Abstract
Resveratrol is a polyphenol that is mainly found in grapes and red wine and has been reported to be a caloric restriction (CR) mimetic driven by Sirtuin 1 (SIRT1) activation. Resveratrol increases metabolic rate, insulin sensitivity, mitochondrial biogenesis and physical endurance, and reduces fat accumulation in mice. In addition, resveratrol may be a powerful agent to prevent age-associated neurodegeneration and to improve cognitive deficits in Alzheimer’s disease (AD). Moreover, different findings support the view that longevity in mice could be promoted by CR. In this study, we examined the role of dietary resveratrol in SAMP8 mice, a model of age-related AD. We found that resveratrol supplements increased mean life expectancy and maximal life span in SAMP8 and in their control, the related strain SAMR1. In addition, we examined the resveratrol-mediated neuroprotective effects on several specific hallmarks of AD. We found that long-term dietary resveratrol activates AMPK pathways and pro-survival routes such as SIRT1 in vivo. It also reduces cognitive impairment and has a neuroprotective role, decreasing the amyloid burden and reducing tau hyperphosphorylation.
Keywords: Senescence, Resveratrol, Sirtuin 1, AMPK, Alzheimer’s disease, β-Amyloid, Tau, Memory impairment
Introduction
Resveratrol (trans-3,4′,5-trihydroxystilbene), a naturally occurring polyphenol mainly found in grapes and red wine, has been reported as a caloric restriction (CR) mimetic with potential anti-aging and anti-diabetogenic properties. Resveratrol increases metabolic rate, insulin sensitivity, mitochondrial biogenesis and physical endurance, and reduces fat accumulation in mice (Lagouge et al. 2006; Baur et al. 2006). The most widely accepted mechanistic hypothesis is that resveratrol’s effects, in the same way as CR, are driven through Sirtuin 1 (SIRT1) regulation (Chung et al. 2010). Although there has been major controversy about whether resveratrol can be an activator of SIRT1, as its ability to interact directly with SIRT1 has been questioned (Beher et al. 2009; Pacholec et al. 2010), it now seems clear that resveratrol activates SIRT1 indirectly (Villalba et al. 2012). It is widely accepted that resveratrol benefits are mediated through AMPK activation (Zang et al. 2006; Baur et al. 2006; Price et al. 2012). Thus, resveratrol leads to increases in the NAD-to-NADH cell ratio, which results in activation of AMPK in vivo, initiating a signaling process that regulates insulin sensitivity and recruits mediators of oxidative metabolism and mitochondrial biogenesis, including PGC1α, PPARδ, and others (Um et al. 2010; Ruderman et al. 2010).
Several findings support the view that longevity can be promoted by CR in mice (Weindruch & Walford, 1988; Selman et al. 2008), along with CR’s broad anti-aging activity (Park et al. 2009). In recent years, interesting studies in nonhuman primates have reported that CR also extended their life span (Colman et al. 2009), but in a very recently published study of the same species CR was not able to do so (Mattison et al. 2012). Though unlikely, the possibility that CR may extend maximum life span has still not been ruled out. Similarly, resveratrol treatment has a range of beneficial effects in mice, but up to now has failed to increase the longevity of ad libitum-fed animals when started midlife (Baur and Sinclair 2006), although in combination with other anti-aging strategies such as CR, it increased mean and maximal life span compared to control animals (Pearson et al. 2008). In addition, dietary resveratrol mimics the effects of CR in insulin-mediated glucose uptake in muscle in aged animals, and gene expression profiling suggests that both CR and resveratrol may retard some aspects of aging through alterations in chromatin structure and transcription (Halagappa et al. 2007; Barger et al. 2008).
Several in vitro and in vivo studies also support the hypothesis that resveratrol may be a powerful agent in preventing age-associated neurodegeneration (Vingtdeux et al. 2008). In in vitro models, resveratrol markedly lowers the levels of secreted and intracellular amyloid-beta (Aβ) peptides (Marambaud et al. 2005). Similarly, with a grape seed polyphenolic extract administered orally to Tg2576 mice, a murine model of Alzheimer’s disease (AD) (Hsiao et al. 1996) improves cognitive deficits. These effects correlate with reductions in the amounts of high molecular weight Aβ assemblies in the brain (Wang et al. 2008). Similar findings have been observed in animals after moderate consumption of red wines (Wang et al. 2006; Ho et al. 2009). Recently, it was shown that resveratrol selectively remodels soluble oligomers, fibrillar intermediates, and amyloid fibrils into alternative aggregated species that are nontoxic (Ladiwala et al. 2010). These studies and others support the theory that resveratrol or polyphenol derivatives could be useful therapeutic agents for AD (Ono et al. 2008). Nevertheless, it is unknown whether resveratrol has similar effects in age-related models of AD.
To this end, we used the age-accelerated mouse (SAMP8). This strain is characterized by deficits in learning and memory (Takeda et al. 1981; Miyamoto et al. 1986; Takeda 2009), emotional disorders such as reduced anxiety-like behavior (Miyamoto et al. 1992; Markowska et al. 1998), impaired immune response, etc. (Yagi et al. 1988; Flood & Morley 1998). More importantly, this strain is increasingly being recognized as a model of age-related AD (Pallas et al. 2008; Morley et al. 2012) as, in addition to age-related learning and memory impairments, the mice show with aging an AD-related pathology such as increases in Aβ (del Valle et al. 2010) and other protein aggregates (Manich et al. 2011), alterations in APP processing by secretases (Morley et al. 2000, 2002), cerebral amyloid angiopathy (del Valle et al. 2011) and increases in tau hyperphosphorylation (Canudas et al. 2005).
Therefore, in this study we sought to clarify the role of dietary resveratrol in the SAMP8 mouse. Previous results in SAMP8 demonstrated that low doses and short-term administration of pterostilbene (polyphenolic derivative of resveratrol) show positive effects on behavior, reductions in tau phosphorylation (Chang et al. 2012) and regulation of cascades associated with PPAR alpha. Based on these encouraging findings, we determined the effects of long-term administration of resveratrol on longevity and signaling cellular processes activated by this polyphenol, namely the SIRT1 pathway and AMPK system. We also extended these studies by examining the resveratrol-mediated neuroprotective mechanism in several specifically AD hallmarks present in SAMP8, such as, Aβ accumulation and tau phosphorylation.
Methods
Animals and resveratrol diet
A total of 216 male SAMP8 and SAMR1 animals were used for the survival study. The animals received a standard diet (2018 Teklad Global 18 % Protein Rodent Maintenance Diet, Harlan) or the same diet supplemented with trans-resveratrol (1 g/kg, Mega Resveratrol, Candlewood Stars, Inc., CT, USA), starting at 2 months of age and divided into four groups of 50 to 60 individuals: SAMR1 control (n = 54), SAMR1 resveratrol (n = 52), SAMP8 control (n = 50), and SAMP8 resveratrol (n = 60). For the neurodegeneration studies, two groups of 10–12 SAMP8 mice were fed with the standard diet or the resveratrol diet, starting the supplements at 2 months and killing the animals to obtain tissue samples at 9 months of age. All the animals had food and water ad libitum and were kept in standard conditions of temperature (22 ± 2 °C) and 12:12-h light–dark cycles (300 lux/0 lux). Studies were performed in accordance with the institutional guidelines for the care and use of laboratory animals established by the Ethical Committee for Animal Experimentation at the University of Barcelona.
Object recognition test
Nine-month SAMP8 control (P8ctl) and SAMP8 resveratrol (P8rsv) animals were placed in a 90° two-arm, 25-cm-long, 20-cm-high, 5-cm-wide black maze. The 20-cm-high walls could be lifted off for easy cleaning. The light intensity in the middle of the field was 30 lux. The objects to be discriminated were made of plastic (5.25-cm high, object A and 4.75-cm high, object B). For the first 3 days, the mice were individually habituated to the apparatus for 10 min. On the 4th day, the animals were submitted to a 10-min acquisition trial (first trial) during which they were placed in the maze in the presence of two identical novel objects (A+A or B+B) placed at the end of each arm. A 10-min retention trial (second trial) occurred 2 h later. During this second trial, objects A and B were placed in the maze, and the time that the animal explored the new object (tn) and the old object (to) were recorded. A discrimination index (DI) was defined as (tn−to)/(tn+to). In order to avoid object preference biases, objects A and B were counterbalanced so that half of the animals in each experimental group were first exposed to object A and then to object B, whereas the other half saw first object B and then object A. The maze and the objects were cleaned with 96° ethanol between different animals, so as to eliminate olfactory cues.
Brain processing
One day after the object recognition test, 9-month animals were intracardially perfused after being anesthetized with 80 mg/kg of sodium pentobarbital. Afterwards, brains were dissected and separated sagitally in two hemispheres, one for immunohistochemistry and the other for protein extraction. Immunohistochemistry brains were frozen by immersion in isopentane, chilled in dry ice and stored at −80 °C until sectioning. Thereafter, frozen brains were embedded in OCT cryostat-embedding compound (Tissue-Tek, Torrance, CA), cut into 20-μm-thick sections on a cryostat (Leyca Microsystems, Germany) at −18 °C and placed on slides. Slides containing brain sections were fixed with acetone for 10 min at 4 °C, allowed to dry at room temperature and then frozen at −20 °C until further staining. The cortex and hippocampus of the other hemisphere were dissected and stored at −80 °C until protein extraction.
Immunohistochemistry
Slides were allowed to defreeze at room temperature and then rehydrated with phosphate-buffered saline (PBS) for 5 min. Then, brain sections were blocked and permeabilized with PBS containing 1 % bovine serum albumin (BSA, Sigma-Aldrich) and 0.1 % Triton X-100 (Sigma-Aldrich) for 20 min. After two 5-min washes in PBS, the slides were incubated with the primary antibody for Aβ40, Aβ42, (see Table 1) overnight at 4 °C. They were then washed again and incubated for 1 h at room temperature in the dark with Alexa Fluor secondary antibody. After washing again, nuclear staining was performed by incubating slides in Hoechst (H-33258, Fluka, Madrid, Spain) at 2 μg/mL in PBS for 10 min at room temperature in the dark. Finally, slides were washed, mounted using Prolong Gold (Invitrogen) anti-fade medium, allowed to dry overnight at room temperature and stored at 4 °C. Image acquisition was performed with a fluorescence laser microscope (BX41, Olympus, Germany).
Table 1.
List of antibodies and dilutions
| Antibody (clone) | Catalog reference | Dilution (1:) | Provider |
|---|---|---|---|
| Acetyl-P53 (acetyl-K382) | ab37318 | 500 | Abcam, Cambridge, UK |
| ADAM-10 | ab39177 | 1,000 | Abcam, Cambridge, UK |
| Beclin-1 | ab16998 | 1,000 | Abcam, Cambridge, UK |
| Cdc2 p34 (17) | sc-54 | 1,000 | Santa Cruz, Santa Cruz, CA, USA |
| Cdk5 (C-8) | sc-173 | 1,000 | Santa Cruz, Santa Cruz, CA, USA |
| GSK-3β (27C10) | #9315 | 1,000 | Cell Signaling, Danvers, MA, USA |
| LC3B | #2775 | 1,000 | Cell Signaling, Danvers, MA, USA |
| p35/p25 (C64B10) | #2680 | 1,000 | Cell Signaling, Danvers, MA, USA |
| p53 (1C12) | #2524 | 1,000 | Cell Signaling, Danvers, MA, USA |
| Phospho-cdc2 (Tyr15) | #9111 | 1,000 | Cell Signaling, Danvers, MA, USA |
| Phospho-GSK-3β (Ser9) | #9336 | 1,000 | Cell Signaling, Danvers, MA, USA |
| Phospho-SAPK/JNK (Thr183/Tyr185) | #9251 | 1,000 | Cell Signaling, Danvers, MA, USA |
| Phospho-Tau (pS396) | 44752G | 1,000 | Invitrogen, Carlsbad, CA, USA |
| SAPK/JNK | #9252 | 1,000 | Cell Signaling, Danvers, MA, USA |
| SIRT1 (SIR11) | ab50517 | 1,000 | Abcam, Cambridge, UK |
| Tau (Tau-5) | AHB0042 | 1,000 | BioSource, Camarillo, CA, USA |
| β-Actin (AC-15) | A5441 | 20,000 | Sigma-Aldrich, St. Louis, MO, USA |
| Aβ40 | ab10147 | 50 | Abcam, Cambridge, UK |
| Aβ42 (12F4) | SIG-39142 | 100 | Covance, CA, USA |
| Alexa Fluor 488 donkey anti-mouse IgG | A-11001 | 250 | Invitrogen, Carlsbad, CA, USA |
| Alexa Fluor 546 donkey anti-rabbit IgG | A-11035 | 250 | Invitrogen, Carlsbad, CA, USA |
| Donkey ECL anti-Rabbit IgG, HRP linked | NA934V | 1,000 | GE Healthcare, UK |
| Goat Anti-Mouse HRP Conjugate | #170-5047 | 1,000 | Bio-Rad, Hercules, CA, USA |
Protein extraction
Cortex and hippocampus were micronized through freezing with liquid nitrogen and grinding with a mortar. For total protein extraction, lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM EDTA, 1 % Triton X-100, pH 7.4) containing complete, Mini, EDTA-free Protease Inhibitor Cocktail (Roche, Mannheim, Germany), and Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich, St. Louis, MO, USA) were added to micronized tissue and left on ice for 30 min. Then, samples were centrifuged at 10,000 × g for 10 min and a supernatant with total protein content was collected. All the protein extraction steps were carried out at 4 °C. Protein concentration was determined by the Bradford protein assay.
Western blot
For Western blot analysis, 20 ug of protein were denatured at 95 °C for 5 min in sample buffer (0.5 M Tris–HCl, pH 6.8, 10 % glycerol, 2 % sodium dodecyl sulfate (SDS), 5 % β-mercaptoethanol, 0.05 % bromophenol blue), separated by SDS-PAGE on 10 % polyacrylamide gels and transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were incubated overnight at 4 °C with the primary antibodies (see Table 1) diluted with Tris-buffered saline containing 0.1 % Tween 20 (TBS-T) and 5 % BSA. Membranes were then washed and incubated with secondary antibodies (see Table 1) with TBS-T for 1 h at room temperature. Protein bands were visualized using a chemiluminescence detection kit (Amersham Biosciences). Band intensities were quantified by densitometric analysis and values were normalized to β-actin.
Statistical analysis
Results were analyzed statistically by GraphPad Prism software. Kaplan–Meier survival curve comparison was performed with the log-rank (Mantel–Cox) test. The other data are presented as mean ± SEM, and means were compared with two-tailed, unpaired Student’s t test or ANOVA following Tukey’s Multiple Comparison Test when necessary. In the object recognition test (ORT) a one-sample t test was used to examine whether single columns were different from zero ones. Statistical significance was attained when P values were <0.05.
Results
Increase in life expectancy due to resveratrol
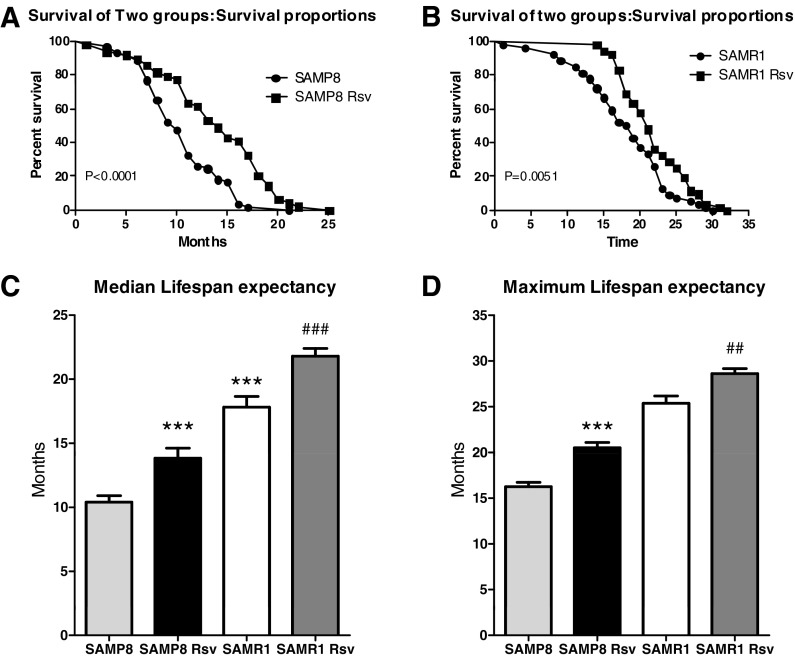
The survival curves were plotted using the Kaplan–Meier estimator. A shift to the right for the resveratrol groups revealed an increased expectancy of life for animals that had been eating the resveratrol diet. The comparison of the groups using the Mantel–Cox log-rank test indicated that there was a significant difference between the survival curves of the control group vs. the resveratrol group, not only in SAMP8 mice (Fig. 1a, P < 0.0001 among groups, Mantel–Cox log-rank test), but also in SAMR1 animals (Fig. 1b, P < 0.01 among groups, Mantel–Cox log-rank test). In addition, the median life expectancy of our control mice was 10.4 months for SAMP8 mice, significantly lower than the 17.8 months of SAMR1 mice (Fig. 1c) in previous studies (Takeda 2009). However, the SAMP8 resveratrol group showed a life expectancy of approximately 14 months, with an increased life expectancy of more than 33 % over the SAMP8 control mice (Fig. 1c). Furthermore, SAMR1 mice fed with resveratrol also showed a median life span of 21.8 months, 22 % more than SAMR1 control mice (Fig. 1c). In addition, maximum life span is the mean of the final 20 % of mice surviving in each group, as determined by the Kaplan–Meier analysis. In comparison with the control groups, both SAMP8 and SAMR1 animals fed with resveratrol significantly increased their maximum life span (Fig. 1d).
Fig. 1.
Kaplan–Meier plot with data expressed as percentage of individuals alive (a, b) and median life span of the four groups studied (c). Mantel–Cox log-rank test analysis reveals a shift to the right for the resveratrol group in SAMP8 (a, P < 0.0001) and SAMR1 (b, P = 0.0051). In the median life span comparison (c) and maximum life span comparison considered as the mean of the final 20 % of mice surviving in each group (d), results are expressed as mean±SEM; ***P < 0.001 vs. SAMP8, ##P < 0.01 vs. SAMR1, ###P < 0.001 vs. SAMR1
Resveratrol decreases cognitive impairment in SAMP8
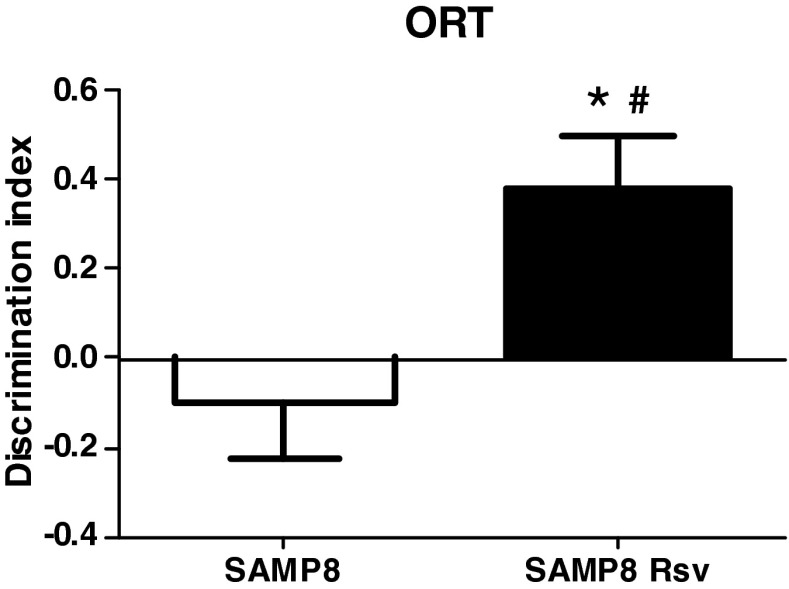
We investigated the effects of a 7-month resveratrol food supplement on 9-month-old SAMP8 mice. This is an age when several alterations such as amyloid deposition or cognitive impairment have been reported (Pallas et al. 2008). We found that, in the ORT, control mice had an impaired memory, as their DI was close to or not different from zero (Fig. 2, P = 0.4665, one-sample t test), revealing that there was no preference for the novel object. On the other hand, resveratrol mice had a positive DI different from zero (Fig. 2, P < 0.05, one-sample t test), revealing that their memory was not impaired as they showed greater preference for the novel object than the one already presented. Furthermore, a comparison of the two groups revealed a more protective effect of resveratrol on their memory than in age-matched SAMP8 mice (Fig. 2).
Fig. 2.
Discrimination index of both groups of SAMP8 animals. Only Rsv group values are positive and different from zero (*P < 0.05). There is a higher DI of Rsv animals than of SAMP8 control mice (#P < 0.05 vs. SAMP8 mice). Bars represent mean±SEM
Resveratrol increases both SIRT1 and AMPK levels while it decreases P53 acetylation
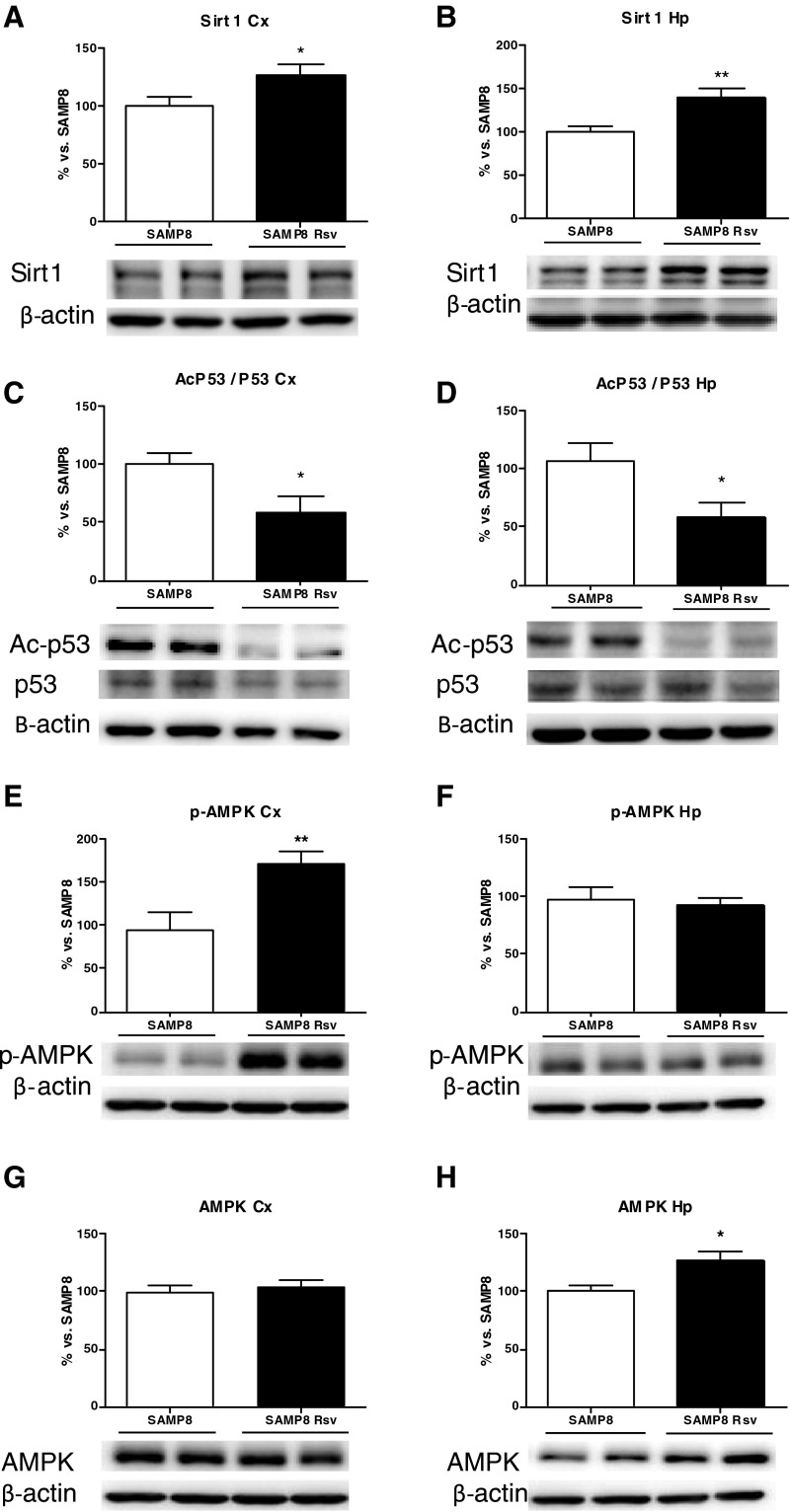
Western blot analysis of the cortex and hippocampus of the two groups revealed higher levels of SIRT1 (Fig. 3a, b) in the animals that had been eating a diet supplemented with resveratrol than in animals eating standard food (control group). In accordance with this observation, the substrate of SIRT1, p53, shows a decrease in its acetylation in these brain areas (Fig. 3c, d). In addition, higher levels of phosphorylated AMPK (p-AMPK) were found in the cortex of the resveratrol group (Fig. 3e) while no modifications were seen in the AMPK levels (Fig. 3g). However, while no increment of p-AMPK levels was found in the hippocampus of the resveratrol mice (Fig. 3f), there were higher AMPK basal levels in these animals than in SAMP8 control mice (Fig. 3h).
Fig. 3.
Levels of sirtuin 1 (a, b), its acetylated substrate p53 (c, d), p-AMPK (e, f), and AMPK (g, h). Bars represent mean±SEM and values are adjusted to 100 % for levels of SAMP8 control mice. Student’s paired t test; *P < 0.05; **P < 0.01 vs. SAMP8. Cortex (Cx), hippocampus (Hp)
Resveratrol reduces amyloid deposition and favors the non-amyloidogenic pathway in the hippocampus of SAMP8 mice
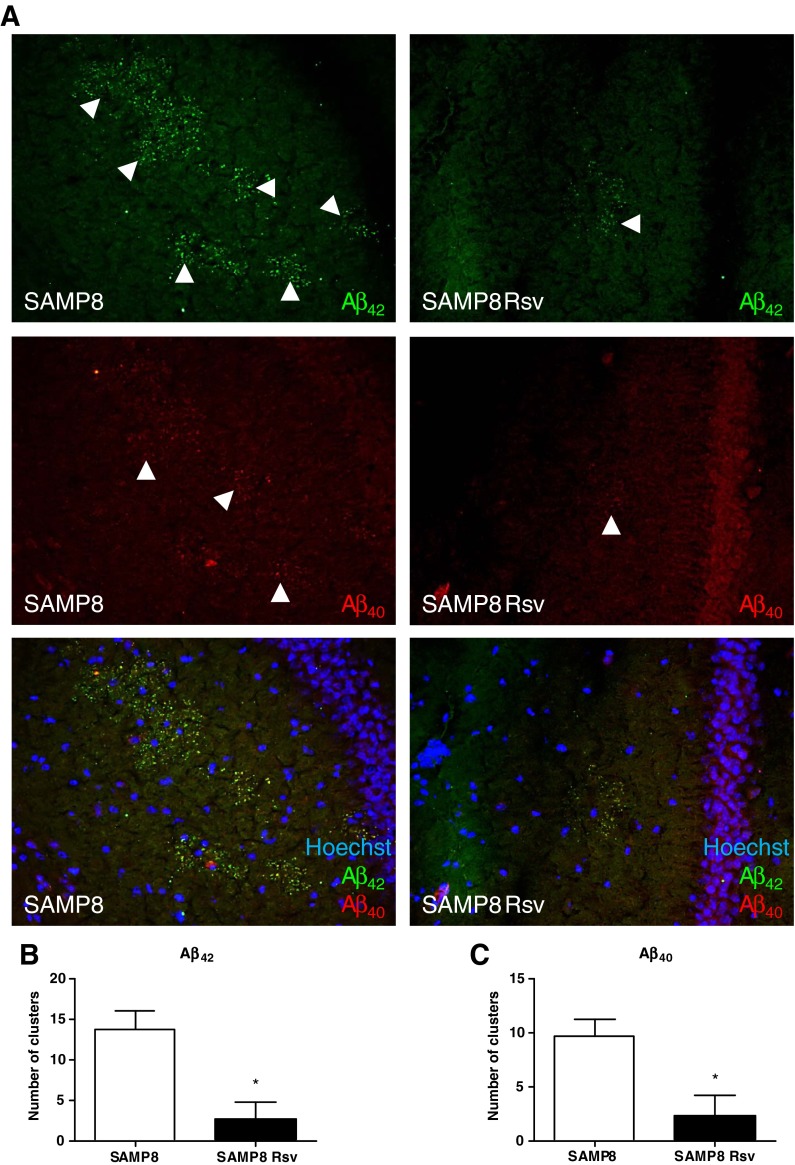
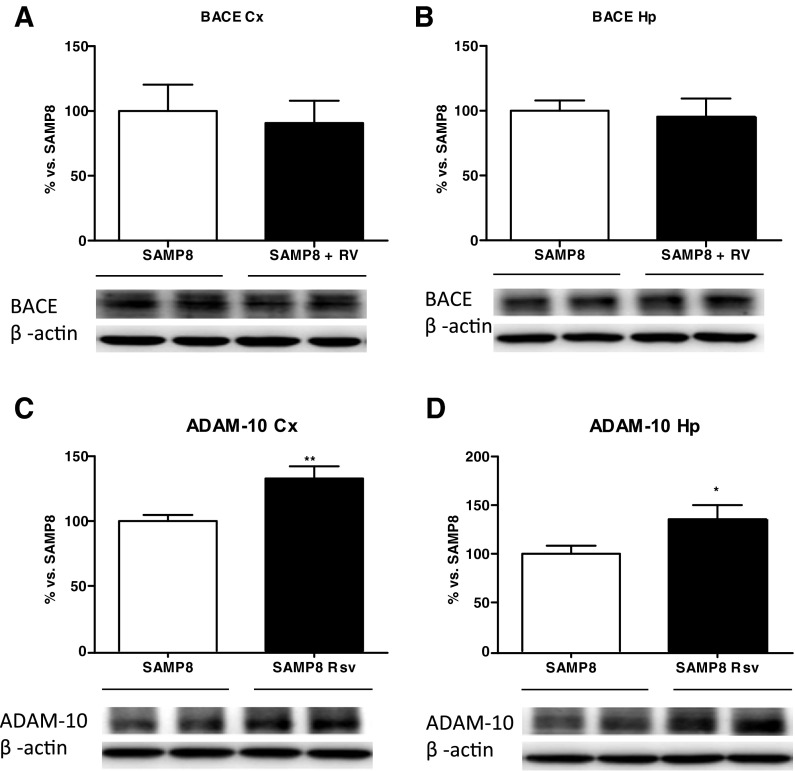
Immunohistochemistry was performed on brain sections with specific antibodies directed against the Aβ42 and Aβ40 to assess whether there were differences between the two groups. Visual analysis revealed amyloid clusters limited only to the hippocampal area, as described before (del Valle et al. 2010). Figure 4 shows that almost no Aβ granules were present in the resveratrol group while several clusters of Aβ42 and Aβ40 granules appeared in the control group (Fig. 4a). Furthermore, we quantified the amount of amyloid clusters that were present in the hippocampus of the two groups. We found that resveratrol decreased the amount of both Aβ42 and Aβ40 accumulations in SAMP8 animals in comparison with SAMP8 control mice (Fig. 4b, c). In addition, Western blot analysis quantified the levels of two enzymes responsible for the amyloidogenic/non-amyloidogenic processing of APP, the α- (ADAM10) and β- (BACE) secretases. We found that while no alterations were seen in the pro-amyloidogenic BACE enzyme (Fig. 5a, b), an increase in the non-amyloidogenic ADAM-10 enzyme was found in both the cortex (Fig. 5c) and the hippocampus (Fig. 5d) of the resveratrol group.
Fig. 4.
Representative hippocampal images of SAMP8 and SAMP8 Rsv animals (a), arrowheads (Aβ40 and Aβ42) indicate some clusters of amyloid granules in both groups. Quantification of the amount of Aβ42 (b) and Aβ40 (c) clusters in the hippocampus of the two groups. Bars represent mean±SEM; values in d–g are adjusted to 100 % for levels of SAMP8 control mice. Student’s paired t test; *P < 0.05 vs. SAMP8. Cortex (Cx), hippocampus (Hp)
Fig. 5.
Cortex and hippocampal levels of BACE (a, b) and ADAM-10 (c, d) of SAMP8 and SAMP8 Rsv animals. Bars represent mean±SEM; values in a–d are adjusted to 100 % for levels of SAMP8 control mice. Student’s paired t test; *P < 0.05; **P < 0.01 vs. SAMP8. Cortex (Cx), hippocampus (Hp)
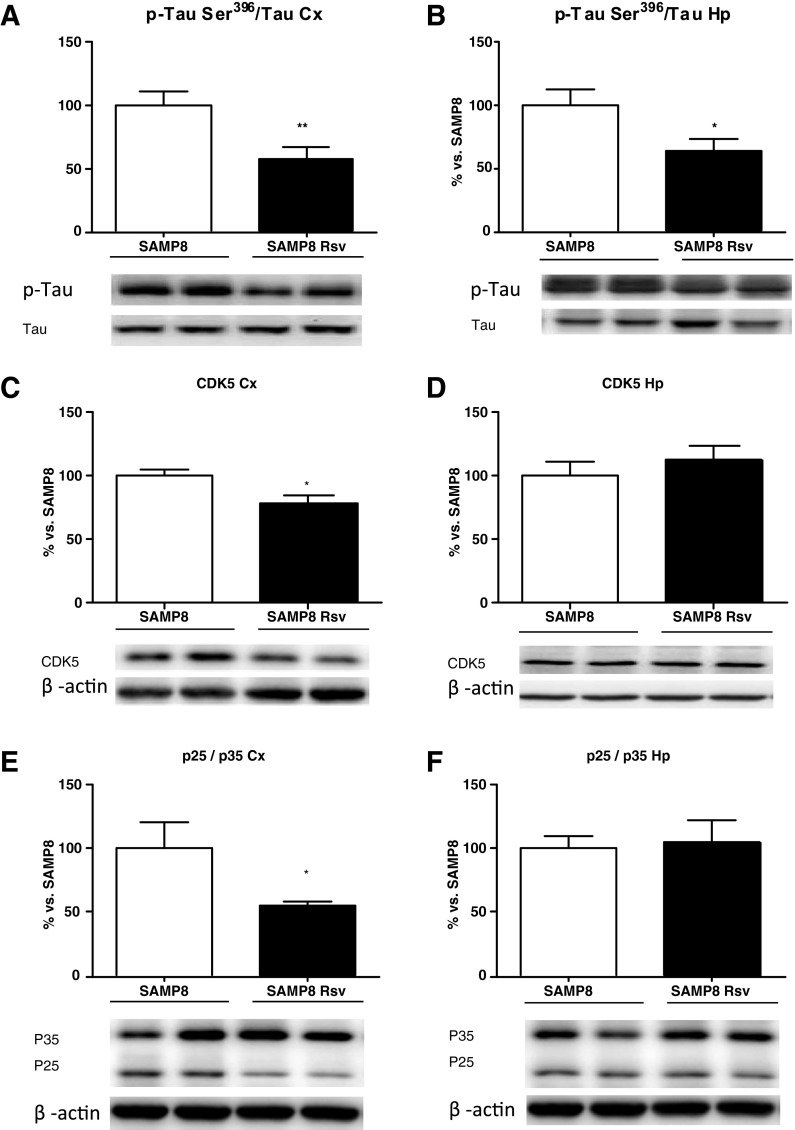
Resveratrol lowers tau hyperphosphorylation at serine 396 and has a differential effect on kinases of the cortex and the hippocampus
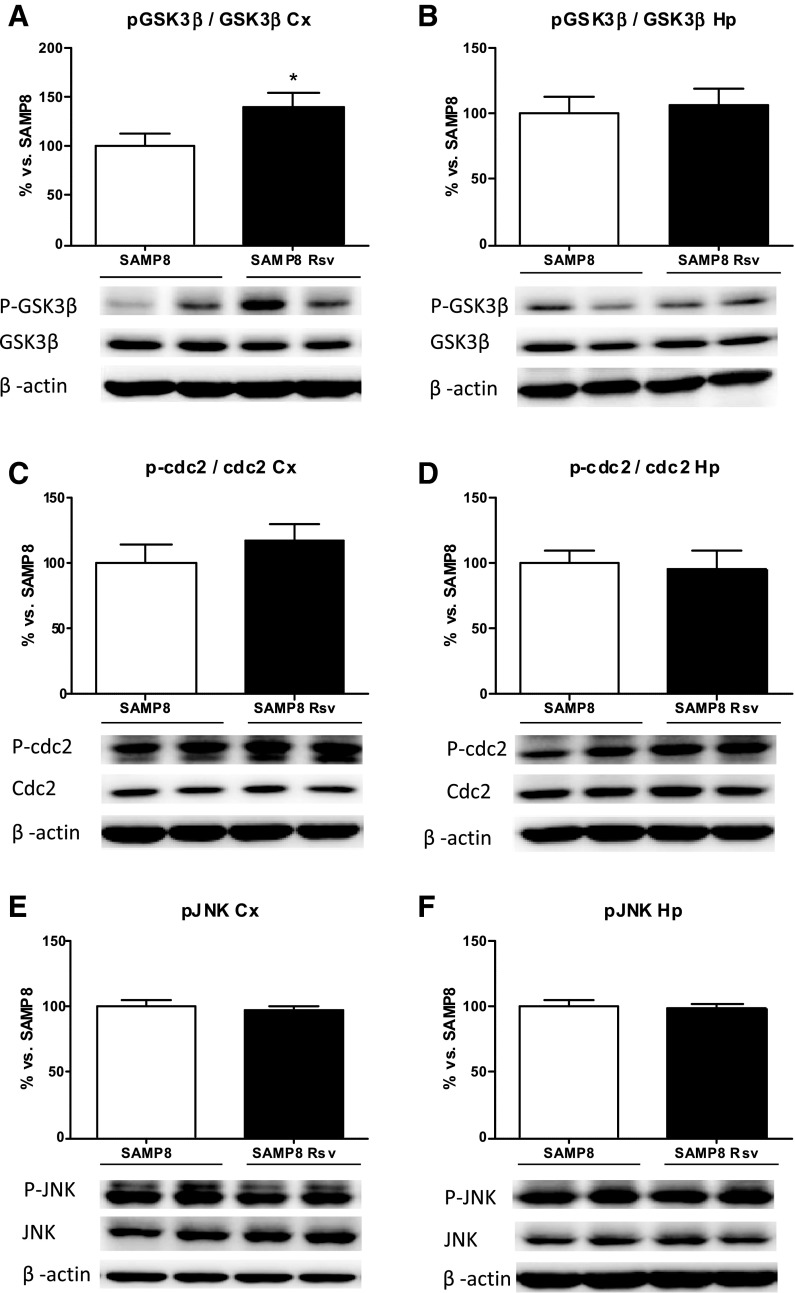
The levels of phosphorylated tau (pTau) at Ser396 have been described as a reliable marker of the severity of AD (Hu et al. 2002). Thus, we evaluated the effect of resveratrol on tau phosphorylation levels in the cortex and hippocampus extracts by Western blot, using a tau antibody that detects only the pTau at Ser396. As can be seen in Fig. 6, not only the cortex but also the hippocampus of animals fed with resveratrol showed lower levels of pTau (Fig. 6a, b). In addition, we investigated the levels of CDK5 and the ratio of its activator p25 to the precursor p35, as well as the phosphorylated levels of GSK3β, CDC2, and JNK. A drop in CDK5 protein levels (Fig. 6c), together with a decrease in the p25/p35 ratio (Fig. 6e), revealed inactivation of this kinase in the cortex of resveratrol animals. In addition, an increase in the levels of phosphorylated GSK3β at Ser9 can be seen (Fig. 7a), which also correlates with the reduced pTau levels, as this enzyme is deactivated when phosphorylated at this residue. However, no modifications were detected in the levels of phosphorylated CDC2 (Fig. 7c) or in the levels of phosphorylated JNK (Fig. 7e). Conversely, there were no changes between resveratrol-treated SAMP8 hippocampus and age-matched SAMP8 control mice in the kinases studied (Figs. 6d, f and 7b, d, f).
Fig. 6.
Levels of phosphorylated tau (pTau) at Ser396 in the cortex (a) and hippocampus (b) of SAMP8 and SAMP8 Rsv groups. Cortex and hippocampal levels of CDK5 (c, d), P25/P35 ratio (e, f). Bars represent mean±SEM and values are adjusted to 100 % for levels of SAMP8 control mice. Student’s paired t test; *P < 0.05; **P < 0.01 vs. SAMP8. Cortex (Cx), hippocampus (Hp)
Fig. 7.
Cortex and hippocampal levels of p-GSK3ß (phosphorylated in Ser9) (a, b). p-cdc2 (phosphorylated in Tyr15) (c, d) and JNK (phosphorylated in Thr183/Tyr185) (e, f). Bars represent mean±SEM and values are adjusted to 100 % for levels of SAMP8 control mice. Student’s paired t test: *P < 0.05 vs. SAMP8. Cortex (Cx), hippocampus (Hp)
Discussion
The results reported here confirm the positive effect of resveratrol on extending mean and maximum life span, memory, and neurodegenerative markers in the SAMP8 mice.
It has been reported that SIRT1 activation by resveratrol increases the life span of Saccharomyces cerevisiae (Howitz et al. 2003), Caenorhabditis elegans (Viswanathan et al. 2005), Drosophila melanogaster (Wood et al. 2004), and the short-lived seasonal fish Nothobranchius furzeri (Valenzano et al. 2006). However, discrepancies between labs remain unexplained. The influence of factors such as interspecies differences in metabolism, genetic variation, diet, physical activity, disease, and mental health should not be underestimated when extrapolating from rodent models (for a review, see Agarwal & Baur 2011). Then, further experimental evidence is needed to clarify the importance of SIRT1 and other mechanisms in the effects of resveratrol.
Here we demonstrate that resveratrol can extend life span in mice. Resveratrol supplement in the diet resulted in a significant increase in mean life expectancy and in maximum life span, in both SAMP8 and SAMR1. At present, resveratrol was reported to prevent early mortality in mice fed with a high-fat diet (Baur et al. 2006) but failed to affect survival significantly in old mice (Miller et al. 2011). A growth hormone releasing hormone antagonist has been shown to extend SAMP8 mice’s median life span (Banks et al. 2010), which was associated with decreased brain oxidative stress. Melatonin has also been reported to increase life span and longevity in SAMR1 and SAMP8 mice (Rodríguez et al. 2008). These authors conclude that the underlying effects of this indoleamine rely on mitochondrial physiology improvement, involving a decrease in reactive oxygen species generation. As old rodents produce more reactive oxygen species than young ones and the rate of mitochondrial reactive oxygen species production is inversely proportional to species’ maximum life span, it would be reasonable to expect that an agent that lowered reactive oxygen species might extend life span (Sohal et al. 1989).
Sirtuins are deacetylases that show anti-aging properties in several animal models and can protect from stress (Donmez et al. 2010). SIRT1 plays a role in regulating different cell processes through deacetylation of important substrates such as p53, FOXO transcription factors, PGC-1α, NFκB, and others, which are closely linked to some age-related diseases (Saunders et al. 2010). SIRT1 activation may play an important role in the life-extending effects of CR (Cohen et al. 2004), and it has been postulated that resveratrol mimics the effect of CR. In this study, we demonstrated an increase in SIRT1 levels in SAMP8 treated with resveratrol in the two brain areas studied, which correlated with a diminution in acetylated forms of p53, one of the main substrates of deacetylase. In addition, SIRT1 pathways are closely related to AMPK signaling as a sensor of energy availability. AMPK is activated by phosphorylation of Thr-172 by LKB1 complex in response to an increase in the AMP/ATP ratio and by calmodulin-dependent protein kinase kinase-beta (CamKKβ) in response to high Ca2+ levels, which contributes to regulating Aβ generation. It has been reported that activation of deacetylase and AMPK are linked through LKB and, when SIRT1 is activated, AMPK is phosphorylated and also activated. Moreover, it has been recently demonstrated that resveratrol’s effects on SIRT1 activation are mediated via the CamKKβ–AMPK pathway by inhibition of cAMP-specific phosphodiesterases (Park et al. 2012a, b). Our results showed that resveratrol activation of SIRT1 in SAMP8 mice correlated with changes in the levels or in the phosphorylation of AMPK, demonstrating again that resveratrol modifies the SIRT1 pathway.
Furthermore, a link between SIRT1 activation, AMPK, and AD is increasingly evident (Gan 2007). Tau phosphorylation and β-amyloid production are sensitive to AMPK inhibition (Greco et al. 2011; Park et al. 2012a, b). SIRT1 activation prevents several signs of neurodegeneration (Bayod et al. 2011), protects against axonal degeneration (Araki et al. 2004), reduces poly-glutamine toxicity (Parker et al. 2005), and diminishes microglia-mediated Aβ toxicity (Chen et al. 2005). AD and Aβ accumulation are inextricably linked with oxidative damage (Smith et al. 1995). Diet supplements with mulberry (a resveratrol-rich fruit) improved not only memory impairment and decreased Aβ accumulation in SAMP8 but also increased antioxidant capacity via the antioxidant response element (ARE)-Nrf2 pathway in the liver and brain (Shih et al. 2010). Furthermore, resveratrol has been reported to improve memory alterations as it preserved cognitive function in aging mice (Oomen et al. 2009) and in transgenic AD mice (Kim et al. 2007). However, although some conflicting results have been obtained on SAMP8 memory alterations (Spangler et al. 2002), we found memory-related deficits at 9 months of age and that resveratrol was able to revert the memory impairment detected.
Part of the beneficial effects described for SIRT1 on Aβ accumulation is the modulation of α-secretases. Transcription of ADAM10 is positively controlled by retinoic acid receptors (RAR), which are activated by their ligand retinoic acid or through deacetylation by SIRT1. Using SIRT1-transgenic and SIRT1-deficient mice, this protein was found to activate the RARb transcription factor, which in turn increased ADAM10 expression (Lichtenthaler 2011). In addition, SIRT1 activation reduced amyloid pathology in a mouse model of AD, and crossing SIRT1 knockout mice with these mice dramatically increased the Aβ burden (Donmez et al. 2010). Moreover, decreased SIRT1 expression has been found in patients with AD, and this decrease correlates with tau and Aβ levels (Julien et al. 2009). Modulation of ADAM10 expression by SIRT1 has also been demonstrated (Gutierrez-Cuesta et al. 2008; Donmez et al. 2010). In our experimental paradigm, we found that resveratrol reduces the Aβ burden in treated SAMP8 brain concomitantly with increases in ADAM10 expression. This effect can be considered specific because no changes were observed in the expression of other secretases, such as, BACE (Donmez et al. 2010). Thus, resveratrol, through SIRT1 activation, specifically induced the non-amyloidogenic processing of non-mutated APP, reducing the presence of previously described amyloid deposits (del Valle et al. 2010).
Furthermore, tau hyperphosphorylation, another hallmark of AD, is mediated by several kinases in the brain. We and others have demonstrated the aberrant phosphorylation of tau in the brain of SAMP8 that is accomplished by activation of several tau kinases such as CDK5, GSK3β, or JNK (Canudas et al. 2005; Chang et al. 2012). Our data show that in the cortex of SAMP8 mice, a diminution in CDK5 and GSK3β activity, both main tau kinases in AD, is induced by resveratrol treatment, and the inhibition of these tau kinases prevented tau phosphorylation in Ser396.
On the other hand, no clear changes in JNK were found. Conversely, with low doses and only 2 months of treatment with pterostilbene, a resveratrol derivative, JNK inhibition was observed in SAMP8, but no changes in tau hyperphosphorylation (measured through PHF antibody) were observed in the cortex (Chang et al. 2011). All these discrepancies are probably due to the different resveratrol doses and also to the long-term treatment by resveratrol that we applied in the present study.
With regard to the hippocampus, although resveratrol was able to prevent tau phosphorylation, we were unable to find changes in the kinases studied. It is plausible to hypothesize that, although long-term treatment by resveratrol prevents tau hyperphosphorylation, detectable by specific phospho-antibodies, the inhibition of intermediate signals under these conditions is lost because of the chronicity of the treatment. On the other hand, oxidative stress is a well-established pathogenic factor in AD (Smith et al. 1995; Markesbery 1997; Perry et al. 1998), and the association of oxidative stress with tau abnormalities is well-known. As such, the resveratrol-driven reductions on tau phosphorylation in the hippocampus could be mediated by the well-known antioxidant effects of this polyphenol rather than through its inhibitory effect on tau kinases. Therefore, our results allow us to conclude that resveratrol inhibits tau phosphorylation in both the cortex and hippocampus.
Finally, we cannot discard the possibly beneficial antioxidant effect of resveratrol in the parameters studied here. More studies should be conducted in different AD models in order to clarify the role of resveratrol in SIRT1 and AMPK pro-survival pathways and other oxidative stress routes such as ARE-Nrf2. However, taking everything into account, in this study we demonstrate that resveratrol alone not only increases mean and maximum life span, and favors AMPK pathways and pro-survival routes such as SIRT1 activation, but also has a neuroprotective role, reducing cognitive impairment in AD and other neurodegenerative parameters such as the amyloid burden and tau hyperphosphorylation.
Acknowledgments
We thank the Language Advisory Service of the University of Barcelona for revising the manuscript. This study was supported by grants SAF-2009-13093, BFU 2010/22149, SAF-2011-23631, and SAF-2012 from the “Ministerio de Educación y Ciencia,” 2009/SGR00893 from the “Generalitat de Catalunya,” 610RT0405 from the Programa Iberoamericano de Ciencia y Tecnologia para el Desarrollo (CYTED), and the Fundación MAPFRE (Spain).
References
- Agarwal B, Baur J. Resveratrol and life extension. Ann NY Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Banks WA, Morley JE, Farr SA, Price TO, Ercal N, Vidaurre I, Schally AV. Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc Natl Acad Sci USA. 2010;107:22272–22277. doi: 10.1073/pnas.1016369107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayod S, Del Valle J, Canudas AM, Lalanza JF, Sanchez-Roige S, Camins A, Escorihuela RM, Pallàs M. Long-term treadmill exercise induces neuroprotective molecular changes in rat brain. J Appl Physiol. 2011;111:1380–1390. doi: 10.1152/japplphysiol.00425.2011. [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Canudas AM, Gutierrez-Cuesta J, Rodríguez MI, Acuña-Castroviejo D, Sureda FX, Camins A, Pallàs M. Hyperphosphorylation of microtubule-associated protein tau in senescence-accelerated mouse (SAM) Mech Ageing Dev. 2005;126:1300–1304. doi: 10.1016/j.mad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging. 2012;33(9):2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I (2010) Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 501(1):79–90 [DOI] [PMC free article] [PubMed]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Sci 325(5937):201–4 [DOI] [PMC free article] [PubMed]
- Del Valle J, Duran-Vilaregut J, Manich G, Casadesús G, Smith MA, Camins A, Pallàs M, Pelegrí C, Vilaplana J. Early amyloid accumulation in the hippocampus of SAMP8 mice. J Alzheimers Dis. 2010;19:1303–1315. doi: 10.3233/JAD-2010-1321. [DOI] [PubMed] [Google Scholar]
- Del Valle J, Duran-Vilaregut J, Manich G, Pallàs M, Camins A, Vilaplana J, Pelegrí C. Cerebral amyloid angiopathy, blood–brain barrier disruption and amyloid accumulation in SAMP8 mice. Neurodegener Dis. 2011;8:421–429. doi: 10.1159/000324757. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses β-Amyloid production by activating the α-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22:1–20. doi: 10.1016/S0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Gan L. Therapeutic potential of sirtuin-activating compounds in Alzheimer’s disease. Drug News Perspect. 2007;20:233–239. doi: 10.1358/dnp.2007.20.4.1101162. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Hamzelou A, Johnston JM, Smith MA, Ashford JW, Tezapsidis N. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem Biophys Res Commun. 2011;414:170–174. doi: 10.1016/j.bbrc.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Cuesta J, Tajes M, Jiménez A, Coto-Montes A, Camins A, Pallàs M. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model. J Pineal Res. 2008;45:497–505. doi: 10.1111/j.1600-079X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Ho L, Chen LH, Wang J, Zhao W, Talcott ST, Ono K, Teplow D, Humala N, Cheng A, Percival SS, Ferruzzi M, Janle E, Dickstein DL, Pasinetti GM. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J Alzheimers Dis. 2009;16:59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hu YY, He SS, Wang X, Duan QH, Grundke-Iqbal I, Iqbal K, Wang J. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer’s disease patients: an ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. Am J Pathol. 2002;160:1269–1278. doi: 10.1016/S0002-9440(10)62554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladiwala AR, Lin JC, Bale SS, Marcelino-Cruz AM, Bhattacharya M, Dordick JS, Tessier PM. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. J Biol Chem. 2010;285:24228–24237. doi: 10.1074/jbc.M110.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–11022. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler SF. Alpha-secretase in Alzheimer’s disease: molecular identity, regulation and therapeutic potential. J Neurochem. 2011;116:10–21. doi: 10.1111/j.1471-4159.2010.07081.x. [DOI] [PubMed] [Google Scholar]
- Manich G, Mercader C, del Valle J, Duran–Vilaregut J, Camins A, Pallàs M, Vilaplana J, Pelegri C. Characterization of amyloid-β granules in the hippocampus of SAMP8 mice. J Alzheimers Dis. 2011;25:535–546. doi: 10.3233/JAD-2011-101713. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Spangler EL, Ingram DK. Behavioral assessment of the senescence-accelerated mouse (SAM P8 and R1) Physiol Behav. 1998;64:15–26. doi: 10.1016/S0031-9384(98)00011-0. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Kiyota Y, Yamazaki N, Nagaoka A, Matsuo T, Nagawa Y, Takeda T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM) Physiol Behav. 1986;38:399–406. doi: 10.1016/0031-9384(86)90112-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Kiyota Y, Nishiyama M, Nagaoka A. Senescence-accelerated mouse (SAM): age-related reduced anxiety-like behavior in the SAM-P/8 strain. Physiol Behav. 1992;51:979–985. doi: 10.1016/0031-9384(92)90081-C. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Bernardo AE, Farr SA, Uezu K, Tumosa N, Flood JF. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/S0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Flood JF. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiol Learn Mem. 2002;78:125–138. doi: 10.1006/nlme.2001.4047. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Kumar VB, Armbrecht HJ. The SAMP8 mouse: a model to develop therapeutic interventions for Alzheimer’s disease. Curr Pharm Des. 2012;18:1123–1130. doi: 10.2174/138161212799315795. [DOI] [PubMed] [Google Scholar]
- Ono K, Condron MM, Ho L, Wang J, Zhao W, Pasinetti GM, Teplow DB. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PGM, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Ag Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Camins A, Smith MA, Perry G, Lee HG, Casadesus G. From aging to Alzheimer’s disease: unveiling “the switch” with the senescence-accelerated mouse model (SAMP8) J Alzheimers Dis. 2008;15:615–624. doi: 10.3233/jad-2008-15408. [DOI] [PubMed] [Google Scholar]
- Park H, Kam TI, Kim Y, Choi H, Gwon Y, Kim C, Koh JY, Jung YK. Neuropathogenic role of adenylate kinase-1 in Aβ-mediated tau phosphorylation via AMPK and GSK3β. Hum Mol Genet. 2012;21:2725–2737. doi: 10.1093/hmg/dds100. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–495. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Sánchez V, Romeu M, Acuña-Castroviejo D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp Gerontol. 2008;43:749–756. doi: 10.1016/j.exger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging. 2010;2:415–431. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, McLaren JS, Mayer C, Duncan JS, Collins AR, Duthie GG, Redman P, Speakman JR. Lifelong alpha-tocopherol supplementation increases the median life span of C57BL/6 mice in the cold but has only minor effects on oxidative damage. Rejuvenation Res. 2008;11:83–96. doi: 10.1089/rej.2007.0586. [DOI] [PubMed] [Google Scholar]
- Shih PH, Chan YC, Liao JW, Wang MF, Yen GC. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J Nutr Biochem. 2010;21:598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Smith MA, Sayre LM, Monnie VM, Perry G. Radical AGEing in Alzheimer’s disease. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Svensson I, Sohal BH, Brunk UT. Superoxide anion radical production in different animal species. Mech Ageing Dev. 1989;49:129–135. doi: 10.1016/0047-6374(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Patel N, Speer D, Hyman M, Hengemihle J, Markowska A, Ingram DK. Passive avoidance and complex maze learning in the senescence accelerated mouse (SAM): age and strain comparisons of SAM P8 and R1. J Gerontol A Biol Sci Med Sci. 2002;57:B61–B68. doi: 10.1093/gerona/57.2.B61. [DOI] [PubMed] [Google Scholar]
- Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Villalba JM, de Cabo R, Alcain FJ. A patent review of sirtuin activators: an update. Expert Opin Ther Pat. 2012;22:355–367. doi: 10.1517/13543776.2012.669374. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield: Charles C Thomas; 1988. [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yagi H, Katoh S, Akiguchi I, Takeda T. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res. 1988;474:86–93. doi: 10.1016/0006-8993(88)90671-3. [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]