Abstract
Gap junction channels and hemichannels formed by the connexin family of proteins play important roles in many aspects of tissue homeostasis in the brain and in other organs. In addition, connexin channels have been proposed as pharmacological targets in the treatment of a number of human disorders. In this review, we describe the connexin-subtype selectivity and specificity of pharmacological agents that are commonly used to modulate connexin channels. We also highlight recent progress made towards identifying new agents for connexin channels that act in a selective and specific manner. Finally, we discuss developing insights into possible mechanisms by which these agents modulate connexin channel function.
Introduction
Connexins are a multi-gene family of proteins that form intercellular gap junction (GJ) channels that mediate direct signaling between cells. GJ channels are formed by the docking of two hemichannels, one from each of two contacting cells and it is now well established that each hemichannel can function in the absence of docking, thereby mediating signaling across the plasma membrane. Both hemichannels and GJ channels play important roles in many aspects of tissue homeostasis in the brain and in other tissues, as exemplified by the association of a growing list of human diseases with mutations in connexin genes. Hereditary human diseases associated with mutations in Cxs include peripheral neuropathies, sensorineural deafness, skin inflammation and erythrokeratodermia, congenital cataractogenesis and oculo-dento-digital dysplasia (Abrams and Scherer, 2012; Kleopa et al.; Lee and White, 2009; Mathias et al., 2010; Paznekas et al., 2009). Studies have also demonstrated a correlation between neoplastic transformation and altered GJ communication (e.g., (Trosko, 2005)) and an important role of GJ communication in the pathogenesis of cardiac arrhythmias (Kalcheva et al., 2007; Saffitz, 2009; Severs et al., 2008) and susceptibility to epileptic seizures (Carlen et al., 2000; Jin and Chen, 2011). Thus, connexin channels in their undocked and docked configurations are important pharmacological targets for modulating cellular behavior, as well as for the treatment of a host of human disorders.
The availability of high-affinity pharmacological tools that specifically affect connexin channels will go a long way towards validating the putative therapeutic utility of targeting connexins. In particular, inhibitors and/or activators that target individual connexin subtypes are desirable because many cells express multiple connexin subtypes. It is also important to discover agents that discriminate between GJ channels and hemichannels; such agents will be of great benefit in pathological situations where excessive opening of hemichannels, which leads to cellular dysfunction or even cell death, is the underlying basis of disease. Examples where hemichannel dysfunction likely plays a significant role include syndromic deafness, neuropathy and neurodegeneration (Liang et al., 2005; Mese et al., 2012; Orellana et al., 2012; Sanchez et al., 2010). In addition to uncovering the physiological and pathological roles of connexin channels, the availability of pharmacological agents would be valuable for structure-function studies aimed at elucidating the molecular bases of gating and permeation. Agents that block or modulate ion channels have been invaluable tools in studies of a number of voltage-gated and ligand-gated ion channels.
At present, there are no inhibitors that bind to connexin channels with affinity in the low nanomolar range. The relative paucity of high-affinity inhibitors is attributable to a number of reasons. Connexin channels are large in diameter and as a consequence it is less likely to find small-molecule inhibitors that act as pore-blockers. There are no known toxins that modulate connexin channel activity, although systematic studies are lacking. There are no robust high-throughput screening assays to easily assess connexin channel function, which has hindered the identification of new lead compounds. In theory, in silico and virtual screening methods may be used to identify new lead compounds, but the success of these methodologies depends heavily on the availability of high resolution atomic structures, preferably with and without the lead compound bound to the channel. A crystal structure of a GJ channel was recently obtained for Cx26 at 3.5 Å resolution (Maeda et al., 2009). However, as with any static crystal structure, the state of the channel, open, closed or otherwise, is unknown and requires validation by experimental studies and the generation of additional structures under conditions promoting various conformations. Molecular dynamic simulations suggest that the Cx26 structure, which was presumed to correspond to the open state of the channel, was, in fact, non-conducting (Kwon et al., 2011). These considerations make structure-based drug design challenging at this point. In addition, compared to other ion channels where there is a wealth of information regarding the structural determinants of drug binding, considerably little is known about the molecular mechanisms by which drugs act on connexin channels and binding sites have not been definitively identified. This relative lack of structure-function information arises partly from the limitations in single channel recordings of GJ channels; direct methods to patch onto junctional membranes in mammalian cells have not been achieved and thus recordings have relied on dual whole cell patch techniques that require one or a few stable active channels between cells or pharmacological intervention to reduce coupling to levels low enough to visualize unitary events. Also, there is a lack of apparent structural similarity with other ion channel proteins whose functional domains have been identified and well-studied.
Despite these deficiencies, it is possible to discover new, more specific inhibitors of connexin channels by the application of a focused medicinal chemistry approach. The development of TRAMs as specific inhibitors of Cx50 channels shows the utility of such an approach (Bodendiek et al., 2012) and it is expected that specific inhibitors of other connexin subtypes can be found. Similarly, electrophysiological recordings of hemichannels, which can be studied in excised patches, which provide high time resolution and rapid exposure of drugs from either side of the membrane, have begun to provide some detail about mechanisms of drug inhibition.
The purpose of this review is not to provide a lengthy survey of the effects of all known pharmacological agents that act on connexins. Interested readers may refer to several recent reviews for more details (Bodendiek and Raman, 2010; Giaume and Theis, 2010; Harris, 2001; Juszczak and Swiergiel, 2009; Salameh and Dhein, 2005; Spray et al., 2002). Rather, we briefly describe the characteristics of pharmacological agents that are commonly used and highlight recent progress made towards identifying new agents. Finally, we discuss developing insights into possible mechanisms of action.
What is known about the current crop of the more commonly used inhibitors?
A large number of small organic molecules have been reported to inhibit GJ or hemichannel currents. These include triarylmethanes (TRAMs) (Bodendiek et al., 2012), antimalarial drugs such as quinine and mefloquine (Cruikshank et al., 2004; Rubinos et al., 2012; Srinivas et al., 2001), fenamates (Harks et al., 2001; Srinivas and Spray, 2003), 2-aminophenoxyborate (2-APB) and derivatives (Bai et al., 2006; Harks et al., 2003; Tao and Harris, 2007), glycyrrhetinic acid and its derivatives (Davidson and Baumgarten, 1988; Davidson et al., 1986), volatile anesthetics such as halothane and ethrane (Burt and Spray, 1989; Johnston et al., 1980), lipophilic compounds such as long-chain alcohols (e.g., heptanol and octanol) (Deleze and Herve, 1983; Spray and Burt, 1990), fatty acid amides including oleamide (Boger et al., 1998), cyclodextrins (Locke et al., 2004), cisplatin (Wang et al., 2010), polyamines (Musa and Veenstra, 2003) and tetraalylammonium ions (Musa et al., 2001). An increasing number of studies also report inhibition of GJ channels and hemichannels using peptides corresponding to specific sequences within extracellular loops E1 and E2 involving the conserved QPG and SHVR motifs of E1 (Gap26 peptide) and the SRPTEK motif in E2 (Gap27 peptide) as well as the cytoplasmic loop (Gap19 peptide) (Boitano and Evans, 2000; Braet et al., 2003a; Braet et al., 2003b; Chaytor et al., 1997; Desplantez et al., 2012; Dora et al., 1999; Isakson et al., 2003; Wang et al., 2012; Wang et al., 2013).
Actions on GJ channels and hemichannels
Most of the Cx inhibitors reported in the literature inhibit both GJ channels and hemichannels (Table 1). They also typically abolish channel activity completely. However, there are exceptions. For example, carbenoxolone does not completely abolish junctional coupling in some cell types, notably those expressing Cx43, even at high concentrations. Similarly, inhibition of junctional or hemichannel currents by Gap 26 and Gap27 peptides is incomplete even at high concentrations (typically 300 to 600 μM) (Desplantez et al., 2012; Wang et al., 2012).
Table 1.
Characteristics of some of the most commonly used connexin channel modulators. See text for references.
| Compound | GJ vs Hemichannel |
Direct action? | Selectivity | Specificity |
|---|---|---|---|---|
| Carbenoxolone | Inhibits both | Unknown | None | Inhibits other ion channels and transporters |
| Flufenamic acid |
Inhibits both | Yes | None | Inhibits other ion channels and transporters |
| Mefloquine | Inhibits both | Yes | Inhibits Cx36 and Cx50 at < 10 μM |
Inhibits other ion channels and transporters |
| Gap19 peptide | Inhibits only hemichannels |
Unknown | Yes | Not determined |
| Gap26, Gap27 peptides |
Inhibits both but with different kinetics |
Unknown | Yes | Inhibits pannexin channels |
| 2-APB | Inhibits both | Unknown | Inhibits Cx36 and Cx50 at < 10 μM |
Inhibits other ion channels and transporters |
The inhibition of both GJ channels and hemichannels by these reagents makes it difficult to selectively discriminate the actions mediated by these two channel configurations. In theory, selective blockade of hemichannels can be achieved by the design of a charged drug or a compound with poor lipid solubility. For example, bath application of BQ+, a quaternary derivative analog of quinine, does not inhibit GJ channel currents, but readily and reversibly reduces hemichannel currents (Rubinos et al., 2012; Srinivas et al., 2001). Alternately, extracellular divalent and trivalent cations, notably Ca2+ and La3+, act extracellularly at binding site(s) that appear to be accessible only in undocked hemichannels and, thus, can be used to separate the effects of hemichannels from GJ channels (Beahm and Hall, 2002; Ebihara et al., 2003; Ebihara and Steiner, 1993; Verselis and Srinivas, 2008). However, their use is limited by poor specificity and secondary effects, most notably Ca2+. More recently, the Cx43 Gap peptides have been reported to inhibit Cx43 hemichannels but not GJ channels (Wang et al., 2013). Specifically, Gap 19 peptides, corresponding to a segment of the cytoplasmic loop sequence, showed little or no effect on Cx43 GJ channels, but produced strong inhibition of Cx43 hemichannels. Gap 26 and Gap 27 peptides, corresponding to segments of extracellular loop sequences, appeared to inhibit Cx43 hemichannels more rapidly than GJ channels (Desplantez et al., 2012; Wang et al., 2012). Although hemichannel inhibition by Gap 19 was not complete even at high concentrations (Wang et al., 2013), this particular peptide may be useful for investigations into the role of Cx43 hemichannels.
Direct vs indirect effects
An important issue regarding connexin modulators is whether they produce their effects by a direct action, i.e. binding to a site on the connexin channel (Table 1). For many of the inhibitors listed above, reduction of GJ or hemichannel currents occurs rapidly upon application. In addition, many of these inhibitors are effective when applied to cell-free excised patches containing one or two hemichannels or in ex vivo assays utilizing purified protein. Such inhibitors include antimalarial drugs, fenamates, 2-APB and lipophiles and cyclodextrins (Eskandari et al., 2002; Rubinos et al., 2012; Tao and Harris, 2007). For two of these inhibitors, BQ+ and polyamines, there is evidence that binding occurs in the aqueous pore and, thus, directly on the connexin protein (Musa et al., 2001; Musa and Veenstra, 2003; Rubinos et al., 2012). No binding sites have been identified for other fast-acting reagents, and indirect actions through associated proteins/lipids or membrane-delimited pathways remains a possibility. In contrast, the low affinity and unusually slow kinetics of inhibition exhibited by the extracellular loop peptides (Gap26 and Gap 27 peptides) and carbenoxolone cast uncertainty regarding their direct actions (Spray et al., 2002; Wang et al., 2012; Wang et al., 2013). For inhibition of GJ channel currents, application of high doses of carbenoxolone (200 μM) and Gap 26 (200 μM) take ~ 10-15 min and 45-60 min, respectively, to reach steady-state (Desplantez et al., 2012; Wang et al., 2012). Inhibition of hemichannel currents is faster, but still requires more than 10 mins. Thus, it may be that these reagents work through protein internalization or turnover, or perhaps an indirect mechanism involving binding to cytoplasmic intermediate molecules. Even though the peptides are complementary to Cx sequence, effects have been reported on pannexins, which are unrelated in sequence (Dahl, 2007)
Connexin subtype-selectivity
Some of these inhibitors listed above have the useful property of acting only on one or few connexin subtypes (Table 1). For example, quinine and mefloquine show a good degree of selectivity for Cx36 and Cx50 over Cx43, Cx32 and Cx26 (Cruikshank et al., 2004; Srinivas et al., 2001), whereas 2-APB at 20 μM showed inhibition of several Cx subtypes, but no appreciable effects on others (Bai et al., 2006). Similarly, peptides corresponding to extracellular loops of Cx43, Cx37, and Cx40 were shown to act in a connexin-selective fashion (Boitano and Evans, 2000; Dora et al., 1999; Isakson et al., 2003; Martin et al., 2005). This selectivity has allowed assessment of the functional contribution of individual connexin subtypes to the coupling conductance in cell types where multiple connexins are expressed. Inhibitors such as carbenoxolone, flufenamic acid and n-alkanols do not appear to discriminate between different connexin subtypes although systematic studies are lacking.
Specificity for connexin channels
Many connexin inhibitors are promiscuous in their effects on other ion channels, receptors and transporters (Table 1), making it difficult to meaningfully interpret the role of connexin channels in physiological and pathophysiological processes (Beaumont and Maccaferri, 2011; Rekling et al., 2000; Rouach et al., 2003; Suadicani et al., 2006; Tovar et al., 2009; Vessey et al., 2004) For example, the putative importance of GJ channels in seizures, which was partly based on the anticonvulsant effects of carbenoxolone in in vitro experimental models of seizure, has recently been questioned as carbenoxolone has been shown to have direct effects on GABA receptors (Connors, 2012). In addition, carbenoxolone has a number of other effects, including a reduction in excitatory and inhibitory synaptic currents, alteration of intrinsic membrane properties and suppression of action potentials (Beaumont and Maccaferri, 2011; Rekling et al., 2000; Rouach et al., 2003; Tovar et al., 2009). Carbenoxolone was also reported to block Ca2+ channels, pannexin channels and P2X7 receptors at concentrations similar to or lower than those that block connexin channels (Bruzzone et al., 2005; Suadicani et al., 2006; Vessey et al., 2004). Similarly, quinine, 2-APB, n-alkanols and fenamates block many ion channels and transporters, and simply cannot be used to assess connexin channel function in neuronal networks, especially in long-term studies. The specificity of mefloquine, the most potent inhibitor found to date for connexin channels, is still marginal at best with reported effects on spontaneous synaptic activity and spiking during long high-frequency trains. Mefloquine also has been reported to block voltage-gated L-type calcium channels, Kir6.2 and KvLQT1 potassium channels, volume-regulated and calcium-activated chloride channels, pannexin channels and P2X7 receptors (Gribble et al., 2000; Kang et al., 2001; Maertens et al., 2000; Suadicani et al., 2006; Traebert et al., 2004). There are no systematic studies on the specificity of connexin mimetic peptides, but they seem to cause a reduction in coupling without exerting major nonjunctional membrane effects (Matchkov et al., 2006). However, these peptides were reported to strongly reduce membrane currents in Xenopus oocytes expressing Pannexin 1 at concentrations similar to those that inhibit connexin channels (Dahl, 2007). Pannexin 1 sequence is unrelated to connexins, which together with the low apparent affinity and curiously slow kinetics of action and, raise questions about non-specific effects of the peptides.
The search for new modulators
It is clear that a systematic approach is necessary to identify new compounds that act on connexins. Development of high throughput-screening (HTS) assays is essential for the identification of high-affinity reagents. At the present time, there are no such cell-based assays for the identification of compounds that produce their effects by a direct action on connexin channels. Although techniques based on fluorescence microscopy imaging have been used for many years to monitor coupling, adapting these methods for HTS, especially for identifying direct-acting modulators, is difficult. The so-called “parachute” assay, which involves measuring dye transfer from donor cells loaded with calcein to dye-free acceptor cells, was adapted for HTS using automated fluorescence microscopy (Li et al., 2003; Picoli et al., 2012). Using this assay, Aventis screened 486,000 compounds and identified 1515 primary positive hits (0.3%) out of which only 103 (6.8%) were confirmed on re-testing (Li et al., 2003). The two most potent compounds identified had IC50 values in the ~3-5 μM range, which is still suboptimal. Also, since compounds in this assay were typically added before plating the donor and acceptor cells, it is unclear how many of these compounds reduced coupling by direct action on connexin channels or acted indirectly by generally altering protein trafficking, insertion, assembly and/or degradation. This assay required considerable optimization in terms of automated focusing, image acquisition, image processing, data storage and data mining. In a more recent assay, a method for detecting the passage of Ca2+ between HeLa cells expressing Cx43 was designed (Haq et al., 2012). Donor cells co-expressing Cx43 and the α1A adrenergic Gα-coupled receptor were co-cultured with recipient cells expressing Cx43 and aequorin, a calcium-sensitive luminescent protein. Application of α1A receptor agonists led to increases in Ca2+ in the donor cells, and in recipient cells via passage through GJs, as detected by measuring the intensity of aequorin luminescence. However, this assay is also indirect; reduction in luminescence intensity may be due to effects of compounds on α1A receptors and on signal transduction mechanisms following receptor activation.
In the absence of robust high-throughput assays, alternate methods are required. An effective (but more time-consuming) method to identify new lead compounds is to screen a limited number of structurally diverse drug molecules (e.g. a library of about 100 compounds enriched with small ion channel modulators) for activity on connexin channels using electrophysiology or dye transfer assays. These so-called “privileged” structures are small molecule pharmacophores that are able to bind to multiple targets and therefore highly likely to exert biological effects (Evans et al., 1988; Horton et al., 2003). Following identification of lead compounds, the hits are optimized through classic medicinal chemistry in order to increase the affinity for connexin channels and/or and decrease the affinity for the other targets (Wermuth, 2004). This approach has enabled the design of highly potent inhibitors of K+ channels (Schmitz et al., 2005; Wulff et al., 2000) and was recently used to identify a specific inhibitor of Cx50 channels (Bodendiek ete al., 2012). Through screening of a small library of compounds enriched in known ion channel pharmacophores, four new small molecule chemotypes that inhibit Cx50 GJ channels in the low micromolar range were identified: triarlymethanes, alkyl substituted dibenzazocinones, flavonoid glycosides and benzimidazoles like astemizole. The triarlymethane (TRAM) clotrimazole, the most potent Cx50 inhibitor of the 4 compounds was used as a template to explore the structure activity relationship (SAR) of the TRAMs for Cx50 inhibition. Clotrimazole is known to block the calcium-activated K+ channel KCa3.1 (a.k.a. IKCa1, SK4) with nanomolar affinity (IC50 ~ 70 nM) and many other K+ and Ca2+ channels at concentrations of 10-50 μM (Wulff et al., 2000). By the selective optimization of the side activity (so-called SOSA approach; (Wermuth, 2004))of clotrimazole, a triarylmethane was designed that inhibited Cx50 channels without affecting other ion channels. Synthesis of analogues of clotrimazole followed by testing on Cx50 GJ channels led to the design of T122 (N-[(2-methoxyphenyl)diphenylmethyl]-1,3-thiazol-2-amine) and T136 (N-[(2-iodophenyl)diphenylmethyl]-1,3-thiazol-2-amine), which inhibit Cx50 with IC50s of 1.2 μM and 2.4 μM. Both compounds exhibited at least 10-fold selectivity over other connexins as well as major neuronal and cardiac voltage-gated K+ and Na+ channels. The structure-activity studies also indicated that the TRAM pharmacophore required for Cx50 inhibition is significantly different from the pharmacophore required for blocking the calcium-activated KCa3.1 channel (Bodendiek et al., 2012). Thus, a connexin subtype specific inhibitor was developed starting with a lead compound that exhibited several fold higher selectivity for K+ channels over Cx50 (Bodendiek ete al., 2012). Application of a similar approach may lead to the development of specific blockers for other connexin channels.
Structure-based methods for design of inhibitors, particularly for ion channels, are still developing. Molecular dynamic simulations have been aimed at obtaining additional Cx26 structures that may be representative of open and closed states (Hung and Yarovsky, 2011; Kwon et al., 2011; Zonta et al., 2012). Such simulations may also prove useful in visualizing potential drug binding pockets. However, assessment of these putative target sites would still need experimental validation. Also crystallization of ligand-bound channels, where details of drug protein interactions are identified, would be most optimal.
Mechanisms by which drugs act on connexin channels
Drugs that rapidly and reversibly inhibit ion channel function act either by blocking the pore or by modulating channel opening/closing, i.e. gating. Slowly-acting drugs may target trafficking and/or protein stability in the membrane, but require new protein synthesis and/or insertion for reversibility. Assessment of the mechanism of drug action is best accomplished using single channel recordings in which pore-blocking or gating events can be visualized. For GJ channels, these experiments are more difficult due to the necessity of using dual whole-cell voltage clamp, which records the activity of all the channels between a cell pair. Cell pairs with few enough active channels to visualize unitary events are infrequent. Hemichannels present a much better preparation due to the ability to patch onto a membrane and isolate single hemichannels for electrophysiological analyses.
Distinguishing pore block from modulation of gating can be difficult because blocking and closing events can appear similar. In addition, agents that inhibit ionic currents by blocking the pore can also modulate gating by obstructing the closing of gates by a classic “foot-in-the door” mechanism (Armstrong, 1971) or conversely by altering the closing rates of gates (Armstrong and Cota, 1999). Introduction of a drug that blocks the pore will cause interruptions of open dwell times in a concentration-dependent manner. If drug occupancy is sufficiently long, transitions to the blocked (non-conducting) state can be observed, but can be difficult to distinguish from normal gating events to the closed (non-conducting) state. However, an advantage of connexin channels is the distinctive features of connexin channel gating. It is now well established that connexin channels possess two distinct gating mechanisms that are voltage sensitive and intrinsic to the hemichannels. One mechanism, termed fast gating, closes hemichannels to a subconducting state. The term fast gating refers to these gating transitions, which are rapid. This gating mechanism is easily distinguished from pore block by the degree of closure, partial vs full. The second gating mechanism, termed slow gating or loop gating completely closes connexin hemichannels, leaving no residual conductance. The term slow refers to the gating transitions, which appear to be slow, taking tens of milliseconds to complete (see Figure 1). The term loop gating refers to evidence that this mechanism involves movement of the extracellular loop domains, specifically E1 (Tang et al., 2009; Verselis et al., 2009). Low-noise recordings from excised patches show that these slow transitions can be seen to be composed of a series of transient substates en route to full opening/closure (Bukauskas and Peracchia, 1997; Srinivas et al., 2005; Trexler et al., 1996).
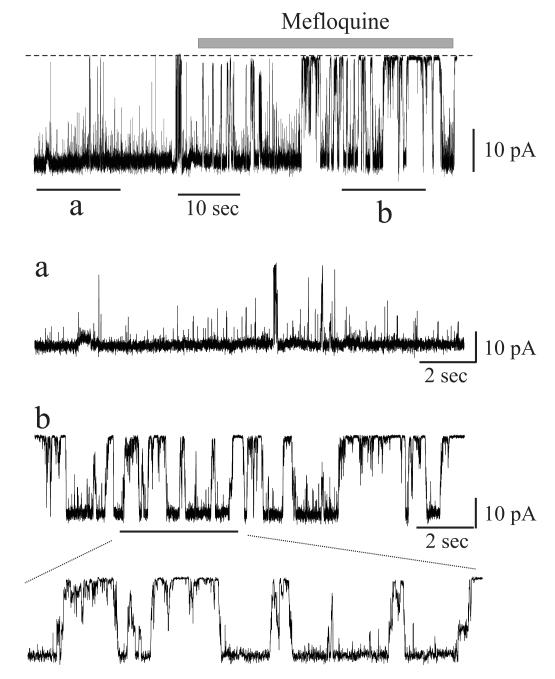
Figure 1.
Mefloquine promotes closure by loop gating. Single channel recordings of Cx50 hemichannels in outside-out patches at a membrane potential of −50 mV. The dotted line indicates the closed state. Insets “a” and “b” are expanded views of the current before and after application of mefloquine (5 μM), respectively. Mefloquine induces loop gating transitions between open and closed states, as shown in the expanded segment in “b”, similar to closing events produced by other modulators.
Connexin channel inhibition generally involves modulation of loop gating
Most studies that report actions of connexin channel inhibitors use intracellular dye spread assays, in the case of GJs, or dye uptake assays, in the case of hemichannels. These methodologies do not address mechanism of action nor can they distinguish direct from indirect effects mediated by cytoplasmic factors. In general, the few studies that have examined the effects of inhibitors electrophysiology have reported increased transitions that resemble loop gating (Bukauskas and Peracchia, 1997; Srinivas et al., 2001; Srinivas and Spray, 2003; Weingart and Bukauskas, 1998) (see Figure 1 for an example). Application of alkanols to coupled cell pairs generally has been reported to produce rapid reductions in conductance to zero, i.e., complete uncoupling (Weingart and Bukauskas, 1998). Prior to complete uncoupling, single GJ channels events are visible and the transitions appear slow. This observation is not connexin specific suggesting it is a mechanism of alkanol action common among to all members of the connexin family (Bukauskas and Verselis, 2004; Weingart and Bukauskas, 1998). Similarly, in a study that examined Cx50 GJ channel inhibition by quinine, and N-benzylquininium (BQ+), a derivative of quinine, recordings of single channels showed evidence that these inhibitors induced slow gating transitions ascribable to loop gating (Srinivas et al., 2001; Rubinos et al., 2012) (Figure 1). Almost all connexin channel inhibitors and modulators, including low pH, halothane, n-alkanols, quinine, and fenamates, also produce these slow transitions, suggesting that the structural elements involved in closure are shared. Recently, studies using recordings of single Cx43 hemichannels reported that Gap26 and Gap19 peptides inhibit hemichannels by shifting loop gating (Wang et al., 2012; Wang et al., 2013). Although not surprising for small molecules, peptides or protein toxins plausibly can block large channels, such as connexins, but to date no such pore blockers have emerged. Thus far, molecular details underlying loop gating have not been elucidated.
What is known about the sites of drug action?
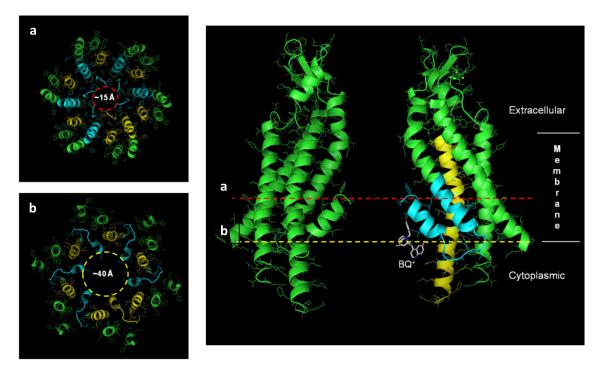
Although loop gating appears to be a the predominant mechanism through which a number of current connexin inhibitors act, little is known about the sites where these drugs bind that lead to modulation of loop gating. An important consideration is that structural elements that mediate loop gating are associated with the pore; this association comes from the fact that gap junction channels are sensitive to the transjunctional voltage, i.e. voltage difference between the cells, and insensitive to absolute membrane potentials (Harris et al., 1981; Spray et al., 1981). There is now wide consensus, from both structural and biophysical studies, that the bulk of the pore, the so-called pore funnel, is formed by the N-terminal (NT), first transmembrane domain (TM1) and the first extracellular loop (E1) domains (Kronengold et al., 2003; Maeda et al., 2009; Oh et al., 2008; Verselis et al., 1994; Verselis et al., 2009). Grossly, the cytoplasmic end of the pore is made by the NT domains, which loop back into the membrane and converge to a narrow point at their amino ends forming the entrance to the pore funnel (Figure 2). Following NT towards the cytoplasm, the pore flares considerably such that the vestibule at the cytoplasmic entrance likely exceeds ~40 Å (Maeda et al., 2009). Towards the extracellular end, the pore funnel continues into a short segment of TM1 followed by E1 (Figure 2).
Figure 2.
Cross sections of the pore at positions indicated by a (red dotted line) and b (yellow dotted line). The diameters corresponding to these two positions are shown on the left. The narrowest part of the pore (~15 Å) is formed by the amino-terminal ends of the NT domains, which converge to form the entrance to the pore funnel (a). Pore diameter at the cytoplasmic vestibule, constituted by the NT extending into TM2, widens to ~40 Å (b). BQ+ (white) is shown in this wide vestibule. The placement and orientation of BQ+ is arbitrary. The image was prepared using PyMOL (http://www.pymol.org).
In agreement with the link between pore and gating, both NT and the EI domains have been shown to be central components of the loop gating machinery (Kronengold et al., 2012; Tang et al., 2009; Verselis et al., 2009). Furthermore, studies of BQ+ action on Cx50 hemichannels unequivocally showed that the binding site is in the pore, towards the cytoplasmic end in the NT domain (Rubinos et al., 2012). Binding of BQ+ in the wide vestibule that is associated with NT could explain why BQ+ does not produce pore block (Figure 2). However, how binding of BQ+ to NT initiates loop gating remains unknown. Interestingly, residues in NT have been shown to be critical for inhibition of Cx40 GJ channels by polyamines (Musa et al. 2003). Thus, the NT domain may be the site of action for a number of chemical agents. We also point out that it is likely that there are other binding locations that could promote loop gating as suggested by the actions of lipophiles such as octanol and heptanol, which more likely produce their effects through perturbation of the lipid-protein interface. Thus, binding of various classes of drugs may occur at different sites on the connexin channel, but all converge to ultimately close the loop gate. The cytoplasmic loop has been identified as a site for the action of aminosulfonates, which have been reported to inhibit Cx26 channels by disruption of a CT-CL interdomain interaction (Locke et al., 2011). Whether this mechanism involves modulation of loop gating or another mechanism of closure is unknown. Elucidation of molecular details of ligand binding and of loop gating should aid in the development modulators exhibiting high affinity and connexin specificity.
We provide an overview of the pharmacology of connexin channels
We discuss the selectivity and specificity of commonly used inhibitors
New methods to discover new modulators are highlighted
The mechanisms and sites of action of drugs are discussed
Acknowledgements
This work was supported by NIH grants EY13869 (to M.S) and GM54179 (to V.K.V).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams CK, Scherer SS. Gap junctions in inherited human disorders of the central nervous system. Biochim Biophys Acta. 2012;1818:2030–2047. doi: 10.1016/j.bbamem.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Cota G. Calcium block of Na+ channels and its effect on closing rate. Proc Natl Acad Sci U S A. 1999;96:4154–4157. doi: 10.1073/pnas.96.7.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB) J Pharmacol Exp Ther. 2006;319:1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys J. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont M, Maccaferri G. Is connexin36 critical for GABAergic hypersynchronization in the hippocampus? J Physiol. 2011;589:1663–1680. doi: 10.1113/jphysiol.2010.201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodendiek SB, Raman G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem. 2010;17:4191–4230. doi: 10.2174/092986710793348563. [DOI] [PubMed] [Google Scholar]
- Bodendiek SB, Rubinos C, Trelles MP, Coleman N, Jenkins DP, Wulff H, Srinivas M. Triarylmethanes, a new class of cx50 inhibitors. Front Pharmacol. 2012;3:106. doi: 10.3389/fphar.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Patterson JE, Guan X, Cravatt BF, Lerner RA, Gilula NB. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc Natl Acad Sci U S A. 1998;95:4810–4815. doi: 10.1073/pnas.95.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L623–630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PE, Evans WH, Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol. 2003a;197:205–213. doi: 10.1002/jcp.10365. [DOI] [PubMed] [Google Scholar]
- Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003b;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Peracchia C. Two distinct gating mechanisms in gap junction channels: CO2-sensitive and voltage-sensitive. Biophys J. 1997;72:2137–2142. doi: 10.1016/S0006-3495(97)78856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JM, Spray DC. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res. 1989;65:829–837. doi: 10.1161/01.res.65.3.829. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Skinner F, Zhang L, Naus C, Kushnir M, Perez Velazquez JL. The role of gap junctions in seizures. Brain Res Brain Res Rev. 2000;32:235–241. doi: 10.1016/s0165-0173(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol. 1997;503(Pt 1):99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW. Tales of a dirty drug: carbenoxolone, gap junctions, and seizures. Epilepsy Curr. 2012;12:66–68. doi: 10.5698/1535-7511-12.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G. Gap junction-mimetic peptides do work, but in unexpected ways. Cell Commun Adhes. 2007;14:259–264. doi: 10.1080/15419060801891018. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM, Harley EH. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun. 1986;134:29–36. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- Deleze J, Herve JC. Effect of several uncouplers of cell-to-cell communication on gap junction morphology in mammalian heart. J Membr Biol. 1983;74:203–215. doi: 10.1007/BF02332124. [DOI] [PubMed] [Google Scholar]
- Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–552. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Dora KA, Martin PE, Chaytor AT, Evans WH, Garland CJ, Griffith TM. Role of heterocellular Gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochem Biophys Res Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- Ebihara L, Liu X, Pal JD. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys J. 2003;84:277–286. doi: 10.1016/S0006-3495(03)74848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RS, et al. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- Giaume C, Theis M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev. 2010;63:160–176. doi: 10.1016/j.brainresrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Davis TM, Higham CE, Clark A, Ashcroft FM. The antimalarial agent mefloquine inhibits ATP-sensitive K-channels. Br J Pharmacol. 2000;131:756–760. doi: 10.1038/sj.bjp.0703638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq N, Grose D, Ward E, Chiu O, Tigue N, Dowell SJ, Powell AJ, Chen MX. A High-Throughput Assay for Connexin 43 (Cx43, GJA1) Gap Junctions Using Codon-Optimized Aequorin. Assay Drug Dev Technol. 2012 doi: 10.1089/adt.2012.469. [DOI] [PubMed] [Google Scholar]
- Harks EG, Camina JP, Peters PH, Ypey DL, Scheenen WJ, van Zoelen EJ, Theuvenet AP. Besides affecting intracellular calcium signaling, 2-APB reversibly blocks gap junctional coupling in confluent monolayers, thereby allowing measurement of single-cell membrane currents in undissociated cells. Faseb J. 2003;17:941–943. doi: 10.1096/fj.02-0786fje. [DOI] [PubMed] [Google Scholar]
- Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Harris AL, Spray DC, Bennett MV. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DA, Bourne GT, Smythe ML. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- Hung A, Yarovsky I. Gap junction hemichannel interactions with zwitterionic lipid, anionic lipid, and cholesterol: molecular simulation studies. Biochemistry. 2011;50:1492–1504. doi: 10.1021/bi1004156. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Seedorf GJ, Lubman RL, Evans WH, Boitano S. Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:552–561. doi: 10.1165/rcmb.2002-0281OC. [DOI] [PubMed] [Google Scholar]
- Jin MM, Chen Z. Role of gap junctions in epilepsy. Neurosci Bull. 2011;27:389–406. doi: 10.1007/s12264-011-1944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MF, Simon SA, Ramon F. Interaction of anaesthetics with electrical synapses. Nature. 1980;286:498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Swiergiel AH. Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:181–198. doi: 10.1016/j.pnpbp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007;104:20512–20516. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Chen XL, Wang L, Rampe D. Interactions of the antimalarial drug mefloquine with the human cardiac potassium channels KvLQT1/minK and HERG. J Pharmacol Exp Ther. 2001;299:290–296. [PubMed] [Google Scholar]
- Kleopa KA, Abrams CK, Scherer SS. How do mutations in GJB1 cause X-linked Charcot-Marie-Tooth disease? Brain Res. 1487:198–205. doi: 10.1016/j.brainres.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronengold J, Srinivas M, Verselis VK. The N-terminal half of the connexin protein contains the core elements of the pore and voltage gates. J Membr Biol. 2012;245:453–463. doi: 10.1007/s00232-012-9457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Pore-lining residues identified by single channel SCAM studies in Cx46 hemichannels. Cell Commun Adhes. 2003;10:193–199. doi: 10.1080/cac.10.4-6.193.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T, Harris AL, Rossi A, Bargiello TA. Molecular dynamics simulations of the Cx26 hemichannel: evaluation of structural models with Brownian dynamics. J Gen Physiol. 2011;138:475–493. doi: 10.1085/jgp.201110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JR, White TW. Connexin-26 mutations in deafness and skin disease. Expert Rev Mol Med. 2009;11:e35. doi: 10.1017/S1462399409001276. [DOI] [PubMed] [Google Scholar]
- Li Z, Yan Y, Powers EA, Ying X, Janjua K, Garyantes T, Baron B. Identification of gap junction blockers using automated fluorescence microscopy imaging. J Biomol Screen. 2003;8:489–499. doi: 10.1177/1087057103257309. [DOI] [PubMed] [Google Scholar]
- Liang GS, de Miguel M, Gomez-Hernandez JM, Glass JD, Scherer SS, Mintz M, Barrio LC, Fischbeck KH. Severe neuropathy with leaky connexin32 hemichannels. Ann Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- Locke D, Kieken F, Tao L, Sorgen PL, Harris AL. Mechanism for modulation of gating of connexin26-containing channels by taurine. J Gen Physiol. 2011;138:321–339. doi: 10.1085/jgp.201110634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke D, Koreen IV, Liu JY, Harris AL. Reversible pore block of connexin channels by cyclodextrins. J Biol Chem. 2004;279:22883–22892. doi: 10.1074/jbc.M401980200. [DOI] [PubMed] [Google Scholar]
- Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- Maertens C, Wei L, Droogmans G, Nilius B. Inhibition of volume-regulated and calcium-activated chloride channels by the antimalarial mefloquine. J Pharmacol Exp Ther. 2000;295:29–36. [PubMed] [Google Scholar]
- Martin PE, Wall C, Griffith TM. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br J Pharmacol. 2005;144:617–627. doi: 10.1038/sj.bjp.0706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchkov VV, Rahman A, Bakker LM, Griffith TM, Nilsson H, Aalkjaer C. Analysis of effects of connexin-mimetic peptides in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H357–367. doi: 10.1152/ajpheart.00681.2005. [DOI] [PubMed] [Google Scholar]
- Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010;90:179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mese G, Sellitto C, Li L, Wang HZ, Valiunas V, Richard G, Brink PR, White TW. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol Biol Cell. 2012;22:4776–4786. doi: 10.1091/mbc.E11-09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H, Gough JD, Lees WJ, Veenstra RD. Ionic blockade of the rat connexin40 gap junction channel by large tetraalkylammonium ions. Biophys J. 2001;81:3253–3274. doi: 10.1016/S0006-3495(01)75960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H, Veenstra RD. Voltage-dependent blockade of connexin40 gap junctions by spermine. Biophys J. 2003;84:205–219. doi: 10.1016/S0006-3495(03)74843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol. 2008;586:2445–2461. doi: 10.1113/jphysiol.2008.150805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, von Bernhardi R, Giaume C, Saez JC. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev Neurosci. 2012;23:163–177. doi: 10.1515/revneuro-2011-0065. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- Picoli C, Nouvel V, Aubry F, Reboul M, Duchene A, Jeanson T, Thomasson J, Mouthon F, Charveriat M. Human connexin channel specificity of classical and new gap junction inhibitors. J Biomol Screen. 2012;17:1339–1347. doi: 10.1177/1087057112452594. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBotzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Segal M, Koulakoff A, Giaume C, Avignone E. Carbenoxolone blockade of neuronal network activity in culture is not mediated by an action on gap junctions. J Physiol. 2003;553:729–745. doi: 10.1113/jphysiol.2003.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinos C, Sanchez HA, Verselis VK, Srinivas M. Mechanism of inhibition of connexin channels by the quinine derivative N-benzylquininium. J Gen Physiol. 2012;139:69–82. doi: 10.1085/jgp.201110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm. 2009;6:S62–65. doi: 10.1016/j.hrthm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Salameh A, Dhein S. Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim Biophys Acta. 2005;1719:36–58. doi: 10.1016/j.bbamem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Sanchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J Gen Physiol. 2010;136:47–62. doi: 10.1085/jgp.201010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Burt JM. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990;258:C195–205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Rozental R, Srinivas M. Prospects for rational development of pharmacological gap junction channel blockers. Curr Drug Targets. 2002;3:455–464. doi: 10.2174/1389450023347353. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci U S A. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Dowd TL, Verselis VK, Bargiello TA. Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J Gen Physiol. 2009;133:555–570. doi: 10.1085/jgp.200910207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Harris AL. 2-aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol Pharmacol. 2007;71:570–579. doi: 10.1124/mol.106.027508. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez-Estevez M, Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci U S A. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko JE. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomed Pharmacother. 2005;59(Suppl 2):S326–331. doi: 10.1016/s0753-3322(05)80065-4. [DOI] [PubMed] [Google Scholar]
- Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008;132:315–327. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis VK, Trelles MP, Rubinos C, Bargiello TA, Srinivas M. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J Biol Chem. 2009;284:4484–4493. doi: 10.1074/jbc.M807430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Wang N, De Bock M, Antoons G, Gadicherla AK, Bol M, Decrock E, Evans WH, Sipido KR, Bukauskas FF, Leybaert L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, You T, Yuan D, Han X, Hong X, He B, Wang L, Tong X, Tao L, Harris AL. Cisplatin and oxaliplatin inhibit gap junctional communication by direct action and by reduction of connexin expression, thereby counteracting cytotoxic efficacy. J Pharmacol Exp Ther. 2010;333:903–911. doi: 10.1124/jpet.109.165274. [DOI] [PubMed] [Google Scholar]
- Weingart R, Bukauskas FF. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 1998;435:310–319. doi: 10.1007/s004240050517. [DOI] [PubMed] [Google Scholar]
- Wermuth CG. Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004;47:1303–1314. doi: 10.1021/jm030480f. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta F, Polles G, Zanotti G, Mammano F. Permeation pathway of homomeric connexin 26 and connexin 30 channels investigated by molecular dynamics. J Biomol Struct Dyn. 2012;29:985–998. doi: 10.1080/073911012010525027. [DOI] [PMC free article] [PubMed] [Google Scholar]