Abstract
LIN28A/B are RNA binding proteins implicated by genetic association studies in human growth and glucose metabolism. Mice with ectopic over-expression of Lin28a have shown related phenotypes. Here we describe the first comprehensive analysis of the physiologic consequences of Lin28a and Lin28b deficiency in knockout (KO) mice. Lin28a/b-deficiency led to dwarfism starting at different ages, and compound gene deletions showed a cumulative dosage effect on organismal growth. Conditional gene deletion at specific developmental stages revealed that fetal but neither neonatal nor adult deficiency resulted in growth defects and aberrations in glucose metabolism. Tissue-specific KO mice implicated skeletal muscle-deficiency in the abnormal programming of adult growth and metabolism. The effects of Lin28b KO can be rescued by Tsc1 haplo-insufficiency in skeletal muscles. Our data implicate fetal expression of Lin28a/b in the regulation of life-long effects on metabolism and growth, and demonstrate that fetal Lin28b acts at least in part via mTORC1 signaling.
Keywords: Lin28a, Lin28b, dwarfism, growth, glucose metabolism, diabetes, let-7, mTOR
INTRODUCTION
Organismal growth is a highly complex process tied to multiple genetic, nutritional, hormonal, and environmental factors. Recent human genome-wide association studies (GWAS) have reported over a hundred loci relevant to height [1–3]. Among these loci are genes encoding the RNA-binding protein LIN28B, which together with its paralog Lin28A regulate biogenesis of the let-7 family of tumor-suppressor microRNAs, as well as mRNA stability and translation via direct binding of various gene transcripts such as the stem cell factor Oct4, the growth factor Igf2, cell cycle regulators and ribosomal subunits [4–11]. In addition to Lin28B, genetic variation around loci for at least three validated let-7 targets (Dot1L, HMGA2, and CDK6) has also been associated with human height in Genome Wide Association Studies [1], and GWAS has also implicated Lin28B in the timing of onset of human puberty [12–15]. Interestingly, lin-28 and let-7 were originally identified as heterochronic regulators of developmental timing in C. elegans [16–18]. Whereas lin-28 controls let-7 biogenesis, let-7 also regulates lin-28 expression by binding its complementary sequences in the 3’ untranslated region. Thus, lin-28 and let-7 regulate each other and comprise a double negative feedback loop. Their expression is tightly regulated during development, and evolutionarily conserved among worms, flies, frogs and mammals [19–21].
We have recently shown that transgenic mice which constitutively overexpress Lin28a manifest increased body size and delayed onset of puberty [22], and that overexpression of both murine Lin28a and human LIN28B promote an insulin-sensitized state that resists diabetes in mice, in direct contrast to overexpression of let-7, which results in insulin resistance and impaired glucose tolerance [23]. Given that Lin28a/b are expressed in distinct spatio-temporal patterns, their precise physiological roles in mammalian development and metabolism remain unclear. Here, we describe Lin28a and Lin28b knockout (KO) mice and characterize the distinct spatio-temporal functions of the Lin28 paralogs.
MATERIALS AND METHODS
Mice
All animal procedures were approved by the Institutional Animal Care and Use Committee. Design of conditional Lin28a knockout mice was reported previously [23]. Design of conditional Lin28b knockout mice is shown in Supplemental Fig. 10. For Lin28a and lin28b conditional KO mice, PCR fragments of both gene loci were cloned into a plasmid having 2 loxP cassetes and a PGK-Neo cassete flanked with frt sequences, and targeting was performed into V6.5 embryonic stem (ES) cells. Homologous recombination was confirmed by Southern blotting. Chimeric mice were generated by injection of ES cells into Balb/c blastocysts, and then bred to Balb/c females to generate germline-transmitted pups. To delete the floxed allele in the germ line, we used a Ddx4-Cre strain (The Jackson Laboratory). LSL-iLet-7 mice were generated by crossing iLet-7 mice [23] and ROSA26-rtTA-IRES-EGFP mice [24]. Ddx4-Cre, Myf5-Cre, Alb-Cre, Ins2-Cre, ubiquitin-Cre/ERT2 and ROSA26-rtTA-IRES-EGFP mice were purchased from The Jackson Labolatory. Tsc1fl/fl mice are kindly provided from Dr. David Kwiatkowski laboratory. For all experiments, littermate controls were used.
Histology
Tissue samples were fixed in 10% buffered formalin or Bouin’s solution and embedded in paraffin.
Glucose and Insulin Tolerance Tests
Overnight-fasted mice were given i.p. glucose (2 mg/g body weight). For insulin tolerance test, 5 hr fasted mice were given 0.75 U insulin/kg body weight by i.p. injection (Humulin). Blood glucose was determined with a Lifescan One Touch glucometer.
Drug treatment
Tamoxifen (Sigma) was dissolved in corn oil at 20 mg/mL. To delete floxed alleles in E15.5 embryos, a dose of 0.1mg/g mouse weight was injected intraperitoneally once to the mothers. For 7 to 9 days old pups, a dose of 0.5 mg/g mouse weight was injected intraperitoneally once. For adult mice, a dose of 0.2 mg/g mouse weight was injected 5 times in consecutive days.
Quantitative RT-PCR
Total RNA was collected by TRIzol and reverse-transcribed. mRNA and microRNA expression were measured by quantitative PCR using the ΔΔCT method as described previously [23].
Targeted liquid-chromatography mass spectrometry (LC/MS/MS)
LC/MS/MS-based metabolomics analysis was performed as described previously [25] Whole embryos were harvested in 80% (v/v) methanol at − 78°C. Insoluble material in lysates was centrifuged at 2,000g for 15 min, and the resulting supernatant was evaporated using a refrigerated speed vac. Samples were resuspended using 20 µL LC/MS grade water. 7 µL was injected and analyzed using a 5500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence HPLC system (Shimadzu) using selected reaction monitoring (SRM) of a total of 252 endogenous water soluble metabolites for analyses of samples. Some metabolites were targeted in both the positive and negative ion mode for a total of 292 SRM transitions. ESI voltage was +4,900 V in the positive ion mode and −4,500 V in the negative ion mode using positive/negative switching. The dwell time was 4 ms per SRM transition, and the total cycle time was 1.82 sec producing 9–12 data points per metabolite peak. Samples were delivered to the MS using hydrophilic interaction chromatography (HILIC) using a 4.6 mm internal diameter × 10 cm Amide XBridge column (Waters) at 300 µL/min. Mobile phase gradients were run starting from 85% buffer B (LC/MS grade acetonitrile) to 35% buffer B from 0–3 min; 35% buffer B to 0% buffer B from 3–12 min; 0% buffer B held from 12–17 min; 0% buffer B to 85% buffer B from 17–18 min; and 85% B held for 7 min to re-equilibrate the column. Buffer A was comprised of 20 mM ammonium hydroxide and 20 mM ammonium acetate in 95:5 water:acetonitrile (pH=9.0). Peak areas from the total ion current for each metabolite SRM transition were integrated using MultiQuant v2.0 software (AB/SCIEX).
Statistical analysis
Data is presented as mean ± SEM. Student’s t test (two-tailed distribution, two-sample unequal variance), Fisher’s exact probability test, or Chi-square test was used to calculate p values, with Mendelian ratios as the expected distribution where appropriate. Statistical significance is displayed as * p < 0.05 or ** p < 0.01.
RESULTS
Lin28a knockout mice manifest dwarfism beginning in embryogenesis
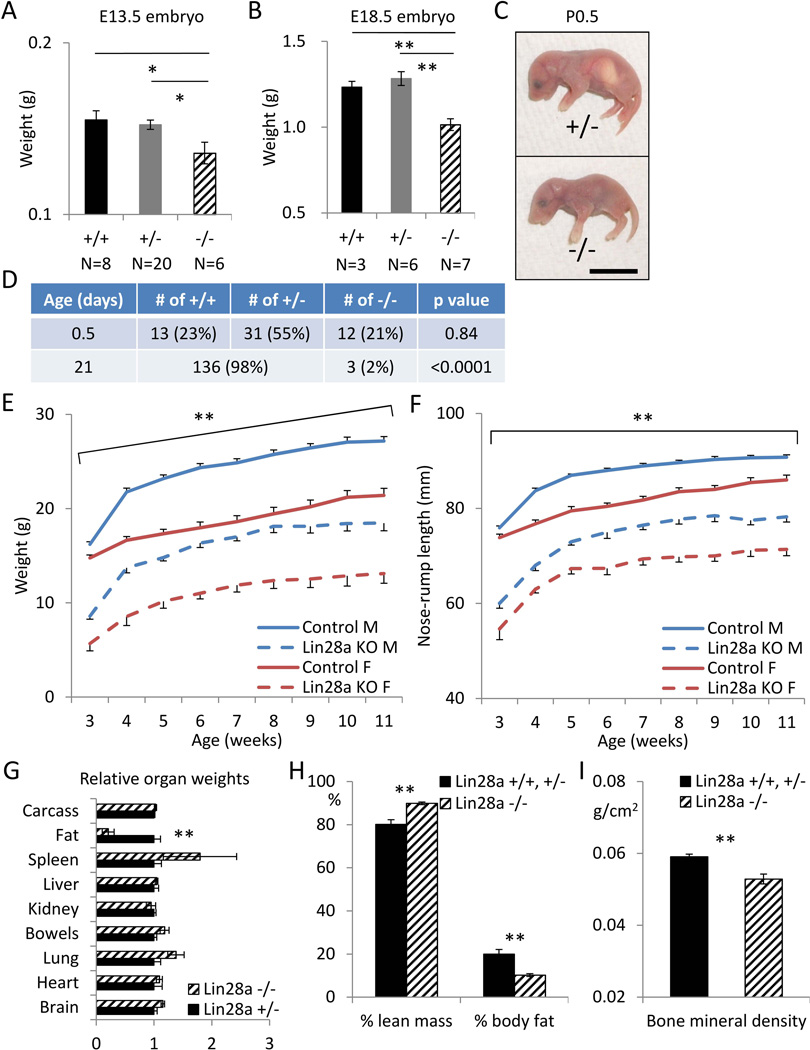
We first generated and analyzed Lin28a KO mice (See Materials and Methods). Lin28a KO mice showed dwarfism as early as E13.5, and by E18.5 showed 20% reduction in weight, relative to heterozygote controls (Fig. 1A and 1B). Although born in the expected Mendelian ratio, over 93% of Lin28a KO mice died within 1 day after birth (Fig. 1C and 1D). By histopathologic analysis, 2 out of 5 KO mice which died perinatally harbored a cardiac ventricular septal defect (Supplementary Fig. 1A), but virtually all other tissues were histologically normal (Supplementary Fig. 1B). The high perinatal lethality remains unexplained.
Figure 1. Constitutive deletion of Lin28a leads to persistent growth retardation from embryogenesis through adulthood.
(A) Body weights of E13.5 Lin28a +/+, +/− and −/− embryos. (B) Body weights of E18.5 Lin28a +/+, +/− and −/− embryos. (C) Representative images of a heterozygous mouse and its Lin28a KO littermate. Bar 1cm. (D) Viability of P0.5 newborns and 21-day-old pups born from an inbreeding cross of Lin28a heterozygotes. P values were calculated by Chi-square test. (E–F) Growth curves for Lin28a heterozygous (solid) vs KO (dashed) mice, both male (blue) and female (red). N=4–15. (E) Body weight. (F) Body length. (G) Organ weights normalized to total body weight from 4 to 5-month-old males. Epididymal fat pads were used to measure the fat weight. N=3. (H) DEXA measurements of % lean and % fat weights, relative to total body weight. N=5–8. (I) DEXA measurement of bone mineral density. N=5–8. * p<0.05, ** p<0.01. Error bars represent SEM.
Because a minority of KO mice survive to adulthood (Fig. 1D), we were able to analyze the postnatal growth of the survivors (Supplementary Fig. 2A). Both male and female KO mice were 30–50% smaller in weight and 20–30% shorter in length, and remained smaller than heterozygote controls throughout the observation period (Fig. 1E and 1F). Organ weights were proportionally lower relative to total body weight, except for fat mass, which was severely decreased in the adult KO mice (Fig. 1G). Dual-energy X-ray absorptiometry imaging confirmed the decreased fat mass and also revealed decreased bone mineral density in the KO animals (Fig. 1H and 1I, Supplementary Fig. 2B), suggesting that Lin28a KO mice suffer from metabolic dysregulation.
Lin28b regulates adult growth in males, but not in females
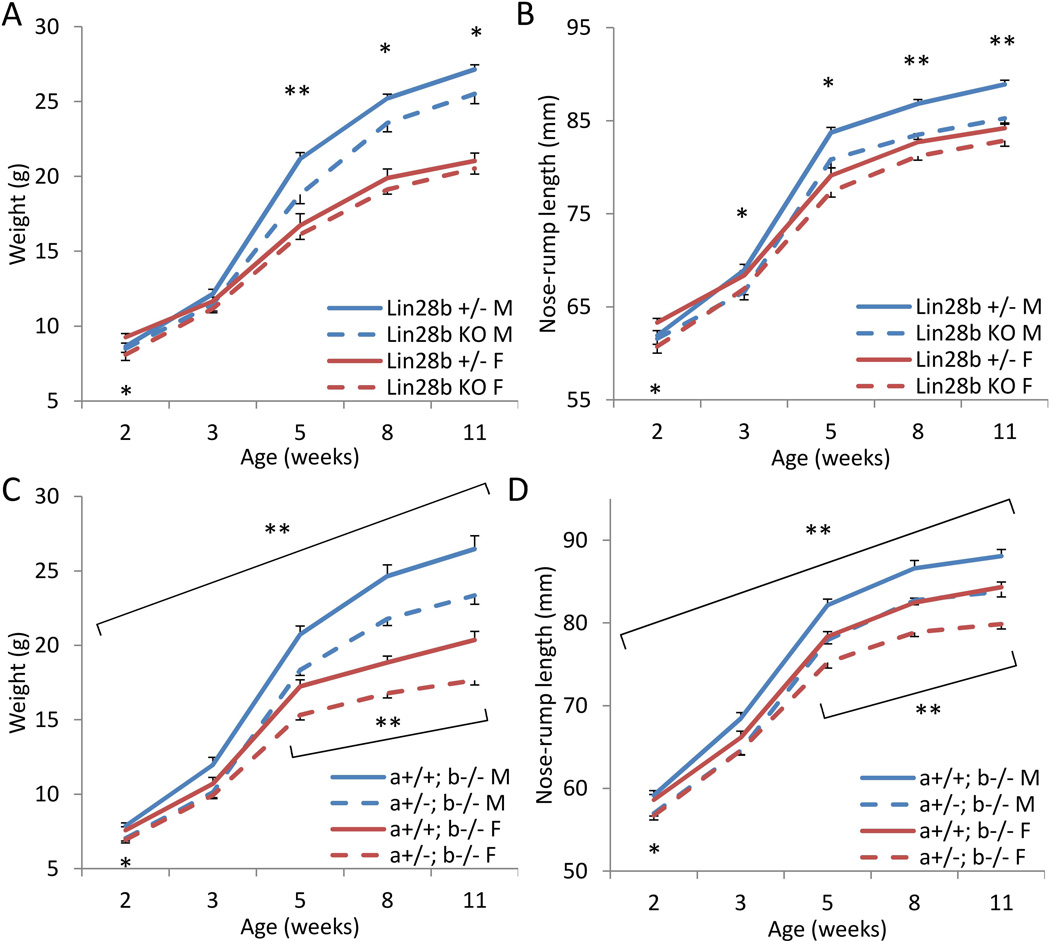
We next generated and analyzed Lin28b KO mice, using a similar gene deletion strategy as for Lin28a KO mice, in which exon 2 encoding the functional cold shock domain was engineered to be flanked by LoxP sites and subsequently deleted by Cre recombinase (See Materials and Methods). Although LIN28B is highly expressed in the placenta [26], we did not observe any difference in weights of Lin28b KO embryos or placentae at E18.5 relative to heterozygote controls, indicating that unlike Lin28a, Lin28b is dispensable for embryonic growth (Supplementary Fig. 3A and 3B). However, Lin28b KO males did show postnatal dwarfism, (Fig. 2A and 2B), with organ weights reduced in relative proportion to total body weight (Supplementary Fig. 3C). In contrast, Lin28b KO females showed no postnatal growth defects, suggesting Lin28b regulates growth in mice in a gender-specific manner.
Figure 2. Lin28a and Lin28b dosage regulates postnatal growth.
(A–B) Postnatal growth curves of Lin28b heterozygous vs. KO mice. N=8–21. (A) Body weight. (B) Body length. (C–D) Postnatal growth curves of Lin28a +/+ vs. +/− mice on a Lin28b KO background. N=11–22. (C) Body weight. (D) Body length. * p<0.05, ** p<0.01. Error bars represent SEM.
Combined dosage of Lin28a/b is critical for postnatal growth
Lin28a and Lin28b are functionally redundant in that they both block biogenesis of let-7 miRNAs and serve as oncogenes [4, 8, 27–29], although the precise mechanism of let-7 repression has been reported to be distinct for Lin28 paralogs [30]. To discern the importance of gene dosage of Lin28a/b on growth phenotypes, we bred Lin28a+/−b−/− mice and examined their progenies. Weights of embryos and placentae at E13.5 were not significantly different between Lin28a+/−b−/− and Lin28a+/+b−/− (i.e. Lin28b KO) mice (Supplementary Fig. 3D). However, in both males and females, Lin28a+/−b−/− mice showed postnatal dwarfism compared to Lin28b KO littermates (Fig. 2C and 2D). Haploinsufficiency of Lin28a affected postnatal growth only in a Lin28b null background, since Lin28a+/− mice grew comparably to Lin28a+/+ (i.e. wild type) controls (Supplementary Fig. 3E), indicating that the combined dose of Lin28a/b alleles is critical to postnatal growth.
Lin28a/b double KO causes developmental delay and embryonic lethality
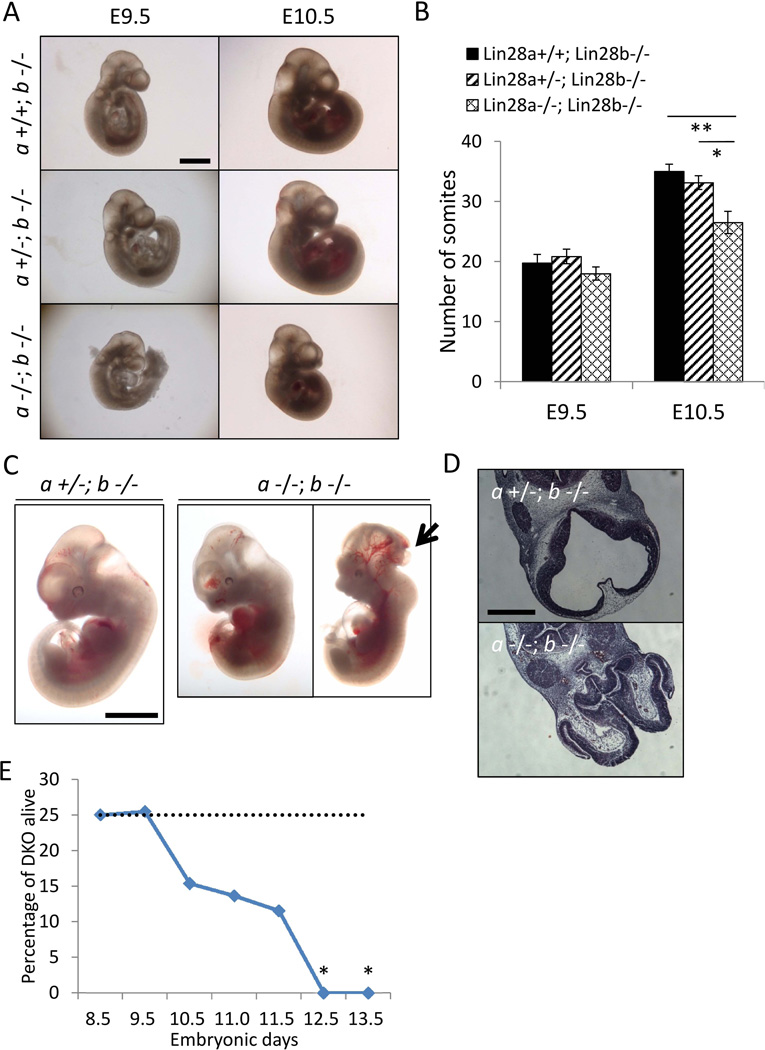
To discern the embryonic phenotypes of dual deficiency of Lin28a/b, we analyzed mid-gestation embryos at different ages and found that double KO (DKO) embryos were smaller in size at E9.5 and E10.5 compared to Lin28a+/+b−/− and Lin28a+/−b−/− embryos (Fig. 3A). Moreover, we observed significantly fewer somites by E10.5 in DKO embryos, suggesting a developmental delay (Fig. 3B). Open neural tubes were found in 2/7 E11.5 DKO embryos, which was also consistent with a developmental delay (Fig. 3C and 3D). Other abnormalities included collapsed ventricles and poor development of the forebrain in some cases. DKO embryos died between E10.5 and E12.5 (Fig. 3E and Supplementary Table).
Figure 3. Double knockout of Lin28a/b leads to synthetic lethality in E10.5–E12.5 embryos.
(A) Representative images, and (B) Number of somites, in Lin28a +/+ vs. Lin28a +/− vs. Lin28a −/− embryos on a Lin28b KO background at E9.5–10.5. N=4–15. (C) Representative images of Lin28a +/− vs. Lin28a −/− embryos on a Lin28b KO background at E11.5. Arrow indicates an open neural tube. (D) Hematoxylin-eosin (H&E) staining of Lin28a +/− vs. Lin28a −/− embryos on a Lin28b KO background at E11.5. (E) Frequency of viable Lin28a −/−; Lin28b −/− embryos at different ages. See the Supplementary Table. Scale bars 1 mm (A and C), 500 µm (D). * p<0.05, ** p<0.01. Error bars represent SEM.
Fetal expression of Lin28a/b is required for regulating postnatal growth
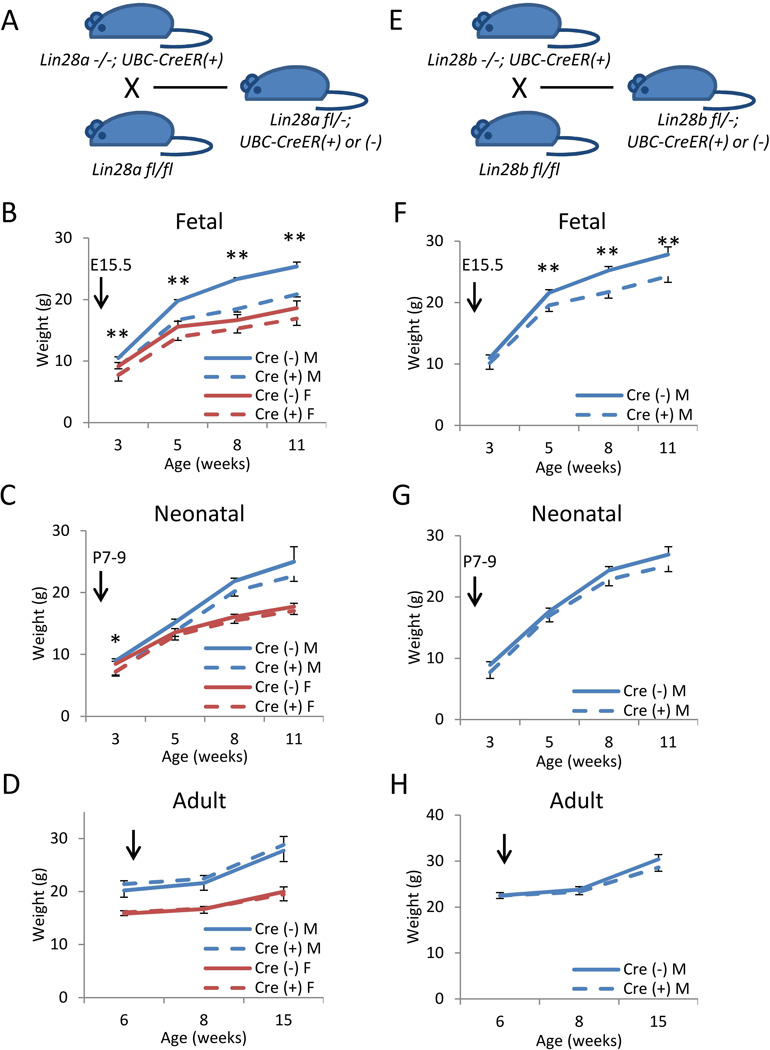
Lin28a and Lin28b are expressed in early embryos but in limited tissues in adults [31]. To test whether embryonic or adult expression regulates postnatal animal growth, we generated tamoxifen-inducible conditional KO mice by crossing a ubiquitin-Cre/ERT2 expressing strain (UBC-CreER) lacking either Lin28a or Lin28b to a strain carrying floxed Lin28afl/fl or Lin28bfl/fl alleles, to generate Lin28a**fl/−; UBC-CreER (+) or Lin28bfl/−; UBC-CreER (+) mice (Fig. 4A and 4E). We injected tamoxifen (TAM) to delete the floxed alleles at different ages. PCR analysis confirmed high efficiency allele deletion in multiple tissues (Supplementary Fig. 4). For Lin28a, Lin28afl/−; UBC-CreER (+) males injected with TAM at E15.5 showed significant postnatal dwarfism, as measured by total body weight and body length (Fig. 4B and Supplementary Fig. 5A), although the degree of dwarfism at 3 wks old is more modest than in constitutive Lin28a KO mice. Lin28afl/−; UBC-CreER (+) females injected with TAM at E15.5 showed no dwarfism phenotype. In contrast, when TAM was injected at 7–9 days (neonate) or 6 weeks of age (adult), their subsequent adult growth was indistinguishable from controls (Fig. 4C–D and Supplementary Fig. 5B–C). For Lin28b, Lin28bfl/−; UBC-CreER (+) males injected with TAM at E15.5 phenocopied the postnatal dwarfism of constitutive Lin28b KO mice (Fig. 4F and Supplementary Fig. 5D). In contrast, no growth phenotype was observed when TAM was injected at 7–9 days (neonate) or 6 weeks old (adult) into Lin28bfl/−; UBC-CreER (+) males (Fig. 4G–H and Supplementary Fig. 5E–F). These results demonstrate that postnatal growth is dictated by fetal expression of both Lin28a and Lin28b, whereas adult expression is dispensable. Interestingly, deletion of Lin28a at E15.5 does not result in the high frequency of perinatal death observed in constitutive Lin28a KO, nor does it phenocopy the full degree of dwarfism due to constitutive loss of Lin28a, whereas deletion of fetal Lin28b at E15.5 fully phenocopies the dwarfism due to constitutive loss of Lin28b. Taken together, our data suggests that Lin28a acts earlier on organismal growth than Lin28b, such that effects of gene deletion are already apparent in utero.
Figure 4. Fetal Lin28a/b, not neonatal or adult Lin28a/b, regulates postnatal growth.
(A) Breeding strategy to test Lin28a fl/− mice at different ages. N=3–12. (B–D) Postnatal growth curves of Lin28a fl/−; UBC-CreER (+) vs. Lin28a fl/− mice. Body weights after tamoxifen (TAM) injections at (B) E15.5, (C) P7–9 and (D) 6 weeks. (E) Breeding strategy to test Lin28b fl/− mice at different ages. N=5–13. (F–H) Postnatal growth curves of Lin28b fl/−; UBC-CreER (+) vs. Lin28b fl/− mice. Body weights after TAM injections at (F) E15.5, (G) P7–9 and (H) 6 weeks. Arrows indicate ages at TAM injection. * p<0.05, ** p<0.01. Error bars represent SEM.
Lin28a KO and Lin28b KO mice suffer from defects in glucose metabolism
We have previously shown that Lin28a and LIN28B transgenic mice are more resistant to diabetes whereas muscle-specific Lin28a KO mice show insulin resistance and impaired glucose uptake [23]. In the present study, we found that Lin28a KO mice show a dramatic loss of fat mass by adulthood (Fig. 1G–H), although we detect no Lin28a/b expression in fat tissues (Supplementary Fig. 6). Thus we further investigated whether Lin28a/b play systemic physiological roles in programming metabolism prior to changes in growth. Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) demonstrated that muscle-specific loss of Lin28b as well as Lin28a [23] led to insulin resistance and impaired glucose uptake (Fig. 5A and 5B). Since Lin28a KO mice already show a significant growth delay as early as E13.5, we performed metabolomic profiling at E10.5 to determine if loss of Lin28a affects embryonic metabolism prior to detectable differences in embryonic growth. We observed a relative accumulation of glucose-6-phosphate and fructose-6-phosphate, and significant decreases in glycolytic intermediates after the phosphofructokinase step, suggesting a lower flux in glycolysis (Fig. 5C). This is consistent with our observations of a lower NADH/NAD ratio, and a higher GSH/GSSG ratio, which are indicative of lower rates of glucose catabolism via glycolysis, Krebs cycle and oxidative phosphorylation, which generate NADH and reactive oxygen species (Fig. 5D). We also found an aberrant drop in dUTP and an accumulation of dTTP, dTDP, dTMP and thymine, suggesting that loss of Lin28a also leads to dysregulation of pyrimidine metabolism (Fig. 5E).
Figure 5. Glucose metabolism dysfunction in Lin28a KO and Lin28b KO mice.
(A) Glucose tolerance tests (GTT) (N=9–10) and (B) Insulin tolerance tests (ITT) (N=8–11) of Lin28b fl/fl; Myf5-Cre mice. (C–E) Metabolomic changes in Lin28a +/− vs. −/− embryos at E10.5. (C) Glycolysis pathway. (D) Bioenergetics and redox balance. (E) Nucleotide synthesis. + p<0.1, * p<0.05, ** p<0.01. Error bars represent SEM.
Fetal muscle-specific expression of Lin28b regulates adult growth
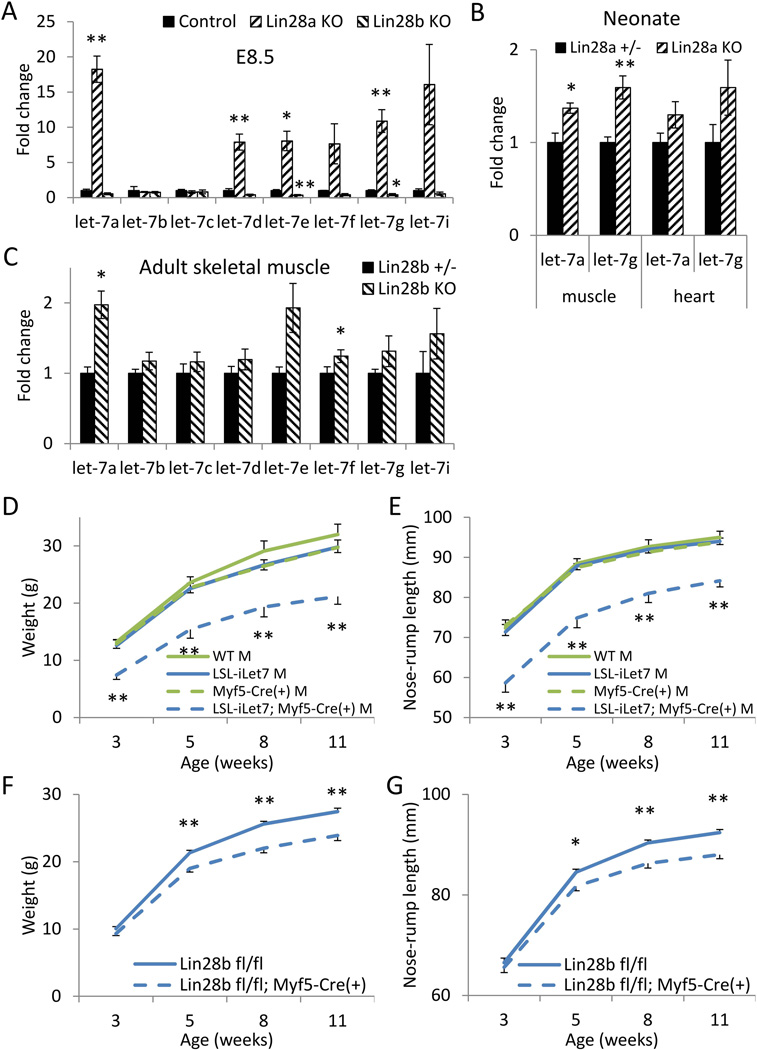
Mice engineered to over-express let-7 miRNA are smaller than controls [23, 32]. To understand how Lin28a and Lin28b deficiency affect let-7 levels, we compared let-7 expression levels by quantitative PCR in E8.5 embryos and various tissues of neonatal and adult mice. Lin28a but not Lin28b KO mice expressed 7- to 18-fold higher levels of let-7a, d, e, f, g, and i but for unclear reasons not b or c, relative to wild-type controls in the E8.5 embryo (Fig. 6A), indicating that Lin28a is the primary regulator of let-7 microRNAs in the early embryo. Let-7 was also higher in the skeletal muscles of neonatal Lin28a KO mice, but no increases in let-7 were observed in adult skeletal muscles nor any other tissue (Fig. 6B and Supplementary Fig. 7A). This result is consistent with the fact that Lin28a is primarily restricted in its expression to fetal tissues. For Lin28b KO mice, some let-7 family members were expressed at modestly higher levels in adult skeletal muscles in Lin28b KO mice (1.3- to 2-fold; Fig. 6C and Supplementary Fig. 7B). These observations indicate that Lin28a potently suppresses let-7 biogenesis during embryogenesis, but its effect decreases postnatally and eventually disappears by adulthood, whereas Lin28b has a smaller but still significant effect on let-7 biogenesis in adulthood, indicating that Lin28a and Lin28b regulate let-7 biogenesis and growth at different developmental stages.
Figure 6. Muscle-specific Lin28b/let-7 regulates postnatal growth.
(A–C) Let-7 levels in (A) Lin28a +/− vs. −/− E8.5 embryos, (B) Lin28a +/− vs. −/− neonatal muscles, and (C) Lin28b +/− vs. −/− adult skeletal muscles. N=3–5. (D–E) Postnatal growth curves of LSL-iLet-7s; Myf5-Cre(+) males given DOX from conception. N=6–13. Statistical significance was shown for LSL-iLet-7s (controls) vs. LSL-iLet-7s; Myf5-Cre(+) mice. (D) Body weights. (E) Body lengths. (F–G) Postnatal growth curves of Lin28b fl/fl; Myf5-Cre males. N=13–17. (F) Body weights. (G) Body lengths. * p<0.05, ** p<0.01. Error bars represent SEM.
Our previous paper reported that iLet-7 mice, in which a Lin28-resistant form of let-7g is induced with doxycycline, manifest growth retardation if let-7g is induced globally from three weeks of age onward [23]. To determine if skeletal muscle-specific let-7 regulates organismal growth, we generated LSL-iLet-7 mice, in which Cre recombinase removes a Lox-Stop-Lox cassette, thereby enabling tissue-specific let-7g induction with doxycycline (See Materials and Methods). We crossed LSL-iLet-7 mice with skeletal muscle specific Myf5-Cre mice, and documented 6 fold more expression of the transgenic let-7g in neonatal skeletal muscle relative to controls (data not shown). Upon induction with doxycycline from conception onward we observed postnatal dwarfism in both males and females, demonstrating that overexpression of let-7 in skeletal muscle is sufficient to cause growth retardation (Fig. 6D and 6E and Supplementary Fig. 7C and 7D).
Furthermore, to determine if the differences in skeletal muscle let-7 between Lin28a and Lin28b KO mice were important for animal growth, we bred skeletal muscle specific Myf5-Cre mice to Lin28afl/fl or Lin28bfl/fl mice. Whereas muscle-specific Lin28a KO mice (Lin28afl/fl; Myf5-Cre+) were comparable in size to control Lin28afl/fl mice (Supplementary Fig. 7E), muscle-specific Lin28b KO (Lin28bfl/fl; Myf5-Cre+) males were smaller than control Lin28bfl/fl males, and phenocopied the dwarfism in constitutive Lin28b KO males (Fig. 6F and 6G, compared to Fig. 2A and 2B). Notably, both Lin28a KO and Lin28bfl/fl; Myf5-Cre+ muscles showed normal histology (Supplementary Fig. 1B and 7F), comparable abundance of fast twitch (type II myosin) muscle fibers, and comparable mitochondrial DNA content and mitochondrial gene expression to controls (Supplementary Fig. 8). In contrast, liver- or pancreatic β cell-specific Lin28b KO did not affect adult growth (Supplementary Fig. 9). Our results indicate that absence of Lin28b in the fetal skeletal muscle is sufficient to dysregulate adult growth, whereas Lin28a must be lacking in additional tissues to manifest a change in adult growth.
Lin28b acts through Tsc1-mTOR signaling in skeletal muscle
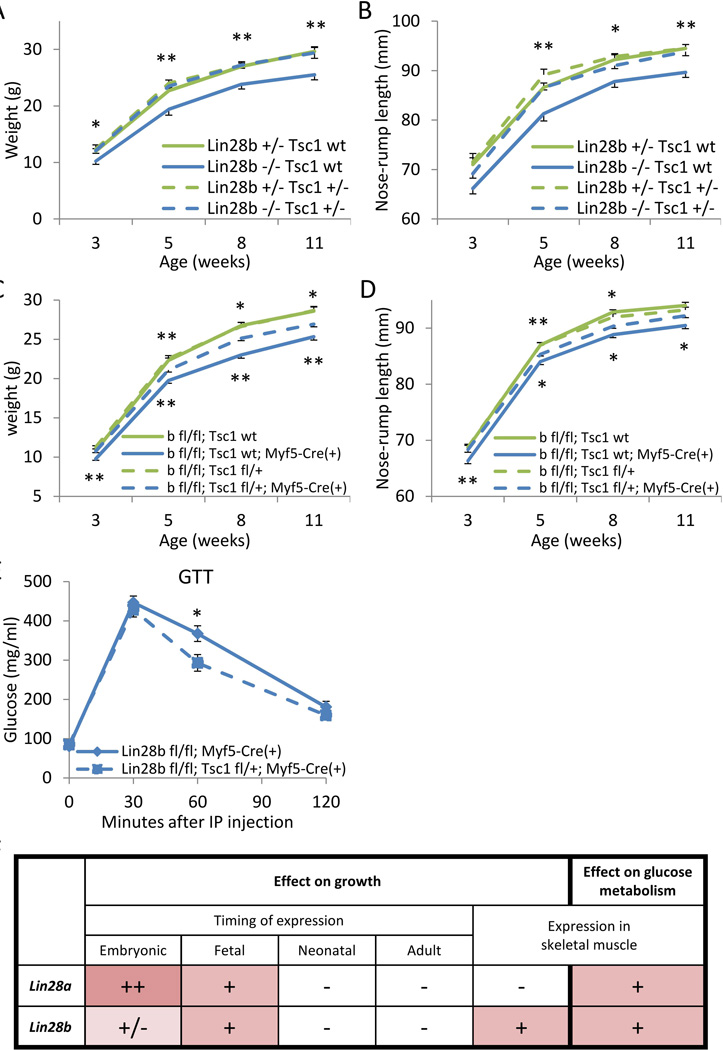
We have previously shown that Lin28a and LIN28B regulate glucose metabolism at least in part via let-7 and the insulin-PI3K-mTOR pathway [23]. Tsc1 is a suppressive regulator of the mTORC1 signaling [33]. Haploinsufficiency of Tsc1 activates mTORC1 signaling. Tsc1 KO mice die during embryonic development, whereas Tsc1+/− mice are viable with increased tumor-susceptibility in various organs [34]. We hypothesized that activation of mTORC1 signaling via Tsc1 haploinsufficiency might rescue the dwarfism of Lin28b KO mice, and thus we crossed Tsc1+/− mice to Lin28b+/− or Lin28b−/− mice and tracked their growth. The growth of Lin28b−/−; Tsc1+/− males was significantly improved compared to Lin28b KO mice, and comparable to that of Lin28b+/− mice (Fig. 7A and 7B). By contrast, Lin28b+/−; Tsc1+/− mice showed no growth advantage relative to Lin28b+/− mice, indicating that the effects of Lin28b deficiency on postnatal growth could be reversed by enhancing mTORC1 signaling. Interestingly, we likewise generated Lin28a−/−; Tsc1+/− mice but found that Tsc1 haploinsufficiency failed to rescue the dwarfism and perinatal lethality phenotypes seen with constitutional Lin28a deficiency.
Figure 7. Tsc1/mTOR signaling in skeletal muscle can partially rescue aberrant programing of glucose metabolism and organismal growth in Lin28b KO mice.
(A–B) Postnatal growth curves of Lin28b KO males, with or without Tsc1 haploinsufficiency. N=6–14. (A) Body weights. (B) Body lengths. Statistical significance was shown for Lin28b −/−; Tsc1 +/+ vs. Lin28b −/−; Tsc1 +/− mice. (C–D) Postnatal growth curves of Lin28b fl/fl; Myf5-Cre males, with or without Tsc1 haploinsufficiency. N=21–29. (C) Body weights. (D) Body lengths. Statistical significance was shown for Lin28b fl/fl; Tsc1 fl/+ vs. Lin28b fl/fl; Tsc1 fl/−; Myf5-Cre(+) mice on the top, and for Lin28b fl/fl; Tsc1 +/+; Myf5-Cre(+) vs. Lin28b fl/fl; Tsc1 fl/−; Myf5-Cre(+) mice on the bottom. (E) Glucose tolerance tests (GTT) of Lin28b fl/fl; Tsc1 fl/+; Myf5-Cre males. N=8–11. (F) Summary of the timing and tissue of Lin28a/b expression and the effect on growth and glucose metabolism. Note that pink color denotes the positive effect on normal growth and glucose metabolism. The intensity of color and the number of + signs reflect the magnitude of the effect. “+/−“ indicates that Lin28b has the effect on growth only if combined with Lin28a loss. * p<0.05, ** p<0.01. Error bars represent SEM.
Next, we tested the hypothesis that Lin28b/Tsc1 effects on growth are skeletal muscle specific. We generated Lin28b fl/fl; Tsc1 fl/+; Myf5-Cre mice, in which Lin28b KO and Tsc1 haploinsufficiency are achieved only in skeletal muscles. Tsc1 haploinsufficiency in skeletal muscles partially rescued the postnatal dwarfism phenotype seen in skeletal muscle specific Lin28b KO mice (Fig. 7C and 7D). Moreover, GTT demonstrated modest but significant improvement in Lin28b fl/fl; Tsc1 fl/+; Myf5-Cre mice compared to Lin28b fl/fl; Myf5-Cre mice (Fig. 7E). Taken together, our results suggest that Lin28b expression in fetal muscle programs adult metabolism and growth in part through the Tsc1-mTORC1 signaling pathway.
DISCUSSION
Heterochronic metabolism and the Lin28a/b-mTOR pathway
Both lin-28 and let-7 were originally identified in a C. elegans mutagenesis screen as heterochronic regulators of developmental timing. We and others have reported that overexpression of Lin28a and let-7 affect organismal size [23, 32] and onset of puberty in mice and humans [12–15]. In searching for the mechanism of growth regulation, we found that mammalian Lin28a/b also regulate glucose metabolism, in part through the let-7-mediated repression of multiple components of the insulin-PI3K-mTOR pathway [23]. In this study, using genetic KO mouse models we have established that Lin28a and Lin28b are physiologically required for normal glucose homeostasis, albeit with distinct spatio-temporal patterns during mammalian development (Fig. 7F). Furthermore, we have found that fetal Lin28a/b exerts long-lasting effects on adult metabolism and growth, long after the normal expression patterns of these paralogs have extinguished in most tissues. Indeed, Lin28afl/−; UBC-CreER (+) or Lin28bfl/−; UBC-CreER (+) mice revealed that when deleted in adulthood, Lin28a or Lin28b are entirely dispensable with regard to the persistent growth observed with organismal aging in mice. Long-standing adult growth and metabolic effects were observed without concomitant gross defects in mammalian development, nor a disproportionate decrease in tissue mass, save for fat, in which Lin28a/b are not expressed. We found that deficiency of Lin28a/b in fetal muscle dysregulates adult metabolism; although we observe no defects in muscle development. Our finding that haploinsufficiency of Tsc1 can partially rescue the growth and glucose metabolism defects of Lin28b KO mice, further suggests that fetal Lin28b’s effects on adult metabolism are mediated, at least in part, by mTORC1 signaling. Loss of embryonic Lin28a led to a general dysregulation of glycolytic flux in early embryos before changes in growth were detected, consistent with previous findings that mTORC1 signaling directly regulates glucose metabolism [33].
Model of differential effects of Lin28a and Lin28b on postnatal growth
Although our results demonstrate that adult growth is altered by fetal but not adult deficiency of Lin28a or Lin28b, we observed important differences in the phenotypes mediated by temporal and tissue-specific deficiency of these closely related paralogs. Conditional deletion of Lin28a at E15.5 failed to phenocopy constitutive loss of Lin28a, which was associated with perinatal lethality and dwarfism, whereas Lin28b deletion at a similar stage fully phenocopied the dwarfism due to constitutive loss of Lin28b. Furthermore constitutive loss of Lin28a caused metabolic dysfunction and dwarfism in embryos, whereas constitutive loss of Lin28b did not influence metabolism until later in adults. These data suggest that Lin28a is relevant at an earlier embryonic stage of development than Lin28b, although both are relevant during mid-gestation as combined loss of both Lin28a and Lin28b led to synthetic lethality first noted around E10.5.
Fetal Lin28a/b and the Barker hypothesis
The “Barker hypothesis”, originally proposed two decades ago, holds that an epigenetic memory of poor fetal and infant nutrition causes permanent changes in metabolism and leads to increased risks in adult life for chronic metabolic diseases such as type 2 diabetes (T2D) [35, 36]. Epidemiological observations support the relationship between fetal metabolism and adult insulin resistance, and it is now generally accepted that environmental factors in early life play a major role in the pathogenesis of T2D [35, 36]. Our conditional KO mice and metabolomics studies here establish that deletion of Lin28a/b during fetal development dictates long-term effects on adult metabolism, resulting in increased insulin resistance and a diabetic phenotype, opposite to the enhanced glucose metabolism which we recently reported as a consequence of hyperfunction of Lin28 in conditional transgenic murine models [23]. The Lin28/let-7 axis thus represents a means by which fetal metabolic regulators can influence life-long growth and metabolism phenotypes. Although we have identified fetal muscle cells as key cellular targets of Lin28 function, and the mTORC1 pathway in the molecular mechanism, how defects in fetal expression of the Lin28/let-7 axis translates into the long-term epigenetic changes that influence adult metabolism remains a mystery. Our several strains of Lin28a/b knockout mice should provide powerful tools to study the fetal programming of adult metabolism, with relevance to the ongoing search for new medical interventions in chronic metabolic diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mathew William Lensch for invaluable discussions and advice, David J. Kwiatkowski for Tsc1 mice, and Roderick Bronson and the Harvard Medical School Rodent Histopathology Core for mouse tissue pathology. We also thank Min Yuan and Susanne Breitkopf for help with mass spectrometry experiments and grants NIH 5P01CA120964-04 and NIH DF/HCC Cancer Center Support Grant 5P30CA006516-46 (J.M.A.) The work was supported by grants from the Alex’s Lemonade Stand Foundation and the Ellison Medical Foundation to GQD. GQD is an affiliate member of the Broad Institute, and an investigator of the Howard Hughes Medical Institute and the Manton Center for Orphan Disease Research.
Footnotes
AUTHOR CONTRIBUTIONS
G.S. conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript. N.S.C. collection and/or assembly of data, and data analysis and interpretation. T.Y.S., H.Z., M.T.S., S.P.S., and N.A.S. collection and/or assembly of data. A.Y., J.P.H., R.I.G., and E.G.M. provision of study materials. J.M.A. collection and/or assembly of data, data analysis and interpretation. L.C.C. data analysis and interpretation. G.Q.D. conception and design, data analysis and interpretation, manuscript writing and final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no competing financial interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes nine figures and one table can be found with this article online.
REFERENCES
- 1.Lettre G, Jackson AU, Gieger C, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40:584–591. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lango Allen H, Estrada K, Lettre G, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanktree MB, Guo Y, Murtaza M, et al. Meta-analysis of Dense Genecentric Association Studies Reveals Common and Uncommon Variants Associated with Height. Am J Hum Genet. 2011;88:6–18. doi: 10.1016/j.ajhg.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heo I, Joo C, Cho J, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piskounova E, Viswanathan SR, Janas M, et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008;283:21310–21314. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 7.Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polesskaya A, Harel-Bellan A. A novel role for an embryonic regulatory protein Lin-28 in adult skeletal muscle differentiation. Med Sci (Paris) 2007;23:796–797. doi: 10.1051/medsci/20072310796. [DOI] [PubMed] [Google Scholar]
- 10.Qiu C, Ma Y, Wang J, et al. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38:1240–1248. doi: 10.1093/nar/gkp1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong KK, Elks CE, Li S, et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009 doi: 10.1038/ng.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulem P, Gudbjartsson DF, Rafnar T, et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet. 2009 doi: 10.1038/ng.383. [DOI] [PubMed] [Google Scholar]
- 14.He C, Kraft P, Chen C, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724–728. doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry JR, Stolk L, Franceschini N, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009;41:648–650. doi: 10.1038/ng.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 17.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 18.Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 19.Nimmo RA, Slack FJ. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma. 2009;118:405–418. doi: 10.1007/s00412-009-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258:432–442. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 21.Schulman BR, Esquela-Kerscher A, Slack FJ. Reciprocal expression of lin-41 and the microRNAs let-7 and mir-125 during mouse embryogenesis. Dev Dyn. 2005;234:1046–1054. doi: 10.1002/dvdy.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu H, Shah S, Shyh-Chang N, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010;42:626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H, Shyh-Chang N, Segre AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belteki G, Haigh J, Kabacs N, et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan M, Breitkopf SB, Yang X, et al. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Chen Y, Ito H, et al. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanathan SR, Powers JT, Einhorn W, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piskounova E, Polytarchou C, Thornton JE, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003;3:719–726. doi: 10.1016/s1567-133x(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 32.Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci U S A. 2011;108:21075–21080. doi: 10.1073/pnas.1118922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwiatkowski DJ, Zhang H, Bandura JL, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 35.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 36.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.