Abstract
Progression of diabetic nephropathy (DN) is manifested by gradual scarring of both the renal glomerulus and tubulointerstitial region. Over the past several years, the general understanding of the pathogenic factors that lead to renal fibrosis in DN has expanded considerably. In this review, some of the important factors that appear to be involved in driving this fibrosing process are discussed, with special emphasis on newer findings and insights. It is now clear that multiple cell types in the kidney contribute to progressive fibrosis in DN. New concepts about bradykinin, TGF-β and eNOS signaling as well as JAK/STAT activation and the central role of inflammation in both glomerular and tubulointerstitial fibrosis are discussed.
Keywords: Diabetes, Kidney, Signaling, Matrix Proteins, Glomerulus
1. Fibrosis in Diabetic Nephropathy
Progression of diabetic nephropathy (DN) to end-stage kidney disease is manifested by the gradual, inexorable scarring of the renal glomerulus followed by a similar fibrosing process in the tubulointerstitial region. Many studies have attempted to elucidate the molecular mechanisms that lead to this chronic, relentless fibrosis in both glomerular and tubulointerstitial compartments so that effective therapies and preventative strategies could be developed. Over the past several years, the general understanding of the pathogenic factors that lead to renal fibrosis in DN has expanded considerably.
Diabetic glomerular fibrosis is caused by accumulation of extracellular matrix proteins in the mesangial interstitial space resulting in fibrosis manifested by either diffuse or nodular changes [1]. The most common matrix proteins detected are collagen types I, III and IV and fibronectin [2]. These accumulate both due to increased synthesis by mesangial cells and to reduced degradation by mesangial matrix metalloproteinases [3]. Over 20 years ago, Mauer and colleagues established the clear link between mesangial matrix expansion and progression of diabetic kidney disease by demonstrating that measures of mesangial expansion strongly predicted the clinical manifestations of DN [4].
The critical role of tubulointerstitial expansion and fibrosis in the progression of DN has also been recognized for at least 2 decades [4, 5]. Tubulointerstitial changes, including fibrosis, appear to be critical for final progression of DN to kidney failure in Type 1 patients, and may play an even more important, though heterogenous, role in Type 2 patients [6]. This expansion differs somewhat from glomerular changes as initial expansion appears to be largely due to increase cellular components which is followed by an increase in interstitial fibrillary collagen [7]. There is a strong positive correlation between the grade of fibrosis of the renal cortical interstitium and the serum creatinine concentration at the time of biopsy in patients with DN. Interstitial expansion appears to contribute to the deterioration of renal function especially during later stages of DN and appears to be particularly important in older patients [7].
Given the strong associations between glomerular and tubulointerstitial fibrosis and the progressive decline of renal function in DN, the critical charge to investigators has been to elucidate the mechanisms that promote fibrosis in DN. In this brief review, the pathogenic processes that appear to be critical to the development of diabetic glomerular and tubulointerstitial fibrosis will be summarized, emphasizing newer findings and insights.
All glomerular cells contribute to fibrosis in DN
Since mesangial matrix expansion is the glomerular lesion that best predicts progressive DN, it would be natural to assume that the mesangial cell is front and center in mediating mesangial sclerosis in DN. This is not exclusively true. Both podocytes and endothelial cells, and crosstalk between all 3 glomerular cell types, play important roles in the evolution of diabetic glomerulosclerosis and a comprehensive approach to understanding glomerular fibrosis requires an analysis of all 3 glomerular cell types. Signaling mechanisms that directly enhance mesangial cell matrix protein expression or reduce matrix metalloproteinase expression are clearly implicated in diabetic glomerular fibrosis. However, more recent reports have indicated that isolated podocyte damage and loss leads to glomerulosclerosis [8] and that podocyte loss appears to be a requisite early event in DN [9]. Indeed, podocyte loss in humans with type 2 diabetes is as good a predictor of progressive nephropathy as is mesangial expansion [10]. Presumably, signals to the mesangium from damaged podocytes, or the hemodynamic factors triggered by podocyte loss could provide stimulus to the mesangial cell to react by increases in ECM synthesis or decreases in ECM degradation. Similarly, attention to glomerular endothelial cells in the pathogenesis of glomerulosclerosis has been revived in the last few years. A growing number of models of endothelial dysfunction have resulted in diabetic fibrosis and sclerosis [11–15] suggesting substantial crosstalk between endothelial and mesangial cells.
Tubulointerstitial Cell Participation in Fibrosis in DN
While the importance of tubulointerstitial fibrosis in DN is well recognized, it has not received as much study as glomerular fibrosis, due in part to lack of good animal models of progressive DN and in vitro systems. Nonetheless, it appears that proximal tubule cells as well as extratubular cells in this compartment contribute to fibrosis, and although controversy remains, there is evidence that proximal tubular cells undergo epithelial to mesenchymal transition (EMT) in DN [16]. While the importance of EMT in the progression of fibrosis in DN is not yet clearly defined, a number of studies have confirmed that critical profibrotic factors are stimulated in proximal tubule epithelia as tubulointerstitial fibrosis proceeds [17, 18] and that accumulation of myofibroblasts occurs in the interstitium [19]. Triggers for tubulointerstitial fibrosis are certainly myriad, but there has been a good deal of attention to the potential role of diabetic glomerular changes in stimulating this process. A number of studies have suggested that albuminuria can trigger a profibrotic response in proximal tubule cells and the surrounding tubulointerstitial compartment. Data derived largely from cultured cell experiments indicate that albumin can bind to receptors on proximal tubular cells and be endocytosed. Both albumin binding and endocytosis appear to trigger proinflammatory and profibrotic responses in proximal tubular cells that can lead to enhanced tubulointerstitial fibrosis via a variety of mechanisms [20–24].
Extrarenal and Systemic Effects in DN Fibrosis
Multiple extrarenal and systemic factors also stimulate fibrotic responses in the glomerular tuft and in the tubulointerstitial compartment in diabetes. There is reason to believe that extraglomerular cells, such as bone marrow-derived mesangial cell progenitors [25] and macrophages [26, 27], may significantly contribute to glomerulosclerosis in DN. In addition, glomerular and systemic hypertension [28] and activation of the renin-angiotensin-aldosterone system [29] are clearly among the most important factors leading to progressive renal fibrosis. Several reports suggest that glomerular hypertension and resultant mesangial cell stretch can also lead to enhanced expression of the GLUT1 facilitative glucose transporter [30] that would in turn trigger off the set of intracellular responses, noted above, leading to glomerulosclerosis. While we will concentrate on specific kidney processes in the following sections, interactions between systemic and local factors will be critical to the ultimate understanding of renal scarring in DN.
2. Mechanisms of glomerular and tubulointerstitial fibrosis: New observations
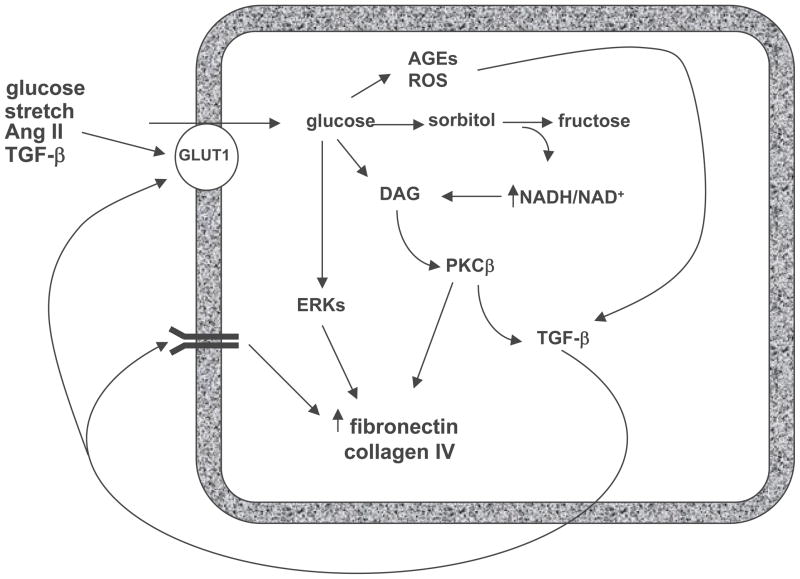
During the 1990s, most studies on pathogenic mechanisms of fibrosis in DN focused on glomerular changes and specifically the role of the mesangial cell in the production of extracellular matrix (ECM) proteins. From these studies a general consensus emerged about major signaling mechanisms involved in this process. In this consensus view, high extracellular glucose induces an increase in glucose uptake via increased expression of the facilitative glucose transporter, GLUT1 [31, 32]. The resultant enhancement in glucose metabolic flux leads to activation of a number of metabolic pathways that result in increased advanced glycation end product (AGE) and oxidative stress generation [33–35], that in turn activate a number of signaling pathways that lead to enhanced ECM production directly via PKC β stimulation [36, 37] of AP-1 transcriptional activation, ERK pathways and, critically, TGF-β1 synthesis [38, 39] which in an autocrine and paracrine fashion stimulates its signaling pathways to stimulate ECM protein synthesis (Fig. 1). These responses triggered by TGF-β1 appear to be the final common pathway by which mesangial fibrosis occurs.
Figure 1. Simplified mesangial model of the pathogenesis of glomerular fibrosis.
High extracellular glucose leads to increased mesangial cell glucose uptake via enhanced expression of the facilitative glucose transporter, GLUT1, which activates metabolic pathways that result in increased reactive oxygen species (ROS) and advanced glycation endproduct (AGE) generation, that in turn activate a number of signaling pathways that augment ECM production directly via PKC β stimulation of AP-1 transcriptional activation, ERK pathways and, critically, TGF-β1 synthesis which in an autocrine and paracrine fashion stimulates its signaling pathways to further enhance ECM protein synthesis. Abbreviations: AGE – Advanced glycation end product; Ang II – Angiotensin II; DAG – Diacylglycerol; ERK – Extracellular signal related kinase; NAD/NADH – Nicotinamide adenine dinucleotide; ROS – Reactive oxidant species; PKC – Protein kinase C; TGF – Transforming growth factor.
There have been a number of interesting insights into mechanisms of diabetic glomerulosclerosis since 2000 that have increased our understanding of the complexities of this process and have focused on events in other cell types in the glomerulus and tubulointerstitium. Some of these mechanisms will be briefly described in this section. It must be stressed that many of these observations have been derived from animal models and cell signaling experiments and have yet to be confirmed in humans. When such confirmation in human disease is available it will be stressed.
Reduction in endothelial nitric oxide synthase and vascular endothelial growth factor
As noted above, the role of endothelial cells in the pathogenesis of diabetic nephrosclerosis has received increased attention. One endothelial abnormality in diabetes is altered regulation of endothelial nitric oxide production [59, 60]. Although there is some controversy [61], most reports suggest higher levels of nitric oxide production early in diabetes but reduced levels in progressive DN [60]. eNOS gene polymorphisms that result in decreased eNOS expression are associated with advanced DN [62, 63]. Several reports that analyzed the effects of diabetes in mice with targeted deletion of the endothelial nitric oxide synthase (eNOS) gene have underlined the critical participation of endothelial responses in diabetic glomerular pathology in animal models [13, 14, 59, 64]. Diabetic eNOS knockout mice developed substantial mesangial expansion and glomerulosclerosis, including nodular sclerosis, as well as other signs of advanced DN including reduction in glomerular filtration rate. The mechanisms by which reduced NO production results in glomerular pathology are not clear. Resultant hemodynamic effects may play a role, but it seems most likely that paracrine effects between endothelial cells, podocytes, mesangial cells and even resident macrophages account for the bulk of the impressive pathology resulting from altered endothelial NO production. A recent report on the effects of eNOS deletion in db/db C57BLKS mice showed that diabetic eNOS knockout mice developed substantial tubular injury as well as cortical scars and increased collagen between tubules. These mice also showed increased interstitial macrophages compared to controls [64]. Thus, knockout of eNOS can contribute to both glomerular and tubulointerstitial fibrosis suggesting that disruption of NO signaling in the kidney may be a major factor in the development of DN. Although no definitive analysis of eNOS activity has been made in human DN, a recent report using a 3-loci haplotype analysis identified eNOS gene variants that were present at higher frequencies among type 2 diabetic patients with DN than among type 2 diabetic patients without nephropathy [65].
Regulation of eNOS in DN may come via vascular endothelial growth factor (VEGF). VEGF has been implicated in podocyte and endothelial alterations in DN [66]. VEGF stimulates eNOS activity in the glomerulus [66] and may exert some of its protective effects through this mechanism. In many chronic kidney diseases VEGF levels are low and are associated with impaired angiogenesis with capillary loss. Conversely, in most animal models of DN, VEGF levels are elevated suggesting an uncoupling of the VEGF-NO axis as postulated by Nakagawa [14, 66]. Interestingly this group has quite recently shown that enhanced VEGF levels in eNOS knockout mice were associated with increased macrophage recruitment into glomeruli, and in vitro studies suggested that exogenous NO could ameliorate VEGF stimulation of macrophage recruitment treatment with an exogenous NO treatment [67].
While such an uncoupling between NO and VEGF may play a role in rodent models, the recent observation that VEGF gene expression is actually decreased in biopsy samples from humans with progressive DN [68] would suggest that diminished VEGF levels could reduce eNOS activity leading to glomerular pathology similar to that seen in the eNOS knockout mice in human diabetic glomerulosclerosis. Both low and high levels of VEGF can have deleterious effects on glomerular endothelium via mechanisms which may be either NO-dependent or NO-independent [69]. Hence, better characterization of the VEGF and eNOS pathways will be essential for understanding podocyte-endothelial crosstalk in diabetic glomerulosclerosis.
Bradykinin 2 receptor blockade
An association between the onset and progression of type 1 DN in humans and the D allele of the Angiotensin Converting Enzyme (ACE) gene has been reported by several groups [40, 41] In addition, ACE inhibitors and angiotenisin receptor blockers are mainstays of renal protection in human DN. However, using knockout and knockin mice and computer modeling Smithies’ group has found that substantial changes in ACE gene dose lead to only modest increases in ACE with minimal effects on blood pressure and angiotensin II levels but interestingly led to substantial decreases in bradykinin suggesting that perhaps bradykinin rather than angiotenisin II was more important in renal responses in diabetes [42, 43]. Therefore, this group studied the contribution of targeted deletion of the bradykinin 2 receptor on the evolution of DN in Akita mice on a C57BL/6 background [44]. In this model, diabetic homozygoous bradykinin 2 receptor knockout mice developed profound mesangial sclerosis that resembled glomerular changes of human diabetic glomerulosclerosis. There were no changes in the glomerular endothelial cells or podocytes. Although the mechanism of this increased glomerulosclerosis phenotype remains unclear, there is normally a high level of bradykinin 2 receptor expression in mesangial cells, and knockout of these receptors was associated with enhanced renal expression of several genes involved in progressive glomerulosclerosis, including TGF-β1, connective tissue growth factor (a TGF-β effector), and p53 [45]. Although these mice have substantial increases in bradykinin 1 receptors [44], this provided no protection from the effects of bradykinin 2 receptor gene deletion. Similar effects to bradykinin 2 receptor knockout were found in diabetic rats treated with a specific nonpeptidic bradykinin 2 receptor antagonist [46]. This agent reversed almost all the salutary effects of ACE inhibitors on albuminuria, glomerular ERK and TGF-β signaling pathways and glomerular gene expression changes. It also enhanced oxidative stress in glomeruli from treated diabetic rats. Hence, activation of bradykinin 2 receptors in the kidney, presumably in mesangial cells, is protective and, through some yet-to-be-determined mechanism(s), must dampen activation of ERK and TGF-β dependent profibrotic pathways.
Plasminogen Activator Inhibitor-1 (PAI-1) activation
Multiple studies have found reductions in plasmin activity due in part to inhibition of tissue plasminogen activator by enhanced levels of PAI-1 in both plasma and kidney of humans with DN and animal models [23, 47]. Decreased plasmin activity leads to enhanced accumulation of ECM proteins such as fibronectin and inhibition of plasminogen activation thus contributes to glomerular changes of DN. Knockout of PAI-1 was shown to ameliorate DN in a mouse model [48] and a recent interesting study of uninephrectomized db/db diabetic mice showed that 2 weeks of treatment with of a mutant PAI-1 molecule that prevented plasminogen from being inactivated also significantly reversed the diabetes-induced decrease in plasmin activity and prevented escalation of albuminuria and ECM accumulation [49]. Together, these data underline the important contribution that PAI-1 and its regulated proteins make to the progression of glomerular fibrosis in DN.
Lipids and Lipid mediators
Increased circulating lipids and enhanced glomerular lipid synthesis have been clearly implicated in diabetic glomerulosclerosis, although the mechanisms by which elevated circulating lipids might contribute to this process remain unclear [50]. In addition, several recent studies by Levi’s group and others have documented enhanced kidney synthesis of triglycerides and cholesterol. This increased local lipid synthesis appears to be stimulated in diabetes due to a number of factors, including increased renal expression of the transcription factor, the sterol regulatory element–binding protein-1 (SREBP-1) which, when overexpressed in mice, causes lipid accumulation, induces expression of TGF-β, plasminogen activator inhibitor (PAI)-1 and vascular endothelial growth factor (VEGF). This endogenous kidney lipid synthesis pathway appears to result directly in enhanced accumulation of extracellular matrix proteins, mesangial expansion and glomerulosclerosis [51–53], suggesting that diabetes induces renal glomerular synthesis of triglycerides and cholesterol which then promote glomerulosclerosis. In effect, local production of triglycerides and cholesterol is analogous to that of local synthesis of other lipid mediators which are generated in glomerular and renal vascular cells and have profound effects on the development of glomerulosclerosis.
These latter mediators, known collectively as eicosanoids (prostanoids, leukotrienes, hydroxyeicosatetraenoic acids [HETEs] and epoxyeicosatrienoic acids [EETs]) exert diverse and complex effects on renal glomeruli, and the specific influence of each eicosanoid varies from cell to cell and depends on specific gene transcription and other machinery [54]. However, many of these effects appear to promote diabetic glomerulosclerosis [54]. For example, selective COX2 inhibition inhibits the development of glomerular sclerosis in rats with streptozotocin diabetes and hypertension [55, 56]. However, the role of COX-derived prostanoids in the pathogenesis of diabetic glomerulosclerosis is not as yet clarified and will require further exploration. Finally, since many of these lipid mediators participate crucially in inflammatory responses, separating out lipid mediators from inflammation as a glomerulosclerosis factor is artificial at best.
MicroRNA regulation of TGF-β signaling
MicroRNAs are short noncoding RNAs of 22 nucleotides that have been shown to play important roles in mammalian gene expression. They induce posttranscriptional gene regulation by blocking protein translation (by binding to the 3′UTR of their target genes) or by inducing mRNA degradation and therefore have the potential to play central roles in gene regulation in both physiologic and pathophysiologic conditions in a number of disease states [57]. More than 500 human microRNAs have been identified and it is predicted that up to 30% of human protein coding genes may be regulated by miRNAs [57]. Recently, Natarajan’s group has found that expression of microRNA-192 is enhanced in glomeruli from mice with both type 1 and type 2 diabetes as well as by TGF-β treatment of cultured mesangial cells [58]. These investigators found that TGF-β-induced miR-192 mediates an increase in collagen 1α2 expression by reducing expression of 2 E-box repressors of collagen 1α2 gene activation. Because miR-192 was increased in tissues from both type 1 and type 2 diabetic mice, the authors felt that hyperglycemia may be a common factor in inducing microRNA-192 expression, but the mechanisms of this regulation remain to be elucidated. This appears to be the first demonstration of a functional role for a microRNA in kidney disease of any type. Whether similar regulation by MicroRNAs occurs in tubular cells in diabetic nephropathy remains to be determined. Since miR-192 is downstream of TGF-β, its stimulation of extracellular matrix synthesis should be a better target for therapy since such interventions could have fewer nonspecific effects than interrupting TGF-β signaling in general. These findings should open up this important regulatory field for further study.
Reduction of Activated Protein C
As another piece of evidence underlining the importance of endothelial cell responses in the evolution of diabetic glomerulosclerosis, a recent study nicely elucidated the effects of the thrombomodulin/Activated protein C (APC) pathway on glomerular endothelial cells in diabetes [12]. In diabetic patients, function of the endothelial thrombomodulin–protein C system is impaired [59] and in diabetic mice, glomerular endothelial thrombomodulin A expression and protein C activation are substantially reduced [12]. APC prevented high glucose induced apoptosis of glomerular endothelial cells and podocytes, but not mesangial cells, in vitro. The role of APC in glomerular injury in diabetes was further explored using 2 mouse models, one with impaired protein C activation and one expressing a hyperactivatable protein C mutation. Mesangial extracellular matrix expansion was enhanced in the diabetic mice with impaired protein C activation and was completely prevented in those with the hyperactivatable protein C mutation. Moreover, mice with impaired protein C activation demonstrated enhanced endothelial and podocyte apoptosis whereas the hyperactivatable protein C mice had fewer apoptotic cells. In an interesting experiment the investigators showed that they could inhibit glomerular mesangial expansion by a generalized apoptosis inhibitor, minocycline. All of these effects were shown to be independent of any changes in coagulation due to altered protein C activation. In summary, this single comprehensive study established that a reduction in protein C activation promotes glomerular capillary dysfunction and apoptosis of endothelial cells and podocytes in experimental diabetes. Moreover, APC seems to be a remarkable mediator of crosstalk between the endothelial cells, podocytes and the mesangium to regulate nephropathy. If these interesting results are confirmed by further analyses and validated in humans with DN it will likely open new pathways for treatment.
JAK/STAT pathway activation
Many growth factors and agonists, including angiotenisin II, act via Janus kinase (JAK)/signal transducers and activation of transcription (STAT) signaling pathways and, therefore, these pathways may be important in the glomerular response to diabetes. Marerro’s group has found that high glucose augments angiotensin II activation of the JAK/STAT pathway in rat kidney glomeruli and that activation of JAK/STAT signaling in mesangial cells enhances TGF-β, collagen IV and fibronectin production [60, 61]. These effects appear to be directly due to hyperglycemia, perhaps mediated by enhanced production of reactive oxygen species, as incubation of cultured mesangial cells in high glucose results in enhanced phosphorylation and hence activation of JAK2 and the downstream substrates of JAK2, STAT1, STAT3 and STAT5A//B [61, 62]. Moreover, inhibition of JAK2, with AG-490, prevented diabetic proteinuria [61, 62] but there has been no report yet on the effects of JAK2 inhibitors on diabetic glomerular fibrosis and sclerosis per se or on effects of JAK2 inhibitors on diabetic tubulointerstitial fibrosis.
We have recently obtained independent confirmation of the potential role of JAK/STAT pathways in diabetic kidney disease. Using a transcriptomic approach with human cDNA samples derived from glomerular and tubulointerstitial regions from humans with both early and more progressive DN, we found that a host of JAK/STAT genes were expressed at higher levels in both these regions [63]. These results were obtained in screenings designed to identify pathways in which gene expression was altered in humans with DN but not in conventional mouse models of DN all of which have failed to recapitulate the progressive glomerulosclerosis and tubulointerstitial fibrosis seen in the human disease. JAK1-3 and STAT1 were each expressed at significantly higher levels in glomeruli of patients with DN. Immunohistochemistry showed a strong JAK2 staining in the glomeruli, as well as in proximal tubules, from patients with DN compared to those from healthy controls. In contrast, there was no increase in JAK2 expression in several common mouse models of DN, suggesting one reason for lack of progressive glomerulosclerosis in these models. We also found that JAK2 expression in mesangial cells, without direct agonist stimulation, leads to activation of JAK/STAT signaling as evidenced by enhanced STAT3 phosphorylation (Berthier, et al., unpublished data), We also found that JAK2 overexpression alone led to enhanced oxidative stress (Berthier, et al., unpublished data). Thus, enhanced JAK2 expression and JAK2 mediated signaling, triggered by high glucose and possibly angiotensin II, appears to occur in progressive diabetic glomerulosclerosis in humans and may result in enhanced glomerulosclerosis. JAK2 signaling has been identified in podocytes [64], but nothing is known about the effects of diabetes on JAK/STAT signaling in this cell type.
JAK/STAT signaling may also play a role in mediating some of the effects of inflammation in DN as these molecules mediate cytokine signaling (see section below and Fig. 2).
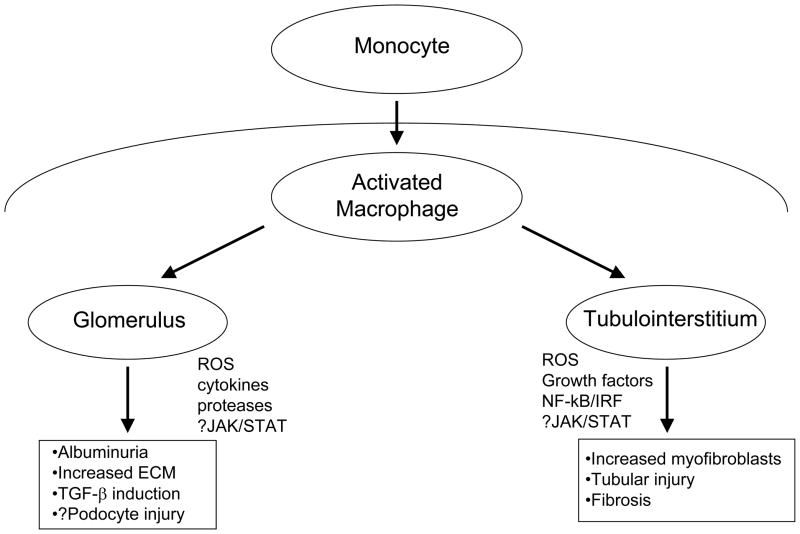
Figure 2. Participation of inflammation and macrophage activation in the progression of fibrosis in diabetic nephropathy.
Increased glucose and other factor in the diabetic milieu drives recruitment of monocytes and their activation into macrophages which accumulate in both the tubulointerstitium and glomerulus of patients with diabetes. Through a variety of mechanisms, a few of which are shown, macrophage activation can stimulate fibrosis in both compartments. ROS – Reactive oxygen species; JAK/STAT – Janus Kinase/Signal Transducers and Activation of Transcription; NΦ–κB/IRF – Nuclear Factor-κB/Interferon Regulatory Factor.
Inflammation
A number of clinical and animal model studies have implicated inflammatory mechanisms as important pathogenic factors in diabetic microvascular complications, including fibrosis in DN. Circulating markers of inflammation are increased in both type 1 and type 2 diabetic patients and a number of these markers (C-reactive protein, fibrinogen, serum amyloid A protein and IL-6) correlate with both albuminuria and increased risk for progression toward end-stage kidney disease [65, 66]. Moreover, an association between decreasing kidney function in type 1 diabetic patients and elevated urinary concentrations of four proinflammatory chemokines (IL-8, IP-10, MCP-1, and MIP-1δ) and IL-6 was recently demonstrated by Krolewski’s group [67]. Importantly, in this latter study, patients with microalbuminuria who did not show progressive declines in kidney function did not have elevated urinary markers.
The best data for the role of inflammation are in progressive tubulointerstitial injury in DN [26, 68–70] which generally correlates best with a progressive decline in kidney function to kidney failure, as noted above. Macrophage accumulation in the tubulointerstitium of animals with diabetes leads to tubular damage and increased numbers of myofibroblasts, ultimately resulting in enhanced fibrosis [26, 70, 71]. A recent study by Kretzler’s group used a sophisticated modular systems biology approach to demonstrate a prominent inflammatory signature in the tubulointerstitial tissue of patients with progressive DN. Using computational promoter analysis the investigators were able to identify a specific set of genes, especially chemokine genes, containing a specific NF-κB promoter module (NFKB_IRFF_01), that were activated in progressive DN. The module NFKB_IRFF_01 has a NF-κB binding site on the plus and interferon regulatory factor (IRF) binding site on the minus DNA strand separated by 14–24 bp. Thus, by integrating gene expression profiling with promoter modeling they were able to define a central role for inflammation in human DN. In addition, this work underlined the central role of the NF-κB/IRF module as a potential “master switch” activating this response [68].
Inflammatory mechanisms also appear to be important in the pathogenesis of diabetic glomerular fibrosis [65, 72, 73]. Recruitment of macrophages into the glomerulus also appears to be critical [74, 75]. These cells generate reactive oxygen species, inflammatory cytokines and proteases that induce glomerular damage. Inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) [69, 74, 76], and other mediators may stimulate glomerular cells to enhance production or reduce degradation of matrix proteins. As evidence for the critical role of the macrophage, targeted deletion of the macrophage scavenger receptor-A ameliorated many of the glomerular changes of experimental DN in mice, including albuminuria, glomerular hypertrophy, mesangial matrix expansion, and overexpression of transforming growth factor-beta at 6 months after induction of diabetes. Moreover, in this model, macrophage infiltration was decreased, proinflammatory genes were suppressed, and attachment of monocytes to type IV collagen was reduced [72]. Evidence over the last few years also implicates inflammatory mechanisms in podocyte injury in diabetic models and suggests that interventions that block inflammation-induced injury can ameliorate diabetic glomerulosclerosis [77]. A simplified schematic of how inflammation may promote DN in both glomerular and tubulointerstitial compartments is illustrated in Figure 2.
The importance of inflammatory mechanisms in the fibrotic responses in DN is also underscored by the evidence that many therapeutic agents that prevent or retard progression of human DN are potent anti-inflammatory agents [73, 78, 79]. In addition, advanced glycation end products and oxidant stress, which are critical factors in the progression of DN, augment and are augmented by inflammatory mechanisms of injury in the kidney [72, 80]. Finally, it should be stressed that both glomerular and tubulointerstitial cells produce a multitude of inflammatory mediators in the diabetic milieu, especially as injury proceeds, which can augment inflammatory damage and even lead to systemic effects.
3. Conclusion
A simple model of the pathogenesis of diabetic glomerular and tubular fibrosis has been confounded by a series of recent reports, each of which underscores the complexity and interrelatedness of the mechanisms of these critical aspects of type 1 and type 2 DN. New pathogenic pathways have been revealed by genetic manipulation of animal models in the last few years which are likely to individually contribute to our understanding and treatment of DN. Undoubtedly, other pathways not yet elucidated or described in this review will be found to contribute importantly to this complication. Conversely, many of the pathways described in this review interact in manifold ways, as noted above in the interrelationships between eNOS, VEGF, JAK/STAT signaling and inflammation. Finally, some of the mechanisms that induce diabetic fibrosis may be the same and some may differ from those involved in other chronic renal diseases. Identifying pathways that are specific for DN may allow for development of specific treatments for this complication, that may add materially to our armamentarium of rather nonspecific therapies. A comprehensive understanding of the pathogenesis of diabetic renal fibrosis, and how it is similar to, and differs from, other causes of nephrosclerosis may require a systems biology and computational approach to knit the new and exciting observations into a coherent if multidimensional paradigm. This complexity, though problematic on one hand, opens up a number of new possibilities for intervention to prevent or forestall DN in patients with diabetes.
Acknowledgments
Relevant research in Dr. Brosius’ laboratory is supported, in part, by National Institutes of Health grant U01DK076139 and Juvenile Diabetes Research Foundation grant 1-2005-347.
References
- 1.Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. Journal of clinical pathology. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–73. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Seminars in nephrology. 2003;23:532–43. doi: 10.1053/s0270-9295(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 4.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. The Journal of clinical investigation. 1984;74:1143–55. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathology, research and practice. 1980;167:204–16. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- 6.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Seminars in nephrology. 2007;27:195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz A, Caramori ML, Sisson-Ross S, Groppoli T, Basgen JM, Mauer M. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002;61:2058–66. doi: 10.1046/j.1523-1755.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 8.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–52. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 9.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. The Journal of clinical investigation. 1997;99:342–8. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–4. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 11.Morcos M, Borcea V, Isermann B, et al. Effect of alpha-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory study. Diabetes Res Clin Pract. 2001;52:175–83. doi: 10.1016/s0168-8227(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 12.Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nature medicine. 2007;13:1349–58. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 13.Zhao HJ, Wang S, Cheng H, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–9. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–50. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte Detachment and Reduced Glomerular Capillary Endothelial Fenestration in Human Type 1 Diabetic Nephropathy. Diabetes. 2007 doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield MD, Bach LA, Forbes JM, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) The Journal of clinical investigation. 2001;108:1853–63. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns WC, Twigg SM, Forbes JM, et al. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17:2484–94. doi: 10.1681/ASN.2006050525. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Advances in chronic kidney disease. 2005;12:177–86. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Pedagogos E, Hewitson T, Fraser I, Nicholls K, Becker G. Myofibroblasts and arteriolar sclerosis in human diabetic nephropathy. Am J Kidney Dis. 1997;29:912–8. doi: 10.1016/s0272-6386(97)90466-2. [DOI] [PubMed] [Google Scholar]
- 20.Zoja C, Donadelli R, Colleoni S, et al. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int. 1998;53:1608–15. doi: 10.1046/j.1523-1755.1998.00905.x. [DOI] [PubMed] [Google Scholar]
- 21.Tang S, Leung JC, Abe K, et al. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. The Journal of clinical investigation. 2003;111:515–27. doi: 10.1172/JCI16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int. 2000;58:618–28. doi: 10.1046/j.1523-1755.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 23.Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995;47:1546–57. doi: 10.1038/ki.1995.218. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Chen J, Chen L, Tay YC, Rangan GK, Harris DC. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol. 1997;8:1537–45. doi: 10.1681/ASN.V8101537. [DOI] [PubMed] [Google Scholar]
- 25.Zheng F, Cornacchia F, Schulman I, et al. Development of albuminuria and glomerular lesions in normoglycemic B6 recipients of db/db mice bone marrow: the role of mesangial cell progenitors. Diabetes. 2004;53:2420–7. doi: 10.2337/diabetes.53.9.2420. [DOI] [PubMed] [Google Scholar]
- 26.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–28. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 27.Okada S, Shikata K, Matsuda M, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586–93. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- 28.Levine DZ. Can rodent models of diabetic kidney disease clarify the significance of early hyperfiltration? : recognizing clinical and experimental uncertainties. Clin Sci (Lond) 2008;114:109–18. doi: 10.1042/CS20070088. [DOI] [PubMed] [Google Scholar]
- 29.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Seminars in nephrology. 2007;27:144–52. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: a trigger for impaired glucose metabolism. J Am Soc Nephrol. 2007;18:2226–32. doi: 10.1681/ASN.2006121362. [DOI] [PubMed] [Google Scholar]
- 31.Heilig CW, Liu Y, England RL, et al. D-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes. 1997;46:1030–9. doi: 10.2337/diab.46.6.1030. [DOI] [PubMed] [Google Scholar]
- 32.D’Agord Schaan B, Lacchini S, Bertoluci MC, Irigoyen MC, Machado UF, Schmid H. Increased renal GLUT1 abundance and urinary TGF-beta 1 in streptozotocin-induced diabetic rats: implications for the development of nephropathy complicating diabetes. Hormone and metabolic research. Hormon- und Stoffwechselforschung. 2001;33:664–9. doi: 10.1055/s-2001-18683. [DOI] [PubMed] [Google Scholar]
- 33.Horie K, Miyata T, Maeda K, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. The Journal of clinical investigation. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skolnik EY, Yang Z, Makita Z, Radoff S, Kirstein M, Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. The Journal of experimental medicine. 1991;174:931–9. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makita Z, Radoff S, Rayfield EJ, et al. Advanced glycosylation end products in patients with diabetic nephropathy. The New England journal of medicine. 1991;325:836–42. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 36.Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Babazono T, Kapor-Drezgic J, Dlugosz JA, Whiteside C. Altered expression and subcellular localization of diacylglycerol-sensitive protein kinase C isoforms in diabetic rat glomerular cells. Diabetes. 1998;47:668–76. doi: 10.2337/diabetes.47.4.668. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1814–8. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. The Journal of clinical investigation. 1994;93:536–42. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marre M, Bernadet P, Gallois Y, et al. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43:384–8. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 41.Doria A, Warram JH, Krolewski AS. Genetic predisposition to diabetic nephropathy. Evidence for a role of the angiotensin I--converting enzyme gene. Diabetes. 1994;43:690–5. doi: 10.2337/diab.43.5.690. [DOI] [PubMed] [Google Scholar]
- 42.Huang W, Gallois Y, Bouby N, et al. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13330–4. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi N, Hagaman JR, Kim HS, Smithies O. Minireview: computer simulations of blood pressure regulation by the renin-angiotensin system. Endocrinology. 2003;144:2184–90. doi: 10.1210/en.2002-221045. [DOI] [PubMed] [Google Scholar]
- 44.Kakoki M, Takahashi N, Jennette JC, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13302–5. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kakoki M, Kizer CM, Yi X, et al. Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. The Journal of clinical investigation. 2006;116:1302–9. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allard J, Buleon M, Cellier E, et al. ACE inhibitor reduces growth factor receptor expression and signaling but also albuminuria through B2-kinin glomerular receptor activation in diabetic rats. Am J Physiol Renal Physiol. 2007;293:F1083–92. doi: 10.1152/ajprenal.00401.2006. [DOI] [PubMed] [Google Scholar]
- 47.Hirano T, Kashiwazaki K, Moritomo Y, Nagano S, Adachi M. Albuminuria is directly associated with increased plasma PAI-1 and factor VII levels in NIDDM patients. Diabetes Res Clin Pract. 1997;36:11–8. doi: 10.1016/s0168-8227(97)01384-3. [DOI] [PubMed] [Google Scholar]
- 48.Nicholas SB, Aguiniga E, Ren Y, et al. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67:1297–307. doi: 10.1111/j.1523-1755.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA. A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. J Am Soc Nephrol. 2008;19:329–38. doi: 10.1681/ASN.2007040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leiter LA. The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract. 2005;68 (Suppl 2):S3–14. doi: 10.1016/j.diabres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–9. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 52.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. The Journal of biological chemistry. 2002;277:18919–27. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 53.Jiang T, Wang XX, Scherzer P, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–93. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 54.Hao CM, Breyer MD. Roles of lipid mediators in kidney injury. Seminars in nephrology. 2007;27:338–51. doi: 10.1016/j.semnephrol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Komers R, Lindsley JN, Oyama TT, Anderson S. Cyclo-oxygenase-2 inhibition attenuates the progression of nephropathy in uninephrectomized diabetic rats. Clinical and experimental pharmacology & physiology. 2007;34:36–41. doi: 10.1111/j.1440-1681.2007.04534.x. [DOI] [PubMed] [Google Scholar]
- 56.Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 2002;62:929–39. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 57.Sassen S, Miska EA, Caldas C. MicroRNA-implications for cancer. Virchows Arch. 2007 doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3432–7. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujiwara Y, Tagami S, Kawakami Y. Circulating thrombomodulin and hematological alterations in type 2 diabetic patients with retinopathy. Journal of atherosclerosis and thrombosis. 1998;5:21–8. doi: 10.5551/jat1994.5.21. [DOI] [PubMed] [Google Scholar]
- 60.Amiri F, Shaw S, Wang X, et al. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61:1605–16. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 61.Banes AK, Shaw S, Jenkins J, et al. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol. 2004;286:F653–9. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 62.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F762–8. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 63.Boucherot AHA, Cohen CD, Schin ML, Burke K, Bayer I, Schmid HRM, Schlondorff D, Brosius F, Kretzler M. Jak/Stat activation in diabetic nephropathy in humans but not mice: transcriptional analysis. JASN. 2006;17:60A. [Google Scholar]
- 64.Logar CM, Brinkkoetter PT, Krofft RD, Pippin JW, Shankland SJ. Darbepoetin alfa protects podocytes from apoptosis in vitro and in vivo. Kidney Int. 2007;72:489–98. doi: 10.1038/sj.ki.5002362. [DOI] [PubMed] [Google Scholar]
- 65.Dalla Vestra M, Mussap M, Gallina P, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16 (Suppl 1):S78–82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- 66.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–65. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 67.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19:789–97. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55:2993–3003. doi: 10.2337/db06-0477. [DOI] [PubMed] [Google Scholar]
- 69.Navarro JF, Milena FJ, Mora C, Leon C, Garcia J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. American journal of nephrology. 2006;26:562–70. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- 70.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19:2987–96. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 71.Tesch GH. Role of macrophages in complications of type 2 diabetes. Clinical and experimental pharmacology & physiology. 2007;34:1016–9. doi: 10.1111/j.1440-1681.2007.04729.x. [DOI] [PubMed] [Google Scholar]
- 72.Usui HK, Shikata K, Sasaki M, et al. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes. 2007;56:363–72. doi: 10.2337/db06-0359. [DOI] [PubMed] [Google Scholar]
- 73.Yozai K, Shikata K, Sasaki M, et al. Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J Am Soc Nephrol. 2005;16:3326–38. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- 74.Furuta T, Saito T, Ootaka T, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–5. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- 75.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16:1711–22. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- 76.Wong CK, Ho AW, Tong PC, et al. Aberrant activation profile of cytokines and mitogen-activated protein kinases in type 2 diabetic patients with nephropathy. Clinical and experimental immunology. 2007;149:123–31. doi: 10.1111/j.1365-2249.2007.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Awad AS, Huang L, Ye H, et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–37. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 78.Mizuno M, Sada T, Kato M, Fukushima Y, Terashima H, Koike H. The effect of angiotensin II receptor blockade on an end-stage renal failure model of type 2 diabetes. Journal of cardiovascular pharmacology. 2006;48:135–42. doi: 10.1097/01.fjc.0000245241.79959.d6. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal R. Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F600–5. doi: 10.1152/ajprenal.00289.2005. [DOI] [PubMed] [Google Scholar]
- 80.Chander PN, Gealekman O, Brodsky SV, et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15:2391–403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]