Abstract
Background
The gastric sling muscle has not been investigated for possible sensory innervation, in spite of the key roles the structure plays in lower esophageal sphincter (LES) function and gastric physiology. Thus, the present experiment used tracing techniques to label vagal afferents and survey their projections in the lesser curvature.
Methods
Sprague Dawley rats received injections of dextran biotin into the nodose ganglia. Fourteen days post-injection, animals were euthanized and their stomachs were processed to visualize the vagal afferent innervation. In different cases, neurons, muscle cells, or interstitial cells of Cajal were counterstained.
Key Results
The sling muscle is innervated throughout its length by vagal afferent intramuscular arrays (IMAs) associated with interstitial cells of Cajal. In addition, the distal antral attachment site of the sling muscle is innervated by a novel vagal afferent terminal specialization, an antral web ending. The muscle wall of the distal antrum is also innervated by conventional IMAs and intraganglionic laminar endings (IGLEs), the two types of mechanoreceptors found throughout stomach smooth muscle.
Conclusions & Inferences
The innervation of sling muscle by IMAs, putative stretch receptors, suggests that sling sensory feedback may generate vago-vagal or other reflexes with vagal afferent limbs. The restricted distribution of afferent web endings near the antral attachments of sling fibers suggests the possibility of specialized mechanoreceptor functions linking antral and pyloric activity to the operation of the LES. Dysfunctional sling afferents could generate LES motor disturbances, or normative compensatory sensory feedback from the muscle could compromise therapies targeting only effectors.
Keywords: interstitial cells of Cajal, lower esophageal sphincter, mechanoreceptor, reflux, vagus
Introduction
Since the gastric sling muscle was identified nearly 350 years ago1,2, it has been the subject of many morphological descriptions (e.g.,3–7) as well as numerous neuropharmacological and physiological assessments of neuromotor function (e.g8–18). In contrast, though, there seem to have been no systematic attempts to examine the muscle for sensory endings. This lack of information about afferents is paradoxical, given the essential role the sling muscle plays in lower esophageal sphincter (LES) function.
Similarly, the distal antral wall where the sling muscle attaches has been examined primarily as a motor and secretory organ19–22, with relatively little attention to any afferent projections to its tissues. The dearth of information about afferents is problematic, given the critical antral functions in emptying reflexes and motor patterning as well as any putative role the organ may play in anchoring and supporting sling muscle functions.
Part of the inertia stalling morphological investigations of afferent projections to the lesser curvature stems from difficulties in distinguishing identified neural projections to the region. These challenges include discriminating sensory endings from motor terminals and intrinsic neurites, obtaining reliable staining solution penetration of the thick antral muscle wall, and preserving the integrity and best orientation of the region during dissection and processing. Recently, neural tracing techniques have been adapted for use in surveying projections to the GI tract23–30, and such protocols have proved effective in distinguishing sensory and motor elements in the gut. Specifically, vagal afferent projections to the stomach have been characterized in the forestomach and corpus--and in a more limited way--in the proximal antrum.23,24,27,28 These surveys have established that the vagus supplies the gastric muscularis with two common types of afferent terminals, intramuscular arrays (or IMAs) and intraganglionic laminar endings (or IGLEs). The analyses have also established that both types of endings are distinctive and complex structures that can be evaluated most readily in whole mounts. While such tissue preparations have proved useful for examinations of much of the stomach as well as the intestines, the gastric sling muscles and antrum have been more refractory to study. The principal sling muscle bundles lie so superficially along the lesser curvature that they are often damaged or inadvertently removed in the process of organ dissection, and the distal antrum has such a thick muscle wall that tissue penetration of staining and labeling agents is difficult.
The present experiment adopted procedures to preserve the sling muscles and prepare the antral wall so that vagal afferent fibers in the regions could be traced and evaluated with an anterograde tracing protocol. The results indicate that (a) the sling muscles are innervated by elongated vagal IMAs, (b) the distal antral attachment sites of the sling muscles are innervated by novel vagal afferent specializations, antral web endings, and (c) the longitudinal and circular muscle wall of the antrum is innervated by the more ubiquitous IGLEs and IMAs.
Materials and Methods
Animals
Male Sprague-Dawley rats (n = 29; Harlan, Indianapolis, IN, USA; weight at perfusion: 285.4 ± 7.3 g; age at perfusion: 13.7 + 0.8 weeks old) were housed individually in an AAALAC-approved colony room with chow (Laboratory Diet No. 5001; PMI Feeds, Inc., Brentwood, MO) and water available ad libitum. The colony was maintained at 22–24°C on a 12:12 hour light:dark schedule. Every effort was made to minimize suffering and the number of animals used, and all procedures were conducted in accordance with the NIH Guide for Care and Use of Animals (NIH Publications No. 80-23) as revised in 1996, and approved by the Purdue University Animal Care and Use Committee.
Neural Tracer Labeling
After an overnight fast, animals were anesthetized with Isoflurane (Isoflo® Abbott Laboratories, North Chicago, IL) and injected with Glycopyrrolate (0.2 mg/ml, s.c.; American Regent, Inc., Shirley, NY). Following a midline incision of the skin of the ventral neck, the left and right nodose ganglia were exposed by blunt dissection and injected with a mixture of dextrans that strongly labels random subsets of all afferents.29,31 Specifically, 1 µl of a 7.5% dextran solution consisting of a 1:1 mixture of 3K and 10K MW lysine-fixable dextran biotin (D7135 and D1956, respectively; Invitrogen, Carlsbad, CA) and containing 0.01 mg/100 µl of Fast Green FCF (Sigma-Aldrich, CO) was injected into each ganglion. [One animal was injected with 10K lysine-fixable dextran, tetramethylrhodamine (D-1817; Invitrogen, Carlsbad, CA).] The skin was then closed with interrupted sutures. The rats recovered on a warming pad prior to being returned to their home cages where they were provided with ad lib access to water and chow mash. For pain management, rats received 0.01 mg/kg of Buprenorphine subcutaneously (Buprenex®, Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) 15 min post-surgery and then the next morning.
Tissue Fixation and Dissection
Fourteen days post-injection, which is an optimal transport time for dextran tracers to label terminals in the stomach following nodose injection29, rats were killed with a lethal dose of sodium pentobarbital (180 mg/kg., i.p.) and transcardially perfused with physiological saline followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4).
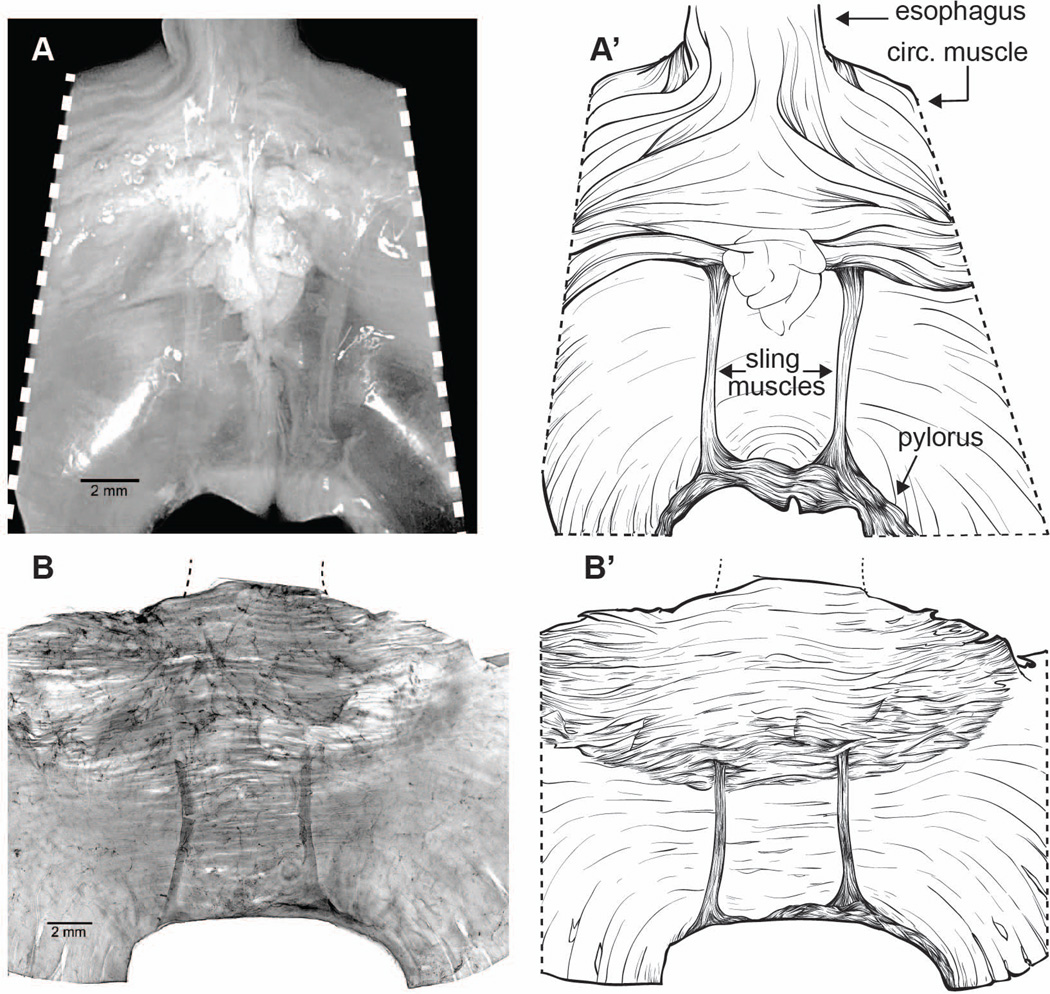
After perfusion, the stomachs were removed. For each antral specimen, the distal stomach was separated from the rest of the organ with a radial cut arching from the greater curvature above the angle of His and continuing through the corpus to where the corpus and antrum meet at the greater curvature. A second radial cut was made through the pyloric torus. The distal stomach specimen was then trimmed to produce a whole mount with the esophagus at one pole, the pylorus at a second pole, and the lesser curvature midline situated centrally (Fig. 1A and 1A’, as well as Fig. 2A). The mucosa and submucosa were removed from the muscle wall. Additionally, other specimens were dissected, trimmed and/or mounted in different orientations to obtain diverse perspectives on the vagal projections to the sling muscle and antrum.
Figure 1.
Lesser curvature specimens illustrating the distal “legs” or principal bands of gastric sling muscle. Panels A and A’: A freshly perfused and trimmed specimen of the lesser curvature that illustrates the region analyzed, prior to final blocking, mucosal removal, staining, and whole mount preparation. (Two small spots of precipitate or surface debris were edited from the whole mount with Photoshop.) B and B’: A lesser curvature whole mount that has been blocked, DAB processed to stain afferents, Cuprolinic Blue counterstained, and flattened on a slide. Scale bars = 2 mm and appear in the lower left of Panel A and Panel B.
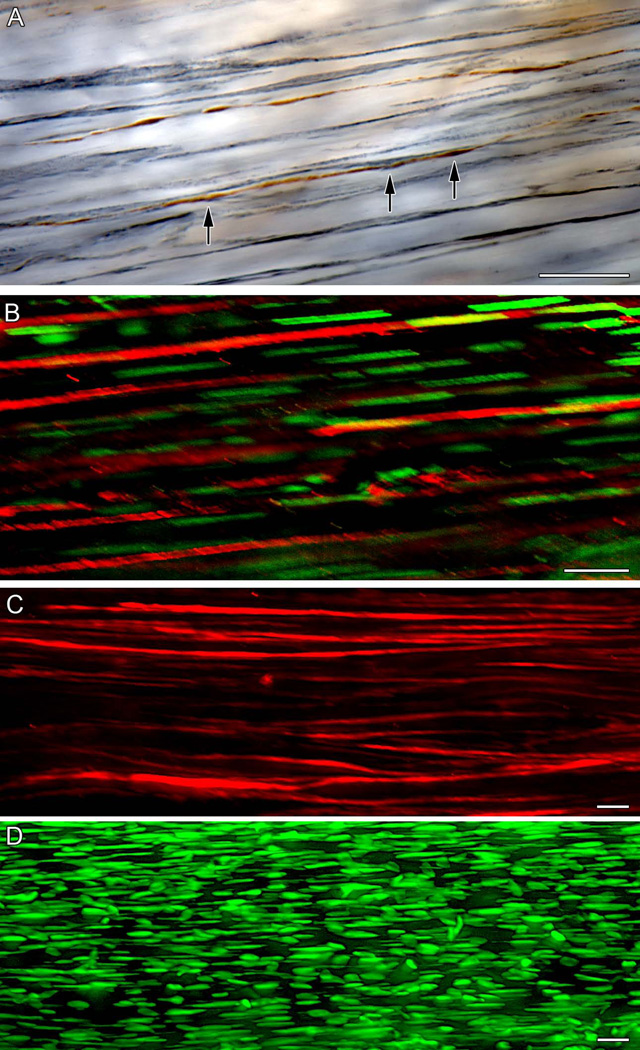
Figure 2.
A vagal intramuscular array (IMA) innervating the gastric sling muscle. Panel A, top left: A Neurolucida countour tracing of a lesser curvature whole mount specimen with the sling IMA tracing superimposed at scale and in its native location. (P = pyloric pole of the whole mount; E = esophageal pole of the whole mount.) Panel A, middle: The full tracing of the IMA innervating the sling muscle. The IMA courses and ramifies over some 8+ mm in one leg of the principal sling muscle bundle. Conforming to the contour of the muscle bundle, the sling IMA is exceptionally long and particularly narrow or compact. [A 500 µm scale bar is located in lower left corner: Letters located near the Neurolucida tracing of the IMA designate the site from which the subsequent panels (B, C, and D) were taken.] Panel B: A segment of the tracing in Panel A produced at higher magnification. (A 250 µm scale bar appears at lower right.) Panels C and D: High power micrographs illustrating the neurites comprising the traced IMA and, in Panel C, the bridging feature that is also characteristic of IMA arbors. (Scale bar at bottom right of Panel D = 20 µm for both Panels C and D.)
To verify that the integrity of the gastric sling was maintained throughout dissection and processing, specimens were photographed serosa-side up: (1) before the blood vessels and adipose tissue were stripped from the lesser curvature (e.g, Fig. 1A and 1A’), (2) after removal of the mucosa and submucosa, (3) once processed to visualize the tracer, and (4) following the various counterstains (e.g., Fig. 1B and 1B’).
Staining
To visualize the vagal fibers labeled with dextran biotin, free-floating whole mounts were treated with a hydrogen peroxide:methanol block (1:4) to quench endogenous peroxidase activity, soaked for 3–5 days in a PBST (PBS containing 0.5% Triton X-100 and 0.08% Na Azide) to facilitate penetration, and then incubated for 1 h in avidin-biotin-horseradish peroxidase complex (PK-6100; Vectastain Elite ABC Kit, Standard; Vector Laboratories, Inc., Burlingame, CA, USA). The specimens were then rinsed in PBS and reacted with diaminobenzidine (DAB) and H2O2 for 5 min to yield a permanent golden-brown stain.
Besides a series of DAB-stained specimens without counterstaining used for differential interference microscopy (n = 5), which helped distinguish between the tissues layers and vessels31, additional DAB-stained whole mounts (n = 13) were assigned to one or more of several counterstaining conditions to better understand the relationship of the vagal sensory terminals to different tissue elements. Briefly, sets of specimens were counterstained with: (A) Cuprolinic Blue (n = 4; quinolinic phthalocyanine; Polysciences, Inc., Warrington, PA), a pan-neuronal marker that labels enteric neurons32,33, (B) SYTOX Green (n = 10; S7020; Invitrogen), a nuclear stain, to define the smooth muscle cells34 or (C) goat, anti-c-Kit (n = 8; AF1356; R&D Systems, Inc., Minneapolis, MN), an antibody to interstitial cells of Cajal, which are cells involved in smooth muscle contraction.22 The antibody to c-Kit was visualized using either Vector SG peroxidase substrate kit (SK-4700; Vector Lab) or Alexa Fluor 594 (A11058; Invitrogen).35,36
Once stained and counterstained, whole mounts were rinsed in distilled water, mounted serosa-up on gelatin-coated slides, air dried, dehydrated in alcohol, cleared in xylene, and coverslipped with D.P.X. (317616; Sigma-Aldrich, St Louis, MO).
Image Analysis and Photography
Specimens were first examined with a Leica DMRE or a Leica DM5500 microscope equipped for brightfield, DIC, and fluorescence microscopy. Labeled afferents were evaluated in a two-step process. In an initial survey, all labeled endings were examined and inventoried. In the second step, endings that were (a) well labeled, (b) isolated from other endings which might otherwise confuse assessments about a particular arbor, and (c) unobscured by tissue folds and artifact were noted and saved for digitizing and quantification. These afferents designated for digital analysis were then traced and quantified at a workstation using Neurolucida software (V.10; MicroBrightField, Inc., Williston, VT) and a Zeiss Axio Imager Z2 microscope with the same illumination options as used in the initial screenings. Each of the microscope stations was equipped with long-working distance objectives (including 40X dry and 63X water immersion lens) to make it practical to image through whole mounts of the thick antral wall.
Single-field images were acquired digitally with a Spot Flex camera controlled using Spot Software (V4.7 Advanced Plus; Diagnostic Instruments, Sterling Heights, MI). To maximize the depth of field of some images, Helicon Focus Pro X64 (Version 5.1.23; HeliconSoft Ltd., Kharkov, Ukraine) was used to merge z-stacked images taken at different focal distances into a single image with an all-in-focus depth of field (i.e. focus stacking).37 Additionally, an Olympus BX61 Spinning Disk confocal microscope operated with SlideBook Digital Microscopy software (Version 5.0.0.31; Intelligent Imaging Innovations, Denver, CO) was used to obtain images of some of the fluorescent counterstaining.
To provide complete images of the extensive terminal arbors, mosaics or multiple-field composites of high magnification images were generated with Surveyor with Turboscan software (V. 6.0.5.3; Objective Imaging, Cambridge, UK) using a Leica DFC310 FX digital CD color camera, running on the Leica DM5500 workstation. Mosaic image z-stacks were also generated, exported to Photoshop CS5 (Adobe Systems, San Jose, CA), and used to trace and reconstruct arbors and then designate and quantify the extent of terminal arbors that were, respectively, lamellar or smooth neurites.
For final figure production, Photoshop CS5 was also used to: (a) apply text and scale bars; (b) adjust brightness, contrast, and sharpness; (c) remove conspicuous artifact (done once: see Fig. 1A); and (d) organize the final layouts of the figures. Additionally, Photoshop CS5 was used to adjust hue to provide the best contrast between the DAB (brown) and the different counterstains.
Statistics
Statistical comparisons consisted of unpaired t-tests generated using GraphPad Prism (version 5.0; GraphPad Software, San Diego, CA).
Results
Since the rat has been used in only a limited number of surveys of the sling muscle38,39 and most anatomical observations of the muscle have been made in other species (for example, human, pig, guinea pig, cat, dog)3,4,5,6,7, we re-assessed the gross morphology of the sling muscle in lesser curvature/antral whole mounts of the stomach from the rat. As illustrated in Fig. 1, the anatomy of the rat sling muscle is comparable to the general pattern described for man and other species.
Specifically, the fibers of the rat sling muscle form a U-shaped muscular yoke in the lesser curvature. Just on either side of a midline or sagittal plane running longitudinally through the lesser curvature, a population of oblique muscle fibers aggregates to form a principal sling muscle bundle that courses proximally from the distal antrum to wrap around the gastroesophageal junction in the angle of His and then return contralaterally to the distal antral wall. More particularly, roughly 2.5 to 3.0 mm lateral to the midline, the muscle fibers of one “leg” of the sling form an attachment subserosally in the stomach wall, at the level of the pyloric torus or the antropyloric junction. The fibers of the leg travel proximally or orally, near the serosal surface, for some 7 or more millimeters, traversing the incisura in a superficial location, before they pass deeper into the smooth muscle wall, fanning out as they run into the muscle layers. The sling fibers then course within the circular muscle layer around the esophageal sphincter region (i.e., looping through the angle of His). Finally, fibers pass distally in the other leg of the sling.
Vagal intramuscular arrays (IMAs) were distributed in the long axial bundles or “legs” of the gastric sling muscle. These IMAs were formed by parent afferent fibers that travelled distally in the lesser curvature to the antropyloric junction to enter a sling muscle bundle at its point of attachment. Once in the sling muscle, the afferent fibers turned to run axially or orally, essentially from pylorus to esophagus, in the sling. As each afferent traveled proximally in the sling, the fiber repeatedly bifurcated to produce an IMA arbor of long neurites running parallel to each other as well as parallel to the muscle fibers of the sling bundle (Fig. 2A, 2B, and 2D). As is the case for IMAs found in other locations in the stomach, short bridging segments of the neurites ran at right angles to and between the parallel arrays of neurites within the sling (Fig. 2C). Individual IMAs occupied either of the legs of the U-shaped sling muscle, but individual afferent fibers did not continue contralaterally to innervate the other leg of the sling muscle.
Samples of sling (n = 6) and antral (n = 20) IMAs that were strongly labeled and relatively free of both artifact and overlapping projections were digitized and evaluated quantitatively with Neurolucida. The sling IMAs were roughly twice the length of the more conventional IMAs located in the circular and longitudinal layers of the wall of the distal antrum (8.7 ± 0.4 mm vs. 3.9 ± 0.3 mm; the difference was significant: p < 0.0001). Whereas the sling IMAs were approximately twice as long, they averaged roughly half as “wide,” measured on their minor axes, as the IMAs in the antral circular and longitudinal muscle layers (0.30 ± 0.04 mm vs. 0.58 ± 0.07 mm; the comparison was again significant: p < 0.05). In more concrete terms, for example, the IMA illustrated in Fig. 2 had an arbor that spanned axially over 8.3 mm. To form the arbor, the terminal IMA neurite bifurcated 220 times, reaching a highest branch order of 28 bifurcations, and generating 440 neurite segments. In total, those 440 neurite segments of the one IMA formed more than 70 millimeters of terminal processes.
Another phenotypic feature of IMA afferents, as reported in previous descriptions of the populations of IMAs that are found in longitudinal and circular muscle layers in the corpus and forestomach, is that their long parallel arrays of neurites run in close association with scaffolds of interstitial cells of Cajal of the intramuscular type (ICC-IMs).24,27,36 In much the way that the vagal afferent innervation of the sling muscle had not been previously investigated, however, the possibility that ICC-IMs might be distributed within the oblique muscle fibers constituting the sling muscle similarly appears not to have been investigated systematically. To evaluate this possibility, the subset of whole mounts in the present series that was immunohistochemically counterstained for c-KIT was assessed. The survey first established that ICC-IMs indeed also course in association with and among the muscle fibers of the sling bundles (Fig. 3B; also 3C and 3D). And then notably, the survey found that, consistent with the common organizational feature of gastric IMAs distributing in conjunction with ICC-IMs, those IMA neurites that innervate the gastric sling muscle bands also are distributed in association with ICC-IMs (Fig. 3A) and have varicosities consistent with those contacts that have been seen in ultrastructural surveys.40 Finally, and instructively, no IGLEs or other types of vagal afferent endings were observed within the bands of muscle fibers forming the sling.
Figure 3.
Organization of vagl IMAs, ICC-IMs, and muscle fibers in the principal gastric sling muscle. Panel A: Dextran-labeled DAB-stained vagal IMA afferent fibers (brown; one designated with arrows) running in association with ICC-IMs (gray-black; c-KIT immunostaining) in the sling muscle. B: A double labeled example of the principal sling muscle bundle illustrating how ICC-IMs (red; c-KIT immunostaining) run in tight association with smooth muscle fibers indicated by their stained nuclei (green; Sytox Green counterstain). C: Interstitial cells of Cajal of the intramuscular type (ICC-IMs) stained with c-KIT immunohistochemistry lie within the sling muscle bundle and run parallel to the oblique muscle fibers of the sling. D: The Sytox Green-stained elongated nuclei of muscle fibers in the principal sling bundle illustrate the orientation of the muscle fibers parallel to the axis of the muscle bundle. The scale bars in the respective panels all equal 20 µm.
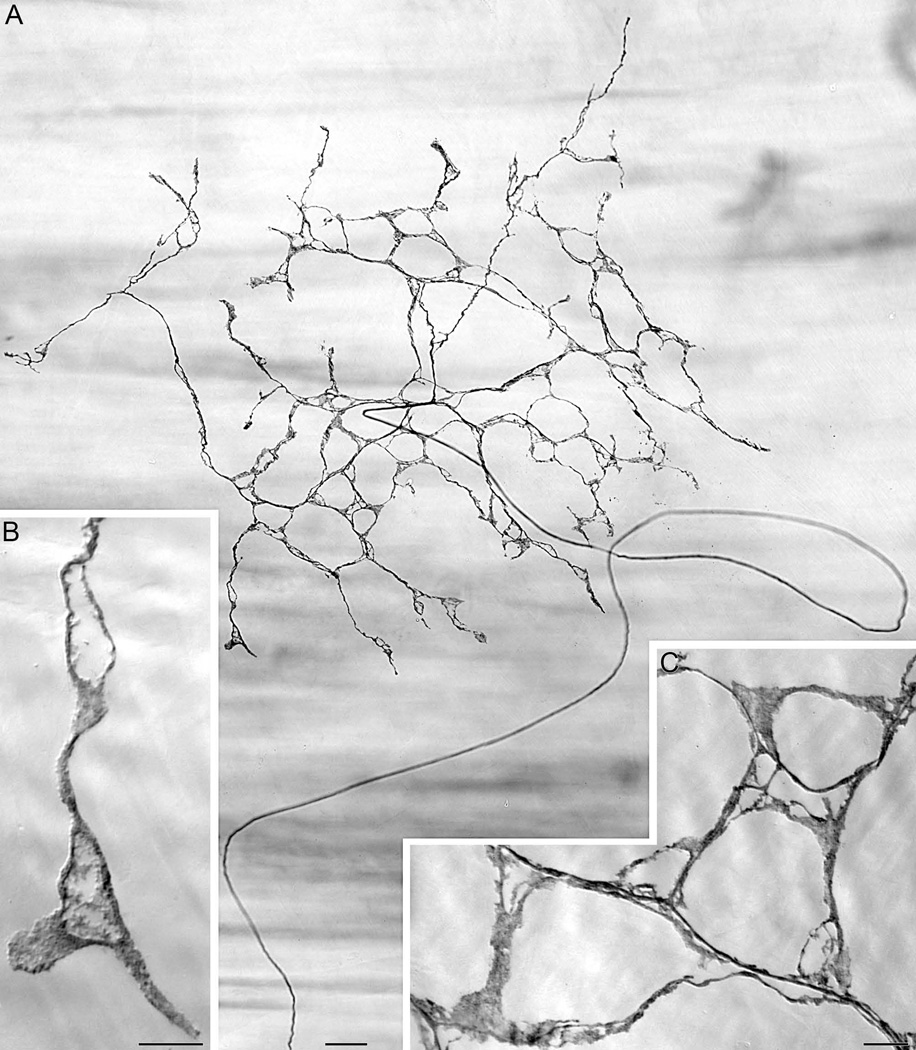
Though not distributed within the band of sling muscle fibers, a second--and novel--vagal afferent terminal specialization, an antral web ending, innervated the attachment sites of the gastric sling muscles. This specialization has not been previously described or illustrated, it appears to occur only in conjunction with the gastric sling, and it is not organized like either IMAs or IGLEs. These specialized web endings, which occur adjacent to the distal antral attachment sites of the sling muscle bundles, are formed by individual vagal afferent fibers terminating as flat plates of lamellar neurites that lie parallel to the serosa and the muscular layers of the antrum. These web endings (Fig. 4) are situated subserosally just (~1 mm) proximal to the distal attachment zones of the sling muscles and immediately lateral to the muscle bundles. Indeed, though the centers of the antral web ending plates are situated lateral to the sling fibers, the medial sides of the plates actually lie immediately below the legs of the sling muscle bundles. It needs to be noted, though, that this apparent location was observed in specimens processed for DAB and flattened as whole mounts, and we cannot eliminate the possibility that prior to the histological processing the sling muscles could be even more tightly juxtaposed with the antral web endings. From the macrophotographic series throughout the processing, there seemed to be some modest medial-lateral movement of the bands of sling muscle in the course of processing that could have displaced the alignment of the slings with the net endings.
Figure 4.
A dextran-labeled vagal afferent antral web ending located subserosally just proximal and lateral to the sling muscle attachment in the distal antrum. Panel A: A wide-field mosaic photomicrograph illustrating the characteristic pattern of a parent neurite (arching up from 6 o’clock) projecting to the region of the antral attachment site and issuing a radial web of laminar neurites to form a flat plate-like ending. Illustrating the superficial location of the web ending plate, the out-of-focus horizontal bands of muscle fibers are deeper, in the smooth muscle layers of the antrum. B and C: Higher power micrographs of processes of the web ending in A, illustrating the neurites and lamellar processes comprising the fascicles and terminals that form the antral web ending. (The process in Panel B is shown in the same orientation it assumes in the very bottom left of the web ending illustrated in Panel A; the processes in Panel C have been rotated approximate 135° counterclockwise from their orientation in their location in the near-bottom left of the web illustrated in Panel A.) The scale bar in Panel A = 40 µm; the scale bar in Panel B = 10 µm; and the scale bar in Panel C = 10 µm.
Each web ending was produced by a parent afferent fiber coursing superficially, from deeper in the muscle wall, to the subserosal level and into the innervation target, the peri-attachment zone, and then forming a central hub from which neurites arborized repeatedly (Fig 4A). These neurites arborized radially in planar fashion immediately below the serosa. The collateralizing neurites tended to radiate away from the parent, forming a net- or web-like ending consisting of a plate of lamellar processes. Since these web ending plates distributed immediately subserosally, superficial to the muscle layers, the lamellar neurites did not course in registration with muscle fibers, ICC-IMs or other types of ICCs (see Fig. 5), blood vessels, or other conspicuous features of the antral wall. Similarly, the antral web endings did not innervate the enteric ganglia, with the exception that occasionally, in a few specimens, a neurite or neurites of a web ending would course deep into the muscle wall to form a basket ending or calyx around some element sandwiched in the muscle tissues (not shown). These infrequently observed calyxes were so dense and the whole mounts so thick, that the elements were not identified with certainty.
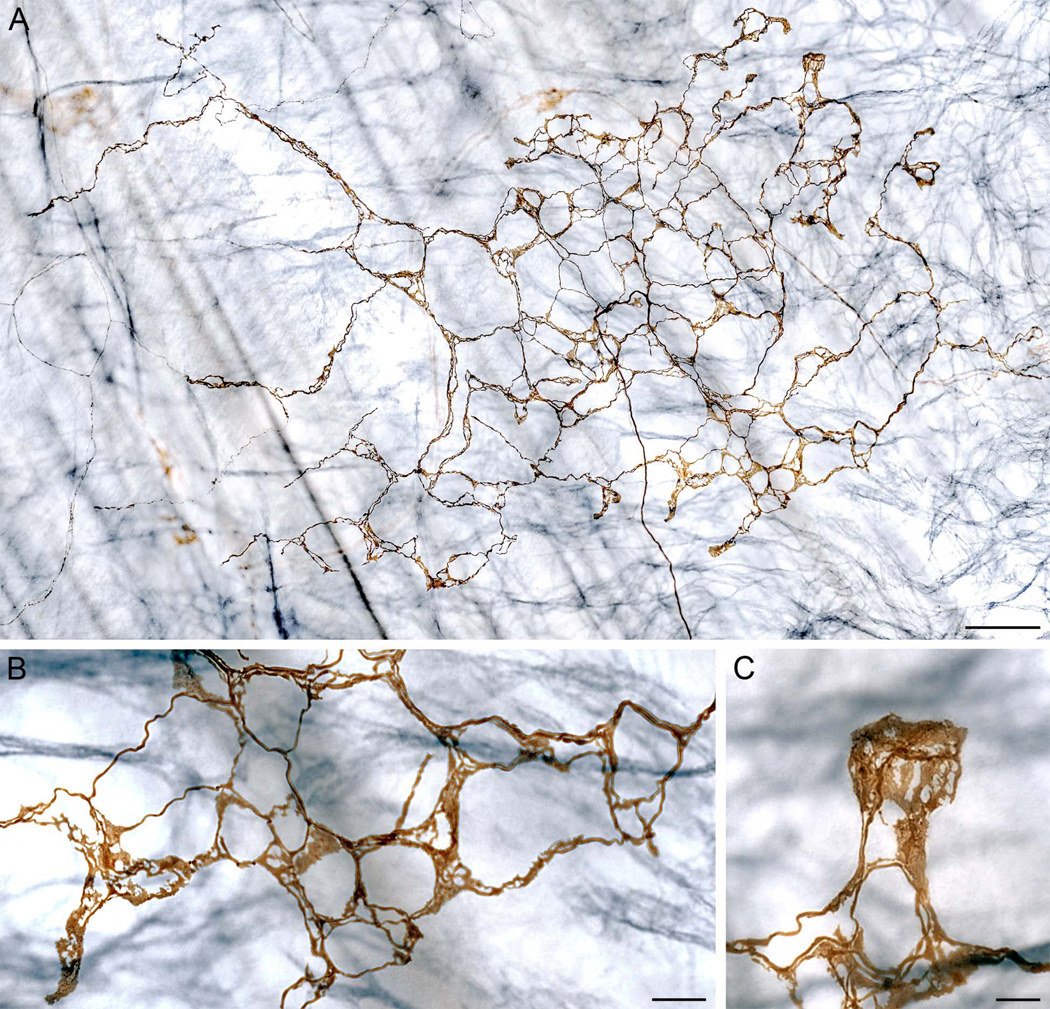
Figure 5.
A dextran-labeled antral web ending (stained brown) in an antral specimen with ICCs counterstained immunohistochemically for c-KIT (blue-grey staining). Panel A: A wide-field mosaic photomicrograph illustrating the architecture of antral web endings. The parent fiber enters at the bottom of the figure at approximate 5:30 o’clock and courses to the center of the ending to begin arborizing. Consistent with the subserosal location of the web endings, the blue-grey muscle-associated ICCs are deeper--in the muscle layers of the antral wall--and out of focus in the image. Panels B and C: High power micrographs of two of the lamellar terminal regions of the web ending illustrated in Panel A. The net of endings in Panel B is located in the lower right part of the web ending, and the laminar terminal region in Panel C is located in the extreme upper right corner of Panel A. Scale bar in panel A = 80 µm; scale bars in B and C = 20 µm.
A conspicuous characteristic of these antral web endings was their highly lamellar pattern of termination. One given parent fiber would branch or bifurcate repeatedly to produce the terminal neurites forming the plate-like antral webs. The neurites forming the web ending tended to aggregate into fascicles of perhaps two to six fibers coursing together, running in something of a looping pattern, to give the ending a honeycomb appearance. In forming the fascicles, the neurites were so tightly intertwined that it was impractical to trace and measure individual neurites reliably. As the fascicles forming the web distributed and radiated to generate the ending, substantial percentages of the overall neurite segments appeared lamellar. Not just the ends of the neurites, but indeed significant portions of the fibers were lamelliform (Figs. 4B, 4C, 5B and 5C). In a series (n = 22) of antral web specimens traced, digitized, and reconstructed, an average of 58.2% of the total lengths of the neurites within the web endings consisted of the lamellar form.
Single vagal afferent parent fibers often elaborated complex and extensive antral webs that individually distributed to form an entire plate of lamellar terminals (Figs. 4 and 5) In a survey of separate web endings (n = 17), the individual plates of afferent terminals occupied on average an area of 0.44 mm2. And more particularly, for example, the antral web ending illustrated in Figure 4 innervated a surface area of 0.31 mm2, while the web ending in Figure 5 had a presumptive “receptive field” of 0.54 mm2. These afferent specializations, the antral web endings, were found exclusively at this distal antral site on the lesser curvature, neighboring the attachment of the sling muscle.
The labeling protocol we employed was chosen in part because it was weighted towards limiting the number of fibers labeled in any one specimen and thus limiting confusion of neurites results from overlapping projection fields. The strategy was effective and generated a majority of cases such as the individual fibers and receptive fields just described. In a few cases, though, two or more parent fibers converged on the same site to form a compound afferent plate and produced webs consisting of two or more abutted “tiles” in mosaic fashion. In three such cases that were digitized, the composite or “tiled” terminal fields occupied an average area of 1.80 mm2.
When the antral web endings were identified in the present survey of the distal antral region, an immediate hypothesis was that the web endings might be either IMAs or IGLEs remodeled to conform to the local antral environment. Whether or not such remodeling does occur remains an open empirical issue, but the present assessment of the antrum did, however, establish that conventional IMAs and IGLEs are found in association with their normal target tissues in the muscularis of the distal antrum, immediately deep to the subserosal antral webs (though exceptionally elongated, the IMA in Fig. 2 illustrates the phenotypic characteristics of IMAs--also see references27,29,36; for an example of an IGLE, see Fig. 6--and also see references23,27,29,35).
Figure 6.
Panel A: A wide-field view of a portion of a dextran-labeled conventional vagal IGLE afferent in the antral muscle wall deep to the oblique sling muscle fibers that are innervated by the exceptionally long IMAs and antral web endings. The parent fiber enters the field at 12:00 o’clock and distributes four conspicuous IGLEs to Cuprolinic Blue stained myenteric ganglia in the muscle wall of antrum. For reference, the field in the micrograph is equivalent in area or total magnification to the field occupied by the antral web ending illustrated in Figure 4A. Panels B–D: Higher power images of three of the IGLEs from Panel A. The IGLEs in these higher power views have been rotated from about 15 to 30 degrees for better framing. For comparing the different afferent specializations, these high power images can be contrasted with the high power laminar processes of the web endings illustrated in Figs. 4B, 4C, 5B, and 5C. The scale bar in Panel D is equivalent to 40 µm in Panel A and 20 µm in Panels B, C and D.
Discussion
The gastric sling muscle forms a critical component of the lower esophageal sphincter (LES)4,10,41 and exhibits disordered contractile activity patterns in gastroesophageal reflux disease (GERD, aka GORD)42. Physiological39,42–44, electrophysiological8,11,12,16, and immunohistochemical14 investigations of the sling and cooperative LES muscle fibers have been focused largely on understanding the motor control of the tissues. Neuropharmacological therapies for GERD are most commonly designed either to ameliorate symptoms (proton pump inhibitors, mucosal protectants, etc.) or to treat efferent and motor disturbances of the functional sphincter with prokinetics.45–48
Little experimental attention has been paid to any possible sensory innervation of the gastric sling, even though it has been recognized that postulated sensory mechanisms of any reflex arcs responsible for LES sphincter function might, in theory, be promising targets for pharmacological management of GERD44 and in spite of evidence that the crural diaphragm, another element contributing to gastroesophageal sphincter function, appears to be innervated by vagal mechanoreceptors that participate in vago-vagal reflexes of the distal esophagus.49 Investigations have evaluated how distension of the whole stomach, which activates mechanoreceptive intramuscular arrays (IMAs) and intraganglionic laminar endings (IGLEs) innervating the entire organ, affects LES motor function.50–52 Indeed, in these experiments, there is also some evidence that the distension of the organ activates stretch receptors52, but again the distension experiments have targeted the whole viscus, not the sling muscle or distal antrum. Similarly, pharmacological blockades of more or less all of the afferent signals from gut have demonstrated effects on the brainstem motor outflow back to the LES15,53, but once again the stimuli have not been delivered to or focused on the sling muscle or its afferents. To address the lack of information about the sling muscle afferent innervation, the present experiment examined the muscle and its antral attachments for vagal sensory innervation.
Our observations establish that (a) both the sling muscle bundles and their attachments in the distal antrum are innervated by vagal afferents and (b) these afferents terminate in two types of highly differentiated and specialized sensory endings. The conclusions can be considered first for the sling muscle IMAs and then for the distal antral web endings.
Sling Muscles are Innervated by Vagal Intramuscular Arrays
Previous surveys of stomach afferents have established that IMAs run in the longitudinal and circular layers of the forestomach, corpus and distal antrum; and the endings are also found in the circular muscle associated with the LES and pylorus.28,35,54 However, in contrast to circular and longitudinal muscle fibers, sling muscles are considered oblique muscles. The present observations now demonstrate that IMAs also innervate the principal bands of oblique sling muscle fibers as they span from the distal antrum to the gastroesophageal junction.
These IMAs in the sling muscle consist of multiple neurites, issued by a single parent fiber, running within the muscle and parallel to the fibers. Like conventional gastric IMAs24,27,36, the sling muscle IMAs branch repeatedly to generate endings of long arbors of parallel neurites interconnected by bridging fibers. On the other hand, in contrast to IMAs commonly found in longitudinal and circular muscle, sling (oblique) muscle IMAs are exceptionally elongated and exceptionally narrow, conforming to the shape of the principal bundle of the sling muscle.
Another new observation emerging from the present survey is the finding that oblique muscle fibers that comprise the sling muscle run in association with interstitial cells of Cajal of the intramuscular type (ICC-IMs). This basic organization of ICC-IMs running in conjunction with sling muscle fibers is noteworthy, with implications for the motor control of the sling muscle fibers, since this architecture apparently has not been previously observed or evaluated in terms of ICC-oblique-muscle operation. Additionally, though, our structural observation of ICC-IMs in the sling muscle also has implications for understanding the sensory innervation of the tissue: like other gastric IMAs, which form functional complexes with ICC-IMs, the sling muscle IMAs run conjointly with scaffolds of ICC-IMs. This structural arrangement suggests that IMAs form complexes or afferent end organs with ICC-IMs.
Functionally, IMAs have not yet been well characterized electrophysiologically. However, in other gastric tissue, both ultrastructural evidence indicating that IMAs form appositions with ICCs40 and additional morphological and functional extrapolations previously reviewed27,36 suggest that IMAs in complexes with ICC-IMs operate like length or stretch detectors, in a manner similar to spindle organs in striated muscle. If this inference is correct, then the sling muscle IMAs may register the lengths or the amount of stretch of the axial bands of muscle linking the distal antrum and gastroesophageal junction. The in vitro observation that strips of sling muscle do not display the rhythmic patterns of contraction or motility seen in strips of longitudinal and circular muscle from other regions of the stomach43 (though in humans they do show transient phasic responses11) seems generally consistent with the idea that sling IMAs may report stretch information rather than phasic cycles of gastric motility.
Distal Antral Attachments of Sling Muscles Contain Specialized Vagal Afferent Antral Web Endings
Previous inventories of vagal afferent terminals in the stomach wall have shown that sensory projection fields consist not only of IMAs within the muscle layers, but also of intraganglionic laminar endings (IGLEs) associated with the myenteric ganglia situated between muscle layers.23,24,27,55 In keeping with this previously reported pattern of dual vagal afferent innervation of the muscle wall in the forestomach, corpus and proximal antrum, the present experiment also verified that the muscle wall of the distal antrum similarly contains conventional IMAs and IGLEs occupying the same respective tissue layers and sites that they innervate in other stomach regions.
In striking contrast to these two widely distributed terminal types, however, the distal antral site of attachment of the sling muscle is also innervated more superficially by a novel, apparently unique, vagal afferent specialization, the antral web ending. The webs differ dramatically from both IMAs and IGLEs in morphology, in location, and in accessory tissues. Morphologically, the antral web endings consist of honeycombed networks of lamellar neurites forming flat terminal plates neighboring the sling attachments. In location, the antral web specializations are situated subserosally, superficial to the layers of smooth muscle, and immediately deep to the serosal membrane. In several ways, then, the antral web endings are phenotypically distinct from both IMAs and IGLEs: In terms of accessory tissues, the antral webs do not distribute within and parallel to muscle fiber bundles (as IMAs do), are not associated with scaffolds of ICC-IMs (as are IMAs throughout circular and longitudinal as well as IMAs in oblique sling muscle), and do not form laminar contacts with myenteric ganglia (as IGLEs do).
Without physiological and electrophysiological analyses of the antral web fibers, it is impractical to ascertain what signals these novel endings transduce or what role(s) the neurites play in reflex circuits. Nonetheless, the characteristic location, restricted distributions, and morphology of the webs offer some provisional suggestions to shape future functional analyses. Though the antral webs are not situated so as to be fully in series with the sling muscle fibers, their distribution immediately lateral and just proximal to the antral attachments of the sling muscles implies that the endings may respond to events either affecting or affected by the gastric sling muscles. Too little is known about the detailed operations of the tissue wall at the exact location of the plates formed by the antral webs, but the terminals of the web afferents may be positioned strategically to transduce the local forces playing in the antral wall and generated either by antropyloric motor programs of emptying or by sling muscle activity on the distal antral attachment (in a manner functionally similar to Golgi tendon organs).
Implications for Antral Afferent Specializations across Species
The IMAs found in the principal bundle of sling muscle had the morphological features, locations within muscle bundles, and associations with ICC-IMs that define IMAs. Hence, it is clear that these sling afferents were indeed IMAs, with their exceptionally long and narrow terminal arbors adapted to and specialized for the particular demands of sling muscle fibers. In keeping with this conclusion, terminal arbors of IMAs in different regions--e.g., forestomach, corpus, and pylorus--are known to vary in length and other parameters.23,24,28,36,54 Furthermore, in an ongoing inventory of afferents associated with the LES, we have noted distinctive and characteristic morphological specializations of circular muscle IMAs associated with clasp muscle fibers (unpublished observations).
The question of the neural source or precursors of the novel antral web afferents is less clear. The observation that conventional IMAs and IGLEs are located in the antral muscularis immediately deep to the antral web ending cannot eliminate the possibility that some IMA or IGLE afferents are redirected in development, become ectopic, and then are differentiated or remodeled by the local subserosal tissue environment near the sling attachment site. Consistent with this idea, the distal antral region immediately adjacent to the site near the sling attachment that was innervated by the web endings often contained afferent terminals that, arguably, appeared transitional in morphology with features of conventional IMAs and features of the web endings. Also, consistent with the complementary idea that the web endings might be remodeled and redistributed IGLEs is the observation that web endings occasionally sent a neurite back deeper into the smooth muscle wall to form a calyx-like cluster. At any rate, the conventional IMAs and IGLEs situated in the muscularis of the antrum offer informative and contrasting frames of reference for assessing the two specialized afferent phenotypes in the distal antrum, namely the exceptionally elongated sling IMA and the conspicuously honeycombed and lamelliform antral web ending.
Issues of afferent specializations and remodeled processes reflecting the unique structure and functions of the antrum raise an additional issue. The stomach generally, and the antrum more particularly, varies considerably in structure from species to species. Such variation occurs in the case of the sling muscle as well, with apparent differences both in the coherence or the compactness of the oblique fiber bundles that constitute the sling and in how far they reach distally2,3,5,6. Presumably, the morphologies and functions of sling muscle IMAs and antral web endings would be expected to vary from species to species in keeping with their gastric adaptations. At the same time, though, it seems both plausible and parsimonious to infer that the afferent terminals we have observed in the rat are not sensory mechanisms found only in one species. Presumably homologous afferent innervation patterns, adapted to the particularities of antral specializations, occur in other species.
Potential Roles of the Vagal Afferent Innervation of the Sling Muscle in Gastric Emptying and GERD
The fact that sling muscle bundles provide direct structural linkages between the antropyloric region and the gastroesophageal junction--and not only direct linkages, but connectives that are likely sensitive to stretch and perhaps reflexively self-calibrating--may call for reassessment of the neurally mediated interactions between the two regions. It is a reasonable hypothesis that the afferent traffic initiated by the vagal sensory specializations associated with the sling muscle may be instrumental in coordinating antral contractions with the transient openings and closings of the pylorus and LES. An understanding of such reflexes should provide a foundation for developing or improving treatments for disorders of gastric emptying.
Additionally, and more particularly, an understanding of the neural controls of the LES may provide a better foundation for treating GERD. Though much success has been achieved in developing therapies to treat the symptoms of GERD, the need to develop preventative strategies that improve LES competence is generally acknowledged.44–48 Such improvements of LES functioning will likely require an understanding of the neural circuits controlling sphincter operation. And given that the gastric sling muscle is innervated by two species of putative mechanoreceptors (i.e. long IMAs and web endings), it may be necessary to consider that the muscle may play a more dynamic and integrative role in coordinating the operation of the LES. Rather than working with an exclusively motor model of the sling muscle as simply an effector contracting to varying degrees to establish LES function, neurogastroenterology may need to recognize that the muscle has the sensory innervation necessary to generate feedback--perhaps closed loop feedback--that can influence the extrinsic and intrinsic motor control of sphincter functions and motility. Thus, the existence of afferents innervating the sling muscle may well have significant therapeutic implications: Distorted afferent feedback could initiate sphincter disturbances or, alternatively, undistorted compensatory sensory feedback might offset or even cancel the effects of therapeutic interventions targeted only to effectors.
Acknowledgments
Acknowledgments and Funding
We thank Jacqueline K. Mason (figure production) and Jennifer McAdams (animal handling and tissue processing) for their expert help on the project.
This work was supported by grants from the National Institutes of Health, USA (DK27627 to TLP; DK61317 to RJP and TLP).
Footnotes
TLP contributed to the design, data analysis and writing of the project. JMG contributed to the design, microscopy, data analysis, and figure preparation. EAB performed surgeries, prepared tissue, and processed specimens. CNB and FNM contributed to the microscopy as well as ending reconstructions and data analyses with Neurolucida. RJP contributed to the design, data analysis, figure preparation, and writing of the project.
No competing interests declared.
References
- 1.Willis T. Pharmaceutice rationalis, sive, Diatriba de medicamentorum operationibus in humano corpore. [Oxford]: E. Theatro Sheldoniano, Prostant apud Robertum Scott Bibliopolam Londinensem, M. DC. LXXIV [1674] [Google Scholar]
- 2.Willis T. In: Pharmaceutice rationalis: Or, an exercitation of the operations of medicines in humane bodies. Shewing the signs, causes, and cures of most distempers incident thereunto. In two parts. As also a treatise of the scurvy. Willis Tho, Dring T, Harper C, Leigh J., editors. Fleetstreet: London; 1679. [Google Scholar]
- 3.Torgerson J. The muscular build and movements of the stomach and duodenal bulb, especially with regard to the problem of the segmental divisions of the stomach in the light of comparative anatomy and embryology. Acta Radiologica Supp. 1942;45:1–191. [Google Scholar]
- 4.Friedland GW, Melcher DH, Berridge FR, Gresham GA. Debatable points in the anatomy of the lower oesophagus. Thorax. 1966;21:487–498. doi: 10.1136/thx.21.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedland GW, Kohatsu S, Lewin K. Comparative anatomy of feline and canine gastric sling fibers. Analogy to human anatomy. Am J Dig Dis. 1971;16:493–507. doi: 10.1007/BF02235539. [DOI] [PubMed] [Google Scholar]
- 6.Vicente Y, Da Rocha C, Yu J, Hernandez-Peredo G, Martinez L, Perez-Mies B, Tovar JA. Architecture and function of the gastroesophageal barrier in the piglet. Dig Dis Sci. 2001;46:1899–1908. doi: 10.1023/a:1010631030320. [DOI] [PubMed] [Google Scholar]
- 7.Yassi R, Cheng LK, Al-Ali S, Sands G, Gerneke D, LeGrice I, Pullan AJ, Windsor JA. Three-dimensional high-resolution reconstruction of the human gastro-oesophageal junction. Clin Anat. 2010;23:287–296. doi: 10.1002/ca.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck CS, Osa T. Membrane activity in guinea pig gastric sling muscle: a nerve-dependent phenomenon. Am J Physiol. 1971;220:1397–1403. doi: 10.1152/ajplegacy.1971.220.5.1397. [DOI] [PubMed] [Google Scholar]
- 9.Liebermann-Meffert D, Allgower M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–38. [PubMed] [Google Scholar]
- 10.Stein HJ, Liebermann-Meffert D, DeMeester TR, Siewert JR. Three-dimensional pressure image and muscular structure of the human lower esophageal sphincter. Surgery. 1995;117:692–698. doi: 10.1016/s0039-6060(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 11.Preiksaitis HG, Diamant NE. Phasic contractions of the muscular components of human esophagus and gastroesophageal junction in vitro. Can J Physiol Pharmacol. 1995;73:356–363. doi: 10.1139/y95-045. [DOI] [PubMed] [Google Scholar]
- 12.Preiksaitis HG, Diamant NE. Regional differences in cholinergic activity of muscle fibers from the human gastroesophageal junction. Am J Physiol. 1997;272:G1321–G1327. doi: 10.1152/ajpgi.1997.272.6.G1321. [DOI] [PubMed] [Google Scholar]
- 13.Giuli R, Jamieson GG, Scarpignato C, editors. The Esophagogastric Junction. Paris, France: John Libbey Eurotext; 1998. [Google Scholar]
- 14.Yuan S, Brookes SJ. Neuronal control of the gastric sling muscle of the guinea pig. J Comp Neurol. 1999;412:669–680. doi: 10.1002/(sici)1096-9861(19991004)412:4<669::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108(Suppl 4a):90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- 16.L'Heureux MC, Muinuddin A, Gaisano HY, Diamant NE. Feline lower esophageal sphincter sling and circular muscles have different functional inhibitory neuronal responses. Am J Physiol Gastrointest Liver Physiol. 2006;290:G23–G29. doi: 10.1152/ajpgi.00303.2005. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu AS, Triadafilopoulos G. Neuro-regulation of lower esophageal sphincter function as treatment for gastroesophageal reflux disease. World J Gastroenterol. 2008;14:985–990. doi: 10.3748/wjg.14.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Mashimo H, Paterson WG. Regional differences in nitrergic innervation of the smooth muscle of murine lower oesophageal sphincter. Br J Pharmacol. 2008;153:517–527. doi: 10.1038/sj.bjp.0707573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewin MJ. Cellular mechanisms and inhibitors of gastric acid secretion. Drugs Today (Barc) 1999;35:743–752. doi: 10.1358/dot.1999.35.10.561693. [DOI] [PubMed] [Google Scholar]
- 20.Schulze K. Imaging and modelling of digestion in the stomach and the duodenum. Neurogastroenterol Motil. 2006;18:172–183. doi: 10.1111/j.1365-2982.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- 21.Parkman HP, Jones MP. Tests of gastric neuromuscular function. Gastroenterology. 2009;136:1526–1543. doi: 10.1053/j.gastro.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- 24.Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- 25.Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Tassicker BC, Hennig GW, Costa M, Brookes SJ. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res. 1999;295:437–452. doi: 10.1007/s004410051250. [DOI] [PubMed] [Google Scholar]
- 27.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–2. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- 29.Powley TL, Phillips RJ. Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D, editors. Advances in vagal afferent neurobiology. Boca Raton: Taylor & Francis; 2005. [Google Scholar]
- 30.Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178:1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holst MC, Powley TL. Cuprolinic blue (quinolinic phthalocyanine) counterstaining of enteric neurons for peroxidase immunocytochemistry. J Neurosci Methods. 1995;62:121–127. doi: 10.1016/0165-0270(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 33.Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: An evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Thacker M, Rivera LR, Cho HJ, Furness JB. The relationship between glial distortion and neuronal changes following intestinal ischemia and reperfusion. Neurogastroenterol Motil. 2011;23:e500–e509. doi: 10.1111/j.1365-2982.2011.01696.x. [DOI] [PubMed] [Google Scholar]
- 35.Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Powley TL, Phillips RJ. Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience. 2011;186:188–200. doi: 10.1016/j.neuroscience.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulbins J, Gulbins R. Photographic multishot techniques : high dynamic range, super-resolution, extended depth of field, stitching. Santa Barbara, CA: Rocky Nook ; Distributed by O'Reilly Media; 2009. [Google Scholar]
- 38.Montedonico S, Diez-Pardo JA, Tovar JA. Gastroesophageal reflux after combined lower esophageal sphincter and diaphragmatic crural sling inactivation in the rat. Dig Dis Sci. 1999;44:2283–2289. doi: 10.1023/a:1026665022685. [DOI] [PubMed] [Google Scholar]
- 39.Montedonico S, Godoy J, Mate A, Possogel AK, Diez-Pardo JA, Tovar JA. Muscular architecture and manometric image of gastroesophageal barrier in the rat. Dig Dis Sci. 1999;44:2449–2455. doi: 10.1023/a:1026678820384. [DOI] [PubMed] [Google Scholar]
- 40.Powley TL, Wang X-Y, Fox EA, Phillips RJ, Liu LWC, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 41.Brasseur JG, Ulerich R, Dai Q, Patel DK, Soliman AMS, Miller LS. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J. Physiol. 2007;580:961–975. doi: 10.1113/jphysiol.2006.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller L, Dai Q, Vegesna A, Korimilli A, Ulerich R, Schiffner B, Brassuer J. A missing sphincteric component of the gastro-oesophageal junction in patients with GORD. Neurogastroenterol Motil. 2009;21:813–852. doi: 10.1111/j.1365-2982.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck CS, Mason GR. Gastric peristalsis. A study of regional rates of contraction. Am J Surg. 1970;119:217–219. doi: 10.1016/0002-9610(70)90037-1. [DOI] [PubMed] [Google Scholar]
- 44.Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 45.Vakil N. New pharmacological agents for the treatment of gastroesophageal reflux disease. Rev Gastroenterol Disord. 2008;8:117–122. [PubMed] [Google Scholar]
- 46.Boeckxstaens GE. Emerging drugs for gastroesophageal reflux disease. Expert Opin Emerg Drugs. 2009;14:481–491. doi: 10.1517/14728210903133807. [DOI] [PubMed] [Google Scholar]
- 47.Hershcovici T, Fass R. Pharmacological management of GERD: where does it stand now? Trends Pharmacol Sci. 2011;32:258–264. doi: 10.1016/j.tips.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Johnson DA, Levy BH., 3rd Evolving drugs in gastroesophageal reflux disease: pharmacologic treatment beyond proton pump inhibitors. Expert Opin Pharmacother. 2010;11:1541–1548. doi: 10.1517/14656566.2010.482932. [DOI] [PubMed] [Google Scholar]
- 49.Young RL, Page AJ, Cooper NJ, Frisby CL, Blackshaw LA. Sensory and motor innervation of the crural diaphragm by the vagus nerves. Gastroenterology. 2010;138:10921–11101. doi: 10.1053/j.gastro.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 50.Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779–784. doi: 10.1016/0016-5085(85)90572-4. [DOI] [PubMed] [Google Scholar]
- 51.Scheffer RC, Akkermans LM, Bais JE, Roelofs JM, Smout AJ, Gooszen HG. Elicitation of transient lower oesophageal sphincter relaxations in response to gastric distension and meal ingestion. Neurogastroenterol Motil. 2002;14:647–655. doi: 10.1046/j.1365-2982.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- 52.Penagini R, Carmagnola S, Cantu P, Allocca M, Bianchi PA. Mechanoreceptors of the proximal stomach: Role in triggering transient lower esophageal sphincter relaxation. Gastroenterology. 2004;126:49–56. doi: 10.1053/j.gastro.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 53.Partosoedarso ER, Young RL, Blackshaw LA. GABA(B) receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G658–G668. doi: 10.1152/ajpgi.2001.280.4.G658. [DOI] [PubMed] [Google Scholar]
- 54.Kressel M, Berthoud HR, Neuhuber WL. Vagal innervation of the rat pylorus: an anterograde tracing study using carbocyanine dyes and laser scanning confocal microscopy. Cell Tissue Res. 1994;275:109–123. doi: 10.1007/BF00305379. [DOI] [PubMed] [Google Scholar]
- 55.Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]