parietal cortex (PPC), respectively, were associated with a higher probability of having initiated substance use in the overall sample.
The cluster of lower GMV involving the superior frontal cortex in the PCE group included the pre-SMA, SMA, and dorsal ACC. These regions contribute to cognitive control through ongoing assessment of actions and outcomes and signal to lateral prefrontal areas when behavioral adjustment is needed (46), including the initiation of a new action or inhibition of a response plan (47). Cognitive control increases with age in typically developing children and facilitates cognitive flexibility relevant to coping with novel situations (48). During adolescence, cognitive control may be particularly important to initiating alternative responses in substance-using situations. Task-switching deficits are associated with lesions in pre-SMA and SMA (47), raising the possibility that reduced cognitive control and substance use initiation may be associated with lower GMVs in these regions. In a VBM study comparing abstinent alcohol-dependent subjects to controls, not only was posterior medial frontal cortex GMV smaller in the alcohol-dependent subjects, but lower GMV in this region was associated with shorter time to alcohol relapse (49), suggesting that lower GMV in this region may increase the risk of substance use.
The cluster of significantly lower GMV in the PPC included bilateral precuneus and the left superior parietal lobe. PPC is important to spatial cognition (50), and performance was lower in PCE relative to NCE children (aged 8–10 years) on a spatial working memory task (2). The possible consequences for risk of substance use initiation of the less efficient learning demonstrated by PCE children, and its relationship with lower GMV, are currently unclear. Abstinent alcohol-dependent individuals had lower PPC GMV and poorer visuospatial test performance than control subjects and seemed to compensate by using frontal lobes for visuoperceptual learning (51,52). PCE adolescents with lower GMV in PPC may similarly divert executive processes to low-level visuoperceptual tasks. By reducing the availability of frontal lobe processing for cognitive control, PCE adolescents may be more vulnerable to impulsivity or dysregulated emotion and consequent substance use. Although these possibilities are currently speculative and warrant direct investigation, a study reporting lower GMV in PPC associating with shorter time to relapse in alcohol-dependent subjects provides additional support for an association between lower GMV in the PPC and increased risk of substance use (49). It is important to note that the inclusion of the dichotomous measure of lifetime substance use initiation in the follow-up analysis reduced the size of this cluster below the threshold for statistical significance. Although levels of substance use were low among adolescents who had initiated use, these data suggest that lower GMV in this region is likely associated with both PCE and substance use.
The ventral-frontal cluster of lower GMV in PCE adolescents includes the IFG, OFC, and ACC, regions involved in emotion regulation and cognitive control. OFC lesions (53) and smaller GMV in the OFC of normal subjects (54) have been associated with higher impulsivity and increased emotionality. Lower ACC GMV has also been associated with impulsivity (54). However, the implications of lower GMV in these regions for adolescent substance use are unclear. In adolescent samples, impulsivity has been associated with substance use (55,56) and substance use initiation (57). Poor emotion regulation has also been linked to substance abuse in adolescents (58). Therefore, lower GMV in the ACC or OFC, which has been associated with impulsivity and emotional dysregulation, suggests increased risk for substance use in PCE adolescents.
Recent studies of GMV in cocaine-dependent adults (59–61) have found lower gray matter relative to controls in ventral-frontal regions that overlap the ventral-frontal cluster reported here. Lower OFC GMV was correlated with cocaine-related compulsivity in Ersche and colleagues, whereas Konova and colleagues (60) found not only lower GMV in the VMPFC in cocaine-dependent subjects, but also greater VMPFC deactivation in a monetary reward task relative to control subjects. With the additional finding that greater VMPFC deactivation correlated with greater striatal activation in the cocaine-dependent sample, Konova and colleagues suggest that frontostriatal abnormalities may reduce self-control of drug use behaviors. Whether lower VMPFC GMV in PCE adolescents reduces this self-control of substance use warrants investigation.
The IFG is part of the interconnected ventromedial and dorso-lateral neural circuitry associated with affective processing (62). The interaction between the ventral and dorsal frontal circuits is important for maintaining executive function during emotional distraction (63). The IFG has also been implicated in impulse control (64). Ersche and colleagues (59) also found lower GMV in the left IFG in a cocaine-dependent sample, and lower GMV in this region correlated with higher scores on the inattention component of an impulsivity measure. As noted earlier, impulsivity has been associated with substance-use initiation in adolescent samples (57). Vulnerability to dysregulated emotional and cognitive processing relating to impaired impulse control, and therefore substance use, could result from lower GMV in the IFG.
The ventral-frontal cluster extended into the limbic system to include the amygdala, hippocampus, parahippocampal gyrus, and limbic-related insular cortex, bilaterally, as well as subcortically into the left ventral striatum. Although altered function in all these regions is associated with addiction and lower amygdala GMV has been found in cocaine-dependent subjects in the amygdala, insula (65), and the hippocampus (66), the role of lower volumes in these regions as risk factors in substance use remains unknown. However, given their roles in the experience of emotion (67), emotion and stress regulation (68,69), and the link between stress and drug abuse (70,71), lower volume in these regions in PCE adolescents may increase the risk of substance use.
We did not find the hypothesized association between the probability of substance-use initiation and GMV in the ventral frontal-limbic cluster. In this region, the use of cluster spatial extent to identify regions of significant GMV difference resulted in a large cluster that involved multiple distinct brain regions. Prospective studies involving larger samples may facilitate identification of specific areas of the ventral-frontal or limbic regions in which lower GMV may represent a marker for the risk of substance use in adolescents.
Substance use within the entire sample was not associated with any regions of lower GMV, but rather with one cluster of larger GMV that included the anterior cerebellum and parts of inferior temporal and occipital cortex in users versus non-users. These results are consistent with reports that marijuana use by adolescents was associated with larger cerebellar volume (72;73), and suggest that PCE and adolescent substance use may have distinct effects on GMV. Further longitudinal studies assessing the effects of PCE and subsequent substance use are needed to fully answer this question.
Conclusions and Limitations
This study found evidence of lower GMV in PCE versus NCE adolescents. Given that lower GMV in two regions was associated with higher probability of having initiated substance use and that substance use was approximately twice as prevalent in PCE adolescents, the results suggest that lower GMV may place PCE adolescents at greater risk for substance use initiation. However, it is important to note that PCE and substance use initiation both preceded the collection of GMV data. The specific effects of PCE and previous substance use on GMV cannot be disentangled, and thus no causal link between PCE and GMV or substance use initiation can be made. However, given that the level of substance use was low, lower GMV may have occurred before use and contributed to the likelihood of initiating use, rather than use affecting GMV. Future research in which adolescents are imaged before substance use initiation is needed to disentangle these effects. Furthermore, although the PCE and NCE adolescents came from the same low-income, inner-city sample, we note that PCE encompasses both effects in utero and of compromised parenting, including, but not limited to, easier access to illicit drugs, potentially increasing the likelihood of substance use. Finally, a larger, more balanced sample is needed to explore gender differences in the effects of prenatal cocaine exposure on GMV. Despite these limitations, the current study is the first to show lower GMV in key regions of emotion, reward, memory and executive function in a comparison of a well-characterized sample of PCE versus NCE adolescents and also to demonstrate an association between lower GMV and substance-use initiation in this high-risk group of adolescents.
Acknowledgments
This study was jointly supported by the National Institutes of Health Office of Research on Women’s Health (ORWH) and the National Institute of Drug Abuse’s Specialized Centers of Research on Women’s Health (Grant No. P50DA016556 to RS).
We thank the staff members at the Yale Magnetic Resonance Research Center for their contribution to this project.
Footnotes
The authors reported no biomedical financial interests or conflicts of interest to disclose with respect to the content of this manuscript. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for Boehringer Ingelheim; has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders, or other health topics; has consulted for law offices on issues related to addictions or impulse control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, continuing medical education events, and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics.
References
- 1.Carmody DP, Bennett DS, Lewis M. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol Teratol. 2011;33:61–68. doi: 10.1016/j.ntt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayes L, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school-aged children exposed in utero to cocaine. Child Neuropsychol. 2007;13:205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- 3.Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33:47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaplin TM, Freiburger MB, Mayes LC, Sinha R. Prenatal cocaine exposure, gender, and adolescent stress response: A prospective longitudinal study. Neurotoxicol Teratol. 2010;32:595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, et al. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol. 2011;33:110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: A mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- 7.Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21:332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- 8.Ren JQ, Malanga CJ, Tabit E, Kosofsky BE. Neuropathological consequences of prenatal cocaine exposure in the mouse. Int J Dev Neurosci. 2004;22:309–320. doi: 10.1016/j.ijdevneu.2004.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001;435:263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- 10.Ismail ZI, Bedi KS. Rats exposed to cocaine during late gestation and early postnatal life show deficits in hippocampal pyramidal and granule cells in later life. J Anat. 2007;210:749–760. doi: 10.1111/j.1469-7580.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buxhoeveden DP, Hasselrot U, Buxhoeveden NE, Booze RM, Mactutus CF. Microanatomy in 21 day rat brains exposed prenatally to cocaine. Int J Dev Neurosci. 2006;24:335–341. doi: 10.1016/j.ijdevneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Dey S, Mactutus CF, Booze RM, Snow DM. Specificity of prenatal cocaine on inhibition of locus coeruleus neurite outgrowth. Neuroscience. 2006;139:899–907. doi: 10.1016/j.neuroscience.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 13.Akbari HM, Azmitia EC. Increased tyrosine hydroxylase immunoreactivity in the rat cortex following prenatal cocaine exposure. Brain Res Dev Brain Res. 1992;66:277–281. doi: 10.1016/0165-3806(92)90093-c. [DOI] [PubMed] [Google Scholar]
- 14.Jones L, Fischer I, Levitt P. Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb Cortex. 1996;6:431–445. doi: 10.1093/cercor/6.3.431. [DOI] [PubMed] [Google Scholar]
- 15.Xiao D, Zhang L. Upregulation of Bax and Bcl-2 following prenatal cocaine exposure induces apoptosis in fetal rat brain. Int J Med Sci. 2008;5:295–302. doi: 10.7150/ijms.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 17.Romano AG, Harvey JA. Prenatal exposure to cocaine disrupts discrimination learning in adult rabbits. Pharmacol Biochem Behav. 1996;53:617–621. doi: 10.1016/0091-3057(95)02061-6. [DOI] [PubMed] [Google Scholar]
- 18.Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106:5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 19.Bechara A. Risky business: Emotion, decision-making, and addiction. J Gambl Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 20.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 21.Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry. 2008;63:214–221. doi: 10.1016/j.biopsych.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, et al. Volumetric MRI study of brain in children with intra-uterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, et al. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Littman B, Parmelee AH. Manual for Obstetric Complications, Infant Studies Project. Los Angeles: Department of Pediatrics, School of Medicine, University of California; 1974. [Google Scholar]
- 27.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging. 2008;27:425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 30.Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- 31.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Grond J. Probabilistic segmentation of white matter lesions in MR imaging. Neuroimage. 2004;21:1037–1044. doi: 10.1016/j.neuroimage.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Bouix S, Martin-Fernandez M, Ungar L, Nakamura M, Koo MS, McCarley RW, et al. On evaluating brain tissue classifiers without a ground truth. Neuroimage. 2007;36:1207–1224. doi: 10.1016/j.neuroimage.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerasa A, Messina D, Nicoletti G, Novellino F, Lanza P, Condino F, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR Am J Neuroradiol. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR. Development of an MRI rating scale for multiple brain regions: Comparison with volumetrics and with voxel-based morphometry. Neuroradiology. 2009;51:491–503. doi: 10.1007/s00234-009-0521-z. [DOI] [PubMed] [Google Scholar]
- 36.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 39.Hayasaka S, Nichols TE. Validating cluster size inference: Random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- 41.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 42.Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 47.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 48.Crone EA, Ridderinkhof KR, Worm M, Somsen RJ, van der Molen MW. Switching between spatial stimulus-response mappings: A developmental study of cognitive flexibility. Dev Sci. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 49.Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, et al. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: A prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sack AT. Parietal cortex and spatial cognition. Behav Brain Res. 2009;202:153–161. doi: 10.1016/j.bbr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Fein G, Shimotsu R, Chu R, Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin Exp Res. 2009;33:1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: Contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- 53.Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- 54.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, et al. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colder C, Chassin L. Affectivity and impulsivity: Temperament risk for adolescent alcohol involvement. Psychol Addict Behav. 1997;11:83–97. [Google Scholar]
- 56.Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Exp Clin Psychopharmacol. 2007;15:264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- 57.Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eshel N, et al. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- 58.Wills TA, Walker C, Mendoza D, Ainette MG. Behavioral and emotional self-control: Relations to substance use in samples of middle and high school students. Psychol Addict Behav. 2006;20:265–278. doi: 10.1037/0893-164X.20.3.265. [DOI] [PubMed] [Google Scholar]
- 59.Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konova AB, Moeller SJ, Tomasi D, Parvaz MA, Alia-Klein N, Volkow ND, Goldstein RZ. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. Eur J Neurosci. 2012;36:2979–2988. doi: 10.1111/j.1460-9568.2012.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parvaz MA, Konova AB, Tomasi D, Volkow ND, Goldstein RZ. Structural integrity of the prefrontal cortex modulates electrocortical sensitivity to reward. J Cogn Neurosci. 2012;24:1560–1570. doi: 10.1162/jocn_a_00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 63.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, et al. Gene × disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 68.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 69.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 72.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 73.Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]