Abstract
Background and aims
Gene variants in CHRNA5-A3-B4, which encode for the α5, α3 and β4 nicotinic receptor subunits, are associated with altered smoking behaviors in European-Americans. Little is known about CHRNA5-A3-B4 and its association with smoking behaviors and weight in Alaska-Native people, which is a population with high prevalence but low levels of tobacco consumption, extensive smokeless tobacco use, and high rates of obesity. We investigated CHRNA5-A3-B4 haplotype structure and its association with nicotine intake and obesity in Alaska-Native people.
Design, Setting, Participants
A cross sectional study of 400 Alaska-Native individuals including 290 tobacco users.
Measurements
CHRNA5-A3-B4 genotype, body weight, and tobacco consumption biomarkers such as plasma cotinine and urinary total nicotine equivalents (TNE).
Findings
Alaska-Native people have a distinct CHRNA5-A3-B4 haplotype structure compared with European/African-Americans. In 290 Alaska-Native tobacco users, the ‘G’ allele of rs578776, which tagged a 30kb haplotype in CHRNA5-A3-B4, was prevalent (16%) and significantly associated with nicotine intake (20% higher plasma cotinine, P<0.001, 16% higher TNE, P=0.076), while rs16969968 was not associated with nicotine intake. Rs578776 acted in combination with CYP2A6, the main nicotine-metabolizing enzyme, to increase nicotine intake by 1.8 fold compared with the low risk group (P<0.001). Furthermore rs2869950, a single nucleotide polymorphism 5′ to CHRNB4, was significantly associated with increased body mass index (P<0.01) in the tobacco users even after controlling for differences in nicotine intake (P<0.01).
Conclusions
Genetic variants in CHRNA5-A3-B4 alter nicotine intake and body mass index in a population of Alaska-Native people, who have a distinct haplotype structure, smoking behaviors and prevalence of obesity.
Keywords: Alaska-Native People, Smoking, CHRNA5-A3-B4, Obesity
Introduction
Smoking and obesity are the greatest preventable causes of premature death. Alaska-Native people experience a higher incidence rate of lung cancer than the United States national average (1), despite reporting on average lower tobacco consumption compared to European-Americans (2, 3). Obesity is also prevalent in Alaska-Native people. In fact, more than 70% of Alaska-Native adults are also considered over-weight or obese (4). Although smoking and obesity appear to be distinct epidemics with different etiologies, smoking and body weight are intertwined in many ways. Here we investigate whether gene variants in the α5, α3, and β4 nicotinic acetylcholine receptor gene cluster (CHRNA5-A3-B4) are associated with nicotine intake and weight in Alaska-Native people as previously observed in smokers of European-ancestry (5, 6)
Nicotine is the major psychoactive component of tobacco. A number of independent genome wide association studies demonstrate a significant association between CHRNA5-A3-B4 gene variants and self-reported smoking quantity as well as with the incidence rate of lung cancer (5, 7–9). Currently, most association studies are conducted in smokers of European-ancestry; little is known about the haplotype structure of CHRNA5-A3-B4 and its relationship with nicotine intake in racial minority groups such as Alaska-Native people.
Multiple independent studies have identified a strong association between tobacco consumption and rs16969968 and its correlated SNP rs1051730 (5, 7, 10, 11). For example, the ‘AA’ genotype of rs16969968 is associated with an increase of roughly one cigarette per day (CPD), 24–100 ng/mL in cotinine, ≈15 nmol in nicotine equivalents and a 1.6 fold higher odds ratio of developing lung cancer when compared to the ‘GG/GA’ genotype group (11–14). In addition to rs16969968-rs1051730, other independent SNPs in CHRNA5-A3-B4 have also been reported to affect self-reported smoking quantity including rs588765 or rs578776 and their correlated SNPs (15, 16).
The first objective of our study was to characterize the haplotype structure of CHRNA5-A3-B4 gene cluster in Alaska-Native people and to evaluate the impact of genetic variants in CHRNA5-A3-B4 on nicotine intake and tobacco-derived carcinogen exposure. Variation in CHRNA5-A3-B4 and CYP2A6, the latter is the main nicotine-metabolizing enzyme, were previously shown in European-Americans to act in combination to alter CPD (and lung cancer risk) (13). Here, we directly compared the quantitative effects of the variation in CHRNA5-A3-B4 and CYP2A6 on self-reported and objective biomarkers of nicotine intake.
Our second objective was to explore the association between CHRNA5-A3-B4 gene variants and body weight. The prevalence of obesity is very high (37.5%) in Alaska-Native people, and another 34.3% are considered overweight (4). Nicotine both increases metabolic rate and suppresses appetite and feeding. Smokers on average weigh less than nonsmokers; and smokers typically gain 4 to 5 kg when they stop smoking (17). This can be attributed to the activation of sympathetic nervous system peripherally and nicotine’s ability to activate α3β4 nicotinic receptors located on POMC (Pro-opiomelanocortin) neurons in the arcuate nucleus of the hypothalamus centrally (18, 19). Since CHNRA5-A3-B4 encodes for the α3β4 nicotinic receptor, it is possible that CHRNA5-A3-B4 gene variants can modulate nicotine’s ability to regulate body weight by altering α3β4 nicotinic receptor function. Previous research suggests that a nicotine intake altering CHRNA5-A3-B4 variant, rs1051730, is associated with altered body weight (6). Yet it is not clear whether this association is an indirect effect of rs1051730 altering nicotine intake or a direct effect of rs1051730 altering nicotine’s effect on body weight, possibly by modulating nicotinic receptor function (6). In this study, we seek to replicate and extend the association between CHRNA5-A3-B4 variants and body weight by looking at the impact of CHRNA5-A3-B4 variants on body weight with, and without, controlling for nicotine intake. Together, these findings will further our understanding of the role of genetic variations in the nicotinic receptor genes in two pressing public health issues, smoking and obesity.
Methods
Study Design
A detailed description of recruitment procedures, participant demographics and the correlation between the tobacco biomarkers has been reported previously (3, 20). Briefly, 400 Alaska-Native individuals were recruited in local villages near Bristol Bay, Alaska. The total current tobacco user group (n=290) included 163 cigarette smokers (mean age=36 years and 56.4% female), 76 commercial (mean age=39.2 years and 54.0% female) and 20 iqmik smokeless tobacco users (mean age=42.9 years and 70.0% female), and 31 mixed products users (mean age=28.8 years and 48.4% female). The non-tobacco user group included 82 former smokers (mean age=45.0 years and 54.9% female) and 28 never users (mean age=45.2 years and 46.4% female)(3). Ethics approval was obtained from Alaska Area IRB, the Bristol Bay Area Health Corporation Board and Ethics Committee, UCSF and the University of Toronto.
CHRNA5-A3-B4 Genotyping
Seventeen SNPs in the CHRNA5-A3-B4 gene cluster were genotyped using Applied Biosystem Taqman genotyping assays. The CHRNA5-A3-B4 SNPs were selected to 1) represent loci which have been previously associated with smoking behavior (e.g. rs16969968-rs1051730; rs588765, and rs7164030-rs578776) and 2) tag the region between CHRNA5 and CHRNA3 (where previous significant associations with smoking behavior have been observed) based on the ‘CHB’ (Han Chinese) and ‘JPT’ (Japanese) datasets of the International Hapmap Project release #28. The position and minor allele frequency of the genotyped SNPs are listed on Table S1.
The Measurement of CYP2A6 activity
We estimated in vivo CYP2A6 activity previously using the plasma ratio of tran-3′-hydroxycotinine to cotinine (also known as nicotine metabolite ratio, NMR) (21). Using a median split of plasma NMR within the total tobacco user group (n=290) as described previously (22), participants who were in the higher NMR stratum were considered the faster CYP2A6 activity group, while those in the lower NMR stratum were considered the lower CYP2A6 activity group.
Plasma and Urinary Biomarkers
We used a panel of biomarkers to assess nicotine intake and tobacco-derived carcinogen exposure. Plasma cotinine and total nicotine equivalents (TNE, the urinary sum of nicotine and 8 of its metabolites and accounts for about 90% of an administered nicotine dose) were used to evaluate nicotine intake (23). Total urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) was used to evaluate tobacco specific nitrosamine exposure (24, 25). 1-Hydroxyfluorene was used in this study as a biomarker of tobacco related polycyclic aromatic hydrocarbons exposure (26). Plasma cotinine and tran-3′-hydroxycotinine levels, as well as urinary TNE, total NNAL, and 1-hydroxyfluorene were quantified by gas/liquid chromatography-tandem mass spectrometry as previously described (27–30).
Statistical Analyses
Statistical analyses were performed using ‘R’ version 2.14 (R foundation for statistical computing). The association between CHRNA5-A3-B4 gene variants and biomarkers of tobacco and tobacco derived carcinogen exposure were assessed by ‘PLINK’ (31). Haplotyping results of CHRNA5-A3-B4 were obtained using the ‘Haploview’ software (32). The biomarkers (plasma cotinine, urinary TNE, NNAL and 1-hydroxyfluorene levels) were normalized by log transformation before statistical analyses. With regard to nicotine intake and tobacco derived carcinogen exposure, no multiple comparison adjustments were used since we considered our analyses a replication and extension of previous findings in a new racial population. Since there were 11 independent loci (i.e. SNPs with r2≤0.9), we considered P values below 0.0045 (i.e. 0.05/11) statistically significant for the assessment of the association between CHRNA5-A3-B4 and BMI.
Results
The CHRNA5-A3-B4 gene cluster haplotype structure in Alaska-Native people
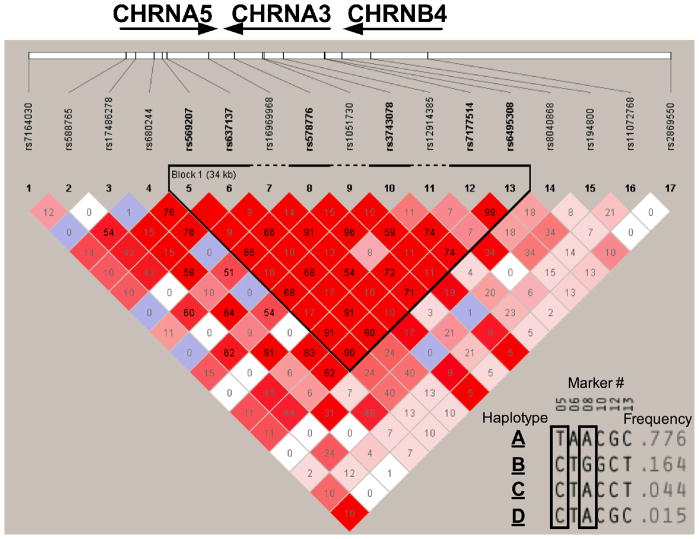
The minor allele frequency of the 17 genotyped SNPs in comparison to other racial groups is listed in Table S1. All SNPs were in Hardy-Weinberg equilibrium. A haplotype analysis revealed a 30 KB region of high linkage disequilibrium (LD) between CHRNA5 and CHRNA3 (i.e. a haplotype block between rs569207 and rs6495308, Fig. 1). Within this haplotype block four prevalent haplotypes were identified (Fig. 1 Insert), and the total prevalence of these four haplotypes was 99.9% in the 400 participants. The most common haplotype, with a 77.6% frequency, was designated as haplotype A. The remaining haplotypes were designated to haplotype B, C and D according to their prevalence respectively. As illustrated by the insert of fig. 1, these four unique haplotypes could be discerned by a few tag SNPs. For example, haplotype A was tagged by rs569207 in our sample, and haplotype B was tagged by rs578776 in our sample.
Figure 1.
Observed CHRNA5-A3-B4 haplotype structure in Alaska-Native people.
The number in the squares indicate the r2 score.
Insert: A haplotype analysis revealed a 30kb region of high linkage disequilibrium between CHRNA5 and CHRNA3.Within this haplotype block, four haplotypes with a prevalence of more than 1% were identified. These four haplotype could be discerned by a few tag SNPs. For example, haplotype A was tagged by rs569207 in our sample (marker #5), while haplotype B was tagged by rs578776 in our sample (marker #8)
The association between gene variants in CHRNA5-A3-B4 and nicotine intake
The associations (by additive models) between CHRNA5-A3-B4 gene variants and nicotine intake and tobacco-derived carcinogen exposure are primarily evaluated in the total tobacco users group (n=290). The associations within the smokers (n=163) and smokeless tobacco users (n=76) are also presented to illustrate the effect in these subgroups. The results are summarized in Table S2 with a few key findings outlined below.
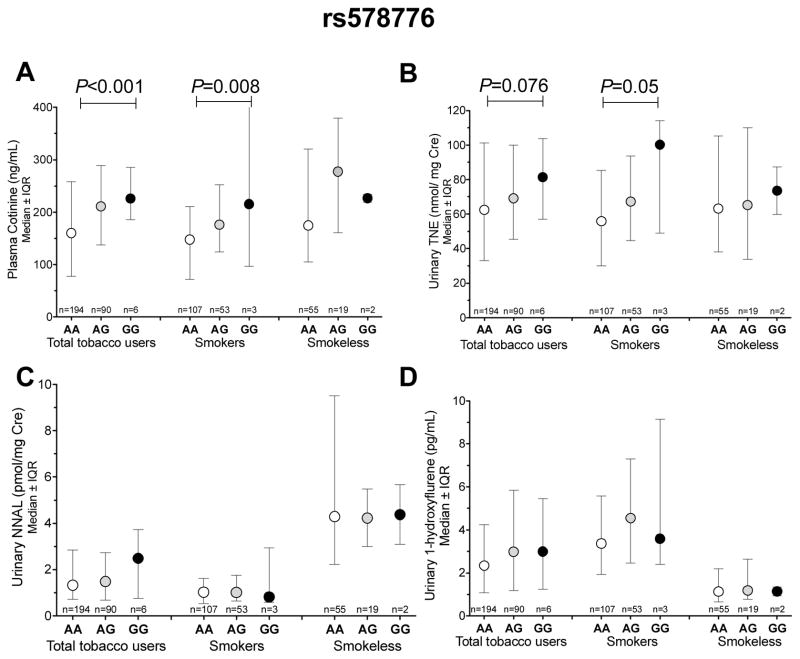
Rs578776
The ‘G’ allele of rs578776, which tagged haplotype B in our sample of Alaska-Native people, was significantly associated with higher cotinine levels in the total tobacco users group and in the cigarette smokers (Fig. 2A and Table S2). As illustrated by fig. 2A, each ‘G’ allele of rs578776 was associated with a roughly 20% (33 ng/mL) increase in plasma cotinine levels in the total tobacco users group and a roughly 22% (33 ng/mL) increase in the smokers (P<0.001 and P=0.008, respectively). When evaluated by a dominant model (i.e. ‘AG’ and ‘GG’ groups combined), rs578776 was weakly associated with plasma cotinine levels in the smokeless tobacco group (P=0.06, Fig. S2). In addition to cotinine, weak associations between rs578776 and urinary TNE were also observed among the total tobacco user group and among the smokers (P=0.076 and P=0.05, respectively. Fig. 2B). Rs578776 was also weakly associated with CPD among the smokers. The average CPD was 7.2 for the ‘AA’ genotype, 8.6 for the ‘AG’ genotype and 11.7 for the ‘GG’ genotype (P=0.02, Table S2). No significant associations between rs578776 and urinary NNAL or 1-hydroxyfluorene levels were observed (Fig. 2C and D). The associations were similar when assessed by dominant models (i.e. pooling the ‘AG’ and ‘GG’ genotypes, Fig. S2). Individuals with the ‘G’ allele of rs578776 exhibited a greater, although non-significant, odds ratio of being a current smoker than a former smoker (OR=1.72, 95%CI=1.00–3.19, P=0.06, n=163 and 82 respectively).
Figure 2.
The association between rs578776 with A) cotinine levels B) Urinary TNE C) Urinary NNAL D) Urinary 1-hydroxypryrene. IQR= interquartile range. The P-values were obtained from additive models.
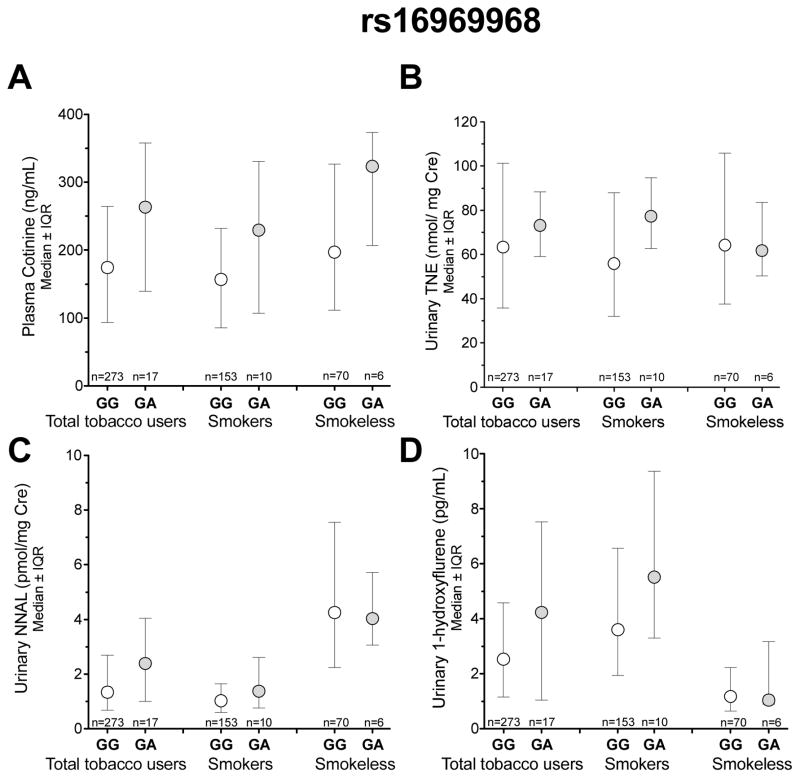
Rs16969968
rs16969968 is a nonsynonymous (amino acid change: D398N) variant previously associated with nicotine intake in smokers of European-ancestry (33, 34). The prevalence of the ‘A’ allele of rs16969968, and the correlated rs1051730, was low (3%) in Alaska-Native people compared to European-Americans (~38%, Hapmap project). Similar to the European-American populations of the international Hapmap project, rs16969968 was in low LD with rs578776 in Alaska-Native people (r2=0.14). While rs16969968 had a large impact on nicotine intake (Fig. 3), this did not reach significance which is at least in part due to the low prevalence and power to detect the association. The ‘GA’ genotype of rs16969968, compared to the ‘GG’ genotype, was non-significantly associated with 1.51 fold (89 ng/mL) higher plasma cotinine in the total tobacco user group and 1.46 fold (73 ng/mL) higher plasma cotinine in the smokers (Fig. 3A). The ‘GA’ genotype of rs16969968 was also non-significantly associated with 16% (10 nmol/mg Cre) higher urinary TNE levels in the total tobacco user group and 38% (21 nmol/mg Cre) higher TNE levels in the smokers (Fig. 3B). Associations of similar directions and magnitudes could also be observed with urinary NNAL and 1-hydroxyflurene (Fig. 3C and Fig. 3D). The ‘A’ allele of rs16969968 was found at a greater frequency in the current smokers compared to the former smokers, although this difference did not reach statistical significance (6.1% vs. 3.7%, n=163 and 82 respectively). Of note, the association between plasma cotinine levels and rs578776 remained significant after adjusting for rs16969968 or excluding the participants with at least one ‘A’ variant allele of rs16969968 (data not shown).
Figure 3.
The association between rs16969968 with A) plasma cotinine levels B) Urinary TNE C) Urinary NNAL D) Urinary 1-hydroxypryrene. IQR= interquartile range
Rs588765
rs588765 and correlated SNPs have previously been associated with increased risk of heavy smoking (15). In our study, no significant associations were observed between rs588765 and nicotine intake with/without adjusting for the effect of rs578776 and/or rs169699698 (Fig. S1).
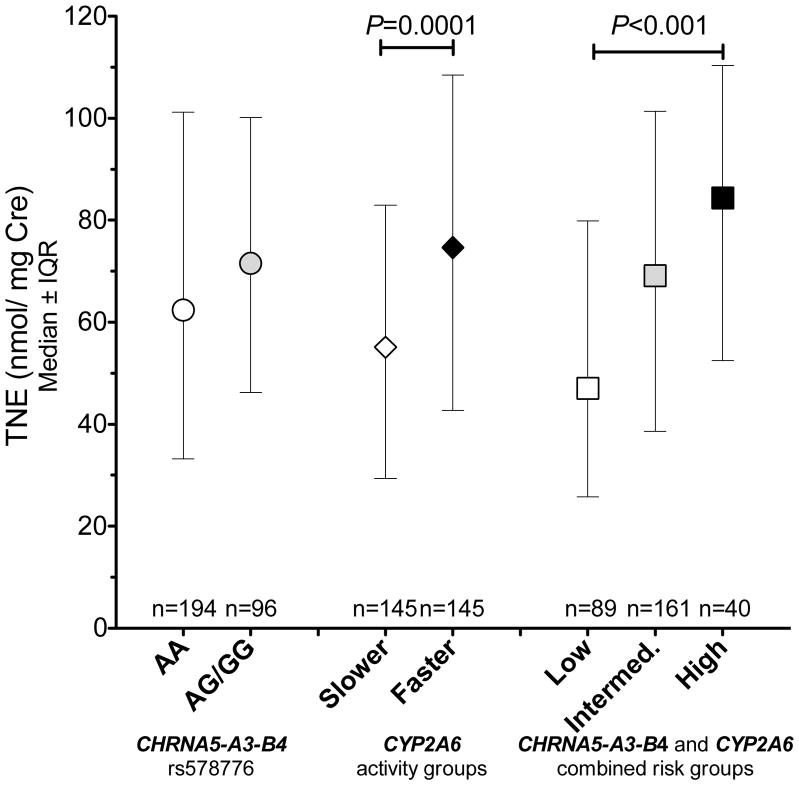
Combined influence of CHRNA5-A3-B4 and CYP2A6 activity on nicotine intake
Next, we examined the combined influence of gene variants in CHRNA5-A3-B4 and altered CYP2A6 activity on nicotine intake. Since CYP2A6 activity can influence the quantitative relationship between nicotine intake and cotinine levels (35), urinary TNE was used as the primary tobacco exposure biomarker. The analyses focused on the total tobacco user group (n=290) for sufficient statistical power. The effects of rs578776 and CYP2A6 activity, alone or together, on tobacco intake are shown in fig. 4. To assess the combined influence of CHRNA5-A3-B4 and CYP2A6, we assigned the tobacco users into one of three risk groups. The low-risk group included participants with both rs578776 AA genotype and slower CYP2A6 activity. Participants with either rs578776 AG/GG genotype or faster CYP2A6 activity were assigned to the intermediate-risk group. Participants with both rs578776 AG/GG genotype and faster CYP2A6 activity were considered the high-risk group. As illustrated in Fig. 4, urinary TNE levels were significantly different between the low risk group (mean=47 nmol TNE/Cre), the intermediate-risk group (mean=69.2 nmol TNE/Cre) and the high-risk group (84.4 nmol TNE/Cre, Kruskal-Wallis test: P=0.0003, Fig 4). Urinary TNE levels also increased with the number of risk genotypes in a linear trend (Jonckheere Trend Test: P=2.6*10−5). Due to the low prevalence of rs16969968, our ability to evaluate the combined effect of rs16969968 and CYP2A6 activity in this study was limited (Fig. S3).
Figure 4.
The combined impact of genetic variants in CHRNA5-A3-B4 and CYP2A6 activity on urinary TNE levels among the total tobacco user group. The low risk group included participants with both rs578776 AA genotype and slower CYP2A6 activity. Participants with either rs578776 AG/GG genotype or faster CYP2A6 activity were assigned to the intermediate risk group. Participants with both rs578776 AG/GG genotype and faster CYP2A6 activity were considered as the high risk group. IQR= interquartile range. The P-values were obtained by non-parametric comparisons (Mann-Whitney or Kruskal-Wallis).
The association between CHRNA5-A3-B4 gene variants and BMI
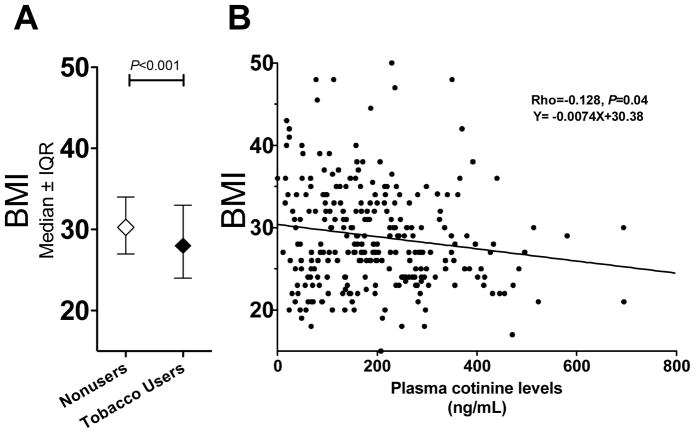
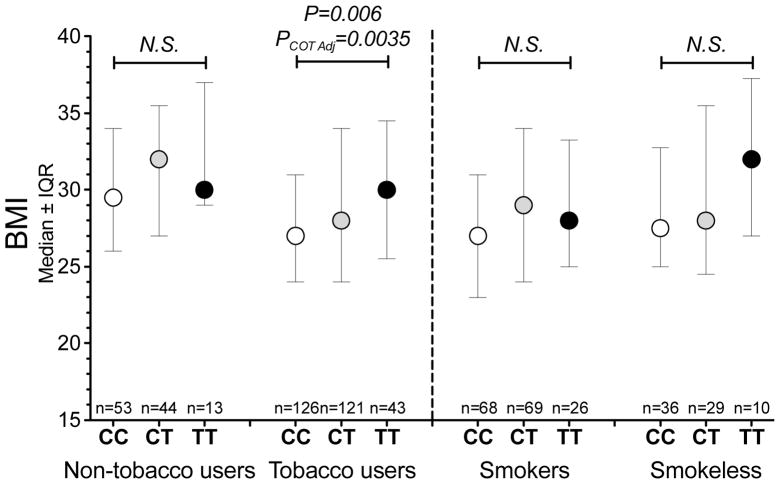
The total tobacco users had significantly lower BMI compared to the non-tobacco group (Fig. 5A, P<0.001). Among the total tobacco user group, there was a significant inverse correlation between plasma cotinine levels and BMI, suggesting nicotine dose dependently reduced body weight (Rho=−0.128, P=0.04, Fig. 5B). Notably, the Y-intercept of the regression line is 30.4 kg/m2, which is the same as the average BMI of the non-tobacco user group. Similar correlation coefficients could be observed when examined in smokers (Rho=−0.117, P=0.15) or the commercial smokeless tobacco users (Rho=−0.133, P=0.27). Neither of the nicotine intake altering SNPs, rs578776 and rs16969968, were associated with BMI in the total tobacco user group or in the smokers only (Table S2). Adjusting for cotinine levels did not improve the associations between rs578776 or rs16969968 with BMI. On the other hand rs2869550, a 36% allele frequency SNP located in the intergenic region 5′ to CHRNB4 which was not associated with altered nicotine intake in Alaska-Native people (Table S2), was associated with BMI in the total tobacco user group (P=0.006, Table S2). The association was even stronger after adjusting for plasma cotinine levels (P=0.0035, Fig. 6). Adjusting for the nicotine intake altering SNPs, such as rs578776, did not change the strength of the association. Rs2869950 was not significantly associated with BMI in the non-tobacco user group (Fig. 6).
Figure 5.
A) Non-tobacco user group have higher BMI than the tobacco users. B) BMI negatively correlated with plasma cotinine levels within the total tobacco user group, suggesting a dose dependent relationship between nicotine intake and body weight.
Figure 6.
Rs2869550 was significantly associated with BMI in the total tobacco user group, but not in the non-tobacco user group. Similar directions of effect were observed between rs2869550 and BMI within the smokers and smokeless users. The P-value of the total tobacco user group was derived from an additive model after adjusting for plasma cotinine levels (Table S3). PCOT adj. = The P value of rs2869950 after adjusting plasma cotinine levels.
Discussion
We characterized the haplotype structure of CHRNA5-A3-B4 among Alaska-Native people. In addition, we identified a significant association between rs578776 and nicotine intake. Secondly, we demonstrated that CHRNA5-A3-B4 gene variants, particularly rs578776, could act in combination with CYP2A6 to modify nicotine intake. Thirdly, we demonstrated that rs2869950, a CHRNA5-A3-B4 gene variant which did not alter nicotine intake, was associated with altered body weight among tobacco users.
Haplotype
The haplotype analyses of CHRNA5-A3-B4 identified both similarities and differences between Alaska-Native people and European-Americans. For example, rs16969968 and rs1051730 were in high degree of linkage disequilibrium in Alaska-Native people as seen in European-Americans. However, the allele frequency of rs16969968-rs1051730 variant SNPs was substantially lower (at 3%) compared to European-Americans (~38%), and was similar to that observed in the Asian populations (at 3%) of the International Hapmap project. In contrast to rs16969968-rs1051730, the LD between rs7163730 and rs578776 was weak in Alaska-Native people compared to the high degree of LD previously observed in European-Americans (15).
Associations with nicotine intake
We demonstrated a significant association between rs578776 (representing haplotype B in Alaska-Native people) and nicotine intake. The ‘G’ allele of rs578776 was associated with increased nicotine intake in Alaska-Native people as previously seen in Asians and European-Americans (15). Of note, the allele frequency of the rs578776 ‘G’ allele was much lower in Alaska-Native people than in European-Americans. In fact, the ‘G’ allele is the major allele of rs578776 in European-Americans whereas it is the minor allele of rs578776 in Alaska-Native peoples.
Rs16969968 and its correlated SNPs generally exhibit the strongest association with nicotine intake in European-Americans and other racial groups (12, 15). In Alaska-Native people, the ‘GA’ genotype of rs16969968 was associated with a roughly 73 ng/mL increase in plasma cotinine, which is within the previously reported range of 24 to 100 ng/mL (11, 12). Due to the low prevalence of rs16969968 in Alaska-Native people, the difference between ‘GG’ and ‘GA’ genotypes was not significant. Overall, while the effect size of rs16969968 on nicotine intake was larger than rs578776 (Plasma cotinine in smokers: 73 vs. 33 ng/mL, respectively), the overall impact on nicotine intake is likely to be smaller than rs578776 in Alaska-Native people due to the allele frequencies.
Associations in combination with CYP2A6 activity
In our study, CYP2A6 activity and CHRNA5-A3-B4 acted in combination to increase nicotine intake. Of note, the combined low-risk group had lower nicotine intake compared to either the ‘AA’ group of rs578776 or the slower CYP2A6 activity group alone and the combined high risk group had higher nicotine intake compared to either the ‘AG/GG’ group of rs578776 or the faster CYP2A6 activity group alone. Consistent with previous observation in European-Americans (13), we observed that CYP2A6 activity had a greater influence on nicotine intake than CHRNA5-A3-B4 gene variants, which was particularly obvious among the smokeless tobacco users. We have previously shown that these Alaska-Native smokeless tobacco users with faster CYP2A6 activity have higher nicotine intake compared to the smokeless tobacco users with slower CYP2A6 activity (22). However, in the present analyses, we did not observe any significant associations between CHRNA5-A3-B4 gene variants and nicotine intake in the smokeless tobacco users. Thus, it is interesting that among European-Americans CHRNA5-A3-B4 gene variants generally have a bigger effect on lung cancer risk than CYP2A6 (13). This suggests that the CHRNA5-A3-B4 gene variants may modulate lung cancer risk by mechanisms in addition to their effects on smoking behavior, such as directly altering cell proliferation and survival (36). The prevalence of the protective CYP2A6 and CHRNA5-A3-B4 alleles were higher in Alaska-Native people than in European-Americans, suggesting the protective CYP2A6 and CHRNA5-A3-B4 alleles may contribute to the low level of self-reported smoking in Alaska-Native people and that other genes or environmental factors may be responsible for the higher risk for lung cancer.
Association with BMI
The Alaska-Native non-tobacco user group had, on average, higher BMI compared to the total tobacco user group. Within the tobacco users, there was a significant negative relationship between nicotine intake and BMI, supporting a negative dose response relationship between nicotine intake and body weight (18). The negative dose response relationship is consistent with the known effects of nicotine to increase metabolic rate and suppress appetite (18). Genetic variants in CHRNA5-A3-B4 have been associated with BMI (6), however, it was not clear whether this genetic association was mediated indirectly by the effect of CHRNA5-A3-B4 genetic variants on nicotine intake or directly by altering nicotine’s effect on the nicotinic receptor pharmacodynamic target. In this study rs578776, which altered nicotine intake, was not signficantly associated with BMI, suggesting the effect of rs578776 on nicotine intake was not strong enough to alter BMI. Interestingly, there was a direct role of another CHRNA5-A3-B4 genetic variants in modulating BMI. We demonstrated that rs2869550, a prevalent SNP located in the intergenic region 5′ to CHRNB4 which was not associated with nicotine intake, could alter nicotine’s ability to modulate body weight even after controlling for plasma cotinine. A possible explanation is that rs2869550 results in altered β4 nicotinic receptor subunit function, reducing nicotine’s ability to suppress eating.
Conclusion
Together, we found an association between CHRNA5-A3-B4 gene variants and nicotine intake in Alaska-Native people. This is novel since the haplotype structure and SNP prevalence in Alaska-Native people differ from European-Americans, and the level and type of nicotine intake also differ. We observed that rs578776, which tagged haplotype B in our sample, was significantly associated with nicotine intake. Due to the high prevalence of rs578776 in Alaska-Native people, it may play a more important role in governing nicotine intake than rs16969968. We also demonstrated that variation in pharmacokinetic (i.e. CYP2A6) and pharmacodynamic (i.e. nicotinic receptors) genes could act in combination to increase nicotine intake. Lastly, we provided evidence that there is a dose response relationship between nicotine intake and body weight, and genetic variants near CHRNA5-A3-B4 tagged by rs2869550 may modulate body weight in smokers directly without altering nicotine intake. A limitation of the current study is that the findings may be only representative of primarily Yupik Alaska-Native peoples; other groups may have different CHRNA5-A3-B4 haplotype structures. We also acknowledge that the moderate sample size could limit our assessments. A larger study would be able to determine the relationship between rs16969968 genotypes and tobacco consumption; we had 58% power with 290 subjects and would need 500 subjects to have 80% power to reject this association.
In summary, our data indicate that genetic variation in CHRNA5-A3-B4 can modulate both nicotine intake and body weight in tobacco users. These findings provide important insights about the contribution of CHRNA5-A3-B4 to variation in both tobacco and obesity, the two most prevalent causes of preventable death and disease.
Supplementary Material
Acknowledgments
The scientific team would like to express their gratitude for the leadership and direction from the members of the Board of Directors of the Bristol Bay Area Health Corporation, the members of the Ethics Committee of that organization and the Community Advisory Board for this study, and the BBAHC Director of Community Health Services, Ms. Rose Loera, and all who contributed their time and expertise to making this study possible. We would also like to acknowledge the contributions of Ms. Kim Hatt, Ms. Helen Peters and Ms. Ana Chartier who were study assistants to the project. In addition, we would like to acknowledge Drs. David Ashley and Tom Bernert for their advice on study design and Ewa Hoffmann and Qian Zhou for assisting DNA sample preparation. This work was co- supported by the Indian Health Service with the National Institute on Drug Abuse and National Cancer Institute (NARCH III U26IHS300012) and funding from National Cancer Institute (HHSN261200700462P and CA114609), National Institute on Drug Abuse (DA012353), National Institutes of Health (DA020830 and DA012353), the Endowed Chair in Addiction for the Department of Psychiatry (RFT), Canadian Institutes of Health Research (MOP86471 and TMH109787), Ontario Graduate Scholarship (for AZZ), CAMH and the CAMH foundation, the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation. We thank Clifford Watson and Connie Sosnoff for performing the analytical chemistry.
Footnotes
Conflicting interests: Dr. Tyndale has done one-day workshop consultations for Novartis and McNeil pharmaceuticals on smoking cessation approaches. Dr. Benowitz has been a paid consultant to pharmaceutical companies that market medications for smoking cessation treatment, and has served as a paid expert witness in litigation against tobacco companies. Dr. Hatsukami has received grant funding from Nabi Biopharmaceuticals to conduct a clinical trial.
References
- 1.Centers. for. Disease. Control. and. Prevention. Racial/Ethnic disparities and geographic differences in lung cancer incidence --- 38 States and the District of Columbia, 1998–2006. MMWR Morbidity and mortality weekly report. 2010;59:1434–1438. [PubMed] [Google Scholar]
- 2.U.S. Department. of. Health. and. Human. Services. African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention; 1998. [Google Scholar]
- 3.Renner CC, Lanier AP, Lindgren B, Jensen J, Patten CA, Parascandola M, et al. Tobacco Use Among Southwestern Alaska Native People. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behavioral. Risk. Factor. Surveillance. System. Health RIsks in Alaska Among Adults. 2008. Alaska Behavioral Risk Factor Survey 2008 Annual Report. [Google Scholar]
- 5.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freathy RM, Kazeem GR, Morris RW, Johnson PC, Paternoster L, Ebrahim S, et al. Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int J Epidemiol. 2011;40:1617–1628. doi: 10.1093/ije/dyr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nature genetics. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 10.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer research. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keskitalo K, Broms U, Heliovaara M, Ripatti S, Surakka I, Perola M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Human molecular genetics. 2009;18:4007–4012. doi: 10.1093/hmg/ddp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munafo MR, Timofeeva MN, Morris RW, Prieto-Merino D, Sattar N, Brennan P, et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst. 2012;104:740–748. doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship Between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 Variation and Smoking Behaviors and Lung Cancer Risk. Journal of the National Cancer Institute. 2011;103:1342–1346. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeMarchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, et al. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer research. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LS, Saccone NL, Culverhouse RC, Bracci PM, Chen CH, Dueker N, et al. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry--a meta-analysis of chromosome 15q25. Genetic epidemiology. 2012;36:340–351. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes, brain, and behavior. 2010;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley RJ, Sandoval DA. Neuroscience: weight loss through smoking. Nature. 2011;475:176–177. doi: 10.1038/475176a. [DOI] [PubMed] [Google Scholar]
- 18.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL, Renner C, Lanier A, Tyndale RF, Hatsukami DK, Lindgren BR, et al. Exposure to Nicotine and Carcinogens among South Western Alaskan Native Cigarette Smokers and Smokeless Tobacco User. Cancer Epidemiol Biomarkers Prev. 2012;21:934–942. doi: 10.1158/1055-9965.EPI-11-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binnington JM, Zhu AZ, Renner CC, Lanier AP, Hatsukami DK, Benowitz NL, et al. CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogenet Genomics. 2012;22:429–440. doi: 10.1097/FPC.0b013e3283527c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu AZ, Binnington MJ, Renner CC, Lanier AP, Hatsukami DK, Stepanov I, et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco specific nitrosamine exposure and lower tobacco specific nitrosamine bioactivation. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 24.Hecht S. S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature reviews Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 25.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le CT, Zhang Y, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.StHelen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P, 3rd, Benowitz NL. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem Res Toxicol. 2012;25:952–964. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Bernert JT, Alexander JR, Sosnoff CS, McGuffey JE. Time course of nicotine and cotinine incorporation into samples of nonsmokers’ beard hair following a single dose of nicotine polacrilex. Journal of analytical toxicology. 2011;35:1–7. doi: 10.1093/anatox/35.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Jacob P, 3rd, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–598. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 30.Jacob P, 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80:8115–8121. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 33.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu AZ, Renner C, Hatsukami DK, Swan G, Lerman CE, Benowitz NL, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race and sex. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-12-1234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brennan P, Hainaut P, Boffetta P. Lancet Oncol. 2010. Genetics of lung-cancer susceptibility. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.