Abstract
In a recent longitudinal study to assess the development of incidental recognition memory processes in monkeys, we showed that the effects of neonatal hippocampal lesions did alter incidental recognition memory only when the animals reached the juvenile period (Zeamer et al., 2010). The current follow-up study tested whether this incidental memory loss was long-lasting, i.e. present in adulthood, or only transitory, due to functional compensation with further brain maturation. The same animals with neonatal hippocampal lesions and their sham-operated controls were re-tested in the visual paired-comparison task when they reached adulthood (48 months). The results demonstrated that, at least for easily discriminable color pictures of objects, the involvement of the hippocampus was only transitory, given that when re-tested as adults, animals with neonatal hippocampal lesions performed as well as sham-operated controls at all delays. Yet, significant recognition memory impairment was re-instated when the discriminability of the stimuli was made more difficult (Black/White pictures of similar objects). The data demonstrate profound functional remodeling within the hippocampus and its interactions with different medial temporal lobe structures from the juvenile period to adulthood, which is substantiated by a parallel morphological maturation of hippocampal intrinsic circuits (Lavenex et al., 2007a; Jabès et al., 2011).
Keywords: incidental recognition, memory, developmental amnesia
The visual paired comparison (VPC) task has proven to be a sensitive paradigm to assess the contribution of the medial temporal lobe structures to recognition memory processes (Zola et al., 2000; Nemanic et al., 2004). In addition, given its incidental nature, this task has been successfully used to assess recognition memory processes from infancy through adulthood (Fagan, 1972, 1990; Gunderson and Sackett, 1984; Gunderson and Swartz, 1984; Bachevalier et al., 1993; Pascalis and De Schonen, 1994; Pascalis et al., 1998). In a recent longitudinal study, a group of infant rhesus macaques were tested in the VPC task at 1, 6, and 18 months of age (Zeamer et al., 2010) using easy discriminable color pictures of objects as stimuli. Recognition memory was present at the youngest age (1 month) even at the longest delay tested (120s) and became more robust by 6 months of age and delay-dependent (better performance at the shorter than longer delays) by 18 months of age. To further investigate the contribution of the hippocampus in the development of recognition memory, novelty preference was also assessed at the same ages in monkeys with selective hippocampal lesions performed at 10–12 days of age. After neonatal hippocampal lesions, recognition memory ability was comparable to that of controls at 1 and 6 months of age, indicating that structures other than the hippocampal formation could support this function in the first postnatal months. However, by 18 months of age, the delay-dependent effect found in the controls was even more pronounced in monkeys with neonatal hippocampal lesions. At this age, animals with neonatal hippocampal lesions did not differ from controls at the short delays, but showed significantly weaker novelty preference than controls at 120s. All together the results demonstrated that critical maturational changes occur within the neural substrate supporting this ability and suggested that, although the temporal cortical areas could support incidental recognition memory in early infancy, the hippocampus began to contribute to it after 6 months of age.
Given that recognition ability in animals with neonatal hippocampal lesions at 18 months was not totally abolished at the longest delay of 120-s as it usually is when the lesions are performed in adulthood (Zola et al., 2000; Nemanic et al., 2004), it became important to assess whether or not novelty preference in animals with neonatal hippocampal lesions will continue to decline with further maturation. Thus, in the present study, we first re-tested the same animals in the VPC task as they reached adulthood using a new set of color pictures. Given that the adult-onset hippocampal lesions did not alter recognition memory at any delays with the use of color objects (Zeamer et al., 2011), we predicted that, when re-tested as adults, animals with neonatal-onset hippocampal lesions may not differ from controls, demonstrating a full recovery of recognition memory. Second, based on recent findings indicating that the magnitude of recognition memory impairment after hippocampal lesions in adult monkeys depends on the types of stimuli used (Zeamer et al., 2011), the second aim of the study was to investigate whether stimulus types will also differently impact recognition memory in animals with neonatal hippocampal lesions. Thus, as for the adult-onset hippocampal lesions, we predicted that the neonatal hippocampal lesions will impair recognition memory when discrimination between the stimuli will be made more difficult.
The subjects were eleven rhesus monkeys (Macaca mulatta), six of which had received sham-operations (Group Neo-C, 3 males, 3 females) and five of which had received neurotoxic lesions of the hippocampus (Group Neo-Hibo, 3 males, 2 females) at 10–12 days of age. At the start of the study, their mean weight was 7.0 kg (range: 5.39 to 9.45) and their mean age was 48 months (range: 44–54 months). All experimental procedures were approved by the Animal Care and Use Committee of the University of Texas Health Science Center, Houston, TX and Emory University, Atlanta, GA. All rearing conditions and neuroimaging and neurosurgical procedures have been detailed previously (Goursaud and Bachevalier, 2007; Zeamer et al., 2010). Neuroimaging investigation of the extent of lesions (Málková et al., 2001; Nemanic et al., 2002) indicated that the hippocampal lesions ranged from 33.2 to 87.4% (average: 56.9%) with minimal unintended damage to parahippocampal areas TH/TF (6.5%) and to the amygdala (2.5%). Two Neo-Hibo cases received extensive bilateral lesions (average: 67% and 87%), whereas the remaining three had more extensive damage on one side (> 60%) but moderate to mild damage on the other (< 21%). Detailed illustrations of all 5 Neo-Hibo cases have been presented elsewhere (Zeamer et al., 2010, see Tables 1 and 2, Figures 2 and 3; Heuer and Bachevalier, 2011a and 2011b, Figures 2 and 1, respectively; Glavis-Bloom et al., 2013, Figure 3).
The behavioral testing was conducted in a testing cubicle located in a sound-attenuated room equipped with a white noise generator to reduce external noise. The animal sitting in a primate chair was positioned approximately 30 cm from a TV monitor on which visual stimuli could be displayed. Monkeys’ eye movements towards stimuli were captured via a video camera (Sony Digital8 TRV-140) mounted above the monitor and fed into a time/date generator connected to a VCR (JVC HR-S4800U) and TV monitor, which allowed the experimenter to measure the animal’s looking time to stimuli during the task. The animals were neither food deprived nor water restricted during behavioral testing that lasted no more than 45 minutes daily. All subjects had previously been tested on VPC at 1.5, 6 and 18 months of age using sets of color pictures of objects that differed at each age (see Zeamer et al., 2010). All animals had received additional cognitive testing including spatial memory (8 and 24 months), reversal learning (36 months) and oddity (3 and 15 months) prior to beginning re-testing on VPC task at 48 months.
To assess the long-term effects of neonatal hippocampal lesions on VPC performance at 48 months, the subjects were given one day of acclimation to the testing apparatus. The stimuli and all experimental procedures were identical to those used at the earlier ages (Zeamer et al., 2011) but the stimuli were entirely new and selected from a pool of 800,000 color clipart of objects (Nova Art Explosion 800,000 Clip Art). For each VPC trial, the animal was familiarized to a single picture that appeared and remained at the center of the monitor for a cumulative looking time of 30 s. After a delay, which varied from 10, 30, 60 and 120 seconds, a retention test was given in which the sample picture and a novel one were displayed side-by-side (12 cm apart) on the monitor for 5 s after the animal initially looked at one of the two images. A second retention test in which the left/right position of the two images was reversed was given after a 5-s delay and then followed by a blank screen which remained for 30 s prior to the start of the next trial. The left/right position of the novel picture on the first retention test varied pseudo-randomly. Ten trials were given at each of the delays (10, 30, 60 and 120 s) and were pseudorandomly intermixed within a daily session. For each trial, several parameters were analyzed according to procedures described elsewhere (Pascalis and Bachevalier, 1999; Zeamer et al., 2010) and included (a) the length of time it took the animal to familiarize to the novel picture (Total Familiarization Phase), (b) the total time looking at both pictures during the two retention tests (Total Retention Time), and (c) the percent time looking to the novel picture across the two retention tests [(time looking at novel / total time looking) × 100)]. To measure developmental progression of performance, novelty preference scores of all animals obtained at 48 months in the current study were compared to those they obtained at 18 months (Zeamer et al., 2010).
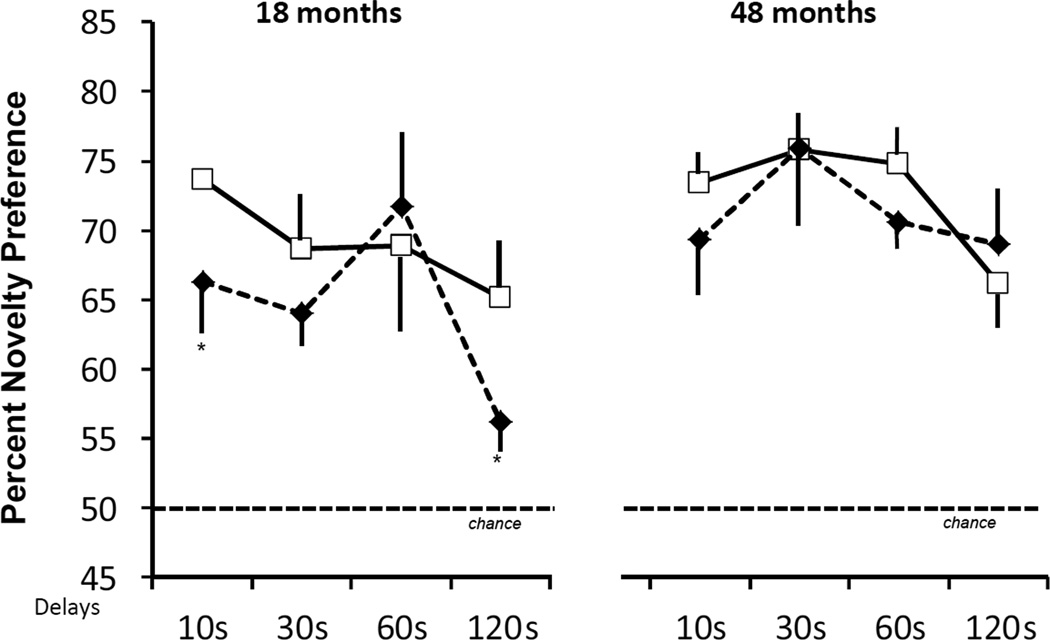
Repeated measure (Group × Age × Delay) ANOVAs indicated that the length of familiarization time and total retention time did not differ between groups and across age and delays (all ps > .05). In addition, the Group × Age × Delay ANOVA for percent looking at novel revealed no significant main effects (Group: F(1, 9) = 2.60, p > .05; Age: F(1, 9) = .01, p > .05; Delay (F(3, 27) = 1.61, p > .05) and no significant interactions between the three factors (all ps > .05). However, as shown in Figure 1, planned comparisons (Group × Delay ANOVA) at each age separately revealed that the significant Group × Delay interaction reported at 18 months (F(3, 27) = 4.12, p < .01) was not reliable when the animals where retested at 48 months of age (F(3, 27) = 0.73, p > .05). Thus, whereas neonatal hippocampal lesions impacted novelty preference at 120 s at 18 months, this group difference was absent at 48 months when animals with neonatal hippocampal lesions performed as well as controls at all delays.
Figure 1.
Scores are mean ± SEM of percent looking at novel color pictures at delays varying from 10s to 120s at 18 months and 48 months. Conventions: animals with neonatal sham-operations (open square, solid line) and animals with neonatal hippocampal lesions (filled diamond, dashed line). Data at 18 months are from Zeamer et al., 2010. * indicates p < .05.
Given our recent demonstration that recognition memory deficits after selective hippocampal lesions in adult monkeys depend on stimulus complexity (Zeamer et al., 2011), we re-tested monkeys with neonatal hippocampal lesions and their sham-operated controls in the VPC task using 30 pairs of black and white pictures of objects that were intentionally selected to be as different as possible and easily discriminable (dissimilar BW stimuli) and 30 pairs of black and white pictures of objects that were, on the opposite, intentionally selected to be similar and share many overlapping features (two planes, two tables, two chairs, two dogs, etc) to impede stimuli discriminability (similar BW stimuli, see examples of each stimulus type in Fig.1, Zeamer et al., 2011). Monkeys were tested first with the dissimilar BW stimuli and then with the similar BW stimuli. As testing with color objects above, the duration of encoding was kept constant at 30 s but only three retention delays (10, 60 and 120s) were intermixed in a pseudo-random order for each stimulus type. Each animal's performance was thus evaluated for a total of 10 trials per delay and per stimulus type. Data obtained with the dissimilar and similar BW stimuli were compared to those obtained above with color pictures of objects at the same delays of 10 s, 60 s, and 120 s.
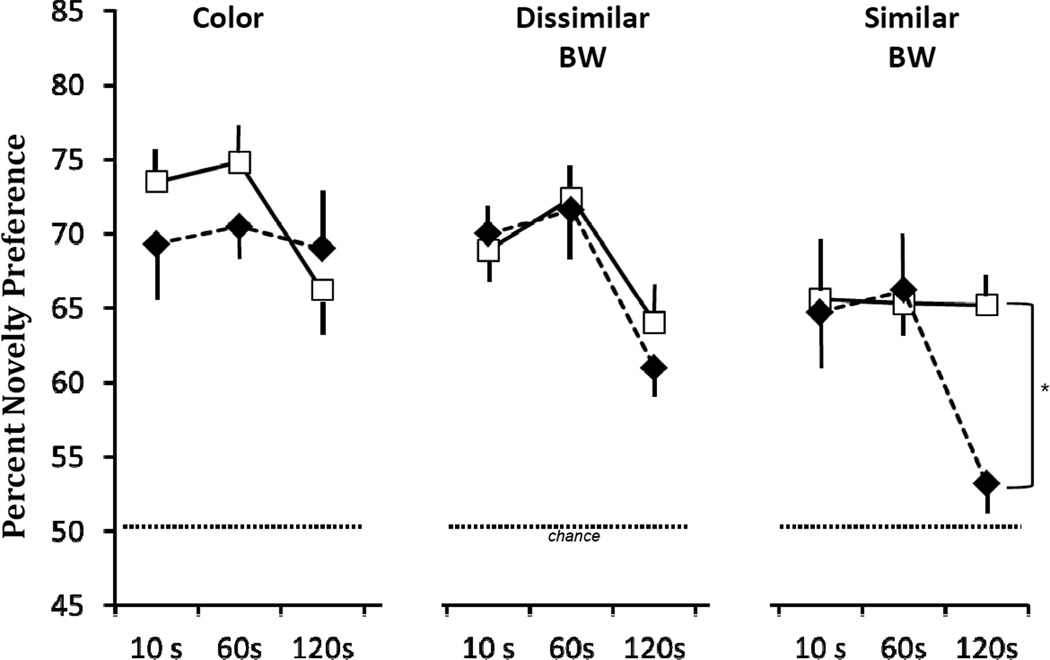
Repeated-measure (Group × Stimulus × Delay) ANOVAs revealed that both groups took longer to familiarize to the Color stimuli than to the BW stimuli (Stimulus effect: FHUYNH-FELDT(1.69, 15.22) = 5.144, p < 0.05), but the two groups did not differ from each other within any stimulus type (p > 0.05). No group differences were noted for the Total Retention Time across any stimulus type (Stimulus effect: FHUYNH-FELDT (1.79, 16.15) = .04, p > .05). Figure 2 illustrates average novelty preference scores across the 3 stimulus types and the three delays for the two groups. The 3-factor ANOVA revealed a significant Stimulus × Delay interaction (FHUYNH-FELDT (3.8, 34.2) = 2.65, p < .05) and a significant Group × Stimulus × Delay interaction [FHUYNH-FELDT (3.8, 34.2) = 3.07; p = .03]. To investigate this interaction, repeated measure (Group × Delay) ANOVAs were run at each stimulus type separately. These analyses indicated no Group X Delay interactions for the color and dissimilar BW stimuli, but a significance interaction for the similar BW stimuli [F(2,18) = 4.13, p < .05; see Fig. 2]. Thus, for the similar BW stimuli, novelty preference for Group Neo-H-ibo was comparable to that of Group Neo-C for the short delays of 10s and 60 s, but not at the longer delay of 120 s, when novelty preference for Group Neo-H did not differ from chance (p > .1) and was significantly different from that of Group Neo-C (t(9) = 3.53, p < .01). It is also important to note that the magnitude of this impairment did not correlate with lesion extent (Pearson r = − 0.16, p > .05; see Tables 1 and 2 in Zeamer et al., 2011 for extent of damage in each case).
Figure 2.
Scores are mean ± SEM of percent looking at novel color (color), dissimilar and similar black-white (BW) pictures at delays varying from 10s to 120s at 48 months. Conventions as in Figure 1. * p < .05.
This study is the first systematic longitudinal investigation of the critical role of the hippocampus in the development of incidental recognition memory processes in monkeys. Together with earlier findings collected on the same animals at earlier stages of development (Zeamer et al., 2010), monkeys with selective neonatal hippocampal lesions performed just as well as sham-operated controls at both 1.5 and 6 months of age when using highly discriminable color picture of objects. However, at 18 months when sham-operated controls displayed a delay-dependent memory decline, those with neonatal hippocampal lesions showed an even sharper decline. The pattern of results indicated that, although incidental recognition memory at the earlier ages could be supported by structures others than the hippocampus, the involvement of the hippocampus to mediate this memory process became essential for the long delays at 18 months. Yet, the current findings demonstrated that, at least for easily discriminable objects, the involvement of the hippocampus was only transitory, given that when re-tested as adults (48 months), animals with neonatal hippocampal lesions performed as well as sham-operated controls at all delays. These data indicate profound functional remodeling within the hippocampus and its interactions with different medial temporal lobe structures from the juvenile period to adulthood. We had already suggested that the normal performance of animals with neonatal hippocampal lesions could be supported by earlier maturating medial temporal cortical areas until an age in the juvenile period when the hippocampus becomes critical for this memory process (Zeamer et al., 2011). The recovery of incidental recognition memory found in the current follow-up study indicates that, with further brain maturation, medial temporal cortical or prefrontal cortical areas could have taken over the function in the absence of a functional hippocampus. Our more recent results are consistent with this prediction since neonatal perirhinal lesions in monkeys altered novelty preference at all ages tested (i.e. 1.5, 6, and 18 months), and this deficit became more profound as the animals matured (Zeamer & Bachevalier, 2009). In addition, the progressive development of hippocampal-dependent memory functions fits well with neuroanatomical reports indicating a differential development of hippocampal intrinsic circuits that extends beyond the first year of age in monkeys (see review Alvarado and Bachevalier, 2000; Lavenex et al., 2007a; Jabès et al., 2011) and with recent neuroimaging studies in children demonstrating strengthening of hippocampal-prefrontal functional connectivity in support of memory processes from adolescence to adulthood (Ofen et al., 2007, 2012; Paz-Alonso et al., 2008; Chai et al., 2010).
Nevertheless, despite the recovery of recognition memory for color stimuli, significant memory impairment was re-instated when the discriminability of the stimuli was made more difficult. This pattern of recognition deficits in adult animals with neonatal hippocampal lesions was strikingly similar to that already reported in adult animals that had received similar hippocampal lesions in adulthood (Zeamer et al., 2011). Both early-onset and adult-onset hippocampal lesions resulted in normal performance when easily discriminable color or black/white pictures were used, but in novelty preference loss when the pictures shared many overlapping features. Thus, the data provide evidence that temporal cortical areas may be sufficient to support incidental memory for easily discriminable stimuli and even for similar stimuli when short retention delays are used. The hippocampus is critically involved when fine-grained comparison of familiar and novel items needs to be maintained for longer delay intervals (Wan et al. 1999; Ranganath et al., 2000; Holdstock et al., 2002; Mumby et al. 2002; Pihlajamaki et al., 2004; Danckert et al., 2007; Hoge and Kesner 2007; Hunsaker et al. 2007; Bakker et al., 2008; Van Elzakker et al. 2008; Zeamer et al., 2011). Although the specific stimulus attributes that engage the hippocampus in recognition memory need to be clarified, that is whether it is related to "ambiguous features" (Bussey and Saksida, 2005, 2007) or to the spatial arrangement of stimulus features (Pihlajamaki et al., 2004; Bachevalier and Nemanic, 2008), the present data are in accordance with some computational models suggesting that the hippocampus may transform similar representations into highly dissimilar, non-overlapping representations, i.e. “pattern separation” (Norman and O’Reilly, 2003; O’Reilly and Rudy, 2001). Interestingly, the dentate gyrus (DG) and CA3 field appear to be primarily involved in this process (Bakker et al., 2008; Hashimoto et al., 2012), and more importantly the intrinsic connectivity pattern of these two hippocampal field continues maturing beyond 1 year of age and into adolescence in monkeys (Jabès et al., 2011).
Furthermore, our data may speak toward the specific medial temporal lobe structure implicated in the incidental recognition memory deficit reported in cases of developmental amnesia in humans. Patients suffering from developmental amnesia due to early hypoxic hippocampal damage show profound deficits in recall but relatively intact recognition memory as assessed by Yes/No recognition tasks (see for review De Haan et al., 2006; Adlam et al., 2009). Despite this saving of recognition memory, a recent report indicated that developmental amnesic patients showed a delay-dependent loss of novelty preference when landscape images were used as stimuli and an overall reduction in novelty preference when fractal images served as stimuli (Munoz et al., 2011). Although in these human cases the impairment could have been associated with abnormal functioning of medial temporal cortical structures known to be critical to support novelty preference (Nemanic et al., 2004), the similarity in the pattern of results in these developmental amnesic patients and those reported in monkeys with known neonatal damage to the hippocampus, strongly favors the view that in these patients as well the deficit in incidental recognition memory is likely due to hippocampal damage.
Finally, it is also notable that, as for developmental amnesia cases (see for review De Haan et al., 2006; Adlam et al., 2009), the deficit in incidental recognition memory following neonatal hippocampal lesions occurs despite normal performance on other recognition tasks. Following training on the VPC tasks, animals of Neo-Hibo lesions were tested in other recognition memory tasks, such as delayed nonmatching-to-sample and object memory span task (Heuer and Bachevalier, 2011a), in which they performed as well as their sham-operated controls. Thus, as reported previously (Reed et al., 1997; Holdstock et al., 2002; Nemanic et al., 2004), the impact of hippocampal lesions on recognition memory processes is task dependent, presumably due to the different memory demands imposed by each task. Together with findings in rodents, monkeys, and humans indicating that neonatal hippocampal lesions permanently alter spatial and working memory abilities (Chambers et al., 1996; Vargha-Khadem et al., 1997; van Praag et al., 1998; Spiers et al., 2001; Burgess et al., 2002; Lipska et al., 2002; Kazama et al., 2003; Killiany et al., 2005; Rehbein et al., 2005; Heuer and Bachevalier, 2011b; Glavis-Bloom et al., 2012, in press; but see Lavenex et al., 2007b for negative findings), the present results demonstrate that the deficits in memory functions after neonatal hippocampal damage are pervasive and enduring.
Acknowledgment
This research was supported by the National Institute for Mental Health (MH58846), National Center for Research Resources (P51RR165 currently supported by the Office of Research Infrastructure Programs/OD P51OD11132), and the National Science Foundation (NSF IBN9876754). We thank Andy Kazama for his help with the behavioral testing of the animals.
References
- Adlam A-L, Malloy M, Mishkin M, Vargha-Khadem F. Dissociation between recognition and recall in developmental amnesia. Neuropsychologia. 2009;47:2207–2210. doi: 10.1016/j.neuropsychologia.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Learn Mem. 2000;7:244–256. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. NeuroReport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Spatial memory in monkeys as measured with the visual paired-comparison task: effects of selective hippocampal, perirhinal and areas TH/TF lesions. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: an alternative approach. Cur Opin Neurobiol. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Jacobs LF, Gabrielli JDE. Scene complexity: influence on perception, memory and development in the medial temporal lobe. Frontiers Hum Neurosci. 2010;4:1–10. doi: 10.3389/fnhum.2010.00021. (article 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy J, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15(6):587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Danckert SL, Gati JS, Menon RS, Köhler S. Perirhinal and hippocampal contribution to visual recognition memory can be distinguished from those of occipio-temporal structures based on conscious awareness of prior occurrence. Hippocampus. 2007;17(11):1081–1092. doi: 10.1002/hipo.20347. [DOI] [PubMed] [Google Scholar]
- de Haan M, Mishkin M, Baldeweg T, Vargha-Khadem F. Human memory development and its dysfunction after hippocampal injury. Trends Neurosci. 2006;29:374–381. doi: 10.1016/j.tins.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Fagan JF., III Infants’ recognition memory for faces. J Exp Child Psychol. 1972;3:453–476. doi: 10.1016/0022-0965(72)90065-3. [DOI] [PubMed] [Google Scholar]
- Fagan JF., III The paired-comparison paradigm and infant intelligence. Ann NY Acad Sci. 1990;608:337–364. doi: 10.1111/j.1749-6632.1990.tb48902.x. [DOI] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado M, Bachevalier J. Neonatal hippocampal damage impairs specific Food/Place associations in adult macaques. Behav Neurosci. doi: 10.1037/a0031498. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdale and orbital frontal cortex. Behav Brain Res. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Gunderson VM, Sackett GP. Development of pattern recognition in infant pigtailed macaques (Macaca nemestrina) Dev Psych. 1984;20:418–426. [Google Scholar]
- Gunderson VV, Swartz KB. Visual recognition in infant pigtailed macaques after a 24-hour delay. Amer J Prim. 1984;8:259–264. doi: 10.1002/ajp.1350080309. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Abe N, Ueno A, Fudji T, Takahashi S, Mori E. Changing criteria for old/new recognition judgments can modulate activity in the anterior hippocampus. Hippocampus. 2012;22:141–148. doi: 10.1002/hipo.20878. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav Neurosci. 2011a;125(2):137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Neonatal hippocampal lesions in rhesus macaques alter the monitoring but not maintenance of information in working memory. Behav Neurosci. 2011b;125(6):859–870. doi: 10.1037/a0025541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O'Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perfornat path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Jabès A, Banta-Lavenex P, Amaral DG, Lavenex P. Postnatal development of the hippocampal formation: A stereological study in macaque monkeys. J Comp Neurol. 2011;519:1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Lay DA, Bachevalier J. 2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Delayed maturation of spatial memory in infant monkeys as assessed by a visual paired-comparison task. Program No. 324.4. 2003. Online. [Google Scholar]
- Killiany R, Rehbein L, Mahut H. Developmental study of the hippocampal formation in rhesus monkeys (Macacca mulatta): II. Early ablations do not spare the capacity to retrieve conditional object-object associations. Behav Neurosci. 2005;119(3):651–661. doi: 10.1037/0735-7044.119.3.651. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Banta-Lavenex PB, Amaral DG. Postnatal development of the primate hippocampal formation. Dev Neurosci. 2007a;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavanex PB, Amaral DG. Spatial relational learning persists following neonatal hippocampal lesions in macaque monkeys. Nature Neurosci. 2007b;10(2):234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27(1):47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Málková L, Bachevalier J, Mishkin M, Saunders RC. Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. NeuroReport. 2001;12:1913–1917. doi: 10.1097/00001756-200107030-00029. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:47–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Chadwick M, Perez-Fernandez E, Vargha-Khadem F, Mishkin M. Novelty preference in patients with developmental amnesia. Hippocampus. 2011;21:1268–1276. doi: 10.1002/hipo.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Ofen N, Chai XJ, Schuil KDI, Whitfield-Gabrieli S, Gabrieli JDE. The development of brain systems associated with successful memory retrieval of scenes. J Neurosci. 2012;32(29):10012–10020. doi: 10.1523/JNEUROSCI.1082-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nature Neurosci. 2007;10(9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal functions. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S. Recognition memory in 3- to 4-day-old human neonates. NeuroReport. 1994;5:1721–1724. doi: 10.1097/00001756-199409080-00008. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month old infants. J Exp Psychol: Learn Mem Cogn. 1998;24:249–260. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso PM, Ghetti S, Donohue SE, Goodman GS, Bunge SA. Neurodevelopmental correlates of true and false recognition. Cereb Cortex. 2008;18:2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Rehbein L, Killiany R, Mahut H. Developmental study of the hippocampal formation in rhesus monkeys (Macaca mulatta): I. Early ablations spare discrimination learning but not recognition memory. Behav Neurosci. 2005;119(3):635–650. doi: 10.1037/0735-7044.119.3.635. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11(6):715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- van Elzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008;15:899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pragg H, Qu PM, Elliod RC, Dreyfus CF, Black IB. Unilateral hippocampal lesions in newborn and adult rats: effects on spatial memory and BDNF gene expression. Behav Brain Res. 1998;92:21–30. doi: 10.1016/s0166-4328(97)00117-4. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer AE, Bachevalier J. Neonatal lesions of the perirhinal cortex alter the development of object recognition memory abilities in monkeys. Soc Neurosci Abstr. 2009;35 doi: 10.1016/j.dcn.2014.07.002. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer EA, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer EA, Meunier M, Bachevalier J. Stimulus similarity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions. Learn Mem. 2011;18:170–180. doi: 10.1101/lm.2076811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]