Abstract
Background
Individuals with a history of colorectal cancer (CRC) have an increased risk of subsequent cancer. We used cancer registry data to evaluate whether this increased risk of cancer after CRC differed by anatomic subsite of a first CRC.
Methods
Individuals diagnosed with first primary CRC between 1992–2009 were identified from 12 Surveillance Epidemiology and End Results (SEER) cancer registries. We calculated standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) comparing the incidence of subsequent cancers in these index CRC cases to cancer incidence rates in the general population. SIRs were calculated for cancers at anatomic sites within and outside the colorectum in analyses stratified by subsite of the index CRC.
Results
Cancer incidence rates were significantly higher in those with prior CRC than in the general population (SIR=1.15, 95% CI: 1.13–1.16). Individuals with an index CRC located between the transverse and descending colon experienced the greatest increased risk both overall (SIR=1.29 to 1.33), and with respect to risk of second CRC in particular (SIR=2.53 to 3.35). Incidence of small intestinal cancer was significantly elevated regardless of index CRC subsite (SIR=4.31, 95% CI: 3.70–4.77); incidence of endometrial cancer was elevated in those with index CRC in the proximal colon (SIR=1.37 to 1.79).
Conclusions
Risk of second cancer after CRC differs by anatomic site of the first tumor, and is particularly pronounced for those with prior CRC located in the transverse to descending colon. The mechanisms underlying this pattern of second cancer risk remain unknown.
Keywords: colorectal cancer, second cancer, metachronous cancer, standardized incidence ratio, tumor subsite
INTRODUCTION
Advances in the early detection and treatment of colorectal cancer (CRC) have contributed to improvements in CRC prognosis over recent decades, such that the population of CRC survivors is growing.1 As the short-term prognosis for CRC improves, longer-term health concerns facing CRC survivors will likely become more prominent.2–4 One such concern is the increased risk of second primary cancers in CRC survivors.5–10 This increased risk may reflect shared genetic or environmental risk factors for different malignancies or the effect of treatment for primary CRC.
Previous studies have reported up to a 1.4-fold increased risk of a second cancer after initial CRC.5,7,8,11 This elevated risk is most consistently observed for risk of second primary CRC.6–8,10–12 Less consistent evidence suggests an increased risk of cancer of the stomach,9 small intestine,5–7,9 breast,5–7 ovaries,6,7 prostate,6,7 bladder,7 kidney,6,7,9 and endometrium5–7,9 after CRC. Based on these prior observations and the fact that biological characteristics of cancer may reflect, to some extent, the embryologic origin of tissue, we hypothesized that the excess risk of second primary cancer after CRC might be high for organs that are developmentally-related with endoderm-derived epithelia, particularly tissues in close proximity to the colorectum.
Recent studies have suggested that risk of second primary cancer after CRC also differs by anatomic site of the first CRC.10,11,13 In particular, it has been suggested that risk of second primary CRC is greater in individuals with a first primary CRC located in the proximal colon compared to individuals with first primary CRC in the distal colon or rectum.10,11,13 In light of differences in tumor biology and potential differences in tumor etiology across the colorectal continuum,14,15 it is plausible that the distribution and risk of second primary cancer could differ according to site of first primary CRC. Thus, we tested the hypothesis that the risk of second primary CRC in bowel subsites may change as a continuum in relation to the subsite of first primary CRC.
Using data from Surveillance Epidemiology and End Results (SEER) cancer registries,16 we estimated the risk of second primary cancer after CRC according to the site of the first and the second primary cancer.
METHODS
We used data from the 12 SEER cancer registries that have contributed cancer incidence data since 1992 to the linked multiple-primary SEER*Stat query database: Hawaii; Iowa; Connecticut; New Mexico; Utah; Atlanta, Georgia; Rural Georgia; Detroit, Michigan; San Francisco-Oakland, California; San Jose-Monterey, California; Los Angeles, California; and Seattle-Puget Sound, Washington.16 Information is collected by SEER on all incident cancers, excluding basal or squamous skin cancer, occurring within these ascertainment regions. SEER also collects follow-up information on deaths and diagnoses of new cancers in individuals with a history of cancer.
To ensure that recurrences and metastases are not recorded as new primary cancers, SEER registrars adhere to a series of coding rules.17 To be classified as a primary cancer, a tumor subsequent to an invasive CRC cannot be described as a metastasis in the clinical record and must be located in a site with different International Classification of Diseases for Oncology third edition (ICD-O-3) topography or histology codes or, if codes are the same, must be diagnosed more than a year after the prior primary CRC. Hereafter we refer to first primary CRC as index CRC.
Index CRC Cases
For the present study, index cases included individuals diagnosed with an index invasive CRC between 1992–2009 within the catchment area for the aforementioned SEER registries. We restricted inclusion to individuals diagnosed at ages 40–79 years with no history of cancer prior to the index CRC. We excluded individuals with index CRC that was identified by death certificate or autopsy only, those with cancers located in the appendix, and those with an index CRC of unknown anatomic subsite. In total, 170,159 index cases met these criteria.
Second Primary Case Criteria
All second primary cancers were diagnosed among index CRC cases between 1992–2009, within the ascertainment area of included SEER registries. Definitions for second primary cancer were based on established SEER criteria.17 Tumors diagnosed during a two month period after the index CRC diagnosis were excluded as likely synchronous tumors. We also conducted sensitivity analyses further excluding tumors diagnosed within six months of index CRC. We restricted our evaluation to second primary cancers diagnosed within 10 years of index CRC; approximately 89% of second cancers were diagnosed within this timeframe. Some individuals were diagnosed with multiple primary cancers within this timeframe; all invasive cancers subsequent to the index CRC were included in calculations of standardized incidence ratios (SIRs), as described below. In situ second cancers were excluded. In total, 16,697 second primary cancers in 14,880 index CRC cases met these inclusion criteria.
Statistical Analyses
We calculated SIRs comparing the observed occurrence of second primary cancers after an index CRC to that which would have been expected based on cancer incidence rates in the general population of the SEER ascertainment areas. Expected incidence rates were stratified by sex, age (five-year intervals), calendar year (five-year intervals), and race (white, black, other); person-years at risk for second cancer were stratified in the same manner. Numbers of expected cancers were calculated by multiplying stratum-specific person-years at risk and corresponding stratum-specific incidence rates, and summing these products across strata. SIRs thus represent the ratio of observed to expected second primary cancers, with adjustment for sex, age, calendar year, and race.
SIRs were calculated separately by index CRC subsite: cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and rectum. For each case group defined by index CRC subsite, we calculated SIRs for second primary cancers at all anatomic sites combined, at all non-CRC sites combined, for each of the most common non-CRC cancer sites, and for each cancer subsite within the colorectum. SIRs based on fewer than five observed second primary cases are not presented. We conducted exploratory analyses stratified by race and by stage at index diagnosis. We restricted SIR calculations to diagnoses and person-years at risk accrued in the 10 years following index CRC diagnosis. A determination of statistical significance was based on a two-sided p-value <0.05.
We also estimated the absolute excess risk for second CRC and for non-CRC second primary cancers with SIRs statistically significantly different from the null. Excess risk was calculated as the number of observed cases minus the number of expected cases divided by person-years at risk. Estimates of excess risk are presented per 10,000 person-years at risk.
All analyses were conducted using SEER*Stat software (Version 7.1.0).
RESULTS
Study Population Characteristics
Characteristics of the index case population are presented in Table 1. The majority of index cases were aged 60–79 years (67%), male (54%), and non-Hispanic White (69%); the proportion of cases exhibiting these attributes was greater among those who subsequently developed a second primary cancer.
TABLE 1.
Characteristics of first primary colorectal cancer (CRC) cases according to occurrence and site of second primary cancer, SEER 13 [1992–2009]
| All 1st Primary CRC Cases N (col %) | Cases who Developed a 2nd Primary Cancer |
||
|---|---|---|---|
| All 2nd Primary Sites N (col %) | 2nd Primary CRC N (col %) | ||
| Age at 1st CRC diagnosis | |||
| 40–49 | 16,834 (10) | 675 (5) | 206 (7) |
| 50–59 | 38,679 (23) | 2,359 (16) | 499 (17) |
| 60–69 | 53,089 (31) | 5,163 (35) | 904 (32) |
| 70–79 | 61,557 (36) | 6,683 (45) | 1,245 (44) |
| Sex | |||
| Male | 92,299 (54) | 9,029 (61) | 1,594 (56) |
| Female | 77,860 (46) | 5,851 (39) | 1,260 (44) |
| Race | |||
| White non-Hispanic | 117,125 (69) | 10,923 (73) | 1,984 (70) |
| Black | 18,493 (11) | 1,618 (11) | 369 (13) |
| White Hispanic | 14,600 (9) | 998 (7) | 246 (9) |
| American Indian/Alaska Native | 778 (0.5) | 67 (0.5) | 10 (0.4) |
| Asian or Pacific Islander | 18,032 (11) | 1,263 (8) | 240 (8) |
| Other/unspecified | 1,131 (1) | 11 (0.1) | 5 (0.2) |
| Stage at 1st CRC diagnosis | |||
| Localized | 71,436 (42) | 8,000 (54) | 1,349 (47) |
| Regional | 64,925 (38) | 5,764 (39) | 1,183 (41) |
| Distant | 29,837 (18) | 801 (5) | 216 (8) |
| Unstaged | 3961 (2) | 315 (2) | 106 (4) |
| Site of 1st CRC | |||
| Cecum | 25,856 (15) | 2,231 (15) | 380 (13) |
| Ascending colon | 18,615 (11) | 1,743 (12) | 267 (9) |
| Hepatic flexure | 5,923 (3) | 549 (4) | 103 (4) |
| Transverse colon | 9,757 (6) | 945 (6) | 218 (8) |
| Splenic flexure | 4,365 (3) | 432 (3) | 130 (5) |
| Descending colon | 7,624 (4) | 784 (5) | 185 (6) |
| Sigmoid colon | 41,829 (25) | 3,880 (23) | 761 (27) |
| Rectosigmoid junction | 16,952 (10) | 1,418 (10) | 275 (10) |
| Rectum | 39,238 (23) | 2,898 (19) | 535 (19) |
| SEER Registry * | |||
| San Francisco-Oakland | 18,826 (11) | 1,553 (10) | 283 (10) |
| Connecticut | 19,001 (11) | 1,812 (12) | 300 (11) |
| Detroit | 20,429 (12) | 2,106 (14) | 425 (15) |
| Hawaii | 7,033 (4) | 536 (4) | 95 (3) |
| Iowa | 17,390 (10) | 1,672 (11) | 314 (11) |
| New Mexico | 7,491 (4) | 539 (4) | 96 (3) |
| Seattle-Puget Sound | 17,277 (10) | 1,579 (11) | 245 (9) |
| Utah | 6,472 (4) | 500 (3) | 100 (4) |
| Atlanta | 10,250 (6) | 731 (5) | 138 (5) |
| San Jose-Monterey | 9,119 (5) | 706 (5) | 117 (4) |
| Los Angeles | 36,203 (21) | 3,093 (21) | 731 (26) |
| Rural Georgia | 668 (0.4) | 53 (0.4) | 10 (0.4) |
Excludes Alaska Native Tumor Registry, which is not included in SEER multiple primary data files.
SIRs for Second Primary Cancer
Regardless of the subsite of index CRC, risk of second primary cancer was significantly elevated (Table 2). Among those with index cancers in the cecum, ascending colon, transverse colon, or descending colon, SIRs remained significantly elevated after excluding second primary CRC from SIR calculations. However, among individuals with an index rectal cancer, the risk of second primary non-colonic cancer was significantly lower relative to the general population [SIR=0.96, 95% confidence interval (CI): 0.92–1.00]. The most consistent and markedly elevated SIRs for non-CRC were for cancer of the small intestine, with statistically significant SIRs ranging from 2.24 (95% CI: 1.47–3.26) for index rectal cancer cases to 7.06 (95% CI: 5.19–9.39) for index ascending colon cancer cases. There was also a significantly elevated incidence of second primary lung (SIR=1.14, 95% CI: 1.10–1.18), bladder (SIR=1.11, 95% CI: 1.04–1.18), kidney (SIR=1.42, 95% CI: 1.30–1.54), stomach (SIR=1.28, 95% CI: 1.16–1.42), and endometrial (SIR=1.26, 95% CI: 1.14–1.40) cancers after index CRC. Associations with elevated incidence for these second primary cancers were consistent by race and stage, with the exception that the elevated SIR for stomach cancer after CRC was largely limited to those with localized-stage index CRC (SIR=1.44, 95% CI: 1.26–1.64 versus SIR=1.09, 95% CI: 0.90–1.29 for regional-stage index cases) (not shown). Associations were not consistent across anatomic subsites of index CRC. In particular, the elevated SIR for second primary endometrial cancers was most pronounced among individuals with index CRC in the proximal colon. A lower than expected incidence of prostate cancer was observed (SIR=0.91, 95% CI: 0.88–0.95), particularly among cases with index cancer in the rectosigmoid junction (SIR=0.82, 95% CI: 0.73–0.92) or rectum (SIR=0.65, 95% CI: 0.59–0.71). However, in analyses stratified by race, this lower incidence of prostate cancer was only evident in Whites (SIR=0.87, 95% CI: 0.84–0.91); incidence of second primary prostate cancer was significantly elevated in Black index cases (SIR=1.13, 95% CI: 1.02–1.24) (not shown).
TABLE 2.
Cancer site-specific standardized incidence ratios (SIRs) for second primary cancer by site of first primary cancer within the rectum, SEER 13 (1992–2009)
| All1st primary subsites | Cecum | Ascending Colon | Hepatic Flexure | Transverse Colon | Splenic Flexure | Descending Colon | Sigmoid Colon | Rectosigmoid Junction | Rectum | |
|---|---|---|---|---|---|---|---|---|---|---|
| All Sites | 1.15* | 1.14* | 1.22* | 1.20* | 1.29* | 1.30* | 1.33* | 1.14* | 1.08* | 1.04* |
| All non-CRC sites | 1.04* | 1.07* | 1.15* | 1.09 | 1.13* | 1.04 | 1.14* | 1.03 | 0.98 | 0.96* |
| Prostate | 0.91* | 1.02 | 1.09 | 0.94 | 1.06 | 1.01 | 1.11 | 0.97 | 0.82* | 0.65* |
| Lung/Bronchus | 1.14* | 1.09 | 1.23* | 1.06 | 1.24* | 1.04 | 1.14 | 1.10* | 1.07 | 1.21* |
| Female Breast | 0.98 | 0.99 | 1.06 | 1.31* | 0.97 | 0.92 | 0.98 | 0.98 | 0.97 | 0.87* |
| Urinary Bladder | 1.11* | 1.20* | 0.88 | 1.08 | 1.04 | 0.65 | 1.29 | 1.10 | 1.34* | 1.11 |
| Non-Hodgkin's Lymphoma | 0.97 | 1.03 | 1.16 | 1.09 | 0.78 | 1.13 | 0.83 | 0.85 | 0.94 | 1.05 |
| Pancreas | 1.04 | 0.88 | 1.36* | 2.02* | 1.02 | 1.23 | 1.08 | 0.93 | 0.97 | 0.91 |
| Kidney | 1.42* | 1.59* | 1.25 | 1.37 | 1.15 | 1.46 | 1.84* | 1.46* | 0.95 | 1.53* |
| Melanoma | 0.93 | 1.05 | 0.79 | 1.49 | 1.03 | 0.95 | 1.12 | 0.86 | 0.95 | 0.85 |
| Stomach | 1.28* | 1.19* | 1.26 | 1.57 | 1.80* | 2.06* | 1.08 | 1.27* | 1.23 | 1.16 |
| Endometrium | 1.26* | 1.57* | 1.37* | 1.49 | 1.79* | 1.37 | 1.25 | 1.24 | 0.96 | 0.89 |
| Non-Lymphocytic Leukemia | 1.08 | 1.14 | 1.14 | 0.84 | 1.33 | 1.01 | 0.81 | 1.07 | 1.28 | 0.96 |
| Esophagus | 1.12 | 1.25 | 1.04 | 0.99 | 1.47 | -- | 1.28 | 1.18 | 0.95 | 1.01 |
| Small intestine | 4.21* | 5.64* | 7.06* | 5.60* | 4.67* | 4.47* | 3.69* | 3.48* | 4.05* | 2.24* |
| Lymphocytic Leukemia | 0.78* | 0.62* | 0.75 | -- | 1.10 | -- | 0.60 | 0.87 | 0.60 | 0.91 |
| Larynx | 1.10 | 1.01 | 1.46 | -- | 0.86 | -- | 1.69 | 1.17 | 1.00 | 0.98 |
| Liver | 0.79* | 0.45* | 1.23 | -- | 1.00 | 1.20 | 1.41 | 0.79 | 0.60 | 0.71 |
p<0.05
-- SIRs based on <5 second primary cases.
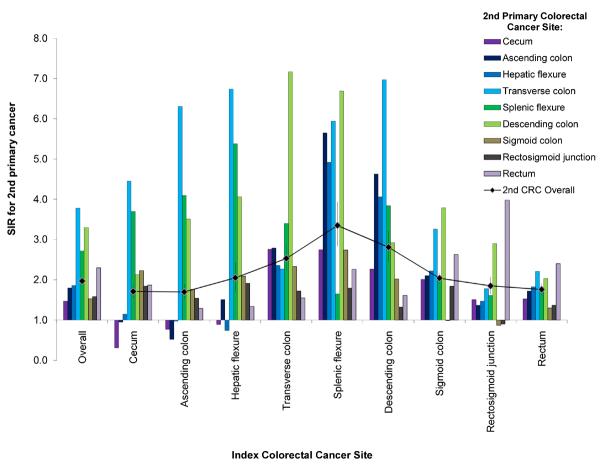
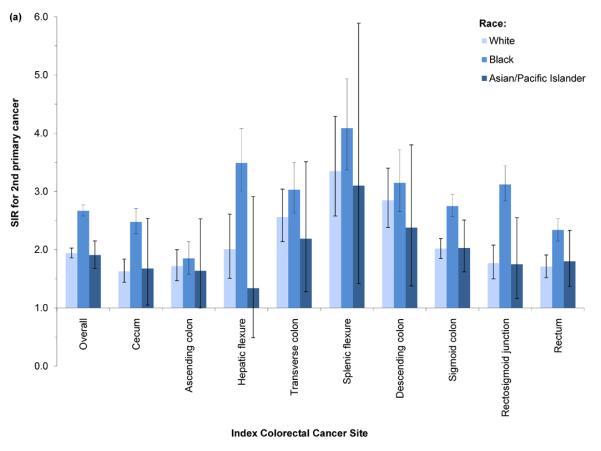
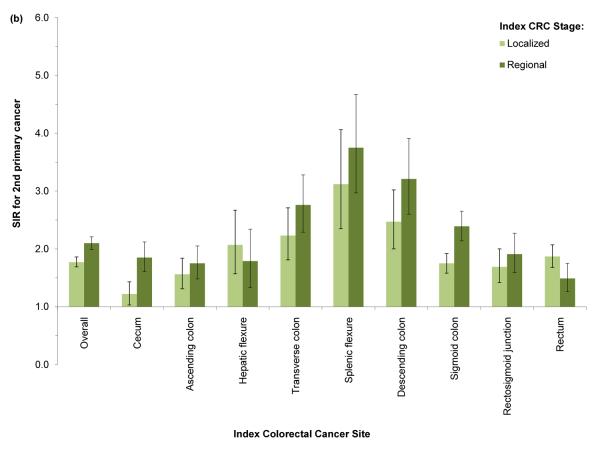
SIRs for second primary CRC were significantly elevated regardless of index CRC subsite (Figure 1). The SIR for second CRC was highest among cases with index CRC in the splenic flexure (SIR=3.35, 95% CI: 2.83–3.94). SIRs were slightly lower in cases with index CRC in the descending (SIR=2.81, 95% CI: 2.44–3.20) and transverse colon (SIR=2.53, 95% CI: 2.22–2.86); SIRs declined with increasing anatomic distance from the splenic flexure. Cases with index cecal or ascending colon cancer experienced significantly lower incidence rates of second cancers in the cecum and ascending colon; however, all other significant SIRs for index/second CRC subsite combinations indicated elevated incidence. In analyses of all index cases combined, SIRs were most elevated with respect to incidence of second primary CRC located in the transverse colon (SIR=3.78, 95% CI: 3.44–4.15), descending colon (SIR=3.30, 95% CI: 2.90–3.75), and splenic flexure (SIR=2.72, 95% CI: 2.26–3.25). Similar patterns of elevated risk were evident across strata defined by race and by stage (Figures 2a–2b); however, the SIR for second primary CRC was higher in Black index cases than in Whites or Asian/Pacific-Islanders, regardless of index CRC subsite.
Figure 1.
Subsite-specific standardized incidence ratio (SIR) for 2nd primary colorectal cancer (CRC) by anatomic site of index CRC, SEER 13 (1992–2009). 2nd CRC diagnosis dates range from 2 months to 10 years after index CRC diagnosis in cases aged 40–79 years.
Figure 2.
Standardized incidence ratio (SIR) for 2nd primary colorectal cancer (CRC) by anatomic site of index CRC and (a) race or (b) stage at index diagnosis, SEER 13 (1992–2009). 2nd CRC diagnosis dates range from 2 months to 10 years after index CRC diagnosis in cases aged 40–79 years.
Excess Risk for Second Primary Cancer
In addition to having the highest SIRs for second primary cancer, cases with index CRC located in the transverse to descending colon had the highest absolute excess risk of second primary cancer: an estimated 52.9 to 58.2 excess cancers per 10,000 person-years were diagnosed in individuals with these index cancers relative to the general population (Table 3). The magnitude of excess risk, in all index cases, was driven by the excess risk of second primary CRC. Overall, 19.7 excess CRCs were diagnosed per 10,000 person-years in individuals with a prior CRC; estimates of excess risk ranged from 13.8 to 49.2 per 10,000 person-years in individuals with index rectal and splenic flexure cancers, respectively.
TABLE 3.
Cancer site-specific absolute excess risk for second primary cancer per 10,000 person-years by site of first primary cancer within the colon or rectum, SEER 13 (1992–2009)
| All 1st primary subsites | Cecum | Ascending Colon | Hepatic Flexure | Transverse Colon | Splenic Flexure | Descending Colon | Sigmoid Colon | Rectosigmoid Junction | Rectum | |
|---|---|---|---|---|---|---|---|---|---|---|
| All 2nd Primary Sites | 26.2 | 27.0 | 41.3 | 38.6 | 52.9 | 56.0 | 58.2 | 25.2 | 13.5 | 7.2 |
| Prostate | −6.2 | 1.4 | 7.4 | −5.0 | 4.8 | 0.5 | 8.1 | −1.8 | −12.5 | −23.2 |
| Lung/Bronchus | 3.8 | 2.8 | 6.7 | 1.7 | 6.8 | 1.1 | 3.9 | 2.7 | 2.0 | 5.1 |
| Urinary Bladder | 1.1 | 2.1 | −1.3 | 0.9 | 0.4 | −3.6 | 2.8 | 1.0 | 3.3 | 1.0 |
| Kidney | 1.8 | 2.7 | 1.1 | 1.7 | 0.7 | 2.1 | 3.7 | 2.0 | −0.2 | 2.3 |
| Stomach | 1.0 | 0.7 | 1.0 | 2.2 | 3.0 | 3.9 | 0.3 | 1.0 | 0.8 | 0.5 |
| Endometrium | 2.1 | 4.7 | 3.0 | 4.0 | 6.4 | 2.9 | 2.0 | 1.9 | −0.3 | −0.9 |
| Small intestine | 2.3 | 3.5 | 4.6 | 3.5 | 2.7 | 2.6 | 1.9 | 1.7 | 2.0 | 0.8 |
| Lymphocytic Leukemia | −0.5 | −0.9 | −0.6 | -- | 0.2 | -- | −0.9 | −0.3 | −0.9 | −0.2 |
| Liver | −0.5 | −1.2 | 0.5 | -- | 0.0 | 0.5 | 1.0 | −0.5 | −0.9 | −0.7 |
| CRC | 19.7 | 15.6 | 15.3 | 22.9 | 32.8 | 49.2 | 36.6 | 20.8 | 16.8 | 13.8 |
| Cecum | 1.7 | −2.8 | −0.9 | −0.4 | 6.7 | 6.4 | 4.4 | 3.4 | 1.7 | 1.6 |
| Ascending colon | 2.2 | −0.1 | −1.5 | 1.5 | 5.2 | 13.0 | 9.7 | 2.9 | 0.9 | 1.7 |
| Hepatic flexure | 0.7 | 0.1 | 0.0 | −0.2 | 1.2 | 3.4 | 2.6 | 1.0 | 0.4 | 0.6 |
| Transverse colon | 3.9 | 5.5 | 8.4 | 9.0 | 1.9 | 7.1 | 8.3 | 3.1 | 1.0 | 1.5 |
| Splenic flexure | 0.9 | 1.6 | 1.8 | 2.6 | 1.4 | 0.4 | 1.6 | 0.6 | 0.3 | 0.3 |
| Descending colon | 2.0 | 1.1 | 2.4 | 2.9 | 5.7 | 5.3 | 1.7 | 2.5 | 1.6 | 0.8 |
| Sigmoid colon | 2.3 | 5.5 | 3.4 | 5.0 | 5.9 | 7.6 | 4.4 | 0.0 | −0.5 | 1.2 |
| Rectosig. junction | 0.9 | 1.4 | 0.9 | 1.5 | 1.2 | 1.3 | 0.5 | 1.3 | −0.2 | 0.5 |
| Rectum | 4.9 | 3.4 | 1.1 | 1.3 | 3.0 | 4.8 | 2.3 | 6.1 | 11.0 | 4.9 |
--Calculation based on <5 second primary cases.
DISCUSSION
In this analysis of cancer registry data, the risk of a second primary cancer after CRC was significantly greater than expected based on cancer incidence rates in the general population. The magnitude of this increased risk differed according to the location of index CRC, and was most pronounced for cases with index CRC of the transverse colon, splenic flexure, and descending colon. These subsites were also the locations at which there was the greatest absolute excess risk of second primary CRC, indicating a generally greater susceptibility to second primary cancer in patients with index cancers in these colonic segments.
Several prior studies have observed an increased risk of second primary cancers after CRC.5–11 In particular, studies have noted an increased risk of bladder,5,7 kidney,5–7 stomach,5,7,9 small intestine,5–7,9 and endometrial5–9 cancer after CRC. In a previous SEER analysis of second primary non-colonic cancers after CRC, Ahmed et al. found that SIRs were most pronounced with respect to risk of small intestinal cancer.9 We similarly found increased SIRs for second primaries to be most pronounced for small intestinal cancer, regardless of the anatomic location of the index CRC.
In considering possible mechanisms responsible for the observed increased risk of non-colonic second primary cancers, it is plausible that some of this increased risk reflects the contribution of genetic predisposition in cases with familial cancer syndromes, such as hereditary non-polyposis colorectal cancer (HNPCC).18 However, HNPCC and other familial syndromes are rare19 and unlikely to fully account for our findings. It is also possible that the increased risk of some cancers reflects misclassification of metastases as new primary cancers, and/or reflects the contribution of shared risk factors. For example, findings of an increased risk of lung cancer after index CRC may reflect the shared contribution of smoking to risk of both malignancies.20,21 Our data also support the hypothesis that the elevated risk of second primary small intestinal, stomach, bladder, and lung cancer reflects the shared origin of the endoderm-derived epithelia at these sites. These embryologically-related tissues might be expected to respond in a similar fashion to environmental exposures and carcinogens, and may be similarly susceptible to aberrant epigenetic changes; such epigenetic changes could, in turn, form a field effect, making these tissues similarly susceptible to the development of primary tumors. This hypothesis may also explain why we observed no elevated risk of leukemia or lymphoma following CRC, as such cancers arise in cells with different embryologic origins.
Similar mechanisms may be responsible for the observed increased risk of second primary CRC. Most prior studies of second primary cancer in CRC survivors have focused on risk of second primary CRC, and have consistently demonstrated significantly elevated incidence rates.5–8,10–13 In a recent registry-based analysis, Ringland et al. reported a cumulative incidence of 2.1% at five years after index diagnosis.10 Estimated SIRs for second primary CRC in previous studies have ranged from 1.4 to 2.2, consistent with our SIR of 2.1.
Few prior studies have considered possible differences in the risk of second primary cancer according to the location of first primary CRC.6,10,11,13 At least three studies, however, have suggested that the risk of second primary CRC is greater after index proximal colon cancer than after index distal colon or rectal cancer.10,11,13 In contrast, we found that the excess risk of second primary cancer, particularly second primary CRC, was greatest among individuals with index CRC located in the splenic flexure, and declined with distance from the splenic flexure. The basis for this pattern is unclear, but may be related to differences in index CRC biology across the CRC continuum.14 Yamauchi et al. recently demonstrated a clear pattern of increasing frequency of CpG island methylation, microsatellite instability, and somatic BRAF mutations across CRC subsites from the rectum to the ascending colon, suggesting that classification of CRC as proximal colon, distal colon, or rectal cancer is an oversimplification.14 It is plausible that other molecular or pathological attributes could follow a pattern of distribution similar to what we observed as the distribution of second primary cancer risk: highest for cases with index CRC in the transverse to descending colon and lowest for cases with index CRC in either end of the colorectum. However, to our knowledge, such a pattern of distribution has not been described for common molecular alterations or clinical characteristics of CRC.
Patterns of risk for second primary CRC by index CRC subsite are also likely related, at least in part, to patterns of surgical procedures for the treatment of index CRC. Less than 14% of first primary CRCs are located in the transverse colon, splenic flexure, or descending colon, and these portions of the colon are likely to be preserved, at least in part, in surgical treatment of index CRC. Right hemicolectomy, for the treatment of proximal colon cancer, generally involves the removal of the cecum, ascending colon, hepatic flexure, and a small portion of the transverse colon; as a result, the SIR for second primary cecal, ascending colon, or hepatic flexure cancer was ≤1.0 for individuals with index CRC at these sites. Sigmoid resection for the treatment of sigmoid colon cancer generally involves the removal of the sigmoid colon and a portion of the descending colon; as a result, the SIR for second primary sigmoid colon cancer after index sigmoid colon cancer is equal to 0.99. Thus, the fact individuals with a history of index CRC experience the greatest increased risk for second primary CRC located in the transverse colon, splenic flexure, or descending colon, may reflect the fact that these portions of the colon are most frequently preserved in the surgical treatment of index CRC.
The results presented here should be interpreted in the context of study limitations. In particular, individual-level information was limited. We lacked data on tumor markers, genetic factors, family history, treatment, and lifestyle characteristics. Additionally, we cannot rule out the possibility that second cancers, particularly second primary CRCs, are recurrences or metastases rather than true second primary cancers; however, we conducted sensitivity analyses excluding all second cancers diagnosed ≤6 months after the index CRC, and found no change to the conclusions drawn from our primary analyses excluding all second cancers diagnosed ≤2 months after index CRC. Some misclassification of CRC subsite is also possible; however, it is unlikely that such misclassification would be differential according to second primary cancer risk. Lastly, despite the very large population of index CRC cases overall within SEER, numbers were limited for SIR calculations specific to less common second primary cancer sites, particularly when stratified by index CRC subsite.
Results presented here confirm previous reports of an elevated cancer risk in CRC survivors relative to the general population, and provide added evidence that this elevated risk differs by location of the index CRC. Although the precise mechanisms underlying this pattern of increased risk are unclear, these results suggest that strategies for cancer surveillance after index CRC may need to be individualized according to the subsite of index CRC.
Acknowledgments
Funding: This work was supported by the National Cancer Institute, National Institutes of Health (R25-CA94880 and K05-CA152715 to A.I.P.; R01-CA151993 to S.O.). The National Institutes of Health had no role in the design or conduct of the study. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Financial Disclosures: None to report.
REFERENCES
- 1.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmack CL, Basen-Engquist K, Gritz ER. Survivors at higher risk for adverse late outcomes due to psychosocial and behavioral risk factors. Cancer Epidemiol Biomarkers Prev. 2011;20:2068–2077. doi: 10.1158/1055-9965.EPI-11-0627. [DOI] [PubMed] [Google Scholar]
- 4.Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105(Suppl 1):S29–37. doi: 10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enblad P, Adami HO, Glimelius B, Krusemo U, Pahlman L. The risk of subsequent primary malignant diseases after cancers of the colon and rectum. A nationwide cohort study. Cancer. 1990;65:2091–2100. doi: 10.1002/1097-0142(19900501)65:9<2091::aid-cncr2820650934>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.McCredie M, Macfarlane GJ, Bell J, Coates M. Second primary cancers after cancers of the colon and rectum in New South Wales, Australia, 1972–1991. Cancer Epidemiol Biomarkers Prev. 1997;6:155–160. [PubMed] [Google Scholar]
- 7.Hemminki K, Li X, Dong C. Second primary cancers after sporadic and familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:793–798. [PubMed] [Google Scholar]
- 8.Evans HS, Moller H, Robinson D, Lewis CM, Bell CM, Hodgson SV. The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut. 2002;50:647–652. doi: 10.1136/gut.50.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed F, Goodman MT, Kosary C, et al. Excess risk of subsequent primary cancers among colorectal carcinoma survivors, 1975–2001. Cancer. 2006;107:1162–1171. doi: 10.1002/cncr.22013. [DOI] [PubMed] [Google Scholar]
- 10.Ringland CL, Arkenau HT, O'Connell DL, Ward RL. Second primary colorectal cancers (SPCRCs): experiences from a large Australian Cancer Registry. Ann Oncol. 2010;21:92–97. doi: 10.1093/annonc/mdp288. [DOI] [PubMed] [Google Scholar]
- 11.Raj KP, Taylor TH, Wray C, Stamos MJ, Zell JA. Risk of second primary colorectal cancer among colorectal cancer cases: a population-based analysis. J Carcinog. 2011;10:6. doi: 10.4103/1477-3163.78114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shureiqi I, Cooksley CD, Morris J, Soliman AS, Levin B, Lippman SM. Effect of age on risk of second primary colorectal cancer. J Natl Cancer Inst. 2001;93:1264–1266. doi: 10.1093/jnci/93.16.1264. [DOI] [PubMed] [Google Scholar]
- 13.Gervaz P, Bucher P, Neyroud-Caspar I, Soravia C, Morel P. Proximal location of colon cancer is a risk factor for development of metachronous colorectal cancer: a population-based study. Dis Colon Rectum. 2005;48:227–232. doi: 10.1007/s10350-004-0805-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–797. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surveillance Epidemiology and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2011 Sub, Vintage 2009 Pops (1992–2009) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch, released April 2012, based on the November 2011 submission. www.seer.cancer.gov
- 17.Johnson CH, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards BK. In: The 2007 Multiple Primary and Histology Coding Rules. National Cancer Institute, Surveillance, Epidemiology; End Results Program, editor. Bethesda, MD: 2007. [Google Scholar]
- 18.StatBite: Lynch syndrome increases the risk of various cancers. J Natl Cancer Inst. 2010;102:1383. doi: 10.1093/jnci/djq369. [DOI] [PubMed] [Google Scholar]
- 19.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 21.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]