Abstract
As prokaryotic models for multicellular development, Stigmatella aurantiaca and Myxococcus xanthus share many similarities in terms of social behaviors, such as gliding motility. Our current understanding of myxobacterial grouped-cell motilities comes mainly from the research on M. xanthus, which shows that filamentous type IV pili (TFP), composed of type IV pilin (also called PilA protein) subunits, are the key apparatus for social motility (S-motility). However, little is known about the pilin protein in S . aurantiaca . We cloned and sequenced four genes (pilA Sa1~4) from S . aurantiaca DSM17044 that are homologous to pilA Mx (pilA gene in M. xanthus DK1622). The homology and similarities among PilASa proteins and other myxobacterial homologues were systematically analyzed. To determine their potential biological functions, the four pilA Sa genes were expressed in M. xanthus DK10410 (ΔpilA Mx), which did not restore S-motility on soft agar or EPS production to host cells. After further analysis of the motile behaviors in a methylcellulose solution, the M. xanthus strains were categorized into three types. YL6101, carrying pilA Sa1, and YL6104, carrying pilA Sa4, produced stable but unretractable surface pili; YL6102, carrying pilA Sa2, produced stable surface pili and exhibited reduced TFP-dependent motility in methylcellulose; YL6103, carrying pilA Sa3, produced unstable surface pili. Based on these findings, we propose that pilA Sa2 might be responsible for the type IV pilin production involved in group motility in S . aurantiaca DSM17044. After examining the developmental processes, it was suggested that the expression of PilASa4 protein might have positive effects on the fruiting body formation of M. xanthus DK10410 cells. Moreover, the formation of fruiting body in M. xanthus cells with stable exogenous TFPSa were compensated by mixing them with S . aurantiaca DSM17044 cells. Our results shed some light on the features and functions of type IV pilin homologues in S . aurantiaca .
Introduction
Myxobacteria belong to a branch of intriguing prokaryotes recognized for their complex social behaviors [1]. A group of myxobacterial cells, including cells from Myxococcus xanthus and Stigmatella aurantiaca , can crawl in swarms on solid surfaces, cooperatively prey on environmental macromolecules or microbial cells, and accumulate at a center to form fruiting bodies when food is exhausted [2,3]. Our current understanding of myxobacterial social cell behaviors comes mainly from research on M. xanthus, which shows that social motility (S-motility) plays a fundamental role in these processes [4,5]. Three constituents, i.e., type four pili (TFP), extracellular polysaccharides (EPS) and lipopolysaccharide (LPS) O-antigens, are known to be essential for S-motility [5,6,7,8,9]. Among them, TFP act as molecular engines to enable S-motility, which are composed of thousands of protein subunits called type IV pilin (or the PilA protein) [6,10]. During S-motility, TFP function by extending at one of the cell poles, attaching to the solid surfaces of the substratum or another cell, and then retracting to pull the cell forward [10,11,12,13,14]. To achieve the cycles of extension and retraction, pilin proteins are assembled into polar filaments mediated by the ATPase PilB, and the extracellular TFP are disassembled into single subunits with the assistance of the ATPase PilT [13,15]. In addition to being the key apparatus for S-motility, TFP also play divergent roles in other physiological aspects of M. xanthus. Extracellular TFP provides proximity signals to the Dif chemosensory pathway to modulate EPS production [16], and the specific cellular pilin localization is required to maintain the normal amount of secreted EPS [17]. Moreover, the TFP apparatus has been proposed to be involved in plasmid natural transformation in M. xanthus [18].
S . aurantiaca and M. xanthus are both in the suborder Cystobacterineae of Myxococcales [1]. They appear very similar to each other in terms of social behaviors and both serve as prokaryotic models for multicellular development [19]. While the morphology of fruiting bodies varies, e.g., M. xanthus fruiting bodies are haystack-shaped and S . aurantiaca elaborate fruiting bodies that consist of tree-like stalks bearing several spore-filled sporangioles at their tops [1], the genetic programs for fruiting body formation and associated characteristics of the two species are very similar [20]. Unlike M. xanthus, relatively little is known about the motility in S . aurantiaca . S . aurantiaca and M. xanthus both require calcium ions for gliding [21], and inhibitors of protein synthesis prevent both the motility in S . aurantiaca and S-motility in M. xanthus [21]. Furthermore, energy-dependent cohesion and motility are suggested to be related phenomena in S . aurantiaca [21,22], which is consistent with the finding in M. xanthus that EPS is involved in both cohesion and S-motility [9,23]. Despite these known similarities between the motility in S . aurantiaca and M. xanthus, the features of the pilin protein, potentially the key component in grouped-cell motility, have not been investigated in S . aurantiaca .
Strain DSM17044 is the type strain of the S . aurantiaca species [24] and is closely related to another lab strain of S . aurantiaca , DW4/3-1. In this study, four genes homologous to the pilA gene in M. xanthus were cloned from S . aurantiaca DSM17044, and subsequently expressed in M. xanthus cells to characterize their products. The motility and development-related phenotypes of M. xanthus cells carrying different S . aurantiaca pilA homologues were systematically investigated. The results obtained in this study could help to understand the potential biological functions of the type IV pilin homologues in S . aurantiaca .
Results
Four genes in S . aurantiaca DSM17044 encode type IV pilin homologues
The genome of S . aurantiaca strain DW4/3-1 was recently sequenced [20], in which five genes were annotated as pilA homologues (the predicted product is a type IV pilus subunit or fimbrial protein), i.e., locus tag STAUR_0004, 1125, 6449, 6450 and 6924 (Genome access No. NC014623.1 in the GenBank database). Because strain DSM17044 is the type strain of the S . aurantiaca species [24] and is closely related to strain DW4/3-1, similar pilA homologues were expected to exist in strain DSM17044. Therefore, five sets of specific primers (listed in Table 1) were designed according to the sequences of the five pilA homologues in strain DW4/3-1, and four genes, pilA Sa1, pilA Sa2, pilA Sa3 and pilA Sa4 (see Material sand Methods), were amplified from DSM17044 genomic DNA with the primer sets targeting genes STAUR_0004, 6449, 6450 and 6924 in the DW4/3-1 genome, respectively. Despite testing several different conditions, PCR using the primer pair Stig pilA-5-F and -R (Table 1) did not result in any specific products (data not shown).
Table 1. Primers used in this study.
| Primer | Sequence (5’~3’) | Description |
|---|---|---|
| DK pilA SP-F | GTGAAGACCCGTGCTGCGGAGTTGC | Used in cloning pilA Mx promoter and signal peptide (PSPMx) sequence from M. xanthus DK1622 genomic DNA |
| DK pilA SP-R | GCCACGGTTGCGGGGGTTGAATC | |
| DK pilA-R | CGAGTTACTGGGCCGCGCCGTCG | Used to amplify PSPMx-pilA Mx |
| Stig pilA-1-F | TTCAACCCCCGCAACCGTGGCTTTCACCCTCATCGAACTCATGATTG | Used in cloning pilA Sa1 gene from S . aurantiaca DSM17044 genomic DNA; designed according to sequence of STAUR_0004 * in DW4/3-1 genome |
| Stig pilA-1-R | TTAGTCGCAGCTGACGTCGTTG | |
| Stig pilA-2-F | TTCAACCCCCGCAACCGTGGCTTCACCCTCATCGAGCTGATGATC | Used in cloning pilA Sa3 gene from S . aurantiaca DSM17044 genomic DNA; designed according to sequence of STAUR_6449 * in DW4/3-1 genome |
| Stig pilA-2-R | TTACTGGCAGTTCACGTCGTTG | |
| Stig pilA-3-F | TTCAACCCCCGCAACCGTGGCTTTACGCTCATCGAGCTGATGATC | Used in cloning pilA Sa4 gene from S . aurantiaca DSM17044 genomic DNA; designed according to sequence of STAUR_6450 * in DW4/3-1 genome |
| Stig pilA-3-R | CTACTCGCAGTCCACGTCATTGTT | |
| Stig pilA-4-F | TTCAACCCCCGCAACCGTGGCTTCACCCTCATTGAGCTCATGATT | Used in cloning pilA Sa2 gene from S . aurantiaca DSM17044 genomic DNA; designed according to sequence of STAUR_6924 * in DW4/3-1 genome |
| Stig pilA-4-R | TTACGGGCAGTTGACGTCGTTG | |
| Stig pilA-5-F | TTCAACCCCCGCAACCGTGGCTTCACCTTTCTCGAAGTGTTGATC | Designed according to sequence of STAUR_1125 * in DW4/3-1 genome |
| Stig pilA-5-R | TCAGAAGTCGCACTGGGTGTCCT | |
| RT-pilA Sa1-F | GCCAGCATCGCCATTCCGAGTTTCA | Used to investigate transcription of pilA Sa1 in DSM17044 |
| RT-pilA Sa1-R | TCGTGCTGCGGTCCTCGTAAGAAGA | |
| RT-pilA Sa2-F | TCTGGCTTTACCCTCATCGAACTCA | Used to investigate transcription of pilA Sa2 in DSM17044 |
| RT-pilA Sa2-R | AGATGCTGCAGTCTCCGAGGTGATA | |
| RT-pilA Sa3-F | TCGTGGTCGCCATCATCGGCATCCT | Used to investigate transcription of pilA Sa3 in DSM17044 |
| RT-pilA Sa3-R | TCAGCGAGACCGTCGGGAAGTTACC | |
| RT-pilA Sa4-F | GGAGCCCCACAACGACGACAACT | Used to investigate transcription of pilA Sa4 in DSM17044 |
| RT-pilA Sa4-R | AACCAGGTATCCGCCGTATCCGAGA |
The locus tag of gene in S . aurantiaca DW4/3-1 genome.
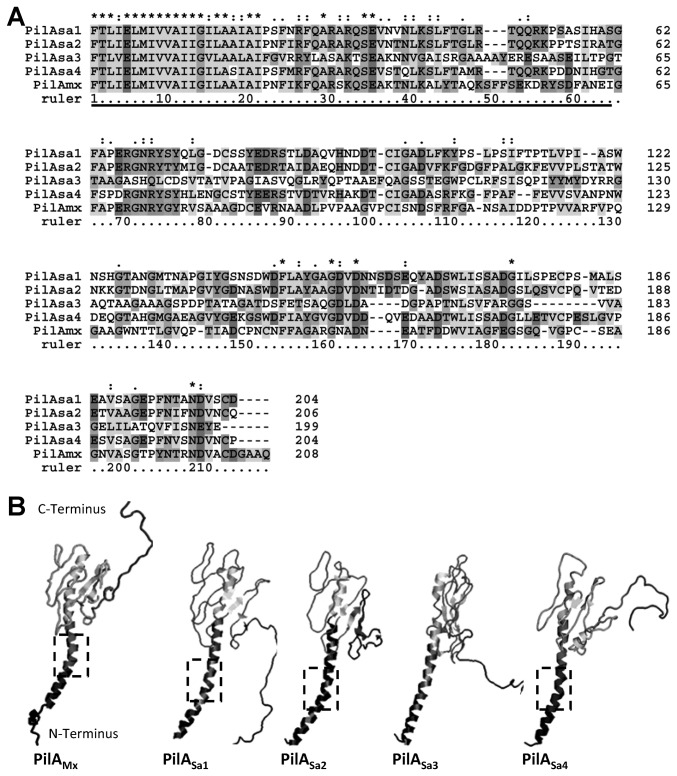
After sequence alignment (Figure 1A), four PilASa proteins from S . aurantiaca DSM17044 were found to share homology with the type IV pilin PilAMx from M. xanthus DK1622. In particular, the N-terminal sequences (1~43 residues) of the five proteins are well conserved, which is consistent with the finding that the first 28 residues of mature pilin are highly conserved among a variety of bacterial species [12,25,26]. Moreover, an N-terminal α-helix has been identified in all crystal structures of type IV pilins, e.g., PilA in Pseudomonas aeruginosa and PilE in Neisseria gonorrhoeae [25,26,27,28,29], which is packed in the filamentous TFP core [29]. As shown in Figure 1B, the simulated three-dimensional conformations of PilAMx and PilASa proteins all exhibit spoon-like structures, in which the highly apolar N-terminal residues form an extended α-helical secondary structure. Interestingly, PilAMx and PilASa1, 2, 4 proteins all show a kink region in the α-helix while PilASa3 has an almost straight α-helical domain (Figure 1B), which may be due to the difference in their primary structures of residues 22~27 (Figure 1A).
Figure 1. Four type IV pilin homologues in S . aurantiaca DSM17044.
(A) Amino acid sequence alignment among type IV pilin in M . xathus DK1622 (PilAMx) and the four homologues in S . aurantiaca DSM17044 (PilASa1~4). The underlined sequences correspond to the predicted N-terminal α-helical structures in panel B. (B) The 3D structures of the PilAMx and PilASa1~4 were predicted using 3D-JIGSAW and Swiss-model as described in the Materials and Methods. The dashed frames indicate the kink regions in α-N-terminal subdomains of the pilin structures.
In the alignment (Figure 1A), the C-terminal sequences of the five proteins are variable, and the low-score segments are mostly in PilASa3 protein sequence. In the putative structures (Figure 1B), the C-terminal globular domain were observed in all five proteins, which is believed to be exposed to the outer surface of TFP and involved in the biological functions of TFP [30,31]. It was also noticed that approximately 20 residues on the C-terminus of all five proteins exhibited random folding, which might be because this part of the sequence was missing in the models of the 3D structure prediction, e.g., PilA in P. aeruginosa and PilE in N. gonorrhoeae. Indeed, a previous study showed that the sequence of PilAMx was at least 17 residues longer than the pilin from P. aeruginosa or N. gonorrhoeae [12]. Despite the random folding portion, PilAMx and PilASa1, 2, 4 proteins were predicted to fold similarly at their C-terminal domains, while PilASa3 formed a more tightly packed C-terminal global structure compared to others.
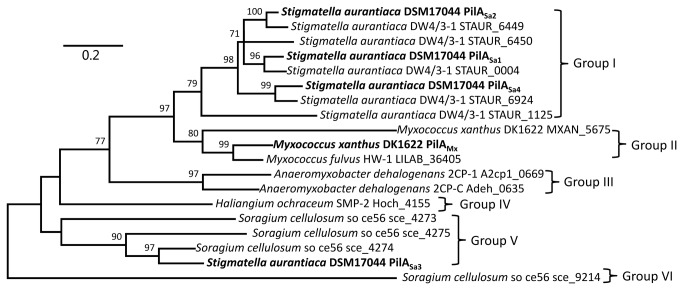
Next, the similarities among PilASa proteins and other myxobacterial homologues were further explored. The amino acid sequences of predicted pilin proteins from different myxobacterial strains were retrieved from the Genbank database and subjected to phylogenetic analysis. The strains belong to Cystobacterineae, Sorangineae and Nannocystineae suborders. As shown in Figure 2, 19 homologous PilA proteins from 8 strains could be divided into 6 deeply branched groups, and proteins from the same or closely related species tended to cluster together. As expected, PilASa1, 2, 4 from S . aurantiaca DSM17044 showed great similarities to proteins STAUR_0004, 6449 and 6924 from S . aurantiaca DW4/3-1, respectively, which is consistent with our initial primer design (Table 1). Surprisingly, PilASa3 is more similar to PilA proteins in Sorangium cellulosum so ce56 (e.g., SCE_4274) rather than its primer-targeted protein STAUR_6450 in S . aurantiaca DW4/3-1.
Figure 2. Phylogenetic analysis of the proteins homologous to type IV pilin from different myxobacterial strains.
The bar indicated the evolutionary distance. The numbers on branch nodes were percentages of 1000 sets of bootstrap supports. The proteins, except for those from S . aurantiaca DSM17044, were denoted as their gene locus tags in the genome of the strain they belonged to.
Expression of four pilA Sa genes in M. xanthus 10410 did not restore S-motility on agar or EPS production
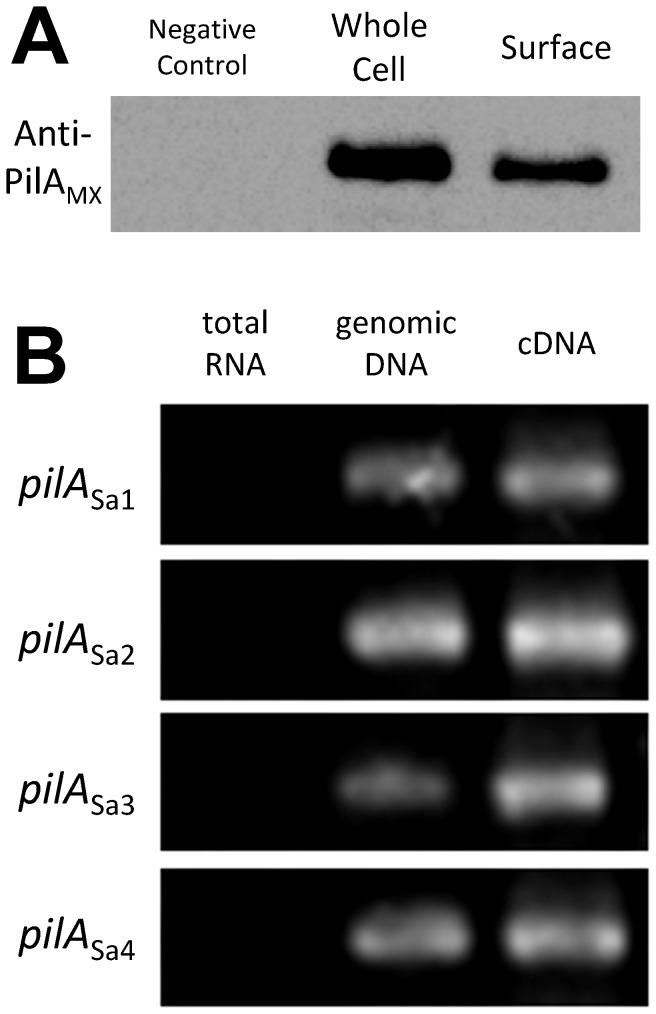
After identifying multiple type IV pilin homologues in S . aurantiaca DSM17044, we sought to determine their potential biological functions. A western blot using an anti-PilAMx antibody was employed to investigate pilin levels in whole cells and surface components of S . aurantiaca DSM17044. As shown in Figure 3A, positive immuno-blot signals were observed in both lanes loaded with whole cell lysates and with isolated extracellular components. This result indicates that the polyclonal anti-PilAMx antibody recognizes the pilin protein from S . aurantiaca DSM17044, which might be due to the similarities between PilAMx and PilASa proteins (Figure 1). Furthermore, the results show that at least one of the PilASa proteins was expressed in S . aurantiaca DSM17044 both intracellularly and extracellularly. Next, the transcription levels of the four pilA Sa genes in S . aurantiaca DSM17044 were determined using RT-PCR. The results show that the mRNA all of four pilA Sa genes could be detected in S . aurantiaca DSM17044 cells during vegetative growth (Figure 3B).
Figure 3. The expression and transcriptions of the pilA Sa genes in S . aurantiaca DSM17044.
(A) Whole-cell pilin (lane 2) and surface pili (lane 3) of S . aurantiaca DSM17044 cells were tested using western-blot probed by anti-PilAMx antibody. The whole-cell lysate of M. xanthus DK10410 (ΔpilA Mx) was loaded in lane 1 as the negative control. (B) The transcriptions of four pilA Sa genes (from top to bottom) in S . aurantiaca DSM17044 vegetative cells were determined with the RT-PCR using specific primers (listed in Table 1). Lanes 1~3 show the agarose gel electrophoresis of RT-PCR products using total RNA, genomic DNA and cDNA as the template, respectively.
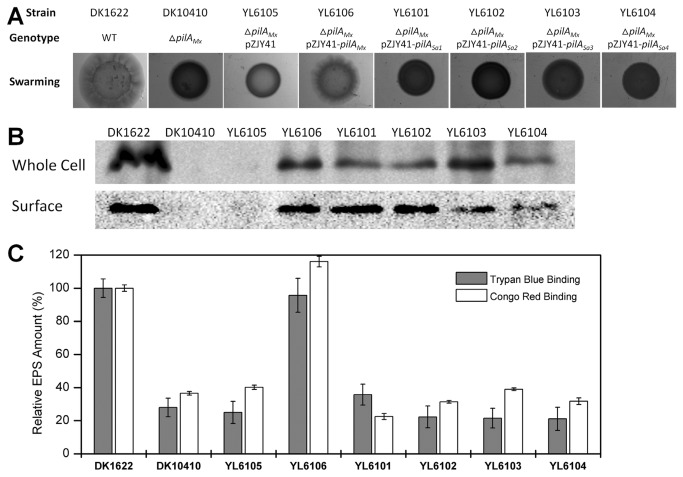
The difficulties of genetic manipulation hindered a deeper investigation of PilASa in S . aurantiaca DSM17044; therefore, the pilA Sa genes were transferred into M. xanthus DK10410 (ΔpilA Mx) using the E. coli-M. xanthus shuttle vector pZJY41 [32]. To prevent the potential influence of upstream sequences, the promoter and signal peptide-coding region of each pilA Sa gene was replaced by its pilA Mx counterpart. The S-motilities of the M. xanthus strains were assayed on CTT medium containing 0.3% agar. As shown in Figure 4A, strains YL6101~4 carrying the pilA Sa1~4 genes exhibited deficient S-motilities and had smooth colony edges, while strain YL6106 (ΔpilA Mx, pZJY41-pilA Mx), the positive control, showed normal S-motility on soft agar and phenotypically resembled wild-type DK1622. The whole cellular and extracellular components of these M. xanthus cells were probed by western-blot using an anti-PilAMx antibody, and positive bands were revealed in all of the samples from YL6101~4 (Figure 4B). These results suggest that although the pilA Sa1~4 genes from S . aurantiaca DSM17044 are expressed by M. xanthus DK10410 (ΔpilA Mx), this does not restore S-motility on a soft agar surface. Therefore, EPS production was examined in these strains, which is another key component for S-motility in addition to TFP [11].
Figure 4. Effects of heterologously expressed pilA Sa genes in M. xanthus DK10410 on S-motility ability, TFP biogenesis and EPS production.
(A) S-motility and surface pili of different M. xanthus strains. Top to bottom rows show swarming on 0.3% CTT agar surfaces after 120 h incubation. (B) Whole-cell pilin (upper row) and surface pili (bottom row) of M. xanthus cells were tested using western-blot probed by anti-PilAMx antibody. (C) Quantitative analysis of EPS production in different M. xanthus strains using trypan blue binding assay (grey columns) and congo red binding assay (white columns). Values for all strains were normalized to the wild-type DK1622, respectively. The data represent triplicate experiments, and mean ± SD is plotted.
Previous studies have shown that the surface pilus (extracellular PilA) is the positive regulator of EPS production in M. xanthus [16]. As shown in Figure 4C, complementary strain YL6106 containing the pilA Mx gene in a ΔpilA Mx fully restored EPS production to levels observed in the wild type DK1622, while the EPS levels in strains YL6101~4 (carrying pilA Sa1~4 genes, respectively) were significantly lower (60~80%) than that of the wild-type strain DK1622 and similar to that of strain DK10410 (ΔpilA Mx). This result shows that the presence of the extracellular PilASa did not up-regulate EPS production in M. xanthus. Meanwhile, several pieces of evidence have shown that PilAMx specifically recognizes and interacts with the EPS of M. xanthus [11,17,31], and that EPS is the trigger for TFP retraction, which enables M. xanthus cells to perform S-motility on agar [11]. Therefore, we hypothesized that the lack of S-motility in DK10410 (ΔpilA Mx) carrying the pilA Sa genes might be due to deficient EPS production or failure of the PilASa proteins to recognize the EPS of M. xanthus.
M. xanthus cells carrying pilA Sa2 demonstrated reduced TFP-dependent motility in 1% methylcellulose solution
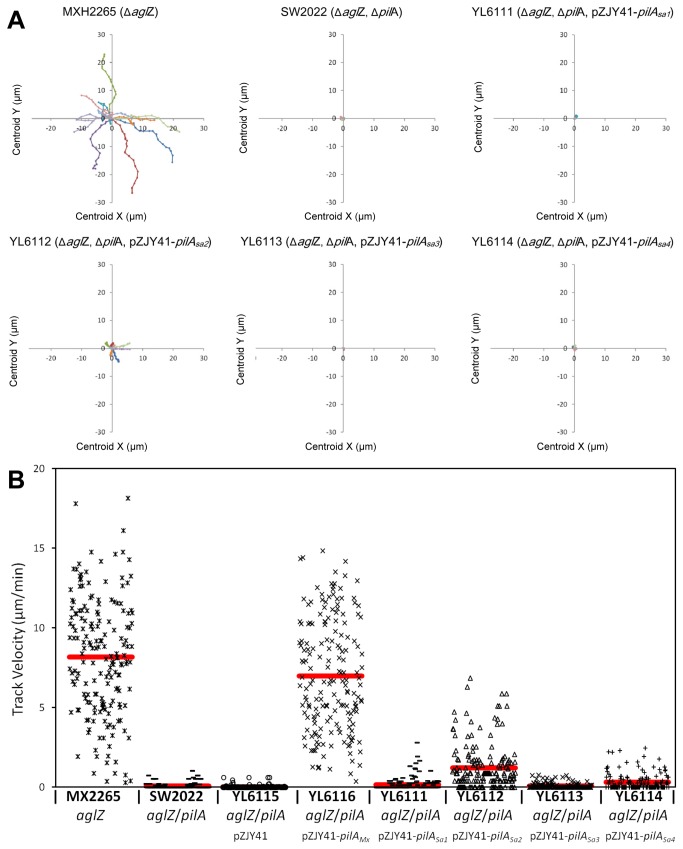
Next, M. xanthus cells were analyzed for motility on a polystyrene surface submerged in a methylcellulose solution because it has been proposed that M. xanthus cells could bypass the need for EPS to anchor their TFP and conduct TFP-dependent single-cell motility under this condition [14]. The aglZ gene was in-frame deleted in strains YL6101~6 to generate strains YL6111~6 (Table 2), respectively, which inactivated the adventurous motility (A-motility) [33] in these strains to eliminate potential motile backgrounds [10]. As shown in Figure 5, and in agreement with previous findings [14], MXH2265 (ΔaglZ) cells and YL6116 cells (containing the pilA Mx gene in a ΔaglZ and ΔpilA Mx mutant background) exhibited similar levels of single-cell motility in the methylcellulose solution, while active motility was totally eliminated in the respective mutant strains defective in surface pilus biogenesis, i.e., SW2022 (ΔaglZ, ΔpilA Mx) and YL6115 (ΔaglZ, ΔpilA Mx, pZJY41). Of the four strains carrying pilA Sa1~4 genes, the YL6112 (ΔaglZ, ΔpilA Mx, pZJY41-pilA Sa2) cells showed relatively active single-cell motility, which was significantly different from the YL6111, YL6113 and YL6114 cells (carrying pilA Sa1, pilA Sa3 and pilA Sa4, respectively), although at a reduced level compared with that of MXH2265 (ΔaglZ) cells.
Table 2. Bacterial strains and plasmids used in this study.
| Designation | Relevant Feature | Ref. or Source |
|---|---|---|
| Strain | ||
| M. xanthus | ||
| DK1622 | Wild type | |
| DK10410 | DK1622, ΔpilA, missing PilA | [41] |
| MXH2265 | DK1622, ΔaglZ, deficient in A-motility | [33] |
| SW2022 | DK1622, ΔaglZ, ΔpilA | [14] |
| YL6101 | DK1622, ΔpilA, containing pTZG-1 | This study |
| YL6102 | DK1622, ΔpilA, containing pTZG-2 | This study |
| YL6103 | DK1622, ΔpilA, containing pTZG-3 | This study |
| YL6104 | DK1622, ΔpilA, containing pTZG-4 | This study |
| YL6105 | DK1622, ΔpilA, containing pZJY41 | This study |
| YL6106 | DK1622, ΔpilA, containing pTZG-5 | This study |
| YL6111 | DK1622, ΔaglZ, ΔpilA, containing pTZG-1 | This study |
| YL6112 | DK1622, ΔaglZ, ΔpilA, containing pTZG-2 | This study |
| YL6113 | DK1622, ΔaglZ, ΔpilA, containing pTZG-3 | This study |
| YL6114 | DK1622, ΔaglZ, ΔpilA, containing pTZG-4 | This study |
| YL6115 | DK1622, ΔaglZ, ΔpilA, containing pZJY41 | This study |
| YL6116 | DK1622, ΔaglZ, ΔpilA, containing pTZG-5 | This study |
| S . aurantiaca | ||
| DSM17044 | Type strain for S . aurantiaca , ATCC 25190 | [24] |
| E. coli | ||
| DH5α | Host for cloning | [61] |
| Plasmid | ||
| pZJY41 | Shuttle vector in E. coli-M. xanthus; Kanr Ampr | [32] |
| pTZG-1 | PSPMx and pilA Sa1 fusion fragment in pZJY41* | This study |
| pTZG-2 | PSPMx and pilA Sa2 fusion fragment in pZJY41 | This study |
| pTZG-3 | PSPMx and pilA Sa3 fusion fragment in pZJY41 | This study |
| pTZG-4 | PSPMx and pilA Sa4 fusion fragment in pZJY41 | This study |
| pTZG-5 | PSPMx and pilA Mx fusion fragment in pZJY41* | This study |
pilA Mx stands for pilA in M. xanthus DK1622, PSPMx stands for promoter and signal peptide sequence of pilA gene in M. xanthus DK1622, and pilA Sa stands for pilA in S . aurantiaca DSM17044.
Figure 5. Tracking motility of M. xanthus strains containing pilA Sa genes in 1% methycellulose solution.
Different M. xanthus cells were submurged in 1% methylcellulose solution and cell movements were recorded by time lapse photography. Motility and trajectories of 10 isolated cells were analyzed. Data are presented as tracking plots (panel A) and as diagrams (panel B). In panel A, a static synthetic view of cell motility tracks was generated as described in the Materials and Methods, and one color was applied for each trajectory. In panel B, the red lines show the average velocitis of respective strains.
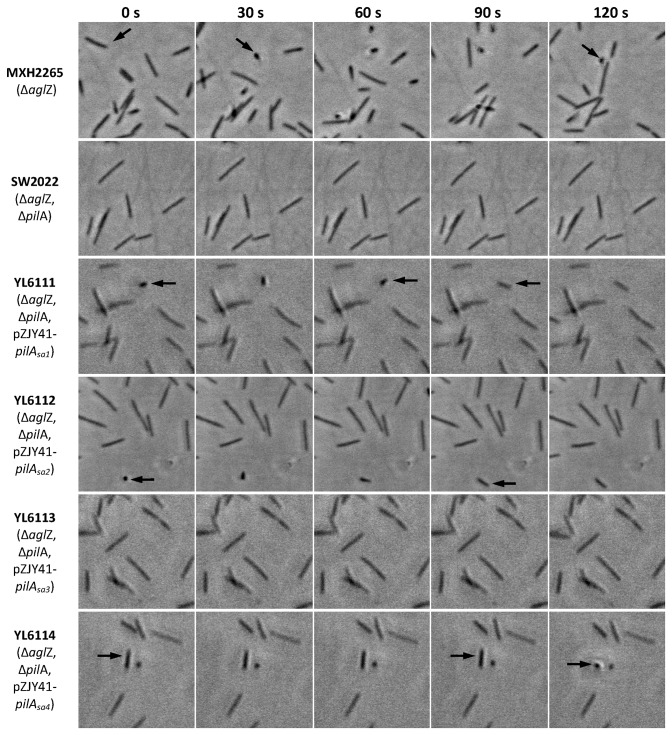
While pilA Sa1~4 genes from S . aurantiaca DSM17044 were all extracellularly expressed in M. xanthus cells (Figure 4B), only the cells carrying pilA Sa2 exhibited reduced motility in methylcellulose (Figure 5), which might be due to differences in the TFP retraction ability of these cells. To further test this possibility, the tethering behavior [10,14] of M. xanthus cells was investigated in the methylcellulose solution. As shown in Figure 6, the motile cells of MXH2265 (ΔaglZ) and YL6112 (ΔaglZ, ΔpilA Mx, pZJY41-pilA Sa2) were occasionally tethered to the surface with their TFP, resulting in the detection of cells with one end attached to the solid surface and lifted-up cell bodies. Cells lacking TFP, e.g., SW2002 (ΔaglZ, ΔpilA Mx), were non-motile and unable to tether. The YL6111 cells (ΔaglZ, ΔpilA Mx, pZJY41-pilA Sa1) and YL6114 cells (ΔaglZ, ΔpilA Mx, pZJY41-pilA Sa4) were not motile while exhibiting occasional tethering behavior, which is similar to the phenotype of the TFP retraction-deficient mutant ΔpilT [10]. This indicates that YL6111 and YL6114 produced stable surface TFP that allow the cells to tether but the pili are unable to retract. As a consequence, S-motility on agar or in methylcellulose is entirely impaired in these two strains (Figures 4A and 5). Interestingly, YL6113 cells (ΔaglZ, ΔpilA Mx, pZJY41-pilA Sa3) showed no motility or tethering in methylcellulose solution (Figure 6), which implies that these cells lack stable surface pili.
Figure 6. Tethering behavior of M. xanthus cells containing pilA Sa genes in 1% methycellulose solution.
M. xanthus cells were deposited onto polystyrene plates and submerged 1% methylcellulose solution, and individual cells were analysed for the tethering behaviour on solid surfaces. Tethered cells appear as dots in the image, indicated by black arrows. Left to right images were taken at 30 s intervals.
Expression of pilA Sa genes affected developmental abilities of M. xanthus host cells
Because it has been shown that the deletion or mutation of pilA Mx compromise the fruiting body formation of M. xanthus on TPM agar [17,34], we wondered if the expression of pilA Sa genes could affect the development of their host M. xanthus cells. As shown in Figure 7 (upper row images), after being incubated on TPM agar for 5 days, YL6101, YL6102 and YL6103 (ΔpilA Mx and carrying pilA Sa1, pilA Sa2 and pilA Sa3, respectively) formed immature fruiting bodies and were all deficient in myxospore production. However, YL6104 (ΔpilA Mx, pZJY41-pilA Sa4) was phenotypically similar to wild type DK1622, exhibiting normal fruiting body formation and reduced sporulation. While S . aurantiaca DSM17044 did not form fruiting bodies on TPM agar, mixing DSM17044 cells with M. xanthus cells significantly affected the development of the latter (Figure 7, images in bottom two rows). The fruiting body formation and sporulation of YL6101, YL6102 and YL6104 (ΔpilA Mx and carrying pilA Sa1, pilA Sa2 and pilA Sa4, respectively) were fully restored compared to those of wild type DK1622 after 1:1 mixing with S . aurantiaca DSM17044 cells. As for YL61103 (ΔpilA Mx, pZJY41-pilA Sa3), these abilities were partially complemented after mixing. Considering Stigmatella has complicated and specific fruiting body structures, which are morphologically different from the round Myxococcus fruiting bodies [2,20], the fruiting bodies on the mixing plates were most likely formed by the M. xanthus cells rather than the S . aurantiaca DSM17044 cells.
Figure 7. Phenotypes of fruiting body formation and sporulation.
Fruiting body formation (1st row) and sporulation (2nd row) of the S . aurantiaca strain DSM17044 and the M. xanthus strains DK1622 (wild-type), YL6101 (ΔpilA, pZJY41-pilA Sa1), YL6102 (ΔpilA, pZJY41-pilA Sa2), YL6103 (ΔpilA, pZJY41-pilA Sa3) and YL6104 (ΔpilA, pZJY41-pilA Sa4) were assayed after incubation of 5.0×106 vegetative cells for 5 d on TPM agar. 2.5×106 cells of S . aurantiaca DSM17044 were pre-mixed with 2.5×106 cells of different M. xanthus strains, respectively, and fruiting body formation (3rd and 4th row) and sporulation (5th row) of the mixing cultures were assayed on TPM agar after 5 d incubation. The images in 4th row exhibit a magified protion of the images in 3rd row, respectively. ‘N.D.’ represents ‘not detected’.
Discussion
In this study, four genes encoding type IV pilin homologues were identified in S . aurantiaca DSM17044 (Figure 1), all of which were transcribed during vegetative growth, and at least one of these genes was expressed in DSM17044 both intracellularly and extracellularly (Figure 3). Moreover, there are five pilA homologues in S . aurantiaca DW4/3-1 [20], two pilA homologues in M. xanthus DK1622 [35], and four pilA homologues in S. cellulosum so ce56 [36], which is consistent with the finding that gene duplicates are common in the genomic sequence of myxobacterial strains as a result of gene diversion and duplication [20,35,36,37]. Some duplicated genes result in a similar protein product, i.e., two genes (MXAN_5430 and MXAN_5432) encode protein S in the M. xanthus DK1622 genome [38], which are assumed to accelerate the biosynthesis of protein S and the formation of myxospores during fruiting body development [39]. Some duplications are assumed to be followed by divergence of the new gene copies, endowing them with new specificities [35]. For example, two copies of the chaperone groEL gene are present in the M. xanthus DK1622 genome; groEL1 (MXAN_4895) is more active in cellular development and sporulation, while groEL2 (MXAN_4467) is important for predation behavior [40]. As for the pilA genes in myxobacteria, the significance of gene duplication remains unclear. In M. xanthus DK1622, the pilA Mx gene encodes the type IV pilin and is responsible for TFP assembly and S-motility [41], while the function of MXAN_5675 (annotated as fimbrial protein) is still unknown.
To determine their potential biological functions, the pilA Sa genes from S . aurantiaca DSM17044 were transferred into M. xanthus DK10410 (ΔpilA Mx) and were successfully extracellularly expressed, which might be because the promoter and signal peptide-coding region of the pilA Mx gene was inserted in front of each pilA Sa gene in every construct. In bacteria, the pilin protein is synthesized as prepilin with an N-terminal hydrophilic signal peptide that is recognized and cleaved by the prepilin peptidase PilD [42]. A previous study has shown that deletion or mutation of the pilA Mx signal peptide significantly compromises PilAMx processing and production [17]; therefore, the whole pilA Mx signal peptide was stitched to each pilA Sa to ensure the gene product could be processed correctly in its M. xanthus host. In addition to the processing, mature pilin proteins are assembled into polar filaments mediated by the PilB ATPase [13,15], which is a key step in pilin protein secretion. Our results suggests that despite the differences in amino acid sequences and predicted protein structures of the PilA proteins (Figure 1), all four PilASa proteins could be exported extracellularly by the PilB ATPase (Figure 4B), indicating that the substrate specificity of PilB in M. xanthus is relatively low.
According to their various motility-related phenotypes (Figure 4~6), the M. xanthus strains carrying different pilA Sa genes were categorized into three distinct types. The type I strains (YL6101 carrying pilA Sa1 and YL6104 carrying pilA Sa4) produced stable surface pili (detected by both western blot and the tethering assay), but were not motile on soft agar or in methylcellulose solution, which indicated that their TFPSa were unable to retract. The type II strain (YL6102 carrying pilA Sa2) also produced stable surface pili and did not display S-motility on soft agar. However, cells in this category showed single-cell motility in methylcellulose solution, albeit at a reduced level compared with the motility of cells carrying pilA Mx. Therefore, it was suggested that M. xanthus cells carrying pilA Sa2 produced retractable TFPSa2 and can perform TFP-dependent motility in the methylcellulose solution, and the nonspecific interactions of TFPSa2 with the polystyrene surface in the methylcellulose solution might compensate for the absence of the TFPSa2-EPS specific interaction. Previous studies showed that swarms of M. xanthus and S . aurantiaca initially merged on an agar surface but subsequently separated and established separate fruiting bodies [43], which implies a potential specific recognition of self-EPS components by the motility systems of these two species during the swarming and development process. The type III strain (YL6103 carrying pilA Sa3) did not exhibit motility or tethering behaviors, indicating that they produced unstable surface pili, which might be attributed to the unique straight α-helical domain of PilASa3 (Figure 1B). The curved structure of the PilAMx α-helical domain has been shown to be essential for stable pili production, and the formation of a kink in the α-N-terminal subdomain has been implicated as in assisting in the tight packing of pilin subunits into TFP [29,44]. In the predicted structure of PilASa3, this kink was missing due to unique residues at positions 22~27 in its primary structure. We also noticed that both counterparts for pilA Sa2 and pilA Sa3 (STAUR_6449 and 6450) were located in a gene cluster in S . aurantiaca DW4/3-1 genome, which is predicted to produce TFP components (from STAUR_6441 to STAUR_6458). It has been shown that the pilin gene in the TFP gene cluster normally encodes the functional type IV pilin for twitching or social motility, e.g., pilA Mx in M. xanthus, pilA in P. aeruginosa and pilE in N. gonorrhoeae [45]. In S . aurantiaca , we propose that pilA Sa2 rather than pilA Sa3 could be responsible for the type IV pilin production to perform group motility.
S . aurantiaca is well known for its complicated and particular fruiting body [2,20], which is quite different from the one formed by Myxococcus cells. However, it has been shown that the expression profile of the development-specific genes in these two species is extramely similar. In particular, the genes involved in signal transduction pathways that are important for fruiting body formation in M. xanthus are conserved in S . aurantiaca [20]. In M. xanthus, the PilAMx protein is thought to be involved in the fruiting body formation process. The deletion of pilA Mx compromises the fruiting body formation of M. xanthus on TPM agar [34], which may be because surface pili serve as a sensor to provide signals to the Dif chemosensory pathway, thereby controlling EPS production [16]. Moreover, a mutation in the PilAMx protein has been shown to diminish the fruiting body formation of M. xanthus by leading to an accumulation of PilAMx in the periplasmic space and reducing surface EPS production [17]. Expression of pilA Sa4 in a M. xanthus ΔpilA Mx background (strain YL6104) phenotypically restored the fruiting body formation and reduced sporulation compared to levels of wild-type DK1622 cells (Figure 7), while YL6104 cells produced a similar amount of EPS compared to DK10410 (ΔpilA Mx) cells (Figure 4C). This suggested that the PilASa4 protein might positively regulate the fruiting body formation of M. xanthus cells through an unknown mechanism rather than by regulating of EPS production. More interestingly, after being mixed with the S . aurantiaca DSM17044 cells, the M. xanthus cells with stable exogenous TFPSa, i.e., cells of YL6101, YL6102 and YL6104, could form mature fruiting bodies and produce wild-type levels of myxospores (Figure 7). Because the specific interaction between TFP and EPS has been suggested in M. xanthus [11,17,31], we favor the hypothesis that TFPSa recognize the EPS from S . aurantiaca and up-regulate the developmental process of the M. xanthus cells. We are currently addressing this hypothesis by examining interactions of PilASa proteins with EPS from M. xanthus and S . aurantiaca .
Materials and Methods
Bacterial strains and cultural conditions
Bacterial stains used in this study were listed in Table 2. M. xanthus cells were grown in CTT medium [46] at 32°C, and S . aurantiaca cells were cultured in VY/2 medium [47] at 32°C. The developmental assay of myxobacterial cells was performed on TPM plates [48]. The S-motility assay was conducted on CTT plates containing 0.3% agar [49]. E. coli cells were cultured in Luria-Bertani (LB) medium [50] at 37°C. When necessary, kanamycin (Kan) was added to the medium to a final concentration of 40 µg/ml.
Amplification of the S . aurantiaca DSM17044 genes homologous to pilA by polymerase chain reaction (PCR)
Five sets of specific primers (Table 1) were designed according to the sequences of the five pilA homologues in the S . aurantiaca strain DW4/3-1 genome [20], and were used in the subsequent PCR with DSM17044 genomic DNA as the template. The DSM17044 genomic DNA was isolated and purified as described previously [51]. For PCR, a 50 µl-volume reaction solution was prepared by mixing 1 µl of template DNA (20 ng/µl), 1 µl of each primer (50 µM), 4 µl of dNTPs (2.5 mM), 1 µl of pfu DNA polymerase (2.5 U/µl, Fermentas), 25 µl of 2×GC Buffer I (Takara Bio) and 17 µl of ddH2O. The conditions for the PCR amplification were as follows: the initial denaturation step was at 94°C for 3 min, annealing was at 65°C for 1 min, polymerization was at 72°C for 1 min, subsequent denaturation was at 94°C for 1 min, and there were 30 cycles. The PCR products were purified with the EZNA Cycle pure kit (Omega). Four genes were amplified from DSM17044 genomic DNA using the primer sets targeting genes STAUR_0004, 6449, 6450 and 6924 in the DW4/3-1 genome (Table 1), which were referred to as the pilA Sa1, pilA Sa2, pilA Sa3 and pilA Sa4 genes in this study (pilA in Stigmatella aurantiaca DSM17044), respectively.
The purified fragments of the pilA Sa genes were ligated into the pGEM-T Easy vector (Promega), electroporated into E. coli DH5α, and the recombinant transformants were screened according to the standard protocol [50]. The recombined plasmids with a proper insertion were extracted and sequenced. The sequences of the four pilA Sa genes (pilA Sa1~4) were deposited in the GenBank database (www.ncbi.nlm.nih.gov) with accession number KF113889, KF113890, KF113891 and KF113892, respectively.
Bioinformatic analysis
The amino acid sequences of PilA in M. xanthus DK1622 (referred to as PilAMx) and PilASa1~4 were compared and aligned using the ClustalX program version 1.83 [52]. The amino acid sequences of the PilA proteins from different myxobacterial strains were retrieved from the Genbank database, and the phylogenetic reconstruction of the sequences was conducted using distance/neighbor joining programs with the Poisson correction distance model in MEGA software package version 4.0 [53]. The interior branch length supports were from 1000 replicates. The putative 3D structures of PilAMx and PilASa1~4 were constructed on-line using 3D-JIGSAW (http://bmm.icnet.uk/~3djigsaw/) [51] and further confirmed by Swiss-Model (http://swissmodel.expasy.org/).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA of S . aurantiaca DSM17044 was extracted using the SV total RNA isolation kit (Promega), and the genomic DNA was removed with the DNA free kit (ABI) following the protocols recommended by the manufacturers. RT-PCR was performed as described previously [54]. The complimentary DNA (cDNA) was synthesized using the downstream primer (RT-R primer, Table 1), and the double stranded DNA was amplified with the proper primer pair (RT-F and RT-R primers, Table 1) for each pilA Sa gene.
Construction and transformation of the pilA-containing plasmids
The promoter and signal peptide fragment of pilA Mx (referred as to PSPMx) was amplified using primers DK pilA-SP-F and DK pilA-SP-R (Table 1) and using M. xanthus genomic DNA as a template. The PSPMx fragment was stitched onto each pilA Sa gene through over-lap PCR as described previously [12]. For the over-lap PCR, DK pilA-F and Stig pilA-R (Table 1) were used as primers, and the fragments of pilA Sa and PSPMx were used as templates. The PSPMx and pilA Mx fusion fragment was directly amplified from M. xanthus genomic DNA using the DK pilA-SP-F and DK pilA-R (Table 1) primers. After purification, the fusion products were ligated into EcoRV-digested plasmid pZJY41 as previously described [32], resulting in the recombinant plasmids pTZG-1~5 (Table 2), which were subsequently transferred into E. coli DH5α and sequenced. The pilA-containing plasmids pTZG-1~5 and empty plasmid pZJY41 were, respectively, electroporated into M. xanthus DK10410 (ΔpilA) or SW2002 (ΔaglZ, ΔpilA) according to the standard protocol [55]. After 7 days, transformants were selected from CTT plates containing 40 µg/ml Kan. The positive transformants were purified, and the plasmids were extracted for confirmation as previously described [32].
S-motility assay
S-motility of M. xanthus cells on agar surfaces was analyzed as described previously [49]. Cells in mid-log phase were collected from CTT broth by centrifugation and resuspended in CTT medium to a final concentration of 5×109 cells/ml. Aliquots of a 2 µl cell suspension were spotted onto swarm plates (CTT medium containing 0.3% agar) and incubated at 32°C for 5 days before record.
Immunoblot analysis of pilin proteins
Cell-surface pili of M. xanthus or S . aurantiaca DSM17044 were isolated from 1010 cells as previously described [41]. Isolated pili were resuspended in SDS-PAGE loading buffer and boiled for 10 min. For whole-cell lysates, 108 M. xanthus or S . aurantiaca DSM17044 cells were directly lysed by boiling in SDS-PAGE loading buffer for 10 min. The samples were then separated by SDS-PAGE (10% gel) and subjected to western-blot analysis using standard methods [56]. Primary anti-PilAMx antibody [12] was used at a 1:4000 dilution, goat anti-rabbit horseradish peroxidase conjugated secondary antibody (Pierce) was used at a 1:4000 dilution. The blots were developed, and the bands were detected using the ECL Chemiluminescence kit (Tiangen).
Examination of extracellular polysaccharides (EPS) production
Two quantitative methods were used to examine EPS production of M. xanthus cells, namely the congo red binding assay [57] and the trypan blue binding assay [16,58]. All strains tested were harvested from CTT broth at the mid-log growth phase and resuspended in MOPS buffer (10 mM MOPS, 8 mM MgSO4, pH 7.6) to a concentration of 5 × 108 cell/ml. The EPS production of all strains was normalized to that of the wild-type strain DK1622, which was arbitrarily set to 1. Experiments were performed in triplicate.
Methylcellulose assay for TFP-dependent motility
The TFP-dependent motility of M. xanthus cells was analyzed using a previously published protocol [10,14]. Polystyrene plates (CostarTM cell culture plates, Fisher) were used as a testing surface. Cell movements were monitored with a Nikon Eclipse TE2000-S inverted microscope through a 40× objective, captured with a Nikon DXM1200F CCD camera and recorded with Nikon ACT-1 software (Version 2.62). Continuous images were taken at 10 s intervals and stored as TIFF image sequence files. The velocity measurements and trajectory tracking were performed as previously described [14] using Manual Tracking [59], a plugin for the ImageJ software (http://rsb.info.nih.gov/ij/). A static synthetic view of cell motility tracks was generated and the recorded coordinates were exported to Microsoft Excel to present the data as plots. The tethering behavior of M. xanthus cells was recorded and analyzed in the same experimental system as previously described [10,14]. When deposited in 1% methylcellulose medium, some wild-type M. xanthus cells were observed to be perpendicular to the polystyrene surface, and appeared to have one of their cell ends tethered to the solid surface with the TFP. Cells with unretractable surface TFP (ΔpilT) were non-motile in this assay while able to be tethered [10], and cells lacking TFP (ΔpilA) or stable surface TFP (SW2031, pilA-A32V) were non-motile and unable to be tethered [44]. The tethered cells were identified in a series of images as those with one end of the cell attached to the solid surface and lifted-up cell bodies.
Development assays
M. xanthus cells were grown in CTT to mid-log phase and concentrated to 5×109 cells/ml in TPM buffer (10 mM Tris-HCl, 1 mM KH2PO4, 8 mM MgSO4, pH 7.6). Ten microliter aliquots of concentrated cells were spotted onto TPM agar and incubated for 5 days at 32°C [60]. Pictures of fruiting body were taken using a Nikon SMZ1500 dissection microscope and recorded by Nikon ACT-1 software (Version 2.62).
For the mixing development experiments, S . aurantiaca DSM17044 cells were grown in VY/2 to mid-log phase and concentrated to 5×109 cells/ml in TPM buffer. The cell suspension of S . aurantiaca DSM17044 was mixed with an equal volume of various M. xanthus cells suspension (5×109 cells/ml) to prepare the mixed inoculums, and 10 µl aliquot of the mixed cells were spotted onto TPM agar and incubated for 5 days at 32°C. The development was recorded as described above.
Sporulation was determined as previously described [60] with minor modifications. The 5-day cultured fruiting bodies were scraped from TPM agar, resuspended in 200 µl of TPM buffer and homogenized by slight sonication. The suspension was incubated at 50°C for 2 hours, serially diluted, mixed with CTT media containing 0.3% agar, poured onto CTT plates with 1.5% agar, and incubated at 32°C for 5 days. The sporulation efficiencies were calculated as the number of colonies that appeared on the CTT plates relative to the original number of cells spotted. Three replicate experiments were performed.
Acknowledgments
We thank Drs. Wenyuan Shi, Rolf Müller, Patricia Hartzell, Heidi Kaplan and Dale Kaiser for providing bacterial strains.
Funding Statement
This work was financially supported by the Chinese Natural Science Foundation Grants 30870020 (to W.H.), 30825001 and 31130004 (to Y.L.), Shandong Natural Science Foundation Grant ZR2012CM003 (to W.H.) and SDU Innovation Grant 2012TS007 (to W. H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shimkets L, Woese CR (1992) A phylogenetic analysis of the myxobacteria: basis for their classification. Proc Natl Acad Sci U S A 89: 9459-9463. doi:10.1073/pnas.89.20.9459. PubMed: 1384053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vasquez GM, Qualls F, White D (1985) Morphogenesis of Stigmatella aurantiaca fruiting bodies. J Bacteriol 163: 515-521. PubMed: 3926747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaiser D (2003) Coupling cell movement to multicellular development in myxobacteria. Nat Rev Microbiol 1: 45-54. doi:10.1038/nrmicro733. PubMed: 15040179. [DOI] [PubMed] [Google Scholar]
- 4. Kaiser D (1979) Social gliding is correlated with the presence of pili in Myxococcus xanthus . Proc Natl Acad Sci U S A 76: 5952-5956. doi:10.1073/pnas.76.11.5952. PubMed: 42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaplan HB (2003) Multicellular development and gliding motility in Myxococcus xanthus . Curr Opin Microbiol 6: 572-577. doi:10.1016/j.mib.2003.10.006. PubMed: 14662352. [DOI] [PubMed] [Google Scholar]
- 6. Wu SS, Kaiser D (1995) Genetic and functional evidence that Type IV pili are required for social gliding motility in Myxococcus xanthus . Mol Microbiol 18: 547-558. doi:10.1111/j.1365-2958.1995.mmi_18030547.x. PubMed: 8748037. [DOI] [PubMed] [Google Scholar]
- 7. Yang Z, Geng Y, Xu D, Kaplan HB, Shi W (1998) A new set of chemotaxis homologues is essential for Myxococcus xanthus social motility. Mol Microbiol 30: 1123-1130. doi:10.1046/j.1365-2958.1998.01160.x. PubMed: 9988486. [DOI] [PubMed] [Google Scholar]
- 8. Bowden MG, Kaplan HB (1998) The Myxococcus xanthus lipopolysaccharide O-antigen is required for social motility and multicellular development. Mol Microbiol 30: 275-284. doi:10.1046/j.1365-2958.1998.01060.x. PubMed: 9791173. [DOI] [PubMed] [Google Scholar]
- 9. Lu A, Cho K, Black WP, Duan XY, Lux R et al. (2005) Exopolysaccharide biosynthesis genes required for social motility in Myxococcus xanthus . Mol Microbiol 55: 206-220. PubMed: 15612929. [DOI] [PubMed] [Google Scholar]
- 10. Sun H, Zusman DR, Shi W (2000) Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr Biol 10: 1143-1146. doi:10.1016/S0960-9822(00)00705-3. PubMed: 10996798. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Sun H, Ma X, Lu A, Lux R et al. (2003) Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus . Proc Natl Acad Sci U S A 100: 5443-5448. doi:10.1073/pnas.0836639100. PubMed: 12704238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Lux R, Pelling AE, Gimzewski JK, Shi W (2005) Analysis of type IV pilus and its associated motility in Myxococcus xanthus using an antibody reactive with native pilin and pili. Microbiology 151: 353-360. doi:10.1099/mic.0.27614-0. PubMed: 15699186. [DOI] [PubMed] [Google Scholar]
- 13. Clausen M, Koomey M, Maier B (2009) Dynamics of type IV pili is controlled by switching between multiple states. Biophys J 96: 1169-1177. doi:10.1016/j.bpj.2008.10.017. PubMed: 19186152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu W, Hossain M, Lux R, Wang J, Yang Z et al. (2011) Exopolysaccharide-independent social motility of Myxococcus xanthus . PLOS ONE 6: e16102. doi:10.1371/journal.pone.0016102. PubMed: 21245931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakovljevic V, Leonardy S, Hoppert M, Søgaard-Andersen L (2008) PilB and PilT are ATPases acting antagonistically in type IV pilus function in Myxococcus xanthus . J Bacteriol 190: 2411-2421. doi:10.1128/JB.01793-07. PubMed: 18223089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Black WP, Xu Q, Yang Z (2006) Type IV pili function upstream of the Dif chemotaxis pathway in Myxococcus xanthus EPS regulation. Mol Microbiol 61: 447-456. doi:10.1111/j.1365-2958.2006.05230.x. PubMed: 16856943. [DOI] [PubMed] [Google Scholar]
- 17. Yang Z, Lux R, Hu W, Hu C, Shi W (2010) PilA localization affects extracellular polysaccharide production and fruiting body formation in Myxococcus xanthus . Mol Microbiol 76: 1500-1513. doi:10.1111/j.1365-2958.2010.07180.x. PubMed: 20444090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Hu W, Lux R, He X, Li Y et al. (2011) Natural transformation of Myxococcus xanthus . J Bacteriol 193: 2122-2132. doi:10.1128/JB.00041-11. PubMed: 21378184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stamm I, Lottspeich F, Plaga W (2005) The pyruvate kinase of Stigmatella aurantiaca is an indole binding protein and essential for development. Mol Microbiol 56: 1386-1395. doi:10.1111/j.1365-2958.2005.04640.x. PubMed: 15882428. [DOI] [PubMed] [Google Scholar]
- 20. Huntley S, Hamann N, Wegener-Feldbrügge S, Treuner-Lange A, Kube M et al. (2011) Comparative genomic analysis of fruiting body formation in Myxococcales. Mol Biol Evol 28: 1083-1097. doi:10.1093/molbev/msq292. PubMed: 21037205. [DOI] [PubMed] [Google Scholar]
- 21. Womack BJ, Gilmore DF, White D (1989) Calcium requirement for gliding motility in myxobacteria. J Bacteriol 171: 6093-6096. PubMed: 2509428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang BY, White D (1992) Cell surface modifications induced by calcium ion in the myxobacterium Stigmatella aurantiaca . J Bacteriol 174: 5780-5787. PubMed: 1522058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimkets LJ (1986) Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus . J Bacteriol 166: 837-841. PubMed: 2940231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia R, Pistorius D, Stadler M, Müller R (2011) Fatty acid-related phylogeny of myxobacteria as an approach to discover polyunsaturated omega-3/6 fatty acids. J Bacteriol 193: 1930-1942. doi:10.1128/JB.01091-10. PubMed: 21317327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parge HE, Forest KT, Hickey MJ, Christensen DA, Getzoff ED et al. (1995) Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature 378: 32-38. doi:10.1038/378032a0. PubMed: 7477282. [DOI] [PubMed] [Google Scholar]
- 26. Craig L, Taylor RK, Pique ME, Adair BD, Arvai AS et al. (2003) Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol Cell 11: 1139-1150. doi:10.1016/S1097-2765(03)00170-9. PubMed: 12769840. [DOI] [PubMed] [Google Scholar]
- 27. Hazes B, Sastry PA, Hayakawa K, Read RJ, Irvin RT (2000) Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J Mol Biol 299: 1005-1017. doi:10.1006/jmbi.2000.3801. PubMed: 10843854. [DOI] [PubMed] [Google Scholar]
- 28. Keizer DW, Slupsky CM, Kalisiak M, Campbell AP, Crump MP et al. (2001) Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J Biol Chem 276: 24186-24193. doi:10.1074/jbc.M100659200. PubMed: 11294863. [DOI] [PubMed] [Google Scholar]
- 29. Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M et al. (2006) Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell 23: 651-662. doi:10.1016/j.molcel.2006.07.004. PubMed: 16949362. [DOI] [PubMed] [Google Scholar]
- 30. Craig L, Pique ME, Tainer JA (2004) Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol 2: 363-378. doi:10.1038/nrmicro885. PubMed: 15100690. [DOI] [PubMed] [Google Scholar]
- 31. Hu W, Yang Z, Lux R, Zhao M, Wang J et al. (2012) Direct visualization of the interaction between pilin and exopolysaccharides of Myxococcus xanthus with eGFP-fused PilA protein. FEMS Microbiol Lett 326: 23-30. doi:10.1111/j.1574-6968.2011.02430.x. PubMed: 22092602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao JY, Zhong L, Shen MJ, Xia ZJ, Cheng QX et al. (2008) Discovery of the autonomously replicating plasmid pMF1 from Myxococcus fulvus and development of a gene cloning system in Myxococcus xanthus . Appl Environ Microbiol 74: 1980-1987. doi:10.1128/AEM.02143-07. PubMed: 18245244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang R, Bartle S, Otto R, Stassinopoulos A, Rogers M et al. (2004) AglZ is a filament-forming coiled-coil protein required for adventurous gliding motility of Myxococcus xanthus . J Bacteriol 186: 6168-6178. doi:10.1128/JB.186.18.6168-6178.2004. PubMed: 15342587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonner PJ, Black WP, Yang Z, Shimkets LJ (2006) FibA and PilA act cooperatively during fruiting body formation of Myxococcus xanthus . Mol Microbiol 61: 1283-1293. doi:10.1111/j.1365-2958.2006.05298.x. PubMed: 16925559. [DOI] [PubMed] [Google Scholar]
- 35. Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS et al. (2006) Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci U S A 103: 15200-15205. doi:10.1073/pnas.0607335103. PubMed: 17015832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneiker S, Perlova O, Kaiser O, Gerth K, Alici A et al. (2007) Complete genome sequence of the myxobacterium Sorangium cellulosum . Nat Biotechnol 25: 1281-1289. doi:10.1038/nbt1354. PubMed: 17965706. [DOI] [PubMed] [Google Scholar]
- 37. Goldman B, Bhat S, Shimkets LJ (2007) Genome evolution and the emergence of fruiting body development in Myxococcus xanthus . PLOS ONE 2: e1329. doi:10.1371/journal.pone.0001329. PubMed: 18159227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inouye S, Franceschini T, Inouye M (1983) Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc Natl Acad Sci U S A 80: 6829-6833. doi:10.1073/pnas.80.22.6829. PubMed: 6316328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inouye S, Ike Y, Inouye M (1983) Tandem repeat of the genes for protein S, a development-specific protein of Myxococcus xanthus . J Biol Chem 258: 38-40. PubMed: 6294106. [PubMed] [Google Scholar]
- 40. Li J, Wang Y, Zhang CY, Zhang WY, Jiang DM et al. (2010) Myxococcus xanthus viability depends on groEL supplied by either of two genes, but the paralogs have different functions during heat shock, predation, and development. J Bacteriol 192: 1875-1881. doi:10.1128/JB.01458-09. PubMed: 20139189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu SS, Kaiser D (1997) Regulation of expression of the pilA gene in Myxococcus xanthus . J Bacteriol 179: 7748-7758. PubMed: 9401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pelicic V (2008) Type IV pili: e pluribus unum? Mol Microbiol 68: 827-837. doi:10.1111/j.1365-2958.2008.06197.x. PubMed: 18399938. [DOI] [PubMed] [Google Scholar]
- 43. Smith DR, Dworkin M (1994) Territorial interactions between two Myxococcus species. J Bacteriol 176: 1201-1205. PubMed: 8106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Z, Hu W, Chen K, Wang J, Lux R et al. (2011) Alanine 32 in PilA is important for PilA stability and type IV pili function in Myxococcus xanthus . Microbiology 157: 1920-1928. doi:10.1099/mic.0.049684-0. PubMed: 21493683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hazes B, Frost L (2008) Towards a systems biology approach to study type II/IV secretion systems. Biochim Biophys Acta 1778: 1839-1850. doi:10.1016/j.bbamem.2008.03.011. PubMed: 18406342. [DOI] [PubMed] [Google Scholar]
- 46. Bretscher AP, Kaiser D (1978) Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol 133: 763-768. PubMed: 415048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reichenbach H, Dworkin M (1992) The myxobacteria. In: Balows A, Trüper H, Dworkin M, Harder W, Schleifer K. The prokaryotes. New York: Springer-Verlag; pp. 3416-3487. [Google Scholar]
- 48. Hagen DC, Bretscher AP, Kaiser D (1978) Synergism between morphogenetic mutants of Myxococcus xanthus . Dev Biol 64: 284-296. doi:10.1016/0012-1606(78)90079-9. PubMed: 98366. [DOI] [PubMed] [Google Scholar]
- 49. Shi W, Zusman DR (1993) The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc Natl Acad Sci U S A 90: 3378-3382. doi:10.1073/pnas.90.8.3378. PubMed: 8475084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Green MR, Sambrook J (2012) Molecular cloning : a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 51. Bates PA, Kelley LA, MacCallum RM, Sternberg MJ (2001) Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins Suppl 5: 39-46 PubMed: 11835480. [DOI] [PubMed] [Google Scholar]
- 52. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ et al. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497-3500. doi:10.1093/nar/gkg500. PubMed: 12824352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5: 150-163. doi:10.1093/bib/5.2.150. PubMed: 15260895. [DOI] [PubMed] [Google Scholar]
- 54. Pan HW, Liu H, Liu T, Li CY, Li ZF et al. (2009) Seawater-regulated genes for two-component systems and outer membrane proteins in Myxococcus. J Bacteriol 191: 2102-2111. doi:10.1128/JB.01556-08. PubMed: 19151139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kashefi K, Hartzell PL (1995) Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF- defect. Mol Microbiol 15: 483-494. doi:10.1111/j.1365-2958.1995.tb02262.x. PubMed: 7783619. [DOI] [PubMed] [Google Scholar]
- 56. Harlow E (1988) Antibodies: a Laboratory Manual. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 57. Arnold JW, Shimkets LJ (1988) Inhibition of cell-cell interactions in Myxococcus xanthus by congo red. J Bacteriol 170: 5765-5770. PubMed: 3142856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Black WP, Yang Z (2004) Myxococcus xanthus chemotaxis homologs DifD and DifG negatively regulate fibril polysaccharide production. J Bacteriol 186: 1001-1008. doi:10.1128/JB.186.4.1001-1008.2004. PubMed: 14761994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cordelieres F (2005) Manual Tracking, a plug-in for ImageJ software. Orsay, France: Institut Curie. [Google Scholar]
- 60. Gorski L, Gronewold T, Kaiser D (2000) A sigma(54) activator protein necessary for spore differentiation within the fruiting body of Myxococcus xanthus . J Bacteriol 182: 2438-2444. doi:10.1128/JB.182.9.2438-2444.2000. PubMed: 10762243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166: 557-580. doi:10.1016/S0022-2836(83)80284-8. PubMed: 6345791. [DOI] [PubMed] [Google Scholar]