Abstract
Cytomegalovirus (CMV) is a frequently encountered infection following hematopoietic cell transplantation, and tissue invasive pneumonia is a dreaded complication of the virus in this population. In this review of CMV pneumonia, we address epidemiology, pathogenesis, diagnostics, current therapy and strategies to prevent the development of CMV disease. We also review emerging treatment and prevention options for this challenging disease.
Keywords: Cytomegalovirus, CMV pneumonia, hematopoietic cell transplant, antiviral, prevention
INTRODUCTION
Cytomegalovirus (CMV) continues to be a major cause of morbidity in hematopoietic cell transplant (HCT) recipients1,2, but of all CMV complications pneumonia is the most associated with significant mortality.3 Prevention strategies aimed at limiting latent reactivation and viral replication have been successful in reducing the incidence of CMV pneumonia to approximately 4% in high-risk seropositive recipients4,5, but changes in transplant practices such as the expanded use of high-risk unrelated and cord blood donor grafts, have also defined new populations more likely to develop CMV invasive disease.6 Unfortunately, even with potent antiviral therapy and advanced critical care management, death from CMV pneumonia remains unacceptably high.3,7
In this review we address the epidemiology, pathogenesis, diagnostics, and review up-to-date treatment and prevention strategies for CMV pneumonia in HCT patients. We also discuss ongoing research focused on novel treatment and prevention options, including antivirals in development. With the continued expansion of transplant programs throughout the world, an increased number of critical care physicians will have exposure to and responsibilities for diagnosing and treating this major post-transplant infectious complication. We hope that this review will serve as a state-of-the-art update on this infrequent yet important HCT complication for those with experience in transplantation and provide a foundation for those new to this unique immunocompromised population.
Epidemiology
Incidence
The incidence of CMV pneumonia in the early years of HCT, prior to the introduction of CMV prevention strategies, was around 10–35% after allogeneic transplantation and 1–6% in autologous transplant recipients.8 The institution of preemptive strategies used at most centers in the US (see prevention section below) have decreased the overall incidence of CMV disease in HCT recipients to around 5–8%.3–5 The burden of disease has also shifted, as gastrointestinal (GI) disease is now considered the most common form of CMV disease in HCT; pneumonia is estimated to make up about 1/3 of disease cases.5,9 The majority of cases of CMV pneumonia still occur in the early post-transplant period (≤ day +100), but the number of those occurring in the late period (after day 100) have increased.3,10 Late CMV disease occurs more frequently in subjects who experienced CMV reactivation within the first 3 months after HCT (27), had graft-versus-host disease (GVHD), have persistent lymphopenia at day 100 (or low CD4 count) and in those seropositive recipients who received of a CMV-seronegative donor graft (28).9–12
Outcomes
Outcomes in patients who develop CMV pneumonia are generally very poor, even with the use of potent antiviral agents and aggressive critical care management. Rates of mortality associated with CMV pneumonia in the pre-treatment era were nearly 100%13, but with the advent of ganciclovir (GCV) and other antiviral therapy options, rates of death have fallen to approximately 30–50%.1,3,14,15 The need for respiratory and critical care support is strongly associated with increased mortality.16 Interestingly, in some retrospective studies rare patients with proven CMV pneumonia survive even without antiviral therapy1, suggesting different host factors may help determine survival post-infection.
Pre and Post-Transplant Risk Factors (Table 1)
Table 1.
Epidemiologic Risk-factors Associated with CMV Disease in Patients Undergoing Hematopoietic Cell Transplantation
| Transplant Associated Risk Factors | Risk | Selected References |
|---|---|---|
|
| ||
| Pre-Transplant Risk Factors | ||
| CMV Serostatus | ||
| CMV R+ Status |

|
3,17,25,152 |
| - if CMV D- Donor Status |

|
21,22,153 |
| CMV R-/D+ |

|
20,24 |
| Donor Graft | ||
| PBMC vs. Bone Marrow | ⇩ | 26 |
| Umbilical Cord Blood |

|
6,19,27,28 |
| Unrelated Donor |

|
14,29,32,154 |
| Donor Mismatch |

|
154,155 |
| Conditioning Regimen | ||
| T-cell depletion |

|
15,18,34–37 |
| Myeloablative vs. Non-myeloablative | ⇳ | 4,30,156,157 |
| Post-Transplant Risk Factors | ||
| CMV detection in Blood* |

|
multiple |
| High initial viral load |

|
50,51 |
| Hematologic recovery | ||
| Lymphopenia† |

|
11,12,50 |
| GVHD | ||
| Acute GVHD |

|
41–43,50,158 |
| Chronic GVHD |

|
10,39,159 |
| GVHD prophylaxis and treatment | ||
| Anti-T-cell therapy |

|
3,160 (see above) |
| Use of MMF |

|
45 – 47 |
| Use of Sirolimus | ⇩ | 48 |
| Steroids >1 mg/kg/day |

|
39,158 |
Abbreviations: CMV, Cytomegalovirus; R+, recipient serpositive; D−, Donor seronegative; R−, recipient seronegative; D+, Donor positive; PBMC, peripheral blood mononuclear cells; GVHD, graft-versus-host disease; MMF, mycophenolate mofetil. Strength of association demonstrated by the number of arrows: indicates increased rate; ⇩indicates decreased rate; ⇳ indicates conflicting data.
indicates increased rate; ⇩indicates decreased rate; ⇳ indicates conflicting data.
Detection in blood increases the risk for the development for both early and late CMV disease.
Likely related to all lymphocyte subsets, but CD4 and CD8 probably most important.
Serologic status (Donor and Recipient)
The most prominent risk factor for CMV pneumonia is the transplant recipient's CMV serologic status prior to transplantation (Table 1). Patients who are known to be seropositive (R+) are at the greatest risk for reactivation of latent virus through the transplant process and have the highest rates of subsequent CMV disease.3,17–20 The relationship of donor serostatus in R+ recipients remains controversial.21–23 In contrast to their high-risk counterparts, seronegative patients (R−) who receive a positive donor graft (D+) have a much lower risk of CMV infection (12–19%) and CMV disease (3–5%).20,24 Less than half of the D+/R− patients that are found to have invasive disease have pneumonia and overall CMV pneumonia is an uncommon complication occurring in only about 1% of patients with preemptive therapy.20 Patients who are CMV D−/R− rarely develop evidence of CMV infection and rates of CMV disease are very low (<1%).3,25
Stem Cell Source
Multiple transplant associated risk factors are linked to the development of CMV disease (see table 1). When considering donor graft source, peripheral blood cells are thought to be associated with a lower risk of CMV reactivation and pneumonia than bone marrow grafts 26, but umbilical cord blood transplant recipients appear to be at even higher risk for CMV disease due to significant delays in immune reconstitution.6,19,27,28 Patients receiving unrelated and mismatched donors are at higher risk of CMV disease, although this increased risk appears to be associated with the development of late disease.14,29–32
Conditioning Regimen
Multiple transplant conditioning regimens have also been associated with the development of CMV disease. Earlier studies suggested CMV complications were delayed in those undergoing non-myeloablative conditioning when compared to myeloablative regimens14, but this has not been confirmed in larger cohort studies.4,31 T cell depletion delays anti-CMV T-cell reconstitution 33 and has been associated with a higher risk of CMV complications in multiple populations.15,18,34–38
Post-transplant complications
Post-transplant complications are also known risk factors for the development of CMV disease. The development of acute and chronic graft GVHD are associated with the development of CMV disease.3,10,39–43 Immunosuppressive strategies for acute and chronic GVHD prophylaxis have been related to higher risk of CMV infection.44 T-cell therapies and mycophenolate mofetil (MMF) delay CMV specific T-cell responses and lead to more frequent CMV complications. 45–47 Interestingly, not all immunosuppressive therapy may have a negative effect on CMV, as sirolimus may have a protective role in CMV infection.48
CMV detection in blood
It is important to note that not all patients who have detectable CMV in their blood develop CMV disease, but that nearly all patients with CMV pneumonia will have evidence of CMV reactivation.49 Determining the risk factors for progression from viral replication in blood to development of tissue invasive disease are challenging in this population, as most risk factors associated with CMV disease are also associated with CMV reactivation. Rates of progression from reactivation to CMV pneumonia have however been associated with leukopenia/lymphopenia at the time of reactivation and the development of a high viral load during preemptive therapy.50,51 Viral kinetics of CMV post-HCT, particularly initial viral load and expansion kinetics, also appear to be strong predictors for the development of CMV disease.51
PATHOPHYSIOLOGY
Following primary infection, CMV becomes latent in the human host avoiding immune detection through multiplicative and diverse mechanisms.52 Data suggests that bone marrow-derived hematopoietic cells, granulocyte-macrophage progenitors, and peripheral blood monocytes serve as reservoirs for reactivation during immunosuppression.20,53–58 In addition, murine models and human studies have demonstrated latency in lung alveolar macrophages and pulmonary epithelial cells.59–61 The allogeneic process likely facilitates reactivation of CMV62, and major alterations in adaptive and innate immunity following transplantation lead to viral escape, replication and eventual tissue invasion. CMV replication is linked to the degree of immunosuppression, and CMV pneumonia is more frequent and severe in HCT recipients than in most other immunosuppressed populations.7 Pathogenesis of CMV pneumonia has been reviewed by others in detail63–65, but the mechanisms by which CMV invades the lung are unclear. In fact,
Immunopathology hypothesis
The immunopathology hypothesis suggests that immune responses to CMV lead to pulmonary complications.63,64 Grundy and colleagues demonstrated that CMV proliferated extensively in the lung in athymic mice without development of pneumonitis, but that once their T-cell immunity had recovered pneumonitis rapidly developed.64,66 This theory is also thought to be supported by the lack of CMV pneumonia events in syngeneic transplants and in HIV patients with more profound CD4 deficiency.64,67
Cytopathological hypothesis
The alternate cytopathological hypothesis suggests that CD8+ T-cells are needed to protect against the development of CMV tissue invasion.64,65 Studies have implicated deficiencies in class I MHC-restricted, CMV-specific CD8+ T-lymphocyte responses in the progression to invasive disease.65 This theory has been supported by clinical observations of severe CMV pneumonia occurring in murine studies and in patients treated by T cell depleting agents.15,68 Furthermore, it has been shown that neutralizing CD8+ T-cells in immunosuppressed CMV infected mice inhibited interstitial pulmonary involvement69, and that adoptive immunotherapy has been shown to abrogate CMV complications.70 In addition, studies have demonstrated that marked reductions in CD4/CD8+ CMV specific T-cells are associated with the development of late disease, and that low dose CMV reactivation and subsequent immune education may prevent this complication.11
Other factors
There are also emerging data that support a role for natural killer cells in CMV complications following HCT, specifically with activating killer immunoglobulin-like receptor (KIR) genotypes of donor cells.71–73 The association between excess NK cells in BALs from immunocompromised patients who died from CMV pneumonia suggests a role in aberrant immune responses to CMV.74 At the same time, herpesvirus infections are much more likely to occur in children who lack NK cells but possess an intact adaptive immune system.75 The endothelium is also though to play a role in the pathophysiology of CMV lung involvement as infected endothelial cells upregulate adhesion molecules, allowing for leukocyte attachment and associated vascular damage.76–78 It is unknown whether the driver of this associated endothelial injury is directly related to CMV, or if toxicity from the transplant process (e.g. total body irradiation) leads to such damage and subsequent CMV reactivation.
DIAGNOSIS
Signs and Symptoms
CMV pneumonia is defined by the presence of clinical signs and symptoms of pneumonia or pulmonary disease combined with the detection of CMV from the lung.79 Patients with CMV pneumonia present with findings consistent with pulmonary dysfunction including a non-productive cough, dyspnea and hypoxia; fever does not always have to be present.80 Clinical examination may demonstrate prominent rales and tachypnea, but may initially be unremarkable. Nevertheless, none of these signs or symptoms are classically associated with CMV pneumonia, and nearly all are only indicative of an ongoing pulmonary process.
Non-Invasive testing
There are no typical laboratory findings that confirm the diagnosis of CMV pneumonia. Patients with CMV pneumonia may have low oxygen saturations on arterial blood gas evaluation, and some patients with may develop leucopenia or leukocytosis; these findings are not specific to CMV pneumonia. CMV detection in blood by CMV PCR or the pp65 antigenemia test can be helpful, but are not diagnostic even in the face of compatible symptoms. Although the majority of patients with CMV pneumonia will have CMV detected in blood, it can rarely occur with without detection in blood.49
Radiology
The most common radiological sign is bilateral interstitial infiltrates on chest x-ray (see Figure 1).80 Focal radiographic signs may also be absent in patients with documented CMV pneumonia.7,13,81 Moreover, neutropenic subjects are less likely to have abnormalities on conventional chest radiography may fail to detect lung infiltrates at an early stage, computed tomography (CT) scan has been shown to higher sensitivity and assist in the early detection of more subtle pulmonary infiltrates.82 The most common findings on CT are bilateral asymmetric ground-glass, air-space opacities and small centrilobular nodules.83 While often a diffuse interstitial process on CT, CMV can also present less frequently as nodules or pulmonary consolidations.84,85 CMV may also present as a co-pathogen to other major infections, further limiting the specificity of radiologic findings.
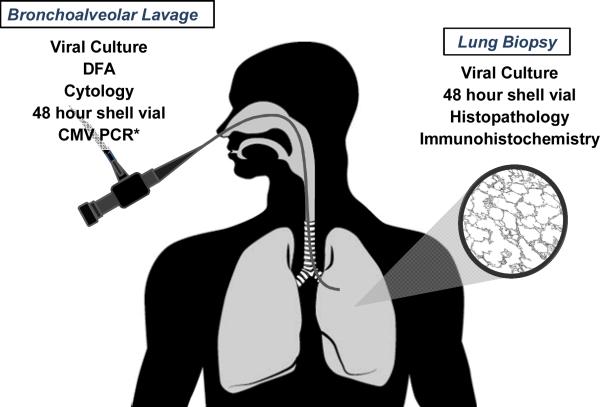
Figure 1. Methods Used to Diagnose CMV Pneumonia.
In order to make a diagnosis of patients must have signs and/or symptoms of pulmonary disease combined with the detection of CMV in bronchoalveolar lavage fluid or lung tissue samples. Detection of CMV can be by any one of the methods described in this figure.79 *A positive PCR test is not considered diagnostic by current guidelines79, but is nonetheless used at many centers for diagnosis of CMV pneumonia.
Abbreviations: CMV, cytomegalovirus; DFA, direct fluorescent antigen; PCR, polymerase chain reaction.
Bronchoscopy/Bronchoalveolar Lavage
Bronchoalveolar lavage has become the most frequent mechanism for the detection of CMV during the clinical management of HCT recipients. A number of laboratory and pathologic analyses are available on BAL fluid, and any one of these tests (Figure 2)can be diagnostic of CMV pneumonia in the presence of appropriate clinical and radiologic findings; histopathology is not required.79 Viral culture is performed by growing CMV in human fibroblastoid cell lines but slow growth may require up to 4 weeks for diagnosis. The required time, low reproducibility and low sensitivity of culture makes this technique impractical for prompt diagnoses. Cytopathologic evaluation for inclusion bodies from BAL fluid has a high specificity but low sensitivity for CMV pneumonia, but immunohistochemical staining with anti-CMV antibodies may enhance sensitivity of this method.86
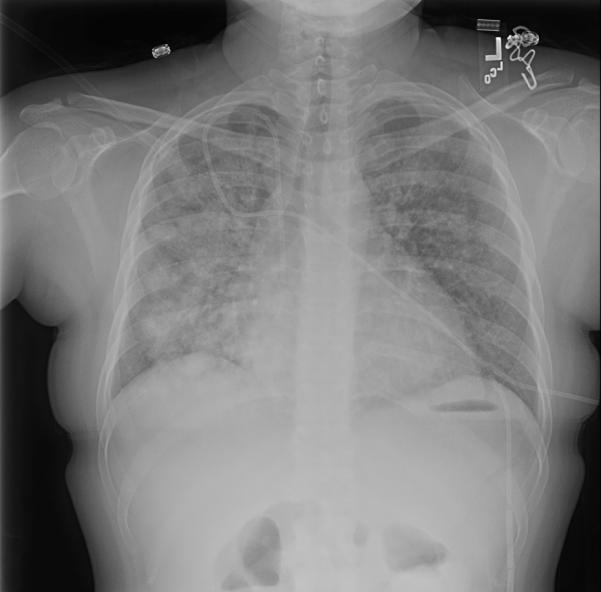
Figure 2. Radiographic Findings of CMV Pneumonia.
A 45 y/o female 34 days post-hematopoietic cell transplantation with CMV pneumonia. Anterior posterior chest radiograph demonstrates a diffuse interstitial process consistent with viral pneumonia.
Rapid detection of CMV can be performed by shell vial culture and/or direct fluorescent antigen testing (DFA). The shell vial method is a rapid culture technique that allows for identification of virus using monoclonal antibodies to immediate-early antigen within 24 hours and has been shown to be highly sensitive for the detection of CMV pneumonia.87 This test however, is operator dependent which limits widespread use in all but the largest centers. DFA is useful as a rapid test (within 1.5 hour), but lacks the sensitivity of other rapid tests.88 Qualitative CMV polymerase chain reaction (PCR) from BAL fluid has been shown to have a good negative predictive value, but poor positive predictive value for the diagnosis of CMV pneumonia.89,90 Quantitative PCR has been suggested to have a higher sensitivity but the absence of established viral load cut-off of CMV for defining CMV disease, limits the use of this technique. Furthermore, studies have demonstrated CMV DNA in BAL samples of asymptomatic HCT recipients, limiting interpretation of positive results.91,92 Still many centers currently utilize CMV PCR as part of their diagnostic strategy although it is not currently considered criteria for CMV pneumonia.79
Lung Biopsy
Histopathological diagnosis of CMV pneumonia from lung biopsy demonstrates typical intranuclear inclusions; CMV within cells may also be detected by immunohistochemical staining or in-situ hybridization.79 All lung biopsies and autopsy samples should also undergo routine shell viral testing for rapid diagnosis. Even though less likely to inform clinical care biopsy samples may also be held for CMV culture as a positive result can be important for epidemiologic studies. Unfortunately in severe cases, lung biopsy is not always feasible, as rapid onset of respiratory failure, mechanic ventilation or thrombocytopenia may limit access to this procedure.93 The patchy nature of the disease suggests that fine needle aspiration and less invasive pathologic sampling may miss clinically relevant disease.94
DIFFERENTIAL DIAGNOSIS
Infectious
Signs and symptoms of CMV pneumonia are not pathognomonic, and clinical manifestations and interstitial lung involvement may be caused by a multitude of other pathogens. Further complicating the picture is the uncertain role of CMV as either pathogen or bystander, as it can be detected in BAL specimens of patients with other confirmed pathogens.24 Bacteria such as Mycoplasma pneumoniae, Chlamydia pneumoniae or Legionella species can all have similar subacute presentations. Respiratory viral pathogens, including influenza, parainfluenza and respiratory syncytial virus, among others, can present with findings that are similar to CMV; these infections may not always be associated with upper respiratory symptoms.95 Other herpesviruses, albeit less frequently, can also present with pulmonary complications following transplantation.96,97 HHV6's role in pulmonary disease remains somewhat unclear, but it can be seen as a co-pathogen in some patients.98–101 Adenovirus, another latent virus, has similar risk factors and can also present during the post-HCT period with similar radiographic and clinical findings.102 The fungus Pneumocystis jiroveci can be challenging to distinguish from CMV pneumonia on clinical and radiographic findings alone, and although infrequent, CMV can also present with nodules or consolidation that resembles fungal pneumonia.84,85
Non-infectious
Non-infectious pulmonary complications can also present with signs and symptoms that may be similar to CMV. Idiopathic pneumonia syndrome presents with cough and tachypnea often seen in CMV pneumonia, and has associated multilobular infiltrates on chest x-ray or CT.103 As a subgroup of these patients, patients with diffuse alveolar hemorrhage (DAH) usually present more acutely.103 Patients who develop non-infectious cryptogenic organizing pneumonia (COP) can also present with low grade fever, non-productive cough, and dyspnea similar to CMV pneumonia.104 Radiologic manifestations can mimic viral pneumonia.105 The development of pulmonary edema or patients who develop chemotherapy associated pulmonary complications can also mimic CMV pneumonia.106 Other medications, such as Sirolimus, can lead to adverse pulmonary complications that may present with interstitial pneumonitis similar to CMV pneumonia or viral process.107
THERAPY
Antiviral therapy
The foundation for CMV pneumonia treatment is the early institution of antiviral therapy. CMV pneumonia following HCT, before the availability of current antivirals, was associated with a high mortality rate (nearly 100%). HCT recipients who receive early antiviral intervention may have improved outcome from CMV pneumonia.108 Early treatment is thought to help control viral replication which may help to limit immune-related lung damage, thereby reducing additional morbidities, such as the development of secondary infections, need for mechanical ventilation and aggressive intensive care management. Still, antiviral therapy will not change the outcome in all patients, as even with active antiviral therapy, death from CMV pneumonia remains an unavoidable outcome in many patients.7,16,109
Therapy is focused on an induction phase (twice daily dosing) and a maintenance phase (once daily dosing) of treatment. At our center patients with CMV pneumonia receive a minimum 3 weeks of induction therapy and at least 2 weeks of maintenance, but patients with more severe disease or slower responses to therapy may need prolonged therapy. First line therapy of CMV pneumonia is intravenous (IV) ganciclovir (GCV). GCV is nucleoside analogue of 2'-deosygaunosine, that undergoes initial phosphorylation by viral kinases encoded by CMV UL97 open reading frame (ORF).110 The active form of the drug, triphosphorylated GCV, competitively inhibits DNA synthesis catalyzed by CMV DNA polymerase (encoded by the UL 54 ORF).110 The use of GCV is limited by hematologic side effects, primarily by neutropenia, which restrict its use in the pre-engraftment phase of transplantation. IV GCV is recommended therapy for CMV pneumonia, although valganciclovir (the L-valyl ester of GCV) is available for oral dosing, it is not typically recommended for HCT patients with CMV pneumonia. Valgancyclovir can be considered for maintenance therapy in lower risk patients who have demonstrated clinical response to therapy.
An alternate to GCV, foscarnet acts by inhibition of CMV viral polymerase.110 Nephrotoxicity is the major adverse side effect of the drug, and can lead to acute renal failure, as well as mineral and electrolyte abnormalities. Because of these serious side effects, foscarnet is considered the second line therapy but is preferred in subjects with myelosuppression and for patients with known resistance to GCV. Cidofovir acts as a competitive inhibitor of DNA polymerase that has been shown to be effective in CMV ocular disease.110,111 Many consider cidofovir a third line agent, due to its significant renal and hematologic toxicities. Combination therapy is sometimes considered in patients with evidence of drug resistance (reviewed in detail elsewhere112) or in those with refractory disease.
Novel antiviral options have generally been studied in the context of CMV prophylaxis, so data on their efficacy in treatment of disease are inadequate to support the use of any of these agents as primary therapy. Maribavir (MBV), is an orally bioavailable drug that interferes with DNA synthesis of CMV, and is also felt to inhibit viral encapsidation and nuclear egress of viral particles by binding to UL97 viral protein kinases.113 MBV has been used as salvage therapy for patients with proven CMV disease under individual emergency investigational new drug applications and the drug was effective at clearing CMV from the blood, but in this study one patient with CMV pneumonia following HCT died from progressive disease despite viral clearance.114 A phase II treatment trial for refractory infections is currently underway.115 Lipid-complex cidofovir (CMX-001) has shown promise against CMV116, but data on its use in CMV pneumonia are not currently available. Letermovir (AIC246), a novel anti-CMV drug that inhibits CMV replication through a mechanism that involves the viral terminase117, has been reported to lead to successful treatment in a patient with disseminated CMV disease.118 Leflunomide, an agent approved for use in rheumatoid arthritis, has CMV activity119 and has also been attempted with mixed success in refractory cases.120,121 None of these agents are currently FDA approved for treatment of CMV or CMV pneumonia.
Immunoglobulins
The use of both CMV-specific and non specific intravenous immunoglobulin (IVIG), are an additional component to consider during therapy. A number of clinical trials have assessed the role of IVIG in the treatment of CMV pneumonia.122 Although early studies did not show effectiveness of IVIG as a stand-alone therapy, studies in the in late 1980's evaluating combination therapy with antivirals and various IVIG formulations demonstrated improved outcomes in patients with CMV pneumonia when compared with historical controls123,124, or to GCV or IVIG alone.125 The small sample size of these early studies and recent evaluations under current preemptive strategies, however, suggest that combination therapy may provide minimal if any additional benefits to standard antiviral treatment.16,108,126,127 The high mortality seen in these patients, the limited side effects of IVIG treatment, and the lack of a randomized clinical trial comparing combination therapy versus antiviral therapy alone, has led most to err on the side of caution and continue the routine use of combined antiviral and immunoglobulin therapy in the treatment of CMV pneumonia. For those centers that choose to use IVIG, it does appear that more costly CMV-specific IVIG may be no better than standard pooled IVIG preparations.128
Adoptive immunotherapy
Newer laboratory techniques to treat severe viral infections include the development of ex-vivo T-cells that are specific for individual and multiple viruses.70,129–131 These therapies have been used to treat patients with severe infections.132 Limited availability, the need for significant preparatory time and high cost of these procedures, limits their use to only major medical centers. The development of novel “off the shelf” methods for improving access and speed of delivery of these therapies should allow for future clinical trials.
PREVENTION
There are numerous reviews that discuss the advantages and disadvantages of different strategies to prevent CMV disease.9,25 In general prevention falls under two principles, primary prophylaxis and preemptive therapy, and both have strengths and weaknesses.
Antiviral prophylaxis
Antiviral prophylaxis has also been evaluated in a number of important clinical trials. GCV prophylaxis has been shown to decrease the risk of early CMV disease, but patients in these trials also had associated increases in neutropenia and subsequent risk for bacterial and fungal infections.133–136 Prolonged antiviral exposure can also lead to selection of resistant CMV,112 and may increase the risk of late disease events by delaying CMV immune recovery.137 Prophylactic strategies among CMV-seropositive allogeneic transplant recipients lowered the incidence of CMV disease to 6% within the first 100 days after HCT but increased late complications (day +100 to 1 year) from 4% to 15% after the end of prophylaxis.138 Acyclovir/Valacyclovir, which have less systemic toxicity, can be used as an alternate to CMV-specific antiviral agents. Use of high-dose valacyclovir reduces the risk of CMV infection139, and in a randomized clinical trial was as effective as GCV as chemoprophylaxis140; others have shown benefits for disease prevention in high-risk populations when combined with preemptive therapy and other more aggressive prevention strategies.6
Newer antiviral agents described in prior sections, may have more tolerable side effect profiles and have been evaluated as options for chemoprophylaxis. Low dose MBV appeared to be beneficial in a phase II trial141, but was ultimately shown to be ineffective as a prophylactic agent in a large multinational trial in HCT.142 CMX001 and Letermovir, appear promising, as both agents have shown reductions in CMV complications in high-risk seropositive allogeneic HCT patients.143,144 Side effects of Letermovir were minimal, while CMX001 had notable GI side effects at high doses; neither drug had adverse effects on hematologic recovery. Although these data are encouraging, future phase III studies are needed to confirm these findings.
Preemptive therapy strategies
Most centers in the US and worldwide use a preemptive approach145, by which patients are screened weekly for CMV in blood and “preemptively” treated with CMV-specific antiviral therapy to prevent the development of disease. This approach has been shown to be effective at decreasing the risk CMV disease (particularly pneumonia) using either a pp65 or CMV PCR based strategies.5,146 Specific cutoffs for institution of preemptive therapy depend on the type of laboratory screening method (pp65 vs. serum PCR vs. whole blood PVR) used at individual centers. Since not all patients who have CMV detected will develop disease20,147, institutions should develop specific thresholds for starting therapy. Once the threshold of detection has been met patients are typically started on preemptive therapy with GCV or foscarnet until they clear CMV from their blood. For example, at our center patients are monitored week by CMV PCR through day 100, and started on therapy if they develop ≥ 500 copies copies/ml plasma. If patients are at high risk (T-cell depleted or on ≥ 1 mg/kg of steroids) they are started at 100 copies31, all cord blood transplant recipients are tested twice weekly and started at any positive value.6 Upon detection patients are started on induction with GCV or foscarnet for at minimum two weeks, followed with at least one week of maintenance. Patients are continued on induction until they have improvement in the viral copy numbers, and therapy is discontinued only after viral clearance. Continued surveillance is recommended in some patients at high-risk for CMV complications after day 100, including those who develop early CMV reactivation and those on high-dose steroids for GVHD.31
Immunoprophylaxis
Immunoprophylaxis has been evaluated in recent metanalysis, which found no benefit to either polyvalent or CMV-specific IVIG in the prevention of CMV pneumonia.148 Since this strategy is both costly and with uncertain benefit, it is not recommended for CMV prevention in this population.
CMV Vaccines
Future options for prevention may include CMV vaccines which are currently in development. The CMV vaccine TransVax was shown to decrease the rates of significant CMV viremia (≥ 500 copies/ml) and the days free from viremia, but the rates of CMV disease did not differ between the vaccine and placebo groups.149 A phase III trial evaluating this vaccine in allogeneic HCT patients is needed. A glycoprotein B vaccine has also been shown to be effective at decreasing the rate and severity of CMV viremia in other immunocompromised patients150, but no current data exists in HCT recipients. A novel tetanus-CMV fusion peptide vaccine has demonstrated immunogenicity in immunocompotent patients, but has not been tested in immunocompromised populations to date.151
CONCLUSIONS
Improved prevention strategies have decreased the incidence of this dreaded transplant complication, but CMV pneumonia remains a problem in patients undergoing HCT. The frequency of CMV reactivation and respiratory complications following transplantation, the diversity of etiologies of pulmonary disease, and the negative side effects associated with CMV-specific antiviral therapy strengthen the argument for an aggressive diagnostic approach in HCT patients with respiratory symptoms and abnormalities on radiologic evaluation. Diagnosis of CMV pneumonia by standard techniques should be done as soon as possible. Future studies are needed to improve currently available diagnostics, including defining thresholds for CMV PCR from BAL, and to develop non-invasive methods of diagnosing CMV disease. There is also a need to develop improved strategies for prevention which are non-toxic, easily administered and effective. The development of the first new CMV antivirals in over 15 years, suggests new treatment options are on the horizon. These novel antiviral agents and emerging CMV vaccines may also help provide improved methods for preventing this important complication.
Acknowledgements
S.A.P. is supported by NIH grant K23HL096831 and an ASBMT/Viropharma New Investigator Award. The authors would like to thank Kyoko Kurosawa for her assistance with the figures and Dr. Michael Boeckh for his review of the manuscript.
Disclosure of Conflicts of Interest S.A.P. has received research support and has served as a consultant for Chimerix, Inc.
REFERENCES
- 1.Konoplev S, Champlin RE, Giralt S, et al. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone Marrow Transplant. 2001 Apr;27(8):877–881. doi: 10.1038/sj.bmt.1702877. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P. CMV infections after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008 Aug;42(Suppl 1):S70–S72. doi: 10.1038/bmt.2008.120. [DOI] [PubMed] [Google Scholar]
- 3.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003 Sep;9(9):543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010 Nov 25;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of Cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012 Jun 6; doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011 Nov 17;118(20):5689–5696. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ison MG, Fishman JA. Cytomegalovirus pneumonia in transplant recipients. Clin Chest Med. 2005 Dec;26(4):691–705. viii. doi: 10.1016/j.ccm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. American journal of respiratory and critical care medicine. 2004 Jul 1;170(1):22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009 Jun 4;113(23):5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003 Jan 15;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 11.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003 Oct 15;102(8):3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 12.Ozdemir E, Saliba RM, Champlin RE, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007 Jul;40(2):125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 13.Ljungman P. Cytomegalovirus pneumonia: presentation, diagnosis, and treatment. Semin Respir Infect. 1995 Dec;10(4):209–215. [PubMed] [Google Scholar]
- 14.Junghanss C, Storb R, Maris MB, et al. Impact of unrelated donor status on the incidence and outcome of cytomegalovirus infections after non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2003 Nov;123(4):662–670. doi: 10.1046/j.1365-2141.2003.04671.x. [DOI] [PubMed] [Google Scholar]
- 15.van Burik JA, Carter SL, Freifeld AG, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007 Dec;13(12):1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 16.Alexander BT, Hladnik LM, Augustin KM, et al. Use of cytomegalovirus intravenous immune globulin for the adjunctive treatment of cytomegalovirus in hematopoietic stem cell transplant recipients. Pharmacotherapy. 2010 Jun;30(6):554–561. doi: 10.1592/phco.30.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010 Aug 1;12(4):322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 18.Buyck HC, Prentice HG, Griffiths PD, Emery VC. The risk of early and late CMV DNAemia associated with Campath use in stem cell transplant recipients. Bone Marrow Transplant. 2010 Jul;45(7):1212–1219. doi: 10.1038/bmt.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomonari A, Takahashi S, Ooi J, et al. Impact of cytomegalovirus serostatus on outcome of unrelated cord blood transplantation for adults: a single-institute experience in Japan. Eur J Haematol. 2008 Mar;80(3):251–257. doi: 10.1111/j.1600-0609.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 20.Pergam SA, Xie H, Sandhu R, et al. Efficiency and Risk Factors for CMV Transmission in Seronegative Hematopoietic Stem Cell Recipients. Biol Blood Marrow Transplant. 2012 Mar 3; doi: 10.1016/j.bbmt.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001 Oct 1;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 22.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003 Dec 15;102(13):4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 23.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004 Mar 15;103(6):2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 24.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002 Feb 1;185(3):273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 25.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010 Jun;24(2):319–337. doi: 10.1016/j.idc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Trenschel R, Ross S, Husing J, et al. Reduced risk of persisting cytomegalovirus pp65 antigenemia and cytomegalovirus interstitial pneumonia following allogeneic PBSCT. Bone Marrow Transplant. 2000 Mar;25(6):665–672. doi: 10.1038/sj.bmt.1702216. [DOI] [PubMed] [Google Scholar]
- 27.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007 Oct 15;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005 Feb;11(2):149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Baron F, Storer B, Maris MB, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006 Nov;12(11):1176–1187. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Pinana JL, Martino R, Barba P, et al. Cytomegalovirus infection and disease after reduced intensity conditioning allogeneic stem cell transplantation: single-centre experience. Bone Marrow Transplant. 2010 Mar;45(3):534–542. doi: 10.1038/bmt.2009.180. [DOI] [PubMed] [Google Scholar]
- 31.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009 Jun;15(6):694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takenaka K, Gondo H, Tanimoto K, et al. Increased incidence of cytomegalovirus (CMV) infection and CMV-associated disease after allogeneic bone marrow transplantation from unrelated donors. The Fukuoka Bone Marrow Transplantation Group. Bone Marrow Transplant. 1997 Feb;19(3):241–248. doi: 10.1038/sj.bmt.1700637. [DOI] [PubMed] [Google Scholar]
- 33.Dodero A, Carrabba M, Milani R, et al. Reduced-intensity conditioning containing low-dose alemtuzumab before allogeneic peripheral blood stem cell transplantation: graft-versus-host disease is decreased but T-cell reconstitution is delayed. Exp Hematol. 2005 Aug;33(8):920–927. doi: 10.1016/j.exphem.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Holmberg LA, Boeckh M, Hooper H, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999 Dec 15;94(12):4029–4035. [PubMed] [Google Scholar]
- 35.Orlandi EM, Baldanti F, Citro A, Pochintesta L, Gatti M, Lazzarino M. Monitoring for cytomegalovirus and Epstein-Barr virus infection in chronic lymphocytic leukemia patients receiving i.v. fludarabine-cyclophosphamide combination and alemtuzumab as consolidation therapy. Haematologica. 2008 Nov;93(11):1758–1760. doi: 10.3324/haematol.13265. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Hieber M, Schwarck S, Stroux A, et al. Immune reconstitution and cytomegalovirus infection after allogeneic stem cell transplantation: the important impact of in vivo T cell depletion. Int J Hematol. 2010 Jun;91(5):877–885. doi: 10.1007/s12185-010-0597-6. [DOI] [PubMed] [Google Scholar]
- 37.Schaenman JM, Shashidhar S, Rhee C, et al. Early CMV viremia is associated with impaired viral control following nonmyeloablative hematopoietic cell transplantation with a total lymphoid irradiation and antithymocyte globulin preparative regimen. Biol Blood Marrow Transplant. 2011 May;17(5):693–702. doi: 10.1016/j.bbmt.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon HS, Lee JH, Choi ES, et al. Cytomegalovirus infection in children who underwent hematopoietic stem cell transplantation at a single center: a retrospective study of the risk factors. Pediatr Transplant. 2009 Nov;13(7):898–905. doi: 10.1111/j.1399-3046.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 39.Asano-Mori Y, Kanda Y, Oshima K, et al. Clinical features of late cytomegalovirus infection after hematopoietic stem cell transplantation. Int J Hematol. 2008 Apr;87(3):310–318. doi: 10.1007/s12185-008-0051-1. [DOI] [PubMed] [Google Scholar]
- 40.George B, Kerridge IH, Gilroy N, et al. A risk score for early cytomegalovirus reactivation after allogeneic stem cell transplantation identifies low-, intermediate-, and high-risk groups: reactivation risk is increased by graft-versus-host disease only in the intermediate-risk group. Transpl Infect Dis. 2012 Apr;14(2):141–148. doi: 10.1111/j.1399-3062.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 41.Olkinuora H, von Willebrand E, Kantele JM, et al. The impact of early viral infections and graft-versus-host disease on immune reconstitution following paediatric stem cell transplantation. Scand J Immunol. 2011 Jun;73(6):586–593. doi: 10.1111/j.1365-3083.2011.02530.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller W, Flynn P, McCullough J, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986 Apr;67(4):1162–1167. [PubMed] [Google Scholar]
- 43.Osarogiagbon RU, Defor TE, Weisdorf MA, Erice A, Weisdorf DJ. CMV antigenemia following bone marrow transplantation: risk factors and outcomes. Biol Blood Marrow Transplant. 2000;6(3):280–288. doi: 10.1016/s1083-8791(00)70010-3. [DOI] [PubMed] [Google Scholar]
- 44.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006 Jan;91(1):78–83. [PubMed] [Google Scholar]
- 45.Onishi C, Ohashi K, Sawada T, et al. A high risk of life-threatening infectious complications in mycophenolate mofetil treatment for acute or chronic graft-versus-host disease. Int J Hematol. 2010 Apr;91(3):464–470. doi: 10.1007/s12185-010-0516-x. [DOI] [PubMed] [Google Scholar]
- 46.Kasper C, Sayer HG, Mugge LO, et al. Combined standard graft-versus-host disease (GvHD) prophylaxis with mycophenolate mofetil (MMF) in allogeneic peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transplant. 2004 Jan;33(1):65–69. doi: 10.1038/sj.bmt.1704299. [DOI] [PubMed] [Google Scholar]
- 47.Hambach L, Stadler M, Dammann E, Ganser A, Hertenstein B. Increased risk of complicated CMV infection with the use of mycophenolate mofetil in allogeneic stem cell transplantation. Bone Marrow Transplant. 2002 Jun;29(11):903–906. doi: 10.1038/sj.bmt.1703583. [DOI] [PubMed] [Google Scholar]
- 48.Marty FM, Bryar J, Browne SK, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007 Jul 15;110(2):490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruell J, Barnes C, Mutton K, et al. Active CMV disease does not always correlate with viral load detection. Bone Marrow Transplant. 2007 Jul;40(1):55–61. doi: 10.1038/sj.bmt.1705671. [DOI] [PubMed] [Google Scholar]
- 50.Jang JE, Hyun SY, Kim YD, et al. Risk factors for progression from cytomegalovirus viremia to cytomegalovirus disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012 Jun;18(6):881–886. doi: 10.1016/j.bbmt.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 51.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000 Jun 10;355(9220):2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 52.Soderberg-Naucler C. Human cytomegalovirus persists in its host and attacks and avoids elimination by the immune system. Crit Rev Immunol. 2006;26(3):231–264. doi: 10.1615/critrevimmunol.v26.i3.30. [DOI] [PubMed] [Google Scholar]
- 53.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000 Jun 15;95(12):3702–3709. [PubMed] [Google Scholar]
- 54.Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondo K, Kaneshima H, Mocarski ES. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondo K, Mocarski ES. Cytomegalovirus latency and latency-specific transcription in hematopoietic progenitors. Scand J Infect Dis Suppl. 1995;99:63–67. [PubMed] [Google Scholar]
- 57.Kondo K, Xu J, Mocarski ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 59.Koffron AJ, Hummel M, Patterson BK, et al. Cellular localization of latent murine cytomegalovirus. J Virol. 1998 Jan;72(1):95–103. doi: 10.1128/jvi.72.1.95-103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurz SK, Rapp M, Steffens HP, Grzimek NK, Schmalz S, Reddehase MJ. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999 Jan;73(1):482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toorkey CB, Carrigan DR. Immunohistochemical detection of an immediate early antigen of human cytomegalovirus in normal tissues. J Infect Dis. 1989 Nov;160(5):741–751. doi: 10.1093/infdis/160.5.741. [DOI] [PubMed] [Google Scholar]
- 62.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997 Oct 3;91(1):119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 63.de Maar EF, Verschuuren EA, Harmsen MC, The TH, van Son WJ. Pulmonary involvement during cytomegalovirus infection in immunosuppressed patients. Transpl Infect Dis. 2003 Sep;5(3):112–120. doi: 10.1034/j.1399-3062.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 64.Barry SM, Johnson MA, Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant. 2000 Sep;26(6):591–597. doi: 10.1038/sj.bmt.1702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riddell SR. Pathogenesis of cytomegalovirus pneumonia in immunocompromised hosts. Semin Respir Infect. 1995 Dec;10(4):199–208. [PubMed] [Google Scholar]
- 66.Grundy JE, Shanley JD, Griffiths PD. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987 Oct 31;2(8566):996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- 67.Squire SB, Lipman MC, Bagdades EK, et al. Severe cytomegalovirus pneumonitis in HIV infected patients with higher than average CD4 counts. Thorax. 1992 Apr;47(4):301–304. doi: 10.1136/thx.47.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shanley JD, Thrall RS, Forman SJ. Murine cytomegalovirus replication in the lungs of athymic BALB/c nude mice. J Infect Dis. 1997 Feb;175(2):309–315. doi: 10.1093/infdis/175.2.309. [DOI] [PubMed] [Google Scholar]
- 69.Steffens HP, Kurz S, Holtappels R, Reddehase MJ. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol. 1998 Mar;72(3):1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Razonable RR. Immune-based therapies for cytomegalovirus infection. Immunotherapy. 2010 Jan;2(1):117–130. doi: 10.2217/imt.09.82. [DOI] [PubMed] [Google Scholar]
- 71.Pergam S, Boeckh M, Hsu K, et al. Donor KIR3DS1 is Associated with Less CMV Disease Following Unrelated Hematopoietic Cell Transplantation in High Risk Subjects. ICAAC 49th Annual Meeting; San Francisco, CA. Sep, 2009. Abstract 2351. [Google Scholar]
- 72.Zaia JA, Sun JY, Gallez-Hawkins GM, et al. The effect of single and combined activating killer immunoglobulin-like receptor genotypes on cytomegalovirus infection and immunity after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009 Mar;15(3):315–325. doi: 10.1016/j.bbmt.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook M, Briggs D, Craddock C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006 Feb 1;107(3):1230–1232. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- 74.Escudier E, Fleury J, Cordonnier C, Vernant JP, Bernaudin JF. Large granular lymphocytes in bronchoalveolar lavage fluids from immunocompromised patients with cytomegalovirus pneumonitis. Am J Clin Pathol. 1986 Nov;86(5):641–645. doi: 10.1093/ajcp/86.5.641. [DOI] [PubMed] [Google Scholar]
- 75.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989 Jun 29;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 76.Persoons MC, Stals FS, van dam Mieras MC, Bruggeman CA. Multiple organ involvement during experimental cytomegalovirus infection is associated with disseminated vascular pathology. J Pathol. 1998 Jan;184(1):103–109. doi: 10.1002/(SICI)1096-9896(199801)184:1<103::AID-PATH964>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 77.Grefte A, Blom N, van der Giessen M, van Son W, The TH. Ultrastructural analysis of circulating cytomegalic cells in patients with active cytomegalovirus infection: evidence for virus production and endothelial origin. J Infect Dis. 1993 Nov;168(5):1110–1118. doi: 10.1093/infdis/168.5.1110. [DOI] [PubMed] [Google Scholar]
- 78.Grefte A, van der Giessen M, van Son W, The TH. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis. 1993 Feb;167(2):270–277. doi: 10.1093/infdis/167.2.270. [DOI] [PubMed] [Google Scholar]
- 79.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002 Apr 15;34(8):1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 80.Crumpacker CS, Zhang JL. Cytomegalovirus. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 7th Edition Churchill Livingstone; Philadelphia: pp. 1971–1987. [Google Scholar]
- 81.Franquet T, Lee KS, Muller NL. Thin-section CT findings in 32 immunocompromised patients with cytomegalovirus pneumonia who do not have AIDS. AJR Am J Roentgenol. 2003 Oct;181(4):1059–1063. doi: 10.2214/ajr.181.4.1811059. [DOI] [PubMed] [Google Scholar]
- 82.Maschmeyer G. Pneumonia in febrile neutropenic patients: radiologic diagnosis. Curr Opin Oncol. 2001 Jul;13(4):229–235. doi: 10.1097/00001622-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Gasparetto EL, Ono SE, Escuissato D, et al. Cytomegalovirus pneumonia after bone marrow transplantation: high resolution CT findings. Br J Radiol. 2004 Sep;77(921):724–727. doi: 10.1259/bjr/70800575. [DOI] [PubMed] [Google Scholar]
- 84.Kang EY, Patz EF, Jr., Muller NL. Cytomegalovirus pneumonia in transplant patients: CT findings. J Comput Assist Tomogr. 1996 Mar-Apr;20(2):295–299. doi: 10.1097/00004728-199603000-00024. [DOI] [PubMed] [Google Scholar]
- 85.Shimada A, Koga T, Shimada M, et al. Cytomegalovirus pneumonitis presenting small nodular opacities. Intern Med. 2004 Dec;43(12):1198–1200. doi: 10.2169/internalmedicine.43.1198. [DOI] [PubMed] [Google Scholar]
- 86.Tamm M, Traenkle P, Grilli B, et al. Pulmonary cytomegalovirus infection in immunocompromised patients. Chest. 2001 Mar;119(3):838–843. doi: 10.1378/chest.119.3.838. [DOI] [PubMed] [Google Scholar]
- 87.Crawford SW, Bowden RA, Hackman RC, Gleaves CA, Meyers JD, Clark JG. Rapid detection of cytomegalovirus pulmonary infection by bronchoalveolar lavage and centrifugation culture. Ann Intern Med. 1988 Feb;108(2):180–185. doi: 10.7326/0003-4819-108-2-180. [DOI] [PubMed] [Google Scholar]
- 88.Gleaves CA, Meyers JD. Rapid detection of cytomegalovirus in bronchoalveolar lavage specimens from marrow transplant patients: evaluation of a direct fluorescein-conjugated monoclonal antibody reagent. J Virol Methods. 1989 Dec;26(3):345–349. doi: 10.1016/0166-0934(89)90117-1. [DOI] [PubMed] [Google Scholar]
- 89.Eriksson BM, Brytting M, Zweygberg-Wirgart B, Hillerdal G, Olding-Stenkvist E, Linde A. Diagnosis of cytomegalovirus in bronchoalveolar lavage by polymerase chain reaction, in comparison with virus isolation and detection of viral antigen. Scand J Infect Dis. 1993;25(4):421–427. doi: 10.3109/00365549309008522. [DOI] [PubMed] [Google Scholar]
- 90.Cathomas G, Morris P, Pekle K, Cunningham I, Emanuel D. Rapid diagnosis of cytomegalovirus pneumonia in marrow transplant recipients by bronchoalveolar lavage using the polymerase chain reaction, virus culture, and the direct immunostaining of alveolar cells. Blood. 1993 Apr 1;81(7):1909–1914. [PubMed] [Google Scholar]
- 91.Ruutu P, Ruutu T, Volin L, Tukiainen P, Ukkonen P, Hovi T. Cytomegalovirus is frequently isolated in bronchoalveolar lavage fluid of bone marrow transplant recipients without pneumonia. Ann Intern Med. 1990 Jun 15;112(12):913–916. doi: 10.7326/0003-4819-112-12-913. [DOI] [PubMed] [Google Scholar]
- 92.Tang YW, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. Journal of clinical microbiology. 2003 Jun;41(6):2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crawford SW, Hackman RC, Clark JG. Open lung biopsy diagnosis of diffuse pulmonary infiltrates after marrow transplantation. Chest. 1988 Nov;94(5):949–953. doi: 10.1378/chest.94.5.949. [DOI] [PubMed] [Google Scholar]
- 94.Myerson D, Hackman RC, Nelson JA, Ward DC, McDougall JK. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984 May;15(5):430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- 95.Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis. 2011 Aug;24(4):333–343. doi: 10.1097/QCO.0b013e3283480440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gasparetto EL, Escuissato DL, Inoue C, Marchiori E, Muller NL. Herpes simplex virus type 2 pneumonia after bone marrow transplantation: high-resolution CT findings in 3 patients. J Thorac Imaging. 2005 May;20(2):71–73. doi: 10.1097/01.rti.0000154072.39497.61. [DOI] [PubMed] [Google Scholar]
- 97.Han CS, Miller W, Haake R, Weisdorf D. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994 Mar;13(3):277–283. [PubMed] [Google Scholar]
- 98.Mariotte E, Schnell D, Scieux C, et al. Significance of herpesvirus 6 in BAL fluid of hematology patients with acute respiratory failure. Infection. 2011 Jun;39(3):225–230. doi: 10.1007/s15010-011-0114-8. [DOI] [PubMed] [Google Scholar]
- 99.Dulery R, Salleron J, Dewilde A, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol Blood Marrow Transplant. 2012 Jul;18(7):1080–1089. doi: 10.1016/j.bbmt.2011.12.579. [DOI] [PubMed] [Google Scholar]
- 100.Cone RW. Human herpesvirus 6 as a possible cause of pneumonia. Semin Respir Infect. 1995 Dec;10(4):254–258. [PubMed] [Google Scholar]
- 101.Cone RW, Hackman RC, Huang ML, et al. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N Engl J Med. 1993 Jul 15;329(3):156–161. doi: 10.1056/NEJM199307153290302. [DOI] [PubMed] [Google Scholar]
- 102.Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006 Aug 1;43(3):331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 103.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. American journal of respiratory and critical care medicine. 2011 May 1;183(9):1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jinta M, Ohashi K, Ohta T, et al. Clinical features of allogeneic hematopoietic stem cell transplantation-associated organizing pneumonia. Bone Marrow Transplant. 2007 Sep;40(5):465–472. doi: 10.1038/sj.bmt.1705768. [DOI] [PubMed] [Google Scholar]
- 105.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007 Jul;13(7):749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 106.Barber NA, Ganti AK. Pulmonary toxicities from targeted therapies: a review. Target Oncol. 2011 Dec;6(4):235–243. doi: 10.1007/s11523-011-0199-0. [DOI] [PubMed] [Google Scholar]
- 107.Patel AV, Hahn T, Bogner PN, et al. Fatal diffuse alveolar hemorrhage associated with sirolimus after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010 Aug;45(8):1363–1364. doi: 10.1038/bmt.2009.339. [DOI] [PubMed] [Google Scholar]
- 108.Machado CM, Dulley FL, Boas LS, et al. CMV pneumonia in allogeneic BMT recipients undergoing early treatment of pre-emptive ganciclovir therapy. Bone Marrow Transplant. 2000 Aug;26(4):413–417. doi: 10.1038/sj.bmt.1702526. [DOI] [PubMed] [Google Scholar]
- 109.Shepp DH, Dandliker PS, de Miranda P, et al. Activity of 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine in the treatment of cytomegalovirus pneumonia. Ann Intern Med. 1985 Sep;103(3):368–373. doi: 10.7326/0003-4819-103-3-368. [DOI] [PubMed] [Google Scholar]
- 110.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006 Sep;71(2–3):154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 111.Ljungman P, Deliliers GL, Platzbecker U, et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2001 Jan 15;97(2):388–392. doi: 10.1182/blood.v97.2.388. [DOI] [PubMed] [Google Scholar]
- 112.Hakki M, Chou S. The biology of cytomegalovirus drug resistance. Curr Opin Infect Dis. 2011 Dec;24(6):605–611. doi: 10.1097/QCO.0b013e32834cfb58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Biron KK, Harvey RJ, Chamberlain SC, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002 Aug;46(8):2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Avery RK, Marty FM, Strasfeld L, et al. Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl Infect Dis. 2010 Dec;12(6):489–496. doi: 10.1111/j.1399-3062.2010.00550.x. [DOI] [PubMed] [Google Scholar]
- 115.Maribavir for Treatment of Resistant or Refractory CMV Infections in Transplant Recipients. ClinicalTrials.gov Identifier NCT01611974. doi: 10.1111/j.1399-3062.2010.00550.x. Accessed from ClinicalTrials.gov on June 2012. [DOI] [PubMed]
- 116.Price NB, Prichard MN. Progress in the development of new therapies for herpesvirus infections. Curr Opin Virol. 2011 Dec;1(6):548–554. doi: 10.1016/j.coviro.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol. 2011 Oct;85(20):10884–10893. doi: 10.1128/JVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaul DR, Stoelben S, Cober E, et al. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant. 2011 May;11(5):1079–1084. doi: 10.1111/j.1600-6143.2011.03530.x. [DOI] [PubMed] [Google Scholar]
- 119.Waldman WJ, Knight DA, Lurain NS, et al. Novel mechanism of inhibition of cytomegalovirus by the experimental immunosuppressive agent leflunomide. Transplantation. 1999 Sep 27;68(6):814–825. doi: 10.1097/00007890-199909270-00014. [DOI] [PubMed] [Google Scholar]
- 120.Avery RK, Bolwell BJ, Yen-Lieberman B, et al. Use of leflunomide in an allogeneic bone marrow transplant recipient with refractory cytomegalovirus infection. Bone Marrow Transplant. 2004 Dec;34(12):1071–1075. doi: 10.1038/sj.bmt.1704694. [DOI] [PubMed] [Google Scholar]
- 121.Battiwalla M, Paplham P, Almyroudis NG, et al. Leflunomide failure to control recurrent cytomegalovirus infection in the setting of renal failure after allogeneic stem cell transplantation. Transpl Infect Dis. 2007 Mar;9(1):28–32. doi: 10.1111/j.1399-3062.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 122.Snydman DR. Cytomegalovirus immunoglobulins in the prevention and treatment of cytomegalovirus disease. Rev Infect Dis. 1990 Sep-Oct;12(Suppl 7):S839–848. doi: 10.1093/clinids/12.supplement_7.s839. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt GM, Kovacs A, Zaia JA, et al. Ganciclovir/immunoglobulin combination therapy for the treatment of human cytomegalovirus-associated interstitial pneumonia in bone marrow allograft recipients. Transplantation. 1988 Dec;46(6):905–907. [PubMed] [Google Scholar]
- 124.Reed EC, Bowden RA, Dandliker PS, Lilleby KE, Meyers JD. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann Intern Med. 1988 Nov 15;109(10):783–788. doi: 10.7326/0003-4819-109-10-783. [DOI] [PubMed] [Google Scholar]
- 125.Emanuel D, Cunningham I, Jules-Elysee K, et al. Cytomegalovirus pneumonia after bone marrow transplantation successfully treated with the combination of ganciclovir and high-dose intravenous immune globulin. Ann Intern Med. 1988 Nov 15;109(10):777–782. doi: 10.7326/0003-4819-109-10-777. [DOI] [PubMed] [Google Scholar]
- 126.Erard V, Guthrie K, Smith J, Chien J, et al. Cytomegalovirus pneumonia after hematopoietic cell transplantation: outcomes and factors associated with mortality. Paper presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. Sep, 2007. Abstract V-1379. [Google Scholar]
- 127.Ljungman P, Engelhard D, Link H, et al. Treatment of interstitial pneumonitis due to cytomegalovirus with ganciclovir and intravenous immune globulin: experience of European Bone Marrow Transplant Group. Clin Infect Dis. 1992 Apr;14(4):831–835. doi: 10.1093/clinids/14.4.831. [DOI] [PubMed] [Google Scholar]
- 128.Ljungman P, Cordonnier C, Einsele H, et al. Use of intravenous immune globulin in addition to antiviral therapy in the treatment of CMV gastrointestinal disease in allogeneic bone marrow transplant patients: a report from the European Group for Blood and Marrow Transplantation (EBMT). Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 1998 Mar;21(5):473–476. doi: 10.1038/sj.bmt.1701113. [DOI] [PubMed] [Google Scholar]
- 129.Gerdemann U, Vera JF, Rooney CM, Leen AM. Generation of multivirus-specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant. J Vis Exp. 2011;(51) doi: 10.3791/2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009 Aug 27;114(9):1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bao L, Dunham K, Stamer M, Mulieri KM, Lucas KG. Expansion of cytomegalovirus pp65 and IE-1 specific cytotoxic T lymphocytes for cytomegalovirus-specific immunotherapy following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008 Oct;14(10):1156–1162. doi: 10.1016/j.bbmt.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O'Reilly RJ, Doubrovina E, Trivedi D, Hasan A, Kollen W, Koehne G. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants. Immunol Res. 2007;38(1–3):237–250. doi: 10.1007/s12026-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 133.Atkinson K, Downs K, Golenia M, et al. Prophylactic use of ganciclovir in allogeneic bone marrow transplantation: absence of clinical cytomegalovirus infection. Br J Haematol. 1991 Sep;79(1):57–62. doi: 10.1111/j.1365-2141.1991.tb08007.x. [DOI] [PubMed] [Google Scholar]
- 134.Winston DJ, Ho WG, Bartoni K, et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993 Feb 1;118(3):179–184. doi: 10.7326/0003-4819-118-3-199302010-00004. [DOI] [PubMed] [Google Scholar]
- 135.Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood. 1997 Sep 15;90(6):2502–2508. [PubMed] [Google Scholar]
- 136.Burns LJ, Miller W, Kandaswamy C, et al. Randomized clinical trial of ganciclovir vs acyclovir for prevention of cytomegalovirus antigenemia after allogeneic transplantation. Bone Marrow Transplant. 2002 Dec;30(12):945–951. doi: 10.1038/sj.bmt.1703770. [DOI] [PubMed] [Google Scholar]
- 137.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994 Apr 1;83(7):1971–1979. [PubMed] [Google Scholar]
- 138.Boeckh M. Current antiviral strategies for controlling cytomegalovirus in hematopoietic stem cell transplant recipients: prevention and therapy. Transpl Infect Dis. 1999 Sep;1(3):165–178. doi: 10.1034/j.1399-3062.1999.010305.x. [DOI] [PubMed] [Google Scholar]
- 139.Ljungman P, de La Camara R, Milpied N, et al. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002 Apr 15;99(8):3050–3056. doi: 10.1182/blood.v99.8.3050. [DOI] [PubMed] [Google Scholar]
- 140.Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC. Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis. 2003 Mar 15;36(6):749–758. doi: 10.1086/367836. [DOI] [PubMed] [Google Scholar]
- 141.Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008 Jun 1;111(11):5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011 Apr;11(4):284–292. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 143.Marty FM, Winston D, Rowley SD, Boeckh M, et al. CMX001 for Prevention and Control of CMV Infection in CMV-Seropositive Allogeneic Stem-Cell Transplant Recipients: A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Trial of Safety, Tolerability and Antiviral Activity. ASBMT Tandem Meeting; San Diego, CA. Feb, 2012. Abstract S203. [Google Scholar]
- 144.Zimmermann H, the AIC246 Study Team Letermovir (AIC246) for prevention of HCMV infections in patients after human blood precursor cell transplantation: a randomised, double-blind, placebocontrolled trial to evaluate the safety, tolerability and antiviral activity of 12 weeks treatment. 23rd European Congress of Clinical Microbiology and Infectious Diseases; London, England. Apr, 2012. Abstract LB2815. [Google Scholar]
- 145.Pollack M, Heugel J, Xie H, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011 May;17(5):664–673. doi: 10.1016/j.bbmt.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996 Nov 15;88(10):4063–4071. [PubMed] [Google Scholar]
- 147.de Gast GC, Boland GJ, Vlieger AM, et al. Abortive human cytomegalovirus infection in patients after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992 Apr;9(4):221–225. [PubMed] [Google Scholar]
- 148.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2009 Feb 10;27(5):770–781. doi: 10.1200/JCO.2008.16.8450. [DOI] [PubMed] [Google Scholar]
- 149.Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012 Apr;12(4):290–299. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
- 150.Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011 Apr 9;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.La Rosa C, Longmate J, Lacey SF, et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis. 2012 Apr 15;205(8):1294–1304. doi: 10.1093/infdis/jis107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bacigalupo A, Tedone E, Sanna MA, et al. CMV infections following allogeneic BMT: risk factors, early treatment and correlation with transplant related mortality. Haematologica. 1992 Nov-Dec;77(6):507–513. [PubMed] [Google Scholar]
- 153.Matthes-Martin S, Lion T, Aberle SW, et al. Pre-emptive treatment of CMV DNAemia in paediatric stem cell transplantation: the impact of recipient and donor CMV serostatus on the incidence of CMV disease and CMV-related mortality. Bone Marrow Transplant. 2003 May;31(9):803–808. doi: 10.1038/sj.bmt.1703927. [DOI] [PubMed] [Google Scholar]
- 154.Ljungman P, Aschan J, Lewensohn-Fuchs I, et al. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation. 1998 Nov 27;66(10):1330–1334. doi: 10.1097/00007890-199811270-00012. [DOI] [PubMed] [Google Scholar]
- 155.Jaskula E, Dlubek D, Sedzimirska M, Duda D, Tarnowska A, Lange A. Reactivations of cytomegalovirus, human herpes virus 6, and Epstein-Barr virus differ with respect to risk factors and clinical outcome after hematopoietic stem cell transplantation. Transplant Proc. 2010 Oct;42(8):3273–3276. doi: 10.1016/j.transproceed.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 156.Bachanova V, Brunstein CG, Burns LJ, et al. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone Marrow Transplant. 2009 Feb;43(3):237–244. doi: 10.1038/bmt.2008.313. [DOI] [PubMed] [Google Scholar]
- 157.Schetelig J, Oswald O, Steuer N, et al. Cytomegalovirus infections in allogeneic stem cell recipients after reduced-intensity or myeloablative conditioning assessed by quantitative PCR and pp65-antigenemia. Bone Marrow Transplant. 2003 Oct;32(7):695–701. doi: 10.1038/sj.bmt.1704164. [DOI] [PubMed] [Google Scholar]
- 158.Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001 Feb 15;97(4):867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- 159.Lonnqvist B, Ringden O, Wahren B, Gahrton G, Lundgren G. Cytomegalovirus infection associated with and preceding chronic graft-versus-host disease. Transplantation. 1984 Nov;38(5):465–468. doi: 10.1097/00007890-198411000-00004. [DOI] [PubMed] [Google Scholar]
- 160.Remberger M, Aschan J, Barkholt L, Tollemar J, Ringden O. Treatment of severe acute graft-versus-host disease with anti-thymocyte globulin. Clin Transplant. 2001 Jun;15(3):147–153. doi: 10.1034/j.1399-0012.2001.150301.x. [DOI] [PubMed] [Google Scholar]