Abstract
Prior work suggests hippocampus-dependent memory undergoes a systems consolidation process such that recent memories are stored in the hippocampus, while older memories are independent of the hippocampus and instead dependent on cortical areas. One problem with interpreting these studies is that memory for the contextual stimuli weakens as time passes between the training event and testing and older memories are often less detailed, making it difficult to determine if memory storage in the hippocampus is related to the age or to the accuracy of the memory. Activity of the mammalian target of rapamycin (mTOR) signaling pathway is known to be important for controlling protein translation necessary for both memory consolidation after initial learning and for the reconsolidation of memory after retrieval. We tested whether p70s6 kinase (p70s6K), a key component of the mTOR signaling pathway, is activated following retrieval of context fear memory in the dorsal hippocampus (DH) and anterior cingulate cortex (ACC) at 1, 10 or 36 days after context fear conditioning. We also tested whether strengthening memory for the contextual stimuli changed p70s6K phosphorylation in these structures 36 days after training. We show that under standard training conditions retrieval of a recently formed memory is initially precise and involves the DH. Over time it loses detail, becomes independent of the DH, and depends on the ACC. In a subsequent experiment we preserved the accuracy of older memories through pre-exposure to the training context. We show that remote memory still involved the DH in animals given pre-exposure. These data support the notion that detailed memories depend on the DH regardless of their age.
Keywords: fear conditioning, p70s6K, rat, remote fear memory, context fear conditioning
Introduction
Damage to the DH disrupts formation or recall of new memories while older memories often remain intact (Kim and Fanselow, 1992; Scoville and Milner, 1957). Several lines of evidence support the idea that after a period of time during which memory relies upon the DH it is transferred to cortical areas, such as the ACC, for long term storage (Frankland and Bontempi, 2005). Other explanations suggest that over time memory loses detail in the process of becoming cortically dependent (Winocur et al., 2007) or requires more effortful recall, resulting in decreased contribution of the DH and greater cortical involvement (Rudy et al., 2005). According to the latter explanation, strengthening contextual memories should preserve the involvement of the DH as the memory ages and cortical areas should not need to become engaged.
Testing whether remote contextual fear memory requires the DH poses a challenge because contextual fear memory becomes more generalized over time. Studies have shown that as the delay between training and testing increases, animals lose the ability to discriminate between the context in which they were trained and a novel one, reflecting a loss of detailed information about the context (Biedenkapp and Rudy, 2007; Riccio et al., 1992; Rudy et al., 2005). However, discrimination at remote time points can be preserved if the animals are exposed to the training context prior to pairing it with shock (Biedenkapp and Rudy, 2007) or are given a brief exposure to the training context prior to test (Zhou and Riccio, 1994). Use of one of these strategies to promote memory for the stimulus attributes of a context would allow one to test whether remote memory relies upon DH or cortical areas in animals with differing abilities to discriminate between contexts.
The present work tested whether activation of p70s6K in the DH and ACC differed after retrieval of a remote contextual fear memory strengthened through pre-exposure to the training context compared to a contextual fear memory without pre-exposure. We chose to look at activation of p70s6K because, as a component of the mTOR pathway, it plays a central role in regulating translation required for synaptic plasticity (Tang et al., 2002), is required for consolidation (Bekinschtein et al., 2007; Belelovsky et al., 2009; Parsons et al., 2006) and reconsolidation of memory (Blum et al., 2006; Gafford et al., 2011; Jobim et al., 2012; Myskiw et al., 2008; Parsons et al., 2006) and most germane to this study has been shown to be activated in the hippocampus following the retrieval of recently formed contextual fear memory (Gafford et al., 2011).
Our work shows that contextual fear memory degrades over time as indicated by the rats inability to distinguish between the training and novel contexts when the animals are tested long after training. Western blot analysis shows that animals tested earlier after contextual fear conditioning showed increased phosphorylation of p70s6K in the DH, but not in the ACC. In contrast, animals tested 36 days after training showed enhanced p70s6K phosphorylation in the ACC, but not in the DH. A separate experiment showed that when memory for the training context was strengthened prior to training there was an increase in p70s6K phosphorylation in the DH after retrieval 36 days later. Our results support the idea that the pattern of activation during recall of a remote memory is related to the accuracy of the memory. Specifically, detailed memories continue to activate p70s6K in the DH even as the memory ages, while less detailed memories activate p70s6K in the ACC.
Materials and Methods
Male Long Evans rats were obtained from Harlan in Madison, WI weighing approximately 300–350 grams. Rats were housed individually in a room maintained on a 14:10 hour light/dark cycle. Experiments took place during the light portion of the cycle. Food and water were available ad libitum. All procedures were carried out with approval of the Institutional Animal Care and Use Committee. Prior to all experiments animals were habituated to the handling and transport procedure for 2 minutes each day over 4 days.
Fear conditioning was conducted in Plexiglas and stainless steel observation chambers (Context A) inside sound attenuated chambers with fans giving 62–64 dB of background noise. Context A was scented with a 5% ammonium hydroxide solution, had shock grid flooring and incandescent lighting. The novel “Context B” was scented with 2% acetic acid solution and had infrared lighting and Plexiglas flooring.
Experiment 1 - Subjects and Procedure
Animals (N=50) were trained and memory was tested 1 (N =17), 10 (N = 11) or 36 (N = 15) days later. Training occurred in Context A (4 minute baseline; three 1.3 mA shocks; 20 sec inter trial interval; 3-minute post-shock period). On their respective testing day animals were placed into their assigned context and given a five minute exposure to Context A: day 1 (A1; N = 10); day 10 (A10; N = 6); day 36 (A36; N=9) or Context B: day 1 (B1; N= 7), day 10 (B10; N = 5); day 36 (B36; N= 6). Freezing behavior (the lack of movement except for respiration) was scored as our measure of fear. A subset of animals (HC; N = 7) remained in their home cage to assess baseline expression of the target proteins. Sixty minutes after testing animals were deeply anesthetized and their brains were quickly removed and kept at −80° C until a 1mm thick section of tissue from the ACC (Target: AP: −2.0–3.0; ML:+/− 2.4 mm;V: −1.6) and DH (Target: AP −3.0 – 4.0; ML +/− 2.6, V −3.0) could be dissected.
Experiment 2- Subjects and Procedure
Rats (N=35) were either pre-exposed (N=14) to the training context or handled (N= 15) for 5 minutes per day over 3 days. Animals were then trained as in Experiment 1 and tested 36 days later for 5 minutes in either Context A: Not pre-exposed A, (N= 7); Pre-exposed A (N = 7) or Context B: Not pre-exposed B (N = 8); Pre-exposed B (N = 7), HC controls (N=6). Sixty minutes after testing brains were removed and processed for western blot analysis along with HC controls.
Western Blot Procedure
Samples were processed for western blot as in (Parsons et al., 2006). Membranes were incubated overnight at 4°C in primary antibody for phosphorylated p70s6K (Thr 412; 1:1000 Millipore, catalog # 07-018) and β actin (1:1000, Cell signaling #4970). β actin was used as a general loading control. Using a similar protocol we have shown there is no change in total p70s6K (Gafford et al., 2011; Parsons et al., 2006). Membranes were incubated in secondary antibody (Millipore, 1:3000 – 1:5000) for 90 minutes, exposed to chemiluminescence solution (Santa Cruz Biotechnology), and exposed to autoradiographic film.
Data analysis
Freezing behavior during training and testing was scored using the automated Freezescan scoring system (Clever Systems Inc, Reston, VA). Film was scanned and optical density was measured using Image J (NIH). Optical density was normalized to HC controls. The resulting values from behavioral testing and western blotting were compared using One-way analysis of variance (ANOVA) and a priori planned comparison tests when appropriate.
Results
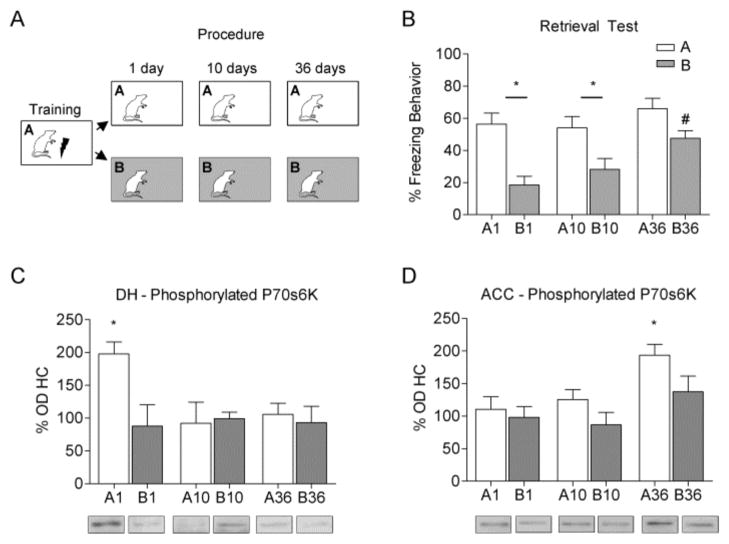
Rats were trained and memory was tested 1, 10 or 36 days later (Procedure: Figure 1A). On their respective testing day animals were either placed in Context A or B for 5 minutes. Figure 1B depicts mean percent freezing behavior during the test session for all groups. An ANOVA showed a significant main effect for Group (F (5, 37) =9.726, p < 0.001). Planned comparisons showed that rats tested 1 day after training in context A froze significantly more than those tested in B (p < 0.001) as did animals tested in A ten days later (p < 0.05). In contrast, animals tested in context A or B 36 days later both showed high levels of freezing, indicating a disruption in contextual discrimination. Although no difference in freezing was found across days in rats tested in Context A (p > 0.05), freezing to Context B was significantly higher in rats tested at 36 days compared to those tested in Context B one day (p < 0.001) or 10 (p < 0.05) days after training. This indicates that over time animals inability to discriminate between the trained and non-trained contexts is driven by increased freezing to the novel Context B.
Figure 1.
(A) Separate groups of rats were trained and tested in either context A or B 1, 10 or 36 days after training. (B) Bars represent average percent time freezing (+/−SEM) in the trained (Context A, white) or untrained context (Context B, grey) during the five minute retrieval test. Rats tested 1 or 10 days after training showed significant discrimination between contexts (* p < 0.05), while those tested 36 days later were unable to discriminate. Animals exposed to Context B on day 36 showed more freezing than those animals exposed to Context B on Day 1 or 10 (# p < 0.05) indicating increased generalization driven by freezing to the untrained context. Western blot analysis shows (C) increased expression of p70s6K in the DH after exposure to Context A one day after training compared to HC, A10, A36 or B1 (* p < 0.05). In contrast, (D) the ACC was activated after exposure to Context A 36 days after training compared to HC, A1, A10 and B36. Representative western blot images are shown below each graph. Bars represent average protein expression (+/−SEM) normalized to home cage controls.
Western blot analysis conducted on DH tissue 60 minutes after testing showed that context exposure significantly increased phosphorylation of p70s6K in the DH (F (6, 43) = 6.911, p < 0.001, Figure 1C). Planned comparisons revealed a significant increase in phosphorylated p70s6K for animals in group A1 compared to HC, B1, A10 and A36 groups (p’s < 0.01).
An identical analysis run on tissue from the ACC (Figure 1D) found a significant main effect of phosphorylated p70s6K (F (6, 43) = 3.755, p < 0.01). Planned comparisons revealed a significant increase in phosphorylated p70s6K in group A36 compared to HC, B36, A1 and A10 groups (p’s < 0.01). Collectively, the changes in p70s6K phosphorylation we observed following retrieval of a recent or remote memory are consistent with the idea that recent contextual fear memory depends on the DH, but at more remote time points the memory relies upon cortical areas.
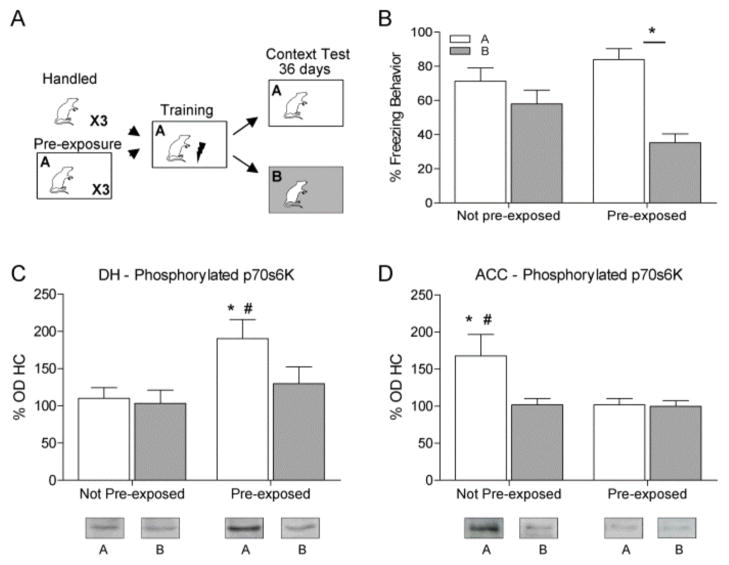
Experiment 2 compared rats that were pre-exposed to the training context to animals that did not receive pre-exposure. Rats were trained and then tested 36 days later in Context A or B (Figure 2A). An ANOVA comparing freezing behavior of pre-exposed animals to those not pre-exposed showed a significant main effect (F (3, 25) = 8.461, p < 0.001, Figure 2B). Planned comparisons showed that rats that were pre-exposed froze significantly more to Context A than B (t, 25 = 4.806, p < 0.001). Planned comparisons also revealed a significant difference in freezing behavior in Context B between animals that received pre-exposure and those that did not (p < 0.05), however freezing to context A was not different between groups (p > 0.05). These data show that pre-exposure to the training context facilitates context discrimination at remote time points due mainly to a difference in freezing to Context B.
Figure 2.
(A) Rats were handled or pre-exposed to the training chamber for 3 days, trained the next day, and tested 36 days later in either context A (white) or B (gray). (B) Only rats given pre-exposure significantly discriminated between contexts (* p < 0.05). (C) Western blot analysis showed a significant p70s6K increase in the DH when animals were pre-exposed and placed in Context A for testing compared to HC, pre-exposed B, or the not pre-exposed Context A group. (D) In contrast there was a significant increase in phosphorylated p70s6K in the ACC in rats not pre-exposed and tested in A compared to HC, animals not pre-exposed and tested in B or pre-exposed and tested in Context A.
A One-way ANOVA on data from western blot analysis of the DH found a significant difference between groups in phosphorylated p70s6K expression (F (4, 30 = 3.406, p< 0.05). Planned comparisons showed that animals pre-exposed and tested in Context A had significantly increased phosphorylated p70s6K in the DH compared to HC (p < 0.01), pre-exposed Context B, (p < 0.05) and not pre-exposed Context A tested rats (p < 0.01) (Figure 2C).
A one-way ANOVA on western blot analysis of the ACC showed a significant difference in phosphorylated p70s6K (F (4, 30) = 2.822, p < 0.05, Figure 2D). Planned comparisons show that the animals not pre-exposed and tested in Context A had increased phosphorylated p70s6K in the ACC compared to HC (p < 0.05), animals not pre-exposed and tested in B (p < 0.05) and animals pre-exposed and tested in A (p < 0.05). This result demonstrates that strengthening of a memory through pre-exposure preserves the involvement of activated p70s6K in the DH and does not engage p70s6K in the ACC during remote memory retrieval.
Discussion
From these data we conclude that remote fear memories involve the DH when detailed information about the context is retained. We showed that over time under standard training and testing conditions animals show poorer discrimination between a novel context and one in which they were shocked as indicated by strong freezing behavior to both Context A and B 36 days after training. As the memory aged the DH showed little p70s6K activity following retrieval, whereas phosphorylation of this kinase was increased over time in the ACC when the rat was unable to discriminate between the training and testing contexts. Rats given the retrieval session 10 days after training were still able to discriminate between contexts although neither the DH nor ACC show significant p70s6K activation. It is possible that a different mechanism of action or a different brain structure is recruited for memory retrieval at this time. Similarly, it should be noted that while animals were unable to discriminate between contexts on day 36, rats exposed to context A showed significantly more p70s6K expression in the ACC compared to those exposed to B. While this shows there is a disconnect between the behavior and protein expression, it should be noted that p70s6K phosphorylation is somewhat elevated in the B36 group compared to B10 and B1. Perhaps with greater statistical power, this would reach significance. Data showing redistribution of activation from DH to ACC have previously been taken to support systems consolidation theory whereby memories initially dependent upon the DH reorganize into cortical circuits and no longer involve the DH (Frankland et al., 2004; Kim and Fanselow, 1992). An alternative explanation for these findings is that increased activation of cortical areas indicates that aged memories require more effort for retrieval (Rudy et al., 2005) or perhaps there is a transformation in the nature of the memory from detailed and hippocampally dependent to imprecise and cortically dependent (Winocur et al., 2007).
To distinguish between these possibilities we improved the accuracy of the memory by using pre-exposure to the training chamber prior to fear learning. Our behavioral findings are consistent with prior studies showing that pre-exposure to the training context rescues the deficit in contextual discrimination at remote time points (Biedenkapp and Rudy, 2007; Riccio et al., 1992). Further, we show that when remote memory is facilitated the pattern of protein expression is altered. Specifically, rats given pre-exposure and tested in Context A show increased expression of phosphorylated p70s6K in the DH compared to controls while they show no concomitant increase in p70s6K in the ACC. Pre-exposure of the animals to the training context prior to training prevented the increase in p70s6K phosphorylation in the ACC seen after remote memory testing. In short, the pattern of protein expression after pre-exposure in the DH and ACC following remote retrieval mimicked the pattern seen after recent memory. These data suggest that the critical variable predicting cortical activity during retrieval of remote memory is not the age of the memory, but rather how detailed the memory is at retrieval. Thus, the results of this experiment are consistent with one of the main predictions of the transformation view (Winocur et al., 2007) which is that when detailed information about the context is retained the DH is involved regardless of the age of the memory.
Our results are generally consistent with a recent study by (Wiltgen et al., 2010) showing that in mice capable of discriminating between contexts at a remote time point, DH inactivation blocked expression of the memory for the training context. However, mice that were unable to discriminate were not affected by inactivation of the DH. These data indicate that in mice which retain detailed information about the context at remote time points, the memory still involves the DH.
Another recent study (Wang et al., 2009) used a contextual discrimination procedure to test if precise context fear memories still depend on the hippocampus at later time points. This study was designed to be a test of the systems consolidation versus transformation views of memory because if mice with hippocampal lesions are capable of discriminating at remote time points it would suggest that remote memories involve extra-hippocampal areas irrespective of their level of detail, supporting systems consolidation theory. Alternatively, if the ability to discriminate was disrupted it would support the idea that precise memories rely on the hippocampus regardless of age and thus support the transformation view. The results showed that context discrimination at remote time points was not affected by hippocampal lesions given prior to test. This argues that precise memories can be expressed in the absence of the hippocampus, a finding consistent with the systems consolidation view. However, other data from the same study found mice with hippocampal lesions showed disrupted freezing to the training context when tested at the remote time point, which is at odds with the systems consolidation view. Nonetheless, the discrepant pattern of results may be related to procedural differences between our study and Wang et al., 2009. In particular the behavioral manipulation used to preserve the precision of memory was quite different between the two studies.
This paper has focused on p70s6K, a target of the mTOR kinase, due to its role in translation initiation underlying synaptic plasticity within the hippocampus (Tang et al., 2002). Other studies assessing recent and remote memory have often measured expression of an immediate early gene to assess engagement of a particular structure during remote memory. This is problematic due to controversy surrounding whether some immediate early genes are engaged during learning and memory per se or simply in response to exposure to novelty or more general cellular activity (Hall et al., 2000). Work from our lab has shown disrupting mTOR signaling in the hippocampus around the time of retrieval results in disruption of a recent contextual fear memory (Gafford et al., 2011). One potential mechanism through which p70s6K might contribute to memory formation and stability at recent and/or remote time points is through effects on local translation at the dendritic spine (Cracco et al., 2005; Vickers et al., 2005). Interestingly, a recent paper has suggested that spine changes evident early on after memory formation also occur after a remote memory has been retrieved (Restivo et al., 2009) offering a strong candidate mechanism through which p70s6K may be involved in remote memory retrieval.
Our study showed that as DH dependent memories age memory for the contextual stimuli is less detailed as evidenced by increased contextual generalization. Phosphorylated p70s6K is increased in the DH after exposure to the training context 1 and 10 days after training and in the ACC 36 days after training. Pre-exposure to the training context strengthens the 36 day old memory for the training context and preserves the activation of p70s6K in the DH. These data offer support for the idea that the DH plays a long term role in the storage of detailed contextual fear memory.
Acknowledgments
Grant sponsor: NIMH; Grant # RO1MH069558
References
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007;87(2):303–7. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Belelovsky K, Kaphzan H, Elkobi A, Rosenblum K. Biphasic activation of the mTOR pathway in the gustatory cortex is correlated with and necessary for taste learning. J Neurosci. 2009;29(23):7424–31. doi: 10.1523/JNEUROSCI.3809-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context preexposure prevents forgetting of a contextual fear memory: implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem. 2007;14(3):200–3. doi: 10.1101/lm.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Runyan JD, Dash PK. Inhibition of prefrontal protein synthesis following recall does not disrupt memory for trace fear conditioning. BMC Neurosci. 2006;7:67. doi: 10.1186/1471-2202-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus. 2005;15(5):551–6. doi: 10.1002/hipo.20078. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–30. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304(5672):881–3. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011;182:98–104. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3(6):533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Jobim PF, Pedroso TR, Christoff RR, Werenicz A, Maurmann N, Reolon GK, Roesler R. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. 2012;97(1):105–12. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89(3):338–51. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26(50):12977–83. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29(25):8206–14. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: methodological implications for assessing associative phenomena. Psychol Bull. 1992;112(3):433–45. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, O’Reilly RC. Prefrontal cortex and the organization of recent and remote memories: an alternative view. Learn Mem. 2005;12(5):445–6. doi: 10.1101/lm.97905. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99(1):467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CA, Dickson KS, Wyllie DJ. Induction and maintenance of late-phase long-term potentiation in isolated dendrites of rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;568(Pt 3):803–13. doi: 10.1113/jphysiol.2005.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Teixeira CM, Wheeler AL, Frankland PW. The precision of remote context memories does not require the hippocampus. Nat Neurosci. 2009;12(3):253–5. doi: 10.1038/nn.2263. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, Silva AJ. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol. 2010;20(15):1336–44. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Sekeres M. Memory consolidation or transformation: context manipulation and hippocampal representations of memory. Nat Neurosci. 2007;10(5):555–7. doi: 10.1038/nn1880. [DOI] [PubMed] [Google Scholar]
- Zhou YL, Riccio DC. Pretest Cuing Can Alleviate the Forgetting of Contextual Stimulus Attributes. Learning and Motivation. 1994;25(3):233–244. [Google Scholar]