Abstract
Bacterial lipopolysaccharides (LPSs) are ubiquitous molecules that are prominent components of the outer membranes of most gram-negative bacteria. Genetic and structural characterizations of Francisella LPS have revealed substantial differences when compared to more commonly studied LPSs of the Enterobacteriaceae. This review discusses both the general characteristics and the unusual features of Francisella LPS.
Keywords: Francisella tularensis, Lipopolysachharide, Lipid A, O-antigen, Kdo hydrolase, Structure, Virulence
1. Introduction
Francisella tularensis is the etiologic agent of tularemia, a potentially fatal human disease. This small, gram-negative, non-motile, facultative intracellular bacterial pathogen has an impressive host range that includes more than two hundred species—vertebrates, invertebrates, and even free-living amoebas.1, 2 Fast adaptation and versatility are key features that enable F. tularensis to survive in diverse environments, including water, insect vectors, and mammalian hosts. The organism’s high degree of infectivity and ease of aerosolization have raised concerns about its potential use as an agent of biological warfare; thus, the Centers for Disease Control and Prevention lists F. tularensis as a category A priority pathogen. F. tularensis subspecies tularensis (type A), holarctica (type B), and mediasiatica have distinct geographical distributions and levels of virulence. F. novicida and F. philomiragia are close relatives of F. tularensis, sharing many of its phenotypical features. F. novicida is considered to be a mouse-adapted member of the genus and F. philomiragia is a muskrat pathogen; both of these species are rarely infectious in humans.2, 3
Bacterial lipopolysaccharides (LPSs) are ubiquitous molecules that are prominent components of the outer membranes of most gram-negative bacteria.4, 5 Like many LPSs, F. tularensis LPS is composed of 1) lipid A, which anchors the LPS to the outer membrane; 2) a core oligosaccharide attached to lipid A by an eight-carbon sugar, 3-deoxy-D-manno-octulosonic acid (Kdo); and 3) an O-polysaccharide (also known as O-antigen), which contains a varying number of tetrasaccharide repeating units. However, several key modifications distinguish Francisella LPS from more commonly studied LPSs of the Enterobacteriaceae. These alterations lead to very low-level toxicity that F. tularensis LPS exhibits. This review discusses both the general characteristics and the unusual features of Francisella LPS.
2.1. Structure and biosynthesis of lipid A
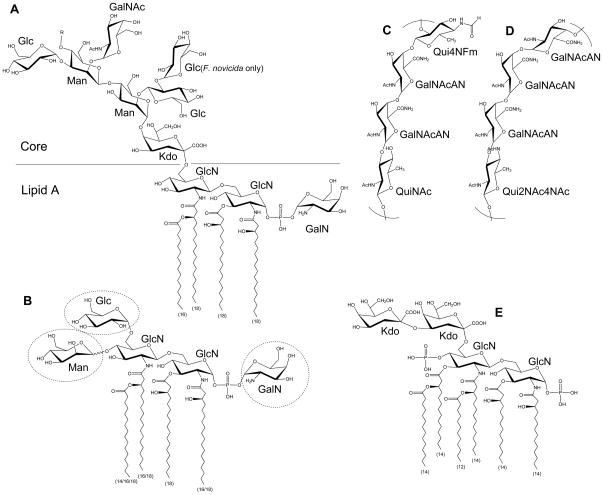
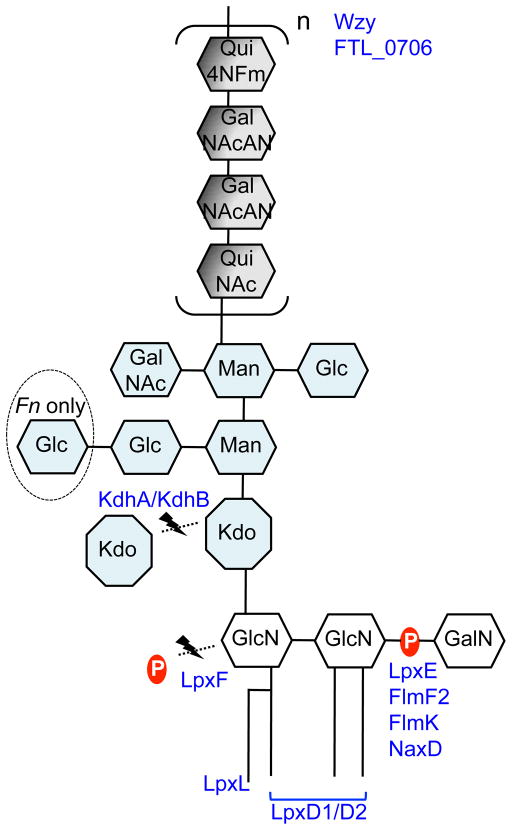
In gram-negative bacteria, LPS is embedded in the outer membrane by a hydrophobic lipid A anchor. The composition of lipid A is well characterized in a variety of organisms, including Escherichia coli and Salmonella species, and substantial differences in Francisella lipid A have been noted. Structural analysis of Francisella lipid A revealed a β-(1–6)-linked diglucosamine (GlcN) backbone with four long fatty acyl groups that are amide-linked with C18:0 (3-OH) at the 2- and 2′-positions and ester-linked with C18:0 (3-OH) at the 3-position (Figure 1A).6–9 The hydroxy group of the 2′-linked fatty acyl chain is further esterified with C16:0. Unlike E. coli lipid A, which has two phosphates at the 1- and 4′-positions (Figure 1E), Francisella lipid A has an α-linked galactosamine (GalN) addition at the 1-position and lacks phosphate at the 4′-position (Figure 1A). This major tetraacyl lipid A, typically observed at m/z 1665 by negative ion MALDI mass spectrometry (MS), is present in all F. tularensis subspecies, F. novicida and F. philomiragia when bacteria were grown at 37°C.10, 11 The absence of negatively charged phosphate groups affects the overall charge of LPS and has implications in the organism’s resistance to antimicrobial peptides. Differences between the presence of phosphate groups in strains of F. tularensis and F. novicida U112 strain lipid A have been reported. An early report8 indicated the lack of a phosphate group at the 1-position of the reducing sugar in the live vaccine strain (LVS) of F. tularensis subsp. holarctica, but later reports revealed that the majority of lipid A did contain the 1-phosphate group.7, 11, 12 A small portion of LVS lipid A was reported to contain galactosamine-1-phosphate, 12, 13 while in strain 1547–57 of F. tularensis subsp. holarctica and F. novicida U112, it is the major lipid A species.7, 11 Reported differences in lipid A structure may arise from differences in the bacterial growth phase when cells are harvested or procedures used for isolation, purification, and analysis of lipid A. Critical proteins involved in modification of the GlcN backbone of F. novicida lipid A have been identified: the inner membrane bound phosphatases LpxE and LpxF are responsible for the removal of phosphate groups from the 1- and 4′-positions, respectively;14–16 FlmK (a homolog of ArnT/PmrK), FlmF2 (ArnC homolog) and NaxD deacetylase are involved in addition of GalN to the phosphate moiety at the 1-position (Figure 2).6, 17–19 Modification of lipid A with a mannose at the 4′-position was also reported (Figure 1B).17, 20 This modification was detected in F. novicida and most isolates of F. tularensis subsp. tularensis, but not in F. tularensis subsp. holarctica.17 FlmF1 and FlmK proteins play roles in mannose addition.17, 18
Figure 1. Structure of Francisella LPS.
Major Francisella lipid A molecule with core oligosaccharide (A). The length of each fatty acyl chain is indicated in parenthesis. Chain length variation (16/18) indicates temperature regulation. Less abundant lipid A variants may have various modifications in sugar composition as shown in dashed circles (B). The structures of F. tularensis O-antigen (C), and F. novicida O-antigen (D) are shown. For comparison, E. coli Kdo2-lipid A is also shown (E). R = O-antigen.
Figure 2. Cartoon representation of Francisella LPS and its modifying proteins.
Proteins are located in their areas of action. See text for further details. Fn: F. novicida.
Another striking difference between Francisella lipid A and the lipid A of most other gram-negative bacteria is the length and number of acyl chains in the molecule. Francisella lipid A has only four long acyl chains with 16 and 18 carbons, whereas most bacterial lipid A has six acyl chains that are usually shorter (12 to 14 carbons) (Figure 1A & E). Temperature-regulated heterogeneity in F. novicida lipid A acyl chains has been reported.20, 21 LpxD1 and LpxD2 were identified as N-acyltransferases whose expression and activity increase at 37°C and 18°C, respectively. Thus, when F. novicida is grown at 18°C (environmental temperature), lipid A acyl chains at the 2- and 2′-positions are composed primarily of C16:0 (3-OH) (Figure 1B). Conversely, when it is grown at 37°C (mammalian host temperature), lipid A has longer acyl chains C18:0 (3-OH). Minor lipid A species with acyl chain variations include C14:0 or C18:O fatty acyl branches instead of a C16:0 secondary chain at the 2′-position.11, 12, 20 LpxL was identified as a Kdo-dependent acyltransferase responsible for modifying the secondary chain on the 2′-acyl group (Figure 2);22 this function was confirmed by demonstration of the ability of Francisella LpxL to complement the acylation of lipid A in an E. coli lpxL mutant.
In E. coli, lipid A with two Kdo sugars (Kdo2–lipid A) is the minimal LPS molecule required for viability.4 While in Francisella, >95% of its lipid A is present in “free” form; it is not attached to Kdo, core oligosaccharide, or O-antigen.6 The sugar and fatty acid composition of this free lipid A is very similar to lipid A with O-antigen. Both F. tularensis and F. novicida are reported to have free lipid A.6, 8, 12, 23 In F. novicida, a small amount of free lipid A contains an α-linked glucose at the 6′-position of distal glucosamine (Figure 1B).6 The biosynthesis of these novel lipid As is discussed in detail by Raetz and colleagues.16
2.2. Structure of core oligosaccharide
Perry and colleagues have made important contributions to the structural elucidation of Francisella LPS.8, 24, 25 Structural analysis of the Francisella core region has revealed an unusual sugar composition. The core is relatively small, and contains two mannose sugars in the inner core instead of the more commonly found heptoses (Figure 1A). F. novicida core oligosaccharide differs from F. tularensis by the presence of α-glucose attached to the β-glucose branch.8, 25 LPS core of LVS was reported to have galactosamine (GalN) instead of N-acetyl galactosamine (GalNAc) as in F. novicida; however, a later report suggested GalNAc is present in the core of LVS.26 In gram-negative bacteria, lipid A is linked to the core region through a Kdo moiety (Figure 1A&E). E. coli and most other bacteria possess a highly conserved bifunctional glycosyltransferase WaaA (KdtA), which transfers two Kdo sugars onto lipid IVA, a lipid A precursor. The addition of Kdo sugars to the lipid A precursor is followed by the addition of the rest of the core unit.4 Francisella LPS has a single non-branched Kdo in its core region (Figure 1A). LPS carrying a single Kdo has also been observed in Bordetella pertussis and Haemophilus influenzae, but in these bacteria WaaA was shown to be a mono-functional Kdo transferase that could transfer only a single Kdo onto lipid IVA.27, 28 Our laboratory and others have recently discovered a Kdo hydrolase system in Francisella composed of kdhA and kdhB gene products.29, 30 It is now known that Francisella initially synthesizes its LPS with two Kdo sugars and this novel Kdo hydrolase is involved in removing the second, side-chain Kdo moiety. KdhA and KdhB are inner-membrane proteins and are both required for Kdo hydrolase activity in Francisella. KdhA catalyzes the cleavage of the side-chain Kdo and has homology to sialidase-family enzymes (Figure 2). KdhB’s function is unknown, but it is predicted to contain a number of alpha-helical transmembrane domains. A similar two-component Kdo hydrolase system has been identified in two other pathogens, Helicobacter pylori and Legionella pneumophila.29, 31
2.3. Structure of O-antigen
NMR and MS analysis by several laboratories identified Francisella O-antigen with its repeating four sugars. LVS, strain 15, OSU10, and SchuS4 strain (members of either subsp. tularensis or subsp. holarctica) have the O-antigen repeating units −2)-β-D-Qui4NFm-(1–4)-α-D-GalNAcAN-(1–4)-α-D-GalNAcAN-(1–3)-β-D-QuiNAc-(1- (Figure 1C)26, 32–34 while F. novicida strain U112 has an antigenically distinct repeating O-antigen unit of −4)-α-D-GalNAcAN-(1–4)-α-D-GalNAcAN-(1–4)-α-D-GalNAcAN-(1–3)-β-D-Qui2NAc4NAc-(1- (Figure 1D).24, 35 Reflecting the structural variation, the O-antigen gene clusters of F. tularensis and F. novicida differ in a number of genes involved in the biosynthesis of individual O-antigen sugars.33 Present in the gene cluster, wzx (FTL_0596) encodes for O-antigen flippase and wzy (FTL_0598) encodes for O-antigen polymerase. The Wzx flippase transports a single O-antigen unit linked to the C55-lipid carrier undecaprenyl phosphate (UDP) from the cytoplasmic to the periplasmic face of the inner membrane, whereas the Wzy glycosyltransferase is responsible for the polymerization of O-antigen of various lengths (Figure 2).4, 36, 37 Similar to O-antigen polymerase in other bacteria, Wzy of F. tularensis LVS is an inner-membrane protein with 11 transmembrane domains.38 The wzy mutant of F. tularensis LVS can synthesize lipid A with a single O-antigen. The wzz gene, whose product determines O-antigen chain length, appears to be missing in F. tularensis and F. novicida. In most gram-negative bacteria, a glycosyltransferase, RfaL/WaaL, serves as the O-antigen ligase, transferring O-antigen from its UDP lipid carrier to the lipid A core. A BLAST search for rfaL in the F. tularensis LVS genome identifies FTL_0706, which is not in the O-antigen gene cluster and displays low homology (41%/25% amino acid sequence similarity/coverage to E. coli RfaL). Consistent with the predicted function of FTL_0706, mutants deficient in this gene product lack LPS with O-antigen (Figure 2).39, 40 Further studies are needed to identify the enzymatic characteristics of these glycosyltransferases.
The transport of fully mature LPS across the outer membrane of gram-negative bacteria is a process that is not well understood. Several Lpt proteins facilitate the transport in E. coli.41 A BLAST search for similar proteins in the Lpt pathway identifies an LptB homolog with high amino acid similarity and LptD, LptF and LptG homologs with very low similarity in F. tularensis. The presence of these proteins suggests a similar pathway may be involved in transporting LPS across the Francisella outer membrane.
Apicella and colleagues identified a large-molecular-weight polysaccharide (100–250 kDa) in capsule extracts of type A and type B strains of F. tularensis.39 The sugar composition was identical to O-antigen. Analysis determined the capsule to be a polymer of −4)-α-D-GalNAcAN-(1–4)-α-D-GalNAcAN-(1–3)-β-D-QuiNAc-(1–2)-β-D-Qui4NFm-(1-. This study did not conclusively reveal the sugar at the reducing end of the molecule, while a tandem MS analysis by Wang et al. suggested the presence of QuiNAc at the reducing end when tetrasaccharide repeats are not attached to LPS core.26 These findings indicate that F. tularensis synthesizes an O-antigen capsule containing approximately 125 to 300 or more O-antigen repeating units. The presence of O-antigen capsules that are not linked to core oligosaccharide and lipid A is also reported in other bacteria.42 Using capsule-specific monoclonal antibodies that do not recognize F. tularensis LPS, Apicella’s group showed that many of the genes in the O-antigen gene cluster are also required for the synthesis of the O-antigen capsule. Among these genes, lpxL, involved in lipid A modification, was required for the synthesis of LPS but not O-antigen capsule.39 Furthermore, the wzy mutant lacked both LPS and O-antigen capsule, indicating a common O-antigen polymerase.38
Francisella LPS is an immunodominant molecule with little diversity among F. tularensis subspecies. Early on, phase/antigenic variation based on colony morphology, immunogenicity, and virulence was noted.43, 44 Analysis of a number of phase variants, which are called “gray” on the basis of colony opacity and morphology, identified alterations in LPS O-antigen (absent or reduced) as well as subtle changes in the core region and lipid A structure.13, 44, 45
3. Biological role of Francisella LPS
LPS molecules of gram-negative bacteria play critical roles in the maintenance of outer-membrane integrity, regulation of cell permeability, regulation of the host’s immune responses, resistance to antimicrobial peptides, resistance to host antibodies, and prevention of complement activation.4 Mutants lacking LPS often exhibit phenotypes that include reduced viability, slow growth, and loss of virulence. Francisella LPS is an important virulence factor that contributes to the organism’s pathogenesis in numerous ways. Lipid A of many gram-negative bacteria are bioactive molecules that interact with the host immune system.46 Unlike others, Francisella lipid A is a very poor stimulant of the host’s innate immunity.10, 47, 48 Recognition of lipid A as foreign is an essential feature of mammalian host defense. Many host cells, such as macrophages and epithelial cells, recognize lipid A by innate immune mechanisms. For example, molecular pattern recognition via the Toll-like receptor 4 (TLR4)/MD2 receptor complex leads to inflammation by stimulating NF-κB-mediated gene transcription.4, 46 The lack of immune recognition of Franciscella lipid A has been attributed to several structural differences compared to E. coli lipid A, a potent immune activator. These include 1) absence of phosphate at the 4′ position as well as the modification of 1-phosphate with GalN and 2) tetraacylation of lipid A with longer acyl chains (16–18 carbons); E.coli’s lipid A is hexaacylated with shorter acyl chains (12–14 carbons) (Figure 1 A & E). The lack of phosphate affects the overall charge of lipid A, a frequent target of cationic antimicrobial peptides. Supporting this notion that charge is important to the biology of lipid A, lpxD1, flmK, and naxD mutants of F. novicida, which are unable to modify 1- or 4′-phosphate, show increased sensitivity to polymyxin B and attenuation in mice.17, 19, 21 Dynamic regulation of lipid A acyl chain length by LpxD1/LpxD2 at varying temperatures appears to be important in maintaining membrane permeability and susceptibility to cationic antimicrobial peptides as well.21 Consistent with LpxD1’s optimum activity at 37 °C, the lpxD1 mutant is unable to modify lipid A with longer acyl groups and is severely attenuated in mice, whereas the lpxD2 mutation has no effect.21
An unusual feature of the Francisella LPS core region is the presence of a single Kdo unit. This LPS modification was shown to be important for virulence. An F. tularensis kdhAB mutant unable to cleave the second Kdo from the remainder of the core oligosaccharide was attenuated in mouse models of infection.49 Interestingly, the kdhAB mutant activated macrophages through the TLR2 signaling pathway as opposed to the TLR4 pathway that recognizes most gram-negative bacterial LPS. Further investigation suggested that kdhAB mutant LPS is not the activator of TLR2 signaling; rather, some other surface components, most likely lipoproteins, are involved in the activation of macrophages. A probable scenario is the presence of an extra Kdo imparts a net negative charge altering the bacterial surface under physiological conditions, resulting in increased access of additional surface molecules to the host’s innate immune system.
As in many other bacterial pathogens, O-antigen of Francisella plays a role in serum resistance. F. tularensis wbtA and wzy mutants, which lack O-polysaccharide, are highly sensitive to killing by serum complement.49–51 These mutants are also defective in intracellular bacterial growth and are highly attenuated in mice. Interestingly, F. novicida core mutants manB and manC, F. novicida wbtA, F. tularensis FTT1238, and F. tularensis wzy mutants, all lacking O-polysaccharide, were hypercytotoxic in the J774 macrophage-like cell line as well as in primary macrophages.40, 52, 53 Cells infected with these mutants secreted high levels of pro-inflammatory cytokines and underwent pyroptosis, a type of programmed cell death. These observations establish a strong connection between Francisella LPS and evasion of the host’s immune system.
Bacterial O-antigens are known to invoke a strong antibody response due to their repetitive structure and constitutive expression. LPS specific antibodies are often protective and thus useful in vaccine design. A number of Francisella O-antigen specific monoclonal antibodies have been shown to be protective in a mouse model of F. tularensis infection.54 X-ray crystal structure of monoclonal antibody Ab52 in a complex with O-antigen further revealed the basis of O-antigen recognition.55 While Francisella O-antigen is a dominant antibody target, Boltje et al. showed that mice immunized with Francisella LPS produced antibodies that recognized a chemically synthesized core hexasaccharide, but not mice immunized with LVS.56 Future studies using these tools will help to better define how the host interacts with Francisella LPS and in the design of effective therapeutic antibodies.
4. Concluding remarks
F. tularensis is a highly infectious pathogen and many studies point to its LPS as a key virulence factor. Francisella LPS has many unusual characteristics and genetic, biochemical, and structural studies have led the way in the exploration of this unique molecule. The presence of free lipid A is an unexpected finding and its biological role is yet to be investigated. Finally, the mechanism underlying LPS transport to the bacterial surface is not well understood. Francisella appears to have several genes in the lpt transport pathway, but the relevance of this LPS transport system has not been studied. Further investigations are needed to confirm the findings of these studies and to identify all the genes involved in the biosynthesis, modification and transport of Francisella LPS.
Highlights.
Francisella has an unusual bacterial LPS in many ways.
Francisella lipid A has only four acyl chains, longer than E. coli lipid A.
Majority of Francisella lipid A are “free”, which are not linked to core+O-antigen.
Francisella LPS core has only one Kdo, the second Kdo is cleaved off by Kdo hydrolase.
Francisella LPS is an important virulence factor.
Acknowledgments
The authors wish to acknowledge Drs. Robin Ross, Fikri Avci, Avner Fink and Julie McCoy for critical reading of the manuscript and helpful discussion.
Abbreviations
- LPS
Lipopolysachharide
- Kdo
3-deoxy-D-manno-octulosonic acid
- Qui4NFm
4,6-dideoxy-4-formamido-D-glucose
- GalNAcAN
2-acetamido-2-deoxy-D-galacturonamide
- QuiNAc
2-acetamido-2,6-dideoxy-D-glucose
- Qui2NAc4NAc
2,4-diacetamido-2,4,6-trideoxy-D-glucose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellis J, Oyston PC, Green M, Titball RW. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLendon MK, Apicella MA, Allen LA. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollis DG, Weaver RE, Steigerwalt AG, Wenger JD, Moss CW, Brenner DJ. J Clin Microbiol. 1989;27:1601–1608. doi: 10.1128/jcm.27.7.1601-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raetz CR, Whitfield C. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trent MS, Stead CM, Tran AX, Hankins JV. J Endotoxin Res. 2006;12:205–223. doi: 10.1179/096805106X118825. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Ribeiro AA, Guan Z, McGrath SC, Cotter RJ, Raetz CR. Biochemistry. 2006;45:14427–14440. doi: 10.1021/bi061767s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. Infect Immun. 2004;72:5340–5348. doi: 10.1128/IAI.72.9.5340-5348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinogradov E, Perry MB, Conlan JW. Eur J Biochem. 2002;269:6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- 9.Gunn JS, Ernst RK. Ann N Y Acad Sci. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, Forsman M, Bystrom M, Pelletier M, Wilson CB, Miller SI, Skerrett SJ, Ernst RK. Infect Immun. 2006;74:6730–6738. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schilling B, McLendon MK, Phillips NJ, Apicella MA, Gibson BW. Anal Chem. 2007;79:1034–1042. doi: 10.1021/ac061654e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beasley AS, Cotter RJ, Vogel SN, Inzana TJ, Qureshi AA, Qureshi N. Innate Immun. 2012;18:268–278. doi: 10.1177/1753425911401054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soni S, Ernst RK, Muszynski A, Mohapatra NP, Perry MB, Vinogradov E, Carlson RW, Gunn JS. Front Microbiol. 2010;1:129. doi: 10.3389/fmicb.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CR. J Biol Chem. 2004;279:49470–49478. doi: 10.1074/jbc.M409078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, McGrath SC, Cotter RJ, Raetz CR. J Biol Chem. 2006;281:9321–9330. doi: 10.1074/jbc.M600435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raetz CR, Guan Z, Ingram BO, Six DA, Song F, Wang X, Zhao J. J Lipid Res. 2009;50(Suppl):S103–108. doi: 10.1194/jlr.R800060-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanistanon D, Hajjar AM, Pelletier MR, Gallagher LA, Kalhorn T, Shaffer SA, Goodlett DR, Rohmer L, Brittnacher MJ, Skerrett SJ, Ernst RK. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song F, Guan Z, Raetz CR. Biochemistry. 2009;48:1173–1182. doi: 10.1021/bi802212t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llewellyn AC, Zhao J, Song F, Parvathareddy J, Xu Q, Napier BA, Laroui H, Merlin D, Bina JE, Cotter PA, Miller MA, Raetz CR, Weiss DS. Mol Microbiol. 2012;86:611–627. doi: 10.1111/mmi.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaffer SA, Harvey MD, Goodlett DR, Ernst RK. J Am Soc Mass Spectrom. 2007;18:1080–1092. doi: 10.1016/j.jasms.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Powell DA, Shaffer SA, Rasko DA, Pelletier MR, Leszyk JD, Scott AJ, Masoudi A, Goodlett DR, Wang X, Raetz CR, Ernst RK. Proc Natl Acad Sci U S A. 2012;109:8716–8721. doi: 10.1073/pnas.1202908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLendon MK, Schilling B, Hunt JR, Apicella MA, Gibson BW. Infect Immun. 2007;75:5518–5531. doi: 10.1128/IAI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker JH, Kaufman JW, Zhang DS, Weiss JP. Innate Immun. 2013 doi: 10.1177/1753425913485308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinogradov E, Conlan WJ, Gunn JS, Perry MB. Carbohydr Res. 2004;339:649–654. doi: 10.1016/j.carres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Vinogradov E, Perry MB. Carbohydr Res. 2004;339:1643–1648. doi: 10.1016/j.carres.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Shi X, Leymarie N, Madico G, Sharon J, Costello CE, Zaia J. Biochemistry. 2011;50:10941–10950. doi: 10.1021/bi201450v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isobe T, White KA, Allen AG, Peacock M, Raetz CR, Maskell DJ. J Bacteriol. 1999;181:2648–2651. doi: 10.1128/jb.181.8.2648-2651.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brabetz W, Muller-Loennies S, Brade H. J Biol Chem. 2000;275:34954–34962. doi: 10.1074/jbc.M005204200. [DOI] [PubMed] [Google Scholar]
- 29.Chalabaev S, Kim TH, Ross R, Derian A, Kasper DL. J Biol Chem. 2010;285:34330–34336. doi: 10.1074/jbc.M110.166314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Raetz CR. Mol Microbiol. 2010;78:820–836. doi: 10.1111/j.1365-2958.2010.07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stead CM, Zhao J, Raetz CR, Trent MS. Mol Microbiol. 2010;78:837–852. doi: 10.1111/j.1365-2958.2010.07304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinogradov EV, Shashkov AS, Knirel YA, Kochetkov NK, Tochtamysheva NV, Averin SF, Goncharova OV, Khlebnikov VS. Carbohydr Res. 1991;214:289–297. doi: 10.1016/0008-6215(91)80036-m. [DOI] [PubMed] [Google Scholar]
- 33.Prior JL, Prior RG, Hitchen PG, Diaper H, Griffin KF, Morris HR, Dell A, Titball RW. J Med Microbiol. 2003;52:845–851. doi: 10.1099/jmm.0.05184-0. [DOI] [PubMed] [Google Scholar]
- 34.Thirumalapura NR, Goad DW, Mort A, Morton RJ, Clarke J, Malayer J. J Med Microbiol. 2005;54:693–695. doi: 10.1099/jmm.0.45931-0. [DOI] [PubMed] [Google Scholar]
- 35.Thomas RM, Titball RW, Oyston PC, Griffin K, Waters E, Hitchen PG, Michell SL, Grice ID, Wilson JC, Prior JL. Infect Immun. 2007;75:371–378. doi: 10.1128/IAI.01241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osborn MJ, Gander JE, Parisi E. J Biol Chem. 1972;247:3973–3986. [PubMed] [Google Scholar]
- 37.Mulford CA, Osborn MJ. Proc Natl Acad Sci U S A. 1983;80:1159–1163. doi: 10.1073/pnas.80.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TH, Sebastian S, Pinkham JT, Ross RA, Blalock LT, Kasper DL. J Biol Chem. 2010;285:27839–27849. doi: 10.1074/jbc.M110.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apicella MA, Post DM, Fowler AC, Jones BD, Rasmussen JA, Hunt JR, Imagawa S, Choudhury B, Inzana TJ, Maier TM, Frank DW, Zahrt TC, Chaloner K, Jennings MP, McLendon MK, Gibson BW. PLoS One. 2010;5:e11060. doi: 10.1371/journal.pone.0011060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindemann SR, Peng K, Long ME, Hunt JR, Apicella MA, Monack DM, Allen LA, Jones BD. Infect Immun. 2011;79:581–594. doi: 10.1128/IAI.00863-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chng SS, Gronenberg LS, Kahne D. Biochemistry. 2010;49:4565–4567. doi: 10.1021/bi100493e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitfield C. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 43.Eigelsbach HT, Braun W, Herring RD. J Bacteriol. 1951;61:557–569. doi: 10.1128/jb.61.5.557-569.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowley SC, Myltseva SV, Nano FE. Mol Microbiol. 1996;20:867–874. doi: 10.1111/j.1365-2958.1996.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 45.Hartley G, Taylor R, Prior J, Newstead S, Hitchen PG, Morris HR, Dell A, Titball RW. Vaccine. 2006;24:989–996. doi: 10.1016/j.vaccine.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 46.Miller SI, Ernst RK, Bader MW. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 47.Sandstrom G, Sjostedt A, Johansson T, Kuoppa K, Williams JC. FEMS Microbiol Immunol. 1992;5:201–210. doi: 10.1111/j.1574-6968.1992.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 48.Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, Cuesta N, Vogel SN. J Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 49.Okan NA, Chalabaev S, Kim TH, Fink A, Ross RA, Kasper DL. MBio. 2013;4:e00638–00612. doi: 10.1128/mBio.00638-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sebastian S, Dillon ST, Lynch JG, Blalock LT, Balon E, Lee KT, Comstock LE, Conlan JW, Rubin EJ, Tzianabos AO, Kasper DL. Infect Immun. 2007;75:2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raynaud C, Meibom KL, Lety MA, Dubail I, Candela T, Frapy E, Charbit A. Infect Immun. 2007;75:536–541. doi: 10.1128/IAI.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai XH, Shirley RL, Crosa L, Kanistanon D, Tempel R, Ernst RK, Gallagher LA, Manoil C, Heffron F. PLoS One. 2010;5:e11857. doi: 10.1371/journal.pone.0011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng K, Broz P, Jones J, Joubert LM, Monack D. Cell Microbiol. 2011;13:1586–1600. doi: 10.1111/j.1462-5822.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Z, Madico G, Roche MI, Wang Q, Hui JH, Perkins HM, Zaia J, Costello CE, Sharon J. Immunology. 2012;136:352–360. doi: 10.1111/j.1365-2567.2012.03589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rynkiewicz MJ, Lu Z, Hui JH, Sharon J, Seaton BA. Biochemistry. 2012;51:5684–5694. doi: 10.1021/bi201711m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boltje TJ, Zhong W, Park J, Wolfert MA, Chen W, Boons GJ. J Am Chem Soc. 2012;134:14255–14262. doi: 10.1021/ja306274v. [DOI] [PMC free article] [PubMed] [Google Scholar]